Abstract
Background
Restorative proctocolectomy (RPC) is the criterion standard surgical treatment for ulcerative colitis (UC). Restorative proctocolectomy is indicated for UC that is refractory to medical treatment, for emergency conditions, and in case of neoplastic transformation. The procedure substantially reduces the risk of UC-associated dysplasia/neoplasia. However, after RPC surgery, even with mucosectomy, cancers of the pouch and/or the anal-transitional zone (ATZ) have been reported with increasing frequency since the first report in 1984. This review highlights pouch-related dysplastic and neoplastic transformation, prevalence and adverse events, risk factors and surveillance following surgery for UC.
Methods
Reports in the literature about patients undergoing pouch surgery from different institutions reported through May 2010 were reviewed to identify patients who developed these complications, and an attempt was made to develop a rational follow-up policy based on the data available.
Results
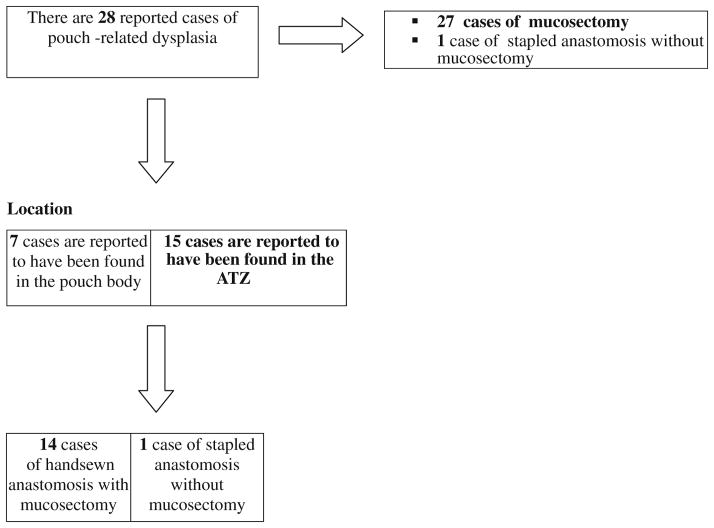
To date, there are 43 reported cancers of the pouch or inlet after RPC for UC: 16 from retrospective series, 1 from a prospective study, and 26 in case reports. Thirty patients underwent mucosectomy and 13 had stapled anastomoses. To date, the number of 28 patients has been diagnosed with dysplasia after RPC for UC. Mucosectomy was performed in 27 of them and in 1 a stapled anastomosis was constructed without mucosectomy. In all cases reviewed, the time interval from the onset of UC to dysplasia/neoplasia was over 10 years.
Conclusion
Neoplastic lesions occurring in UC patients after RPC have been shown to be absolutely inevitable. Even mucosectomy does not completely eliminate the risk. There is little evidence to support routine biopsy of the ileal mucosa or the anal-transition zone except in patients with histological type C changes, sclerosing cholangitis, and unremitting pouchitis in the ileal mucosa. Such patients should be selected for endoscopic surveillance to detect dysplasia preceding pouch adenocarcinoma.
Keywords: Ulcerative colitis, Restorative proctocolectomy, Mucosectomy/stapled anastomosis, Dysplasia/adenocarcinoma etiology
Background
One-third of patients with ulcerative colitis (UC) will eventually require surgery [1, 2] because of either disease refractory to medical treatment, dysplasia or cancer (found during screening colonoscopy) or, in children or adolescents, growth retardation. In any of the aforementioned circumstances, three surgical options are recommended: conventional total proctocolectomy with permanent ileostomy (TPC), restorative proctocolectomy (RPC) with ileal pouch anal anastomosis (IPAA), and total abdominal colectomy with ileorectal anastomosis (IRA) [3]. IIleorectal anastomosis is only suitable for the few patients whose rectum is relatively free of inflammation and who do not have dysplasia or established cancer in the rectum [4]. Conventional proctocolectomy leaves the patient with a permanent ileostomy [5]. Restorative proctocolectomy is now the standard criterion surgical procedure for treating UC [6]. The indications for each procedure and the surgical options usually chosen are colectomy with IRA or RPC [7]. The success of RPC is largely dependent on careful patient selection, and accurate diagnosis with meticulous surgical technique is of the utmost importance [3]. Excision of the entire colon and rectum with mucosectomy of the residual anorectal stump is intended to achieve complete removal of all disease-prone mucosa while maintaining transanal fecal continence [3, 7]. The procedure, however, may inadvertently leave small islands of residual mucosa [3, 7]. It is therefore indisputable that ileo-anal pouch mucosa and the anorectal mucosa below the ileo-anal anastomosis at the anal-transitional zone (AZT) are at risk of developing cancer [8–10]. This review highlights the experience in the literature about the incidence of ileal pouch-related dysplasia and/or cancer following RPC for UC.
Methods
A systematic literature search was conducted to identify retrospective studies and reports of similar cases, and comparative studies reporting post-operative early and late ileal pouch adenomas and adenocarcinoma adverse events were reviewed. Patients undergoing RPC surgery for UC from 1975 through June 2009 were prospectively enrolled. Institutional Review Board approval was obtained by each of the participating research institutions. The US National Library of Medicine database (MEDLINE), the Excerpta Medica database (EMBASE), the Cochran Library, and Google® search engine were searched for published articles on “ulcerative colitis,” “colectomy,” “restorative proctocolectomy,” “ileoanal anastomosis,” “ileal pouches,” “villous adenomas,” “adenocarcinoma,” “dysplasia,” “metaplasia,” “pouch dysplasia,” “pelvic pouch,” and “pouch neoplasia.” The initial search covered the period from January 1975 through December 2007. A second search was performed for the period from January 2008 through May 2010 to update the initial search. The search excluded articles not in English and non-human studies, as well as editorials. Additional articles were identified by cross-referencing papers retrieved in the initial search. Papers were included on the basis of most recent available evidence for each specific point of interest. Agreement about classification was assessed with the k value during the title review and abstract review. If the k value was ≥0.6, the titles were reviewed, divided into 2 sets; each was reviewed by only 1 of the 2 researchers. If the k value was <0.6, reviewers discussed discrepancies followed by other assessments of agreement. A similar process for abstract review was conducted, with an increased k value of 0.7 required for acceptance. The same authors involved in the original title, abstract, and article review process conducted hand searches of bibliographies from accepted articles and review articles. These hand searches resulted in the retrieval of a limited number of additional articles for review.
Results
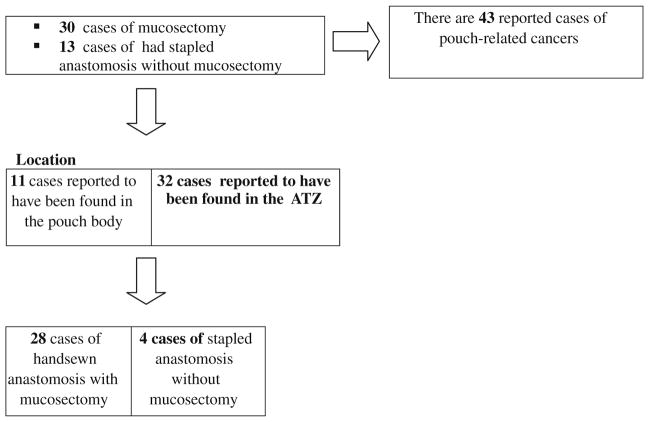
The summary of all reported pelvic pouch-related cancer and dysplasia following RPC for UC is shown in Figs. 1 and 2, Tables 1 and 2. Dr. Alan Park performed the first RPC in 1978. Since then, pouch-related cancers have been reported during follow-ups. To date, there are 43 reported cases of adenocarcinoma of the pouch, or outflow tract, following RPC for UC (Fig. 1) [11–34]. Out of the 43 known patients with pouch-related cancer, 11 had cancer originating from the pouch body and 32 had cancer originating from the AZT [11–33]. Malignancy following RPC appeared after mucosectomy in 30 patients and in 13 patients following stapled anastomosis (Fig. 1).
Fig. 1.
The number of reported cases of pouch-related neoplasia, the nature of the anastomosis (whether hand-sewn or stapled with or without mucosectomy), and the location of the cancer. This table underscores the fact that mucosectomy does not always prevent the development of pouch-related cancer of the Anal transition zone (ATZ)
Fig. 2.
Pouch-related dysplasia, the nature of the anastomosis (whether hand-sewn or stapled with or without mucosectomy), and the location of the cancer. This table underlines the fact that that mucosectomy does not always prevent the development of pouch-related dysplasia in the ATZ
Table 1.
Reported ileal pouch neoplasia following restorative proctocolectomy for ulcerative colitis
| Author, published [reference] | Nature of study | Age at diagnosis of UC | Interval: diagnosis of UC to cancer (years) | Surgical procedure | Interval: surgery to cancer (years) | Age at diagnosis of cancer (years) | Number of Patients | Location | Histology |
|---|---|---|---|---|---|---|---|---|---|
| Ravitch [12] | Case report | Not reported | Not reported | RPC | Not reported | Not reported | 1 | Rectal segment | T4N0M0 |
| Stern [13] | Case report | 21 | 35 | RPC stapled | 4 | 56 | 1 | Rectal cuff | T2N2M0 |
| Puthu [11] | Case report | 34 | 11 | RPC stapled | 6 | 45 | 1 | Pouch | T4N0M1 |
| Sanjuan [14] | Case report | 30 | 22 | RPC stapled | 1 | 52 | 1 | Pouch | T1N0M0 |
| Sequens [15] | Case report | 45 | 9 | RPC stapled | 1,3 | 54 | 1 | ATZ | T3N0M0 |
| Vieth [16] | Case report | 18 | 16 | RPC MUC | 2 | 34 | 1 | Pouch | T2N0M0 |
| Iwama [17] | Case report | 27,5 | 21 | RPC MUC | 1,5 | 50 | 1 | IAA | T4N2M1 |
| Rotholtz [19] | Case report | 59 | 6 | RPC stapled | 7 | 65 | 1 | ATZ | T3N0M0 |
| Heuschen [18] | Case report | 11,8 | 23 | RPC MUC | 3,2 | 38 | 1 | Pouch | T3N0M0 |
| Hyman [20] | Case report | 23 | 18 | RPC stapled | 5 | 41 | 1 | Rectal stump | T2N2M0 |
| Laureti [21] | Case report | 26 | 28 | RPC MUC | 2 | 48 | 1 | IAA | T3N1M0 |
| Baratsis [22] | Case report | 23 | 26 | RPC stapled | 2 | 49 | 1 | ATZ | T4N0M0 |
| Hassan [27] | Case report | 38 | 10 | RPC MUC | 1,8 | 48 | 1 | Pouch | Adenocarcinoma |
| Bell [24] | Case report | 24 | 27 | RPC stapled | 12 | 51 | 1 | IAA | T3N1M0 |
| Negi [23] | Case report | Not reported | Not reported | RPC MUC | 5 | 28 | 1 | Rectal stump | T4N0M0 |
| Bentrem [26] | Case report | 19 | 30 | RPC MUC | 14 | 63 | 1 | Pouch | T3N0M0 |
| Lee [25] | Case report | Not reported | 18 | RPC MUC | 15-ott | Not reported | 3 | IAA | T4N2M0 |
| Knupper [28] | Case report | 12 | 20 | RPC stapled | 2 | 34 | 1 | Pouch | T4N2M0 |
| Walker [30] | Case report | 37 | 15 | RPC MUC | 5 | 52 | 1 | Pouch | Adenocarcinoma |
| Das [5] | Case report | Not reported | 27 | RPC MUC | 25 | Not reported | 1 | Rectal stump | Adenocarcinoma |
| Schaus [47] | Perspective study | Not reported | Not reported | RPC stapled | Not reported | Not reported | 1 | Pouch | Malignant |
| Chia [32] | Case report | Not reported | 11 | RPC stapled | Not reported | Not reported | 1 | ATZ | T4N0M0 |
| Koh [46] | Case report | Not reported | 54 | RPC stapled | 14 | Not reported | 1 | Pouch inlet | Adenocarcinoma |
| Branco [54] | Case report | 8 | 29 | RPC MUC | 6 | 37 | 1 | Pouch | Adenocarcinoma |
| Pedersen [33] | Case report | 45 | 11 | RPC MUC | 9 | 56 | 1 | ATZ | Adenocarcinoma |
| Zmora [34] | Prospective study | 18 | 28 | Proctectomy & IPAA | 10 | 46 | 1 | Rectal cuff | Cancer cells |
| IRA Stapled | 28 | ||||||||
| Kariv [38] | Routine surveillance | 34.4 ± 14.4 | 23.3 ± 12.5 | IPAA MUC | 9.7 ± 6.4 | 57.6 ± 13.6 | 15 | 11 in the pouch or AZT | Adenocarcinoma |
Table 2.
Reported ileal pouch-related dysplasia following restorative proctocolectomy for ulcerative colitis (UC)
| Author, published [reference] | Nature of study | Age at diagnosis of UC (years) | Interval: diagnosis of UC to Dysplasia (years) | Surgical procedure | Interval: surgery to Dysplasia (years) | Age at diagnosis of Dysplasia (years) | Number of patients | Location | Histology |
|---|---|---|---|---|---|---|---|---|---|
| Herline [53] | Routine surveillance | Not reported | 10 (mean, 12.7 ± 2 | RPC MUC | 8.4 ± 4.6 | Not reported | 1 | Pouch | Dysplasia |
| Hassan [27] | Case report | 28 | 10 | RPC MUC | 2 | 40 | 1 | Pouch | Dysplasia |
| Walker [30] | Case report | 26 | 20 | RPC MUC | 12 | 52 | 1 | Pouch mucosa | High-grade dysplasia |
| Schaus [47] | Prospective study | Not reported | Not reported | RPC stapled | Not reported | 1 | Pouch | Dysplastic | |
| Nilubol et al. [75] | Prospective study | Not reported | Median 5.4 | RPC MUC | Median 36.1 | Median 41.6 | 1 | Pouch | Dysplasia |
| Kariv [38] | Routine surveillance | 34.4 ± 14.4 | 23.3 ± 12.5 | IPAA MUC | 9.7 ± 6.4 | 57.6 ± 13.6 | 23 | 1 in the pouch 1 in pouch and ATZ 15 in ATZ 6 non-specific |
Dysplasia |
UC ulcerative colitis, RPC restorative proctocolectomy, IPAA ileal pouch anal anastomosis, ATZ anal-transitional zone, MUC mucosectomy
Branco et al. observed that in patients with dysplasia or cancer at the original resection and/or those who presented at an older age, there was a shorter interval to the development of post-UC-RPC cancer than in other patients [35]. The median time until dysplasia/cancer was 3 years (mean 4.3, range 1.3–14) in the 9 patients who originally had cancer versus 6.5 years (mean 7.7, range 2–18) in the 12 patients who originally had low- or high-grade dysplasia and 6.5 (mean 8.6, range 2–19) in the 9 patients who had no neoplasia. Among the 21 patients who underwent surgery on account of cancer or dysplasia, 23% (n = 5) of the original lesions had occurred above the rectum [35]. Most studies have shown that the incidence of dysplasia or cancer after RPC is strongly associated with the duration of UC disease prior to colectomy and/or the presence of dysplasia or cancer in the proctocolectomy specimen [36, 37]. In all reported cases (Fig. 1), the interval from diagnosis of UC until RPC was over 10 years. Other factors observed to be associated with dysplasia and cancer of the pouch included long-term chronic inflammation (pouchitis, proctitis, and cuffitis) [38], histological type C changes [39, 40], and the presence of sclerosing cholangitis [41].
Ileal pouch cancer risk after RPC for UC
Conventional hand-sewn anastomosis after mucosectomy and double-stapled anastomosis without mucosectomy are the most common techniques used in fashioning intestinal anastomoses [3, 42]. Controversy persists over the techniques used in ileo-anal anastomosis (IAA). The majority of surgeons [42] feel that with the double-stapled anastomosis, there are fewer anastomotic complications and better rectal continence is achieved. However, a cuff of rectal mucosa is retained [43] (Fig. 3a), which is the main argument used by the opponents of the double stapling technique [15, 19]. It seems clear though, despite controversies, that mucosectomy (Fig. 3b) does not always rule out the subsequent development of pouch-related cancer [28, 30]. With the hand-sewn anastomosis, little to no rectal mucosa is left behind [44]. Microscopic islands of retained rectal mucosa, however, have been reported in 20% of patients [44]. This technique has largely been abandoned in patients not at high risk and replaced by the double-stapled technique, which is said to offer better functional results [3, 45]. Reports have shown that the remaining rectal tissue [41, 46], the ileal pouch itself [43, 47], and the pouch outlet after mucosectomy are the sites where cancer is most likely to develop. One should bear in mind that a hand-sewn IPAA is more difficult to survey than a stapled one and that there is often stenosis and leakage that can cause excoriation and tenderness of the perianal skin. In a recent case report [48], an anal-canal mucinous adenocarcinoma with lymph node metastasis was diagnosed 7 years after ileal pouch excision.
Fig. 3.
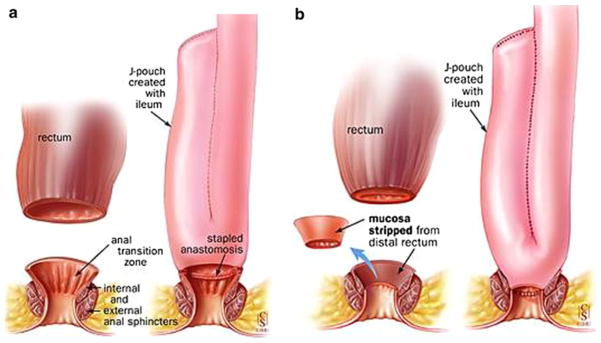
The first restorative proctocolectomy, also known as the pouch operation, was performed at St Mark’s, by Sir Alan Parks in 1978. This is a modified pouch known as “J-shaped pouch” invented by Utsunomyia. a Shows a J-shaped ileal pouch and anal anastomosis without stripping and b a J-shaped ileal pouch and anal anastomosis with stripping (mucosectomy). Adapted from M’Koma et al. [3]
Dysplasia and neoplasia of the pouch mucosa
The incidence of dysplasia in pouch mucosa has been reported [49]. Studies from Karolinska University [40, 50] followed 94 patients prospectively over a median period of 6.3 years (range 3–14 years). Eight patients developed low-grade dysplasia. All had a type C mucosal pattern with chronic pouchitis and deoxyribonucleic acid (DNA) aneuploidy, which raised the possibility of future malignant transformation. Barrett [51] studied 30 UC-RPC patients with regular endoscopic review and multiple biopsies taken from the afferent limb, mid-pouch anteriorly, mid-pouch posteriorly, and the anastomotic area. Biopsies from 4 patients demonstrated inflammation. All 4 had mild-to-moderate chronic inflammatory changes, and one patient had low-grade dysplasia with a background history of chronic pouchitis. Thompson-Fawcett et al. [49] followed 106 high-risk UC-RPC patients, including 29 with a Kock ileostomy for more than 14 years, 42 UC-RPC for more than 12 years, and 34 patients with chronic pouchitis from a cohort of 1,221 patients. Eleven had a history of dysplasia or cancer in the original proctocolectomy specimen. One of the 106 patients had dysplasia, which was multifocal and low-grade. Deoxyribonucleic acid analysis by flow cytometry demonstrated aneuploidy in this patient and in other two patients [49]. A collaborative study from Sweden and United Kingdom [8] reported long-term mucosal adaptation patterns and the incidence of dysplasia in 40 patients at a mean interval of 30 years following a Kock continent ileostomy for UC. Type A and type B mucosal patterns, based on the criteria described by Lofberg et al. [50] and Setti Carraro et al. [52], were found in 29 patients and a type C pattern was observed in 11 patients. There were 3 cases of dysplasia, which were low grade and found exclusively in the type C group. No patients were found to have high-grade dysplasia or adenocarcinoma. Due to a significant disagreement among the pathologists in reporting low-grade and indefinite categories of dysplasia, the incidence of indefinite and low-grade dysplasia of 27.5 and 7.5% reported by one group of pathologists was reported by the second group to be 7.5 and 5%, respectively. Herline et al. [53] reviewed 222 UC-RPC pouch biopsy specimens from 160 patients for an average follow-up of 8.4 years. Surveillance of over 1,800 pouches identified only one case of focal, low-grade dysplasia. A group from Mount Sinai School of Medicine, New York, NY [54], reports adenocarcinoma arising in an ileal pouch 29 years after UC diagnosis and 6 years after RPC.
In an Italian study, pouchoscopies were performed with biopsies taken from the pouch [55] during follow-up of 55 UC-RPC patients for a median of 14 years (range 10.7–19.8 years). Interestingly, there were no patients with dysplasia in the 440 biopsy samples obtained and all were negative for p53 antigen [55]. Similar observations were made in a study on 45 UC-RPC patients followed up with clinical examination and pouch endoscopy, including mucosal biopsies [56]. The median duration of UC until surgery was 6 years (range 1–28), and the median time interval from diagnosis of UC until follow-up was 24.8 (range 17–46) years. Neither high-grade dysplasia nor invasive carcinoma was diagnosed in these patients. It appears that cancer in the UC-RPC population is relatively rare and related to the duration of disease rather than to the interval from RPC [41].
Dysplasia and neoplasia of the residual anorectal mucosa
The IAA is made between the ileal pouch and either the anal canal or the lower rectum [57]. The level depends on whether a manual anastomosis with mucosectomy or a stapled anastomosis with mucosectomy has been performed [3]. Using the former technique, the level can be controlled directly by the level at which the mucosectomy is made, while this is more difficult when carrying out a stapled anastomosis [58]. Therefore, in some patients, there may be a considerable length of anorectal mucosa below the anastomosis, which is considered at risk of neoplastic transformation [41]. Zmora et al. [34], in their recent paper, reported that one patient was found to have cancer cells in random biopsies from the rectal cuff. Ten years prior, she had undergone proctectomy and IPAA due to T2NO rectal cancer and 28 years prior total abdominal colectomy with ileorectal anastomosis due to a right-sided T2NO colon cancer. Inflamed anorectal mucosa following stapled anastomosis may be symptomatic in up to 25% of patients [59], indicating that the so-called strip proctitis or cuffitis in the residual mucosa may be clinically important. The length of this segment will vary according to the level of the IAA, and it may be referred to as the ATZ. Thompson Fawcett et al. [60] showed considerable variation in the position and extent of the ATZ both from individual to individual and to some extent within the same individual. In almost all patients, the IAA is more proximal leaving a varying length of columnar epithelium. When referring to the epithelium below the ileo-anal anastomosis, “ATZ” is therefore not accurate and should be replaced by the phrase “residual anorectal mucosa.” Using this definition, the risk of dysplasia or cancer occurring below the IAA after both stapled and/or hand-sewn anastomosis reaches 16% [61, 62].
In a study from St. Mark’s Hospital [63] on the incidence of dysplasia in the mucosectomy specimen taken from the anorectal stump during RPC of 118 patients with UC, 12 patients (10%) had dysplasia in the colon specimen and dysplasia in the anorectal mucosa was found in 3 patients. There was a positive correlation between these data and the presence of carcinoma in the surgical specimen and the duration of disease. A group from the Cleveland Clinic Foundation [64–66] reported a significant incidence of dysplasia in the ATZ of UC-RPC patients. Low-grade dysplasia was found in 3.1% of patients and had developed over a median period of 1.3–6.4 years, postoperatively. No association was found, however, between dysplasia and the duration of UC, the use of double-stapled versus single-stapled technique or the distance of the anastomosis from the pectineal line. Dysplasia developed in 7 (3%) of 210 patients. High-grade dysplasia was seen in one patient, and the risk of dysplasia was significantly increased in patients with prior cancer or dysplasia in the colon. In a larger series [66], from the United States, 289 patients were followed by regular examinations and biopsies of the ATZ. Dysplasia was found in 8 (2.76%) patients at a median period of 9 months post-RPC. High-grade dysplasia was seen in two patients, one with a history of chronic pouchitis and the other with preoperative dysplasia in the colon. All 8 of the patients with dysplasia were either followed closely or underwent mucosectomy with pouch advancement and re-anastomosis via an endo-anal approach. No patient developed carcinoma. There was no association between the occurrence of dysplasia and gender, age, preoperative disease duration, or extent of colitis. However, dysplasia was significantly associated with cancer or dysplasia in the colon or rectum in the proctocolectomy specimen. A study conducted at John Radcliffe Hospital, in the United Kingdom [62], assessed the risk of dysplasia and the presence of aneuploidy in the columnar cuff epithelium after stapled IAA in 113 patients with UC. The reported mean follow-up after pouch formation was 2.5 years. Successful columnar cuff biopsies were performed in 93% of patients, and no patient with dysplasia was found. Two biopsy specimens from one patient showed aneuploidy. Another study [67] reviewed 135 UC-RPC patients for a median of 56 months and the median interval from the diagnosis of disease to RPC was 8.8 years. There was no evidence of either dysplasia or carcinoma in the anorectal mucosa up to 12 years after surgery, and the authors suggested that cuff surveillance in the first decade after stapled RPC, in the absence of dysplasia or carcinoma in the original colectomy specimen, may be unnecessary.
Possible associated factors for neoplastic transformation
Five factors associated with neoplastic transformation following RPC for UC have been observed, namely dysplasia or cancer in the operative specimen; interval from the diagnosis of UC; type C mucosal changes; extra intestinal manifestations (EIM); and manual versus stapled anastomotic technique.
Dysplasia or cancer in the operative specimen
Most studies have shown that the incidence of dysplasia following RPC is increased with a preoperative history of dysplasia or carcinoma, or a finding of the same in the original surgical specimen [49]. Twelve of the 17 patients (71%%) who were reported to have developed cancer in the anorectal mucosa or in the ileo-anal pouch had dysplasia or cancer identified before RPC or found subsequently in the surgical specimen. This appears to be the most important predisposing factor for the transformation and/or development of neoplasia following RPC.
Interval from the diagnosis of UC
Studies [68] have shown that the vulnerability of colorectal cancer in patients with long-standing UC increases with time and is related to the anatomical extent of the disease in the surgical specimen [69]. Pouch-related cancers did not occur in any of the patients before 10 years from the diagnosis of UC, and the median interval was 20 years. The interval from RPC (median 5 years; range 1.3–18 years) varied more, and it seems likely that the time of UC diagnosis is the vital interval when considering surveillance.
Type C Mucosal change
A type C mucosal pattern of the pouch mucosa is observed to be associated with chronic pouchitis and also with dysplasia and aneuploidy. While Setti Carraro et al. [52] observed that these patients can be identified within months of RPC by histopathological examination of the biopsy material, other authors [49] did not demonstrate an association between chronic pouchitis and dysplasia and some have not found any dysplasia at all in patients undergoing pouch surveillance [70].
Extraintestinal manifestations
The association between type C changes and extraintestinal manifestations (EIM), including primary sclerosing cholangitis (PSC) and their apparent relationship with dysplasia, suggests that patients with type C changes are at risk of developing pouch-related neoplastic transformation [50].
Manual versus stapled anastomotic technique
To date, there are reports of 43 patients with carcinoma in the anorectal mucosa [19] following RPC for UC. In 13 of these patients, cancer developed after stapled anastomosis without mucosectomy [15, 19, 71] and in 30 after mucosectomy and hand-sewn anastomosis, indicating that malignancy can develop after either form of anastomosis. There has been no prospective study that reported the cumulative risk of carcinoma in the anorectal mucosa based on life table analysis. It is possible that patients who had received a stapled anastomosis [48, 63, 67, 72] have a significant risk in the long term when there is residual rectal mucosa, particularly if dysplasia was present preoperatively.
Surveillance for neoplastic transformation
Recommended guidelines have recently been developed at St. Mark’s Hospital [7] to provide consistent evidence-based care by pouch specialist practitioners. Surveillance flexible pouchoscopy is recommended annually in patients at high risk of neoplastic transformation and every 5 years in others [41]. Delaini et al. [73] suggest that intense follow-up and research-based evidence are important in maintaining RPC as the criterion standard procedure for surgical treatment of UC. Although RPC risk surveillance is costly [41], the good news is that the incidence of carcinoma in the ileal pouch or anorectal mucosa appears to be rare during the first 10 years after RPC. It is not clear whether there is a metaplasia—dysplasia—carcinoma sequence [18, 56] following RPC, or whether there is simply sporadic cancer in the ileal pouch in certain, susceptible individuals. The literature, however, suggests that patients with preoperative neoplastic transformation, type C pouch mucosal changes, PSC, and antecedent dysplasia/carcinoma are at higher risk of developing dysplasia or invasive adenocarcinoma than the normal population undergoing RPC. These features merit concern and both the pouch and the anorectal mucosa should be monitored by endoscopic biopsy [41] to identify the appearance of type C changes. This is particularly important in patients who have had UC for 10 years, in which case, pouchoscopy with multiple biopsies and anorectal mucosal biopsies should be considered. Alternative forms of surveillance have been proposed by Elkowitz et al. [74], including testing for p53 over-expression and aneuploidy of biopsies, in addition to histopathological assessment for dysplasia. A method of surveillance of the anorectal mucosa using high-magnification chromatoscopic pouchoscopy has been described [17]. The procedure gives an accurate assessment of the microanatomy of the anorectal, permitting accurate biopsy targeting. It is possible that these approaches may become part of future surveillance.
Conclusion
Although RPC in UC patients with or without cancer or dysplasia can safely be performed with a reasonably high success rate, these patients may later develop cancer in the ATZ. Even mucosectomy does not rule this out. A preoperative diagnosis of dysplasia or cancer of the colon or rectum is an associated factor for pouch-related dysplasia or adenocarcinoma. There is little evidence to support routine biopsy of the ileal and the anorectal mucosa in UC-RPC patients, except in those with dysplasia or carcinoma in the original specimen. Patients with type C ileal mucosal changes and those with sclerosing cholangitis should be selected for surveillance. Patients with long-standing pouchitis, proctitis, and cuffitis are at risk of developing adenocarcinoma if inflammation is found. Surveillance may involve multiple biopsies of the ileal reservoir and the anorectal mucosa below the ileo-anal anastomosis.
Acknowledgments
This study was supported by 3U54CA091408-09S1 (to MMC-VICC cancer partnership). We acknowledge all the scientists who made contributions to the areas of research that are reviewed here but were not cited owing to space constraints. The authors declare their views have not been influenced by the source of funding.
Footnotes
J. W. Um, A. E. M’Koma contributed to conception and design and participated in the acquisition of data, analysis and interpretation of data, and drafting the manuscript.
Contributor Information
J. W. Um, Department of Surgery, Korea University College of Medicine, Seoul, South Korea
A. E. M’Koma, Email: amkoma@mmc.edu, Department of Biochemistry and Cancer Biology, Meharry Medical College School of Medicine, 1005 Dr. D. B. Todd Jr. Blvd, Nashville, TN 37208-3599, USA. Vanderbilt Ingram-Cancer Center, Vanderbilt School of Medicine, Nashville, TN, USA
References
- 1.Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103:1444–1451. doi: 10.1016/0016-5085(92)91163-x. [DOI] [PubMed] [Google Scholar]
- 2.Bach SP, Mortensen NJ. Ileal pouch surgery for ulcerative colitis. World J Gastroenterol. 2007;13:3288–3300. doi: 10.3748/wjg.v13.i24.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.M’Koma AE, Wise PE, Muldoon RL, Schwartz DA, Washington MK, Herline AJ. Evolution of the restorative proctocolectomy and its effects on gastrointestinal hormones. Int J Colorectal Dis. 2007;22:1143–1163. doi: 10.1007/s00384-007-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bülow S, Büow C, Vasen H, Jarvinen H, Björk J, Christensen IJ. Colectomy and ileorectal anastomosis is still an option for selected patients with familial adenomatous polyposis. Dis Colon Rectum. 2008;51:1318–1323. doi: 10.1007/s10350-008-9307-3. [DOI] [PubMed] [Google Scholar]
- 5.Das P, Smith JJ, Tekkis PP, Heriot AG, Antropoli M, Nicholls RJ. Quality of life after indefinite diversion/pouch excision in ileal pouch failure patients. Colorectal Dis. 2007;9:718–724. doi: 10.1111/j.1463-1318.2007.01216.x. [DOI] [PubMed] [Google Scholar]
- 6.Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978;2:85–88. doi: 10.1136/bmj.2.6130.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin SD, Clark SK, Tekkis PP, Ciclitira PJ, Nicholls RJ. Review article: restorative proctocolectomy, indications, management of complications and follow-up–a guide for gastroenterologists. Aliment Pharmacol Ther. 2008;27:895–909. doi: 10.1111/j.1365-2036.2008.03643.x. [DOI] [PubMed] [Google Scholar]
- 8.Hultén L, Willén R, Nilsson O, Safarani N, Haboubi N. Mucosal assessment for dysplasia and cancer in the ileal pouch mucosa in patients operated on for ulcerative colitis–a 30-year follow-up study. Dis Colon Rectum. 2002;45:448–452. doi: 10.1007/s10350-004-6218-9. [DOI] [PubMed] [Google Scholar]
- 9.Farouk R, Pemberton JH, Wolff BG, Dozois RR, Browning S, Larson D. Functional outcomes after ileal pouch-anal anastomosis for chronic ulcerative colitis. Ann Surg. 2000;231:919–926. doi: 10.1097/00000658-200006000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paterson CA, Dozois RR. The ileal pouch-anal anastomosis: success and failure. Chirurgie. 1998;123:545–549. doi: 10.1016/s0001-4001(99)80001-4. [DOI] [PubMed] [Google Scholar]
- 11.Puthu D, Rajan N, Rao R, Rao L, Venugopal P. Carcinoma of the rectal pouch following restorative proctocolectomy. Report of a case. Dis Colon Rectum. 1992;35:257–260. doi: 10.1007/BF02051019. [DOI] [PubMed] [Google Scholar]
- 12.Ravitch MM. The reception of new operations. Ann Surg. 1984;200:231–246. doi: 10.1097/00000658-198409000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern H, Walfisch S, Mullen B, McLeod R, Cohen Z. Cancer in an ileoanal reservoir: a new late complication? Gut. 1990;31:473–475. doi: 10.1136/gut.31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Sanjuan JC, Polavieja MG, Naranjo A, Castillo J. Adenocarcinoma in an ileal pouch for ulcerative colitis. Dis Colon Rectum. 1995;38:779–780. doi: 10.1007/BF02048042. [DOI] [PubMed] [Google Scholar]
- 15.Sequens R. Cancer in the anal canal (transitional zone) after restorative proctocolectomy with stapled ileal pouch-anal anastomosis. Int J Colorectal Dis. 1997;12:254–255. doi: 10.1007/s003840050100. [DOI] [PubMed] [Google Scholar]
- 16.Vieth M, Grunewald M, Niemeyer C, Stolte M. Adenocarcinoma in an ileal pouch after prior proctocolectomy for carcinoma in a patient with ulcerative pancolitis. Virchows Arch. 1998;433:281–284. doi: 10.1007/s004280050248. [DOI] [PubMed] [Google Scholar]
- 17.Iwama T, Kamikawa J, Higuchi T, et al. Development of invasive adenocarcinoma in a long-standing diverted ileal J-pouch for ulcerative colitis: report of a case. Dis Colon Rectum. 2000;43:101–104. doi: 10.1007/BF02237251. [DOI] [PubMed] [Google Scholar]
- 18.Heuschen UA, Heuschen G, Autschbach F, Allemeyer EH, Herfarth C. Adenocarcinoma in the ileal pouch: late risk of cancer after restorative proctocolectomy. Int J Colorectal Dis. 2001;16:126–130. doi: 10.1007/s003840000276. [DOI] [PubMed] [Google Scholar]
- 19.Rotholtz NA, Pikarsky AJ, Singh JJ, Wexner SD. Adenocarcinoma arising from along the rectal stump after double-stapled ileorectal J-pouch in a patient with ulcerative colitis: the need to perform a distal anastomosis. Report of a case. Dis Colon Rectum. 2001;44:1214–1217. doi: 10.1007/BF02234647. [DOI] [PubMed] [Google Scholar]
- 20.Hyman N. Rectal cancer as a complication of stapled IPAA. Inflamm Bowel Dis. 2002;8:43–45. doi: 10.1097/00054725-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Laureti S, Ugolini F, D’Errico A, Rago S, Poggioli G. Adenocarcinoma below ileoanal anastomosis for ulcerative colitis: report of a case and review of the literature. Dis Colon Rectum. 2002;45:418–421. doi: 10.1007/s10350-004-6194-0. [DOI] [PubMed] [Google Scholar]
- 22.Baratsis S, Hadjidimitriou F, Christodoulou M, Lariou K. Adenocarcinoma in the anal canal after ileal pouch-anal anastomosis for ulcerative colitis using a double stapling technique: report of a case. Dis Colon Rectum. 2002;45:687–691. doi: 10.1007/s10350-004-6268-z. [DOI] [PubMed] [Google Scholar]
- 23.Negi SS, Chaudhary A, Gondal R. Carcinoma of pelvic pouch following restorative proctocolectomy: report of a case and review of the literature. Dig Surg. 2003;20:63–65. doi: 10.1159/000068855. [DOI] [PubMed] [Google Scholar]
- 24.Bell SW, Parry B, Neill M. Adenocarcinoma in the anal transitional zone after ileal pouch for ulcerative colitis: report of a case. Dis Colon Rectum. 2003;46:1134–1137. doi: 10.1007/s10350-004-7293-7. [DOI] [PubMed] [Google Scholar]
- 25.Lee SW, Sonoda T, Milsom JW. Three cases of adenocarcinoma following restorative proctocolectomy with hand-sewn anastomosis for ulcerative colitis: a review of reported cases in the literature. Colorectal Dis. 2005;7:591–597. doi: 10.1111/j.1463-1318.2005.00794.x. [DOI] [PubMed] [Google Scholar]
- 26.Bentrem DJ, Wang KL, Stryker SJ. Adenocarcinoma in an ileal pouch occurring 14 years after restorative proctocolectomy: report of a case. Dis Colon Rectum. 2003;46:544–546. doi: 10.1007/s10350-004-6597-y. [DOI] [PubMed] [Google Scholar]
- 27.Hassan C, Zullo A, Speziale G, Stella F, Lorenzetti R, Morini S. Adenocarcinoma of the ileoanal pouch anastomosis: an emerging complication? Int J Colorectal Dis. 2003;18:276–278. doi: 10.1007/s00384-002-0452-1. [DOI] [PubMed] [Google Scholar]
- 28.Knupper N, Straub E, Terpe HJ, Vestweber KH. Adenocarcinoma of the ileoanal pouch for ulcerative colitis–a complication of severe chronic atrophic pouchitis? Int J Colorectal Dis. 2006;21:478–482. doi: 10.1007/s00384-005-0063-8. [DOI] [PubMed] [Google Scholar]
- 29.Ota H, Yamazaki K, Endoh W, et al. Adenocarcinoma arising below an ileoanal anastomosis after restorative proctocolectomy for ulcerative colitis: report of a case. Surg Today. 2007;37:596–599. doi: 10.1007/s00595-006-3452-x. [DOI] [PubMed] [Google Scholar]
- 30.Walker M, Radley S. Adenocarcinoma in an ileoanal pouch formed for ulcerative colitis in a patient with primary sclerosing cholangitis and a liver transplant: report of a case and review of the literature. Dis Colon Rectum. 2006;49:909–912. doi: 10.1007/s10350-006-0517-2. [DOI] [PubMed] [Google Scholar]
- 31.Sagar P. Adenocarcinoma in a pouch without a preceeding history of dysplasia. Colorectal Dis. 2006;8:526–527. doi: 10.1111/j.1463-1318.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 32.Chia CS, Chew MH, Chau YP, Eu KW, Ho KS. Adenocarcinoma of the anal transitional zone after double stapled ileal pouch-anal anastomosis for ulcerative colitis. Colorectal Dis. 2008;10:621–623. doi: 10.1111/j.1463-1318.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen ME, Rahr HB, Fenger C, Qvist N. Adenocarcinoma arising from the rectal stump eleven years after excision of an ileal J-pouch in a patient with ulcerative colitis: report of a case. Dis Colon Rectum. 2008;51:1146–1148. doi: 10.1007/s10350-008-9238-z. [DOI] [PubMed] [Google Scholar]
- 34.Zmora O, Spector D, Dotan I, Klausner JM, Rabau M, Tulchinsky H. Is stapled ileal pouch anal anastomosis a safe option in ulcerative colitis patients with dysplasia or cancer? Int J Colorectal Dis. 2009;24:1181–1186. doi: 10.1007/s00384-009-0744-9. [DOI] [PubMed] [Google Scholar]
- 35.Branco BC, Sachar DB, Heimann T, Sarpel U, Harpaz N, Greenstein AJ. Adenocarcinoma complicating restorative proctocolectomy for ulcerative colitis with mucosectomy performed by Cavitron ultrasonic surgical aspirator. Colorectal Dis. 2009;11:428–429. doi: 10.1111/j.1463-1318.2008.01651.x. [DOI] [PubMed] [Google Scholar]
- 36.Ziv Y, Fazio VW, Strong SA, Oakley JR, Milsom JW, Lavery IC. Ulcerative colitis and coexisting colorectal cancer: recurrence rate after restorative proctocolectomy. Ann Surg Oncol. 1994;1:512–515. doi: 10.1007/BF02303617. [DOI] [PubMed] [Google Scholar]
- 37.Remzi FH, Fazio VW, Delaney CP, et al. Dysplasia of the anal transitional zone after ileal pouch-anal anastomosis: results of prospective evaluation after a minimum of ten years. Dis Colon Rectum. 2003;46:6–13. doi: 10.1007/s10350-004-6488-2. [DOI] [PubMed] [Google Scholar]
- 38.Kariv R, Remzi FH, Lian L, et al. Preoperative colorectal neoplasia increases risk for pouch neoplasia in patients with restorative proctocolectomy. Gastroenterology. 2010;139:806–812. doi: 10.1053/j.gastro.2010.05.085. [DOI] [PubMed] [Google Scholar]
- 39.Gullberg K, Stählberg D, Liljeqvist L, et al. Neoplastic transformation of the pelvic pouch mucosa in patients with ulcerative colitis. Gastroenterology. 1997;112:1487–1492. doi: 10.1016/s0016-5085(97)70029-5. [DOI] [PubMed] [Google Scholar]
- 40.Veress B, Reinholt FP, Lindquist K, Lofberg R, Liljeqvist L. Long-term histomorphological surveillance of the pelvic ileal pouch: dysplasia develops in a subgroup of patients. Gastroenterology. 1995;109:1090–1097. doi: 10.1016/0016-5085(95)90566-9. [DOI] [PubMed] [Google Scholar]
- 41.Das P, Johnson MW, Tekkis PP, Nicholls RJ. Risk of dysplasia and adenocarcinoma following restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 2007;9:15–27. doi: 10.1111/j.1463-1318.2006.01148.x. [DOI] [PubMed] [Google Scholar]
- 42.Kayaalp C, Nessar G, Akoglu M, Atalay F. Elimination of mucosectomy during restorative proctocolectomy in patients with ulcerative colitis may provide better results in low-volume centers. Am J Surg. 2003;185:268–272. doi: 10.1016/s0002-9610(02)01376-4. [DOI] [PubMed] [Google Scholar]
- 43.Heppell J, Weiland LH, Perrault J, Pemberton JH, Telander RL, Beart RW., Jr Fate of the rectal mucosa after rectal mucosectomy and ileoanal anastomosis. Dis Colon Rectum. 1983;26:768–771. doi: 10.1007/BF02554744. [DOI] [PubMed] [Google Scholar]
- 44.O’Connell PR, Pemberton JH, Weiland LH, et al. Does rectal mucosa regenerate after ileoanal anastomosis? Dis Colon Rectum. 1987;30:1–5. doi: 10.1007/BF02556908. [DOI] [PubMed] [Google Scholar]
- 45.Heald RJ, Allen DR. Stapled ileo-anal anastomosis: a technique to avoid mucosal proctectomy in the ileal pouch operation. Br J Surg. 1986;73:571–572. doi: 10.1002/bjs.1800730719. [DOI] [PubMed] [Google Scholar]
- 46.Koh PK, Doumit J, Downs-Kelly E, et al. Ileo-anal j-pouch cancer: an unusual case in an unusual location. Tech Coloproctol. 2008;12:341–345. doi: 10.1007/s10151-008-0420-z. [DOI] [PubMed] [Google Scholar]
- 47.Schaus BJ, Fazio VW, Remzi FH, Bennett AE, Lashner BA, Shen B. Clinical features of ileal pouch polyps in patients with underlying ulcerative colitis. Dis Colon Rectum. 2007;50:832–838. doi: 10.1007/s10350-006-0871-0. [DOI] [PubMed] [Google Scholar]
- 48.Gullberg K, Liljeqvist L. Stapled ileoanal pouches without loop ileostomy: a prospective study in 86 patients. Int J Colorectal Dis. 2001;16:221–227. doi: 10.1007/s003840100289. [DOI] [PubMed] [Google Scholar]
- 49.Thompson-Fawcett MW, Marcus V, Redston M, Cohen Z, McLeod RS. Risk of dysplasia in long-term ileal pouches and pouches with chronic pouchitis. Gastroenterology. 2001;121:275–281. doi: 10.1053/gast.2001.26442. [DOI] [PubMed] [Google Scholar]
- 50.Löfberg R, Liljeqvist L, Lindquist K, Veress B, Reinholt FP, Tribukait B. Dysplasia and DNA aneuploidy in a pelvic pouch. Report of a case. Dis Colon Rectum. 1991;34:280–283. doi: 10.1007/BF02090171. discussion 283–284. [DOI] [PubMed] [Google Scholar]
- 51.Barrett M, Schoetz D, Semple J, et al. Long-term risk of neoplastic changes in ileoanal pouches in patients with ulcerative colitis. Dis Colon Rectum. 1998;41:A26. [Google Scholar]
- 52.Setti Carraro P, Talbot IC, Nicholls RJ. Longterm appraisal of the histological appearances of the ileal reservoir mucosa after restorative proctocolectomy for ulcerative colitis. Gut. 1994;35:1721–1727. doi: 10.1136/gut.35.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herline AJ, Meisinger LL, Rusin LC, et al. Is routine pouch surveillance for dysplasia indicated for ileoanal pouches? Dis Colon Rectum. 2003;46:156–159. doi: 10.1007/s10350-004-6517-1. [DOI] [PubMed] [Google Scholar]
- 54.Branco BC, Sachar DB, Heimann T, Sarpel U, Harpaz N, Greenstein AJ. Adenocarcinoma complicating restorative proctocolectomy for ulcerative colitis with mucosectomy performed by Cavitron Ultrasonic Surgical Aspirator(R) Colorectal Dis. 2009;11:428–429. doi: 10.1111/j.1463-1318.2008.01651.x. [DOI] [PubMed] [Google Scholar]
- 55.Tarroni D, Wilkinson KH, Saunders B, Talbot I, Nicholls RJ. Long-term histological assessment of the ileal reservoir following restorative proctocolectomy for ulcerative colitis and dysplasia. Dis Colon Rectum. 2002;45:A7. [Google Scholar]
- 56.Börjesson L, Willén R, Haboubi N, Duff SE, Hultén L. The risk of dysplasia and cancer in the ileal pouch mucosa after restorative proctocolectomy for ulcerative proctocolitis is low: a long-term term follow-up study. Colorectal Dis. 2004;6:494–498. doi: 10.1111/j.1463-1318.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- 57.Geiger JD, Teitelbaum DH, Hirschl RB, Coran AG. A new operative technique for restorative proctocolectomy: the endorectal pull-through combined with a double-stapled ileo-anal anastomosis. Surgery. 2003;134:492–495. doi: 10.1067/s0039-6060(03)00087-4. [DOI] [PubMed] [Google Scholar]
- 58.Seow-Choen F, Ho YH, Goh HS. The ileo-anal reservoir: results from an evolving use of stapling devices. J R Coll Surg Edinb. 1994;39:13–16. [PubMed] [Google Scholar]
- 59.Bernstein CN. Natural history and management of flat and polypoid dysplasia in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:573–579. doi: 10.1016/j.gtc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 60.Thompson-Fawcett MW, Warren BF, Mortensen NJ. A new look at the anal transitional zone with reference to restorative proctocolectomy and the columnar cuff. Br J Surg. 1998;85:1517–1521. doi: 10.1046/j.1365-2168.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 61.Schmitt SL, Wexner SD, Lucas FV, James K, Nogueras JJ, Jagelman DG. Retained mucosa after double-stapled ileal reservoir and ileoanal anastomosis. Dis Colon Rectum. 1992;35:1051–1056. doi: 10.1007/BF02252995. [DOI] [PubMed] [Google Scholar]
- 62.Thompson-Fawcett MW, Rust NA, Warren BF, Mortensen NJ. Aneuploidy and columnar cuff surveillance after stapled ileal pouch-anal anastomosis in ulcerative colitis. Dis Colon Rectum. 2000;43:408–413. doi: 10.1007/BF02258310. [DOI] [PubMed] [Google Scholar]
- 63.Tsunoda A, Talbot IC, Nicholls RJ. Incidence of dysplasia in the anorectal mucosa in patients having restorative proctocolectomy. Br J Surg. 1990;77:506–508. doi: 10.1002/bjs.1800770510. [DOI] [PubMed] [Google Scholar]
- 64.Ziv Y, Fazio VW, Sirimarco MT, Lavery IC, Goldblum JR, Petras RE. Incidence, risk factors, and treatment of dysplasia in the anal transitional zone after ileal pouch-anal anastomosis. Dis Colon Rectum. 1994;37:1281–1285. doi: 10.1007/BF02257797. [DOI] [PubMed] [Google Scholar]
- 65.Brown CJ, Maclean AR, Cohen Z, Macrae HM, O’Connor BI, McLeod RS. Crohn’s disease and indeterminate colitis and the ileal pouch-anal anastomosis: outcomes and patterns of failure. Dis Colon Rectum. 2005;48:1542–1549. doi: 10.1007/s10350-005-0059-z. [DOI] [PubMed] [Google Scholar]
- 66.Shen B, Remzi FH, Lavery IC, Lashner BA, Fazio VW. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clin Gastroenterol Hepatol. 2008;6:145–158. doi: 10.1016/j.cgh.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 67.Coull DB, Lee FD, Henderson AP, Anderson JH, McKee RF, Finlay IG. Risk of dysplasia in the columnar cuff after stapled restorative proctocolectomy. Br J Surg. 2003;90:72–75. doi: 10.1002/bjs.4007. [DOI] [PubMed] [Google Scholar]
- 68.Shelton AA, Lehman RE, Schrock TR, Welton ML. Retrospective review of colorectal cancer in ulcerative colitis at a tertiary center. Arch Surg. 1996;131:806–810. doi: 10.1001/archsurg.1996.01430200016003. [DOI] [PubMed] [Google Scholar]
- 69.Stählberg D, Veress B, Tribukait B, Broomé U. Atrophy and neoplastic transformation of the ileal pouch mucosa in patients with ulcerative colitis and primary sclerosing cholangitis: a case control study. Dis Colon Rectum. 2003;46:770–778. doi: 10.1007/s10350-004-6655-5. [DOI] [PubMed] [Google Scholar]
- 70.Sarigol S, Wyllie R, Gramlich T, et al. Incidence of dysplasia in pelvic pouches in pediatric patients after ileal pouch-anal anastomosis for ulcerative colitis. J Pediatr Gastroenterol Nutr. 1999;28:429–434. doi: 10.1097/00005176-199904000-00015. [DOI] [PubMed] [Google Scholar]
- 71.Sierra-Montenegro E, Fernández-Rivero JM, Villanueva-Sáenz E, Peňa-Ruiz Esparza JP, Martinez-Hernández Magro P, Solo-Quirino R. Quality of life after restorative proctocolectomy with ileo-anal J pouch in patients with ulcerative colitis. Cir Cir. 2007;75:449–452. [PubMed] [Google Scholar]
- 72.Memon AA, Marks CG. Stapled anastomoses in colorectal surgery: a prospective study. Eur J Surg. 1996;162:805–810. [PubMed] [Google Scholar]
- 73.Delaini GG, Scaglia M, Colucci G, Hultén L. The ileoanal pouch procedure in the long-term perspective: a critical review. Tech Coloproctol. 2005;9:187–192. doi: 10.1007/s10151-005-0225-2. [DOI] [PubMed] [Google Scholar]
- 74.Elkowitz D, Daum F, Markowitz J, et al. Risk factors for carcinoma of the pelvic ileal pouch/anal canal in ulcerative colitis. Ann Clin Lab Sci. 2004;34:143–149. [PubMed] [Google Scholar]
- 75.Nilubol N, Scherl E, Bub DS, Gorfine SR, Marion J, Harris MT, Kornbluth A, Lichtiger S, Rubin P, George J, Chapman M, Harpaz N, Present D, Bauer JJ. Mucosal dysplasia in ileal pelvic pouches after restorative proctocolectomy. Dis Colon Rectum. 2007;50:825–831. doi: 10.1007/s10350-007-0217-6. [DOI] [PubMed] [Google Scholar]