Abstract
Study Design
Histological features of the intervertebral disc (IVD)-endplate interface were analyzed.
Objective
To define cartilaginous and bony vertebral endplate in commonly used laboratory animals and compare with that of the humans.
Summary of Background Data
Endplates are crucial for the IVD nutrient supply: the IVDs have limited blood supply; most nutrients diffuse through endplates to nourish the discs. Various animal models of IVD and endplate degeneration have been used to study the etiology and treatments of spinal disorders. However, because humans are biped, the spine mechanics differ significantly from other mammals. Translation of animal research findings requires a characterization and comparison of the vertebral endplate in the respective species. In this study, we compared the endplate structure of laboratory animal species at the age range commonly used for modeling spine degeneration with that of an adult human.
Methods
Mouse, rat, rabbit, goat, and human IVDs and the adjacent vertebral bodies were isolated from the lower lumbar spine. Tissues were stained with Alcian Blue, counterstained with hematoxylin and eosin.
Results
Structure of the vertebral endplate varied significantly between the adult animal species and that of the humans. Growth plates persisted in all adult animals studied, whereas the growth plate is absent in the adult humans. In the mice and rats, the cartilaginous endplates are in continuation with the growth plates, with only a small bony center. Rabbits and goats have a bony layer between cartilaginous endplate and the growth plate. The human endplate consist of a cartilaginous layer and the bony endplate.
Conclusion
Significant differences exist in histological features of the endplate across animal species and that of the humans. Consideration should be given when animal models are used to study IVD degeneration and surgical treatments.
Keywords: endplate, intervertebral disc, mammals, human
Pathology of the intervertebral disc (IVD) and vertebral endplate play important roles in IVD degeneration,1 and ultimately, the development of back pain. However, data obtained from animal models should be interpreted in context of the endplate structures because the endplate is an important structure in nutrition transport for the IVD.
A clear definition of endplate is lacking, although the end-plates serve important physiological functions, such as dispersing the compressive load experienced by the vertebral body (VB)2 and providing nutrition to the disc via diffusion.3–5 Numerous animal models of disc degeneration exist, including mouse, rat, sand rat, rabbit, goat, dogs, and apes.6 Although animal models serve a useful purpose, there are several differences among species that make extrapolation of results from animals to humans difficult. Differences in morphology, biomechanical properties, and biochemical processes of the disc/ endplate complex must be considered.2,7,8
Studies have demonstrated similarities in disc biomechanics and biochemical makeup across species, after geometry and size are normalized.9,10 However, during our previous studies using animal models, significant histological variation among species in both the cartilaginous and osseous endplates have been observed. Furthermore, there is no consensus on the definition and structure of the vertebral endplate across multiple species. Our goal was to perform a histological comparison of the IVD-endplate junction among multiple animal species and human. The information will be valuable to researchers and clinicians seeking to apply the findings in animal models to understand human spinal disorders.
MATERIALS AND METHODS
Animal and Human Tissue Acquisition
Mice (FVB/NJ strain; University of Illinois at Chicago transgenic produce service, Chicago, IL; n = 3) aged 7 to 9 months; females were donated by another investigator according to Rush Institutional Animal Care and Use Committee (IACUC) guidelines. Lumbar (L) spine was isolated and L6–L7 level was photographed.
Rats (Sprague-Dawley breed, both males and females; Harlan Laboratories, Indianapolis, IN; n = 3) aged 8 to 12 months were donated by another investigator according to Rush IACUC guidelines. L6–L7 level was photographed.
Rabbits (New Zealand White; Myrtle’s Rabbitry, Nashville, TN; n = 3) aged 4 months were isolated for another project (IACUC #08-039). L6–L7 level was photographed.
Male Nubian goats (Thomas Morris Inc., Reisterstown, MD; n = 6) approximately 4 years of age were used. The Nubian goats are sexually mature at an age as early as 6 months. The use of these animals was approved by the IACUC of Thomas D. Morris, Inc. (Reisterstown, MD; #06-052). This was used for another project.11,12 L5–L6 level was photographed.
Human L4–L5 IVD with adjacent bony endplates were obtained from a 48-year-old female and a 67-year-old male cadaveric organ donor. Tissues were procured within 24 hours from time of death from the Gift of Hope Organ Donor Network, according to Rush University Institutional Review Board (approval no. 00482) guidelines. L4–L5 discs with approximately 1 cm of adjacent vertebral bodies were obtained with a saw. The L4–L5 level was photographed.
Tissue Processing
The IVDs and a portion of the adjacent bony VB were isolated. The disc with its surrounding endplates was fixed with 10% buffered formalin for 24 to 72 hours, depending on the size of the tissue. The endplate-disc-endplate segments were decalcified with decalcification solution consisting of 10% citric acid with 22% formic acid, with a daily change of solution for 24 to 72 hours until the bony portion was completely decalcified. The tissues were then dehydrated and embedded in paraffin and sectioned to 5-μM thickness, and stained with Alcian Blue with hematoxylin and eosin counter stain. Specifically, the tissue sections were deparaffinized for 30 minutes, stained with 1% Alcian Blue Solution (Poly Scientific R&D Corp., Bay Shore, NY) for 5 minutes, followed with hematoxylin for 5 minutes and eosin for 20 seconds.
Histological Evaluation
All samples were examined under a light microscope (Nikon, Japan) and photographed by an experienced orthopedic surgeon. Representative sections from each species were chosen for presentation. The tissues were further examined by an experienced histologist and a clinician scientist.
RESULTS
Mouse Endplate
The mouse endplate (Figure 1) consists primarily of a cartilaginous endplate (CEP) containing 3 to 7 layers of chondrocytes, stained light blue with Alcian Blue. Most of the CEP is separated from the VB by a layer of growth plate (stained dark blue). A tidemark-like structure is invariably present between the growth cartilage and the bony VB (black arrow). Tidemarks are also present between the CEP and growth cartilage, most notably at posterior aspect of the spine where there are multiple layers of tidemarks (green arrows in Figure 1B). The CEP seems to be continuous with those of the annulus fibrosus (AF), with some of the annulus fibers extending into the CEP.
Figure 1.
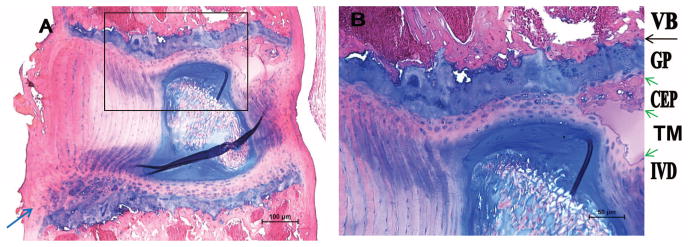
The mouse endplate consists of cartilaginous endplate and the adjacent growth plate. A, Low magnification. B, High magnification. IVD indicates intervertebral disc; TM, tidemark; CEP, cartilaginous endplate; GP, growth plate; VB, vertebral body. Green arrows denote TMs; black arrow, tidemark-like structures; blue arrow, a small secondary ossification center.
The mouse only have a CEP, there is no bony endplate. There is only a very small secondary ossification center in the ventral-caudal aspect of the spine (blue arrow in Figure 1A; lower end in Figure 1A is the caudal aspect of the spine). This likely represents the zone of Ranvier, which contributes to growth of the vertebral column in width.
Rat Endplate
Similar to the mouse endplate, the rat endplate (Figure 2) contains a CEP between the IVD and the growth plate. Compared with the mouse CEP, the rat endplate is thicker, consisting of 10 to 15 layers of cells. The CEP adjoins the growth plate, with a tidemark (green arrow in Figure 2C) in between. The growth plate in turn connects with VB, with a tidemark-like structure connecting the growth plate and VB (black arrow in Figure 2C). Similar to the mouse, the CEP is continuous with the AF, with some of the anterior annulus fibers inserting into the CEP.
Figure 2.
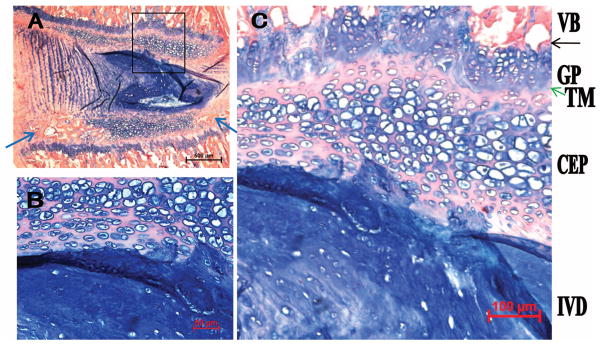
The rat endplate consists of CEP, secondary ossification centers (blue arrows), and the GP. A, Low magnification. B and C, High magnification. Blue arrows identify the small secondary ossification centers; green arrow, TM; black arrow, the tidemark-like structure. TM indicates tidemark; CEP, cartilaginous endplate; GP, growth plate; VB, vertebral body; IVD, intervertebral disc.
Compared with that of mouse, a more pronounced secondary ossification center is seen in the ventral-caudal aspect of the spine. In addition, a smaller one is present at the dorsal-caudal aspect (blue arrows in Figure 2A; bottom of Figure 2A is the caudal aspect of the spine).
Rabbit Endplate
The rabbit CEP (Figure 3) features a thin chondrocyte layer containing only 1 to 3 cell layers. Unlike the mouse or rat endplate, the rabbit endplate contains a robust bony end-plate with multiple vascular channels (thick blue arrows in Figure 3C). This bony layer may be analogous to the secondary ossification centers seen in the mouse and rat because the rabbit growth cartilage persists into adulthood. This bony endplate layer abuts the growth plate, which in turn transit to the VB. There is a tidemark between the bony endplate and the growth plate (green arrow). The CEP is in continuity with the AF.
Figure 3.
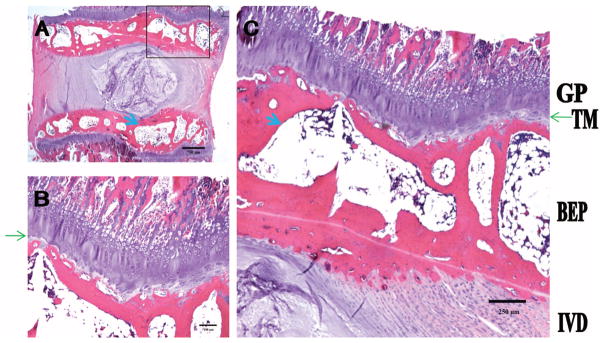
The rabbit endplate consists of a thin layer of cartilaginous endplate, pronounced secondary ossification center (equivalent to the BEP), and GP. A, Low magnification. B and C, High magnification. Thin green arrows denote TM; thick blue arrow, vascular channel. TM indicates tidemark; GP, growth plate; BEP, bony endplate.
Goat Endplate
The goat endplate (Figure 4) architecture is strikingly similar to that of the rabbit endplate. The CEP contains 1 to 3 layers of chondrocytes, which are stained blue. There are 1 to 2 tidemark-like structures bridging the IVD to the bony end-plate (black arrow). In addition, there are sporadic tidemark-like structures between the growth plate and the VB. Similar to that of the rabbit, a significant bony endplate layer exists (analogous to secondary ossification center) with multiple vascular channels (thick blue arrows in panel C). The bony endplates lie between the CEP and the growth plate (green arrow in panel A).
Figure 4.
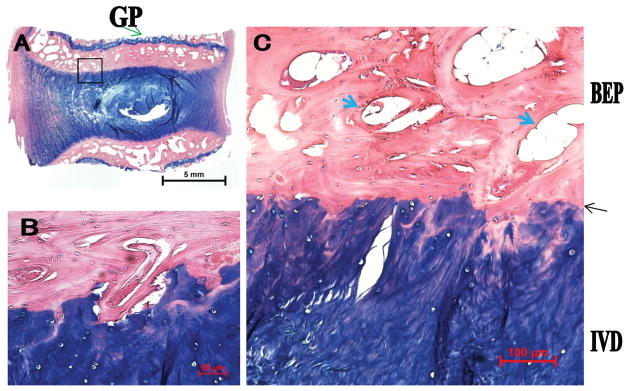
The goat endplate consists of a thin layer of cartilaginous endplate, pronounced secondary ossification center (equivalent to the BEP), and GP. A, Low magnification. B and C, High magnification. Thin black arrow denotes tidemark-like structure; thick blue arrow, vascular channel; green arrow, GP. GP indicates growth plate; BEP, bony endplate; IVD, intervertebral disc.
Human Endplate
The adult human endplate is unique from the other species in several ways (Figure 5). There is a thick CEP with only few chondrocytes. This layer is stained pink with areas of light blue, and seems to contain calcified fibrocartilage based on gross morphology, although future immunostaining for type I and type II collagen is needed to confirm this. Second, there is a bony endplate that serves as the transition from the cartilaginous layer and the VB. The bony endplate only contain relatively few vascular channels (thick blue arrows in Figure 4B, C). There are 1 to 3 layers of tidemark-like structures identified between the calcified cartilage and bony endplate (black arrows).
Figure 5.
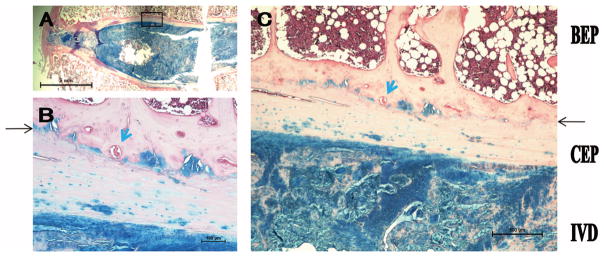
The human endplate is composed largely of a calcified CEP and a laminated BEP layer. A, Low magnification. B and C, High magnification. Thin black arrow denotes tidemark-like structure; blue arrow, vascular channel. CEP indicates cartilaginous endplate; BEP, bony endplate; IVD, intervertebral disc.
In summary, we have shown in this study that the small animal (mouse and rat) endplates primarily consist of thick CEPs, with only small secondary ossification centers. In the medium-sized animals (rabbit and goat), there are thinner CEPs, full-length bony endplates (secondary ossification centers), and persisting growth plates. In the humans, the CEP is thick and most likely consists of calcified fibrocartilage. Growth plate is fused in adult humans, whereas growth plate persists in all adult animal species studied.
DISCUSSION
In this study, several morphological and histological features in commonly used laboratory animals were distinct from those of the human. First, the endplate is thought to contain both a cartilaginous and an osseous component.13 However, we have shown that in both the mouse and rat, the endplate consisted primarily of a CEP adjacent to the growth plate, with only small secondary ossification centers. Although the rabbit and goat endplates have both CEPs and bony endplates, they contain growth plates adjacent to the bony endplates. The adult human endplate contains a thick CEP and a bony endplate, but the growth plate has been replaced by trabecular bone.
All the animals studied here (mouse, rat, rabbit, and goat) have a full thickness growth plate that lies adjacent to the vertebral bone. It is unclear if the growth plate will ever fuse in these animals as the growth plate in human adults. For example, the Nubian goat studied here is already 48 months in age, and considered to have reached adulthood (the Nubian goats have a normal life span of 8 to 12 years and are sexually mature at an age as early as 6 mo). Mouse has a growth plate up to the age of 1 year (data not shown). Sexually mature animals still have growth plates, suggesting that the animal may still have growth capacity.
Not surprisingly, histological features of the human are distinct from those of rodents and goat, but bears striking similarities to those of the cynomolgus monkey. Both the human and monkey endplates contain a cartilaginous zone near the IVD, and an intermediate zone with calcified cartilage.14 Interestingly, there is a thin layer of growth cartilage in the 3- to 4-year-old cynomolgus monkey (these monkeys reach sexual maturity at about 5 yr of age and have a life span of 30 yr in captivity). It is not clear whether this difference is due to the fact that humans stand upright for a more prolonged period, whereas monkeys spend significant time as quadrupeds. In the future, it would be informative to examine human infant and adolescent specimens to further understand the evolution of secondary ossification centers and growth plates in the human. Our study clearly shows that the human IVDs seem relatively mature compared with the animals with regard to the persistence of a growth plate in the quadrupeds. However, the monkey endplate structures bear striking similarities to that of the humans.14 These findings suggest that novel biological treatments developed on the basis of testing in quadruped animals ideally should be tested in monkeys or other primates before clinical trials.
The contour of the endplate is uneven. A biomechanical study on human cadaveric spine found that the peripheral endplate is stronger than the central endplate.15 Although decreased bone mineral density of the adjacent vertebrae and disc degeneration correlates with weaker endplates, the location of weakness is always in the center.16 Bone remodeling may explain, in part, why the peripheral endplate is stronger than the central endplate.17 A magnetic resonance imaging (MRI) study has indeed shown that the endplate of the human lumbar spine is thinner in the center.18 In this study, we have only examined the midsagittal section of the endplates. Future studies using micro-computed tomography and micro-MRI will better delineate the 3-dimensional structure of the endplates.
The human endplate undergoes several changes in response to aging and disc degeneration. The CEP undergoes calcification, which may manifest itself as endplate sclerosis on plain radiographs. This process may lead to the development of early disc degeneration.19 As the CEP calcifies, nutrient diffusion into the disc may further decrease, accelerating disc degeneration.20 This was elegantly demonstrated in a human serial MRI study that documented the 24-hour temporal pattern of diffusion in human normal and degenerated discs.21 Furthermore, there is evidence suggesting that with increased age, the rate of channel occlusion increases.22 This degenerative process seen with advanced aging and severe disc degeneration is controversial; other studies suggest that the end-plate actually becomes more porous and thinner, contributing to decreased stiffness and strength in the endplates.23 Fields et al24 have observed that the presence of a double layer of bony endplate on computed tomographic scan may improve biomechanical and nutritional function, thus protecting disc from degeneration. Taken together, endplate structure changes may contribute to IVD degeneration. Furthermore, endplate changes, especially Modic type I changes, are also correlated with low back pain.25 Future studies of the endplate with high-resolution MRI in patients with back pain may also shed light on the relationship of structure and symptoms.
CONCLUSION
Although the human lumbar endplate shares similarities in histological characteristics with commonly used laboratory animals, significant differences exist. The human endplate is likely to contain calcified cartilage, whereas the animal endplates likely consist of hyaline cartilage. In addition, the human spine differs from those of the animals in that its growth plate and secondary ossification centers are absent. These findings suggest that no single animal model provides a complete representation of the human endplate. As such, although animal models serve as valuable tools for research on human IVD-endplate pathology, one must be cautious in extrapolating data.
Key Points.
CEPs are present adjacent to the AF in all mammals and humans.
Mouse and rat CEPs and growth plates are largely in continuation, with only a small secondary ossification center in the ventral-caudal aspect.
Distinct secondary ossification centers completely separate the CEP and the growth plate in the rabbit and goat.
Adult humans have lost their growth plates, whereas all other mammalian species have persistent growth plates.
Endplate structure varies among animal species and human. Data in animal models should be interpreted in context with the endplate structures.
Acknowledgments
The authors thank Dr. Qiping Zheng, Dr. Yaojuan Lu, for providing mouse tissues, Dr. Amarjit S. Virdi, for providing rat tissues, and Dr. Thomas Shannon, for providing rabbit tissues. The authors also thank Irving Shapiro, DDS, PhD, Jill P. Urban, PhD, and Harvey Smith, MD, for their valuable insights and discussions.
References
- 1.Moore RJ. The vertebral endplate: disc degeneration, disc regeneration. Eur Spine J. 2006;15(suppl 3):S333–7. doi: 10.1007/s00586-006-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacLean JJ, Owen JP, Iatridis JC. Role of endplates in contributing to compression behaviors of motion segments and intervertebral discs. J Biomech. 2007;40:55–63. doi: 10.1016/j.jbiomech.2005.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urban JP, Holm S, Maroudas A, et al. Nutrition of the intervertebral disc: effect of fluid flow on solute transport. Clin Orthop Relat Res. 1982;170:296–302. [PubMed] [Google Scholar]
- 4.Urban JP, Holm S, Maroudas A, et al. Nutrition of the intervertebral disk. an in vivo study of solute transport. Clin Orthop Relat Res. 1977;129:101–14. [PubMed] [Google Scholar]
- 5.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 2004;29:2700–9. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 6.Lotz JC. Animal models of intervertebral disc degeneration: lessons learned. Spine (Phila Pa 1976) 2004;29:2742–50. doi: 10.1097/01.brs.0000146498.04628.f9. [DOI] [PubMed] [Google Scholar]
- 7.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205:357–62. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine (Phila Pa 1976) 2007;32:328–33. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- 10.Beckstein JC, Sen S, Schaer TP, et al. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine (Phila Pa 1976) 2008;33:E166–73. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Drapeau S, An HS, et al. Histological features of the degenerating intervertebral disc in a goat disc-injury model. Spine (Phila Pa 1976) 2011;36:1519–27. doi: 10.1097/BRS.0b013e3181f60b39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Drapeau S, Howard SA, et al. Transplantation of goat bone marrow stromal cells to the degenerating intervertebral disc in a goat disc injury model. Spine (Phila Pa 1976) 2011;36:372–7. doi: 10.1097/BRS.0b013e3181d10401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twomey LT, Taylor JR. Age changes in lumbar vertebrae and intervertebral discs. Clin Orthop Relat Res. 1987;224:97–104. [PubMed] [Google Scholar]
- 14.Longo UG, Ripalda P, Denaro V, et al. Morphologic comparison of cervical, thoracic, lumbar intervertebral discs of cynomolgus monkey (Macaca fascicularis) Eur Spine J. 2006;15:1845–51. doi: 10.1007/s00586-005-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou Y, Yuan W. Influences of disc degeneration and bone mineral density on the structural properties of lumbar endplates. Spine J. 2012;12:249–56. doi: 10.1016/j.spinee.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Grant JP, Oxland TR, Dvorak MF, et al. The effects of bone density and disc degeneration on the structural property distributions in the lower lumbar vertebral endplates. J Orthop Res. 2002;20:1115–20. doi: 10.1016/S0736-0266(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 17.Frei H, Oxland TR, Rathonyi GC, et al. The effect of nucleotomy on lumbar spine mechanics in compression and shear loading. Spine (Phila Pa 1976) 2001;26:2080–9. doi: 10.1097/00007632-200110010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Moon SM, Yoder JH, Wright AC, et al. Evaluation of intervertebral disc cartilaginous endplate structure using magnetic resonance imaging. Eur Spine J. 2013;22:1820–8. doi: 10.1007/s00586-013-2798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutges JP, van der Jagt OP, Oner FC, et al. Micro-CT quantification of subchondral endplate changes in intervertebral disc degeneration. Osteoarthr Cartilage. 2011;19:89–95. doi: 10.1016/j.joca.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Bernick S, Cailliet R. Vertebral end-plate changes with aging of human vertebrae. Spine (Phila Pa 1976) 1982;7:97–102. doi: 10.1097/00007632-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Rajasekaran S, Babu JN, Arun R, et al. ISSLS Prize winner: A study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine (Phila Pa 1976) 2004;29:2654–67. doi: 10.1097/01.brs.0000148014.15210.64. [DOI] [PubMed] [Google Scholar]
- 22.Benneker LM, Heini PF, Alini M, et al. 2004 Young Investigator Award winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine (Phila Pa 1976) 2005;30:167–73. doi: 10.1097/01.brs.0000150833.93248.09. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez AG, Rodriguez-Soto AE, Burghardt AJ, et al. Morphology of the human vertebral endplate. J Orthop Res. 2012;30:280–7. doi: 10.1002/jor.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fields AJ, Sahli F, Rodriguez AG, et al. Seeing double: a comparison of microstructure, biomechanical function, and adjacent disc health between double- and single-layer vertebral endplates. Spine (Phila Pa 1976) 2012;37:E1310–7. doi: 10.1097/BRS.0b013e318267bcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahme R, Moussa R. The Modic vertebral endplate and marrow changes: pathologic significance and relation to low back pain and segmental instability of the lumbar spine. AJNR Am J Neuroradiol. 2008;29:838–42. doi: 10.3174/ajnr.A0925. [DOI] [PMC free article] [PubMed] [Google Scholar]


