Abstract
Background and Purpose
Motor recovery after ischemic stroke in primary motor cortex is thought to occur in part through training-enhanced reorganization in undamaged premotor areas, enabled by reductions in cortical inhibition. Here we used a mouse model of focal cortical stroke and a double-lesion approach to test the idea that a medial premotor area (medial agranular cortex [AGm]) reorganizes to mediate recovery of prehension, and that this reorganization is associated with a reduction in inhibitory interneuron markers.
Methods
C57Bl/6 mice were trained to perform a skilled prehension task to an asymptotic level of performance after which they underwent photocoagulation-induced stroke in the caudal forelimb area. The mice were then retrained and inhibitory interneuron immunofluorescence was assessed in prechosen, anatomically defined neocortical areas. Mice then underwent a second photocoagulation-induced stroke in AGm.
Results
Focal caudal forelimb area stroke led to a decrement in skilled prehension. Training-associated recovery of prehension was associated with a reduction in parvalbumin, calretinin, and calbindin expression in AGm. Subsequent infarction of AGm led to reinstatement of the original deficit.
Conclusions
We conclude that with training, AGm can reorganize after a focal motor stroke and serve as a new control area for prehension. Reduced inhibition may represent a marker for reorganization or it is necessary for reorganization to occur. Our mouse model, with all of the attendant genetic benefits, may allow us to determine at the cellular and molecular levels how behavioral training and endogenous plasticity interact to mediate recovery.
Keywords: inhibition, parvalbumin, poststroke reorganization, premotor, recovery
With improved acute care, more patients with ischemic stroke survive and up to 60% of them have disability in arm or leg use, and up to one third need placement in a long-term care facility.1 In addition to the personal impact, the economic cost of disability translates to more than $30 billion in annual care.2 Most recovery at both the impairment and functional level occurs in the first 3 months after stroke3–5 but little is known about how this early spontaneous biological recovery is achieved. A similar window of maximal recovery is also seen in rodent models of stroke6–8 and, therefore, these models could provide insight into the mechanisms of spontaneous biological recovery and how such recovery interacts with training protocols. In rodent models, cortical reorganization is associated both with reduction in impairment and adoption of compensatory behavior, both of which can lead to recovery of function.6,7,9–11 For example, cortex adjacent to infarcted cortex (peri-infarct cortex) has been shown to reorganize so as to re-establish function.10–13 Similarly, premotor areas, defined as frontal areas that have direct access to primary motor cortex as well as to the spinal cord,14 have been shown to reorganize in days to weeks after a focal primary motor stroke.15–18 Reorganization is assumed to have occurred in a premotor region if there is a change in its motor output pre- compared with post-stroke measured with intracortical stimulation or if destruction of this area leads to loss of recovered motor function.10,17,19
One mechanism for reorganization is a change in the balance between excitation and inhibition in surviving cortex.20 Although acute ischemia triggers cell death in part from excessive neuronal depolarization, which can be abrogated by GABA-inhibitors,20 tonic GABA-ergic neurotransmission is deleterious to recovery in the chronic phase.21 The inhibitory cortical interneurons that mediate GABA-ergic neurotransmission are a diverse group of cells which can express different calcium-binding proteins including parvalbumin (PV), calretinin (CR), and calbindin (CB), each serving as a marker for the identity22,23 and activity24–27 of an inhibitory interneuron.
We created a rodent model of focal motor stroke to study recovery of skilled prehension. We chose the mouse over the rat for reasons pertinent to both the current experiment and follow-up investigations. These reasons included small size, ease of stroke induction, and the possibility of future studies in widely available transgenic lines. In pilot data we noted a reduction in inhibitory interneuron marker expression in what appeared to be in medial agranular cortex (AGm), a previously described anatomic area in the mouse with characteristics consistent with it being a premotor area.28–30 We tested the hypothesis that reorganization in AGm mediates recovery of prehension after focal motor stroke by creating a second stroke in AGm after recovery from an initial CFA stroke, with the prediction that this would reinstate the motor deficit. We also wished to confirm that AGm consistently shows reductions in PV, CR, and CB.
Materials And Methods
Subjects
Adult male C57bl/6 mice 70 to 120 days old were singly housed in custom-made chambers and kept on a 12/12 hour light/dark cycle. Behavioral tasks were carried out in the same room and same chambers in which the animals were housed to reduce the stress of new surroundings. Two to 3 days before learning the prehension task, animals were placed on a scheduled administration of 2.5 g Bioserv dustless precision pellet mouse chow per day with water ad libitum. Animals were food restricted to 85% of their starting weight. All animal handling and use was performed according to the protocols set by the Johns Hopkins University Animal Care and Use Committee.
Skilled Motor Prehension Task
Training was conducted similarly to that described before31 but in standard mouse cages modified with a sealable, vertical 16 cm × 0.9 cm slit through which the mice would stick their paw. Cages were additionally modified with a standing steel stage measuring 1.5 cm × 11.5 cm × 8 cm directly in front of the slit. Once the mice were familiarized to the pellets and had lost 15% of their body weight, they were trained on the prehension task. On sticky tape on a movable steel bar, 45 mg Bioserv dustless precision pellets were placed and maintained at the same height as the standing steel cage. The pellets were positioned 0.5 cm away from the standing steel stage and aligned with the edge of the cage slit contralateral to the preferred paw. This configuration required the animal to reach with their preferred paw for pellets 1 at a time. Successful grasps occurred when the animal reached its forelimb through the slit, grabbed the pellet, and ate the pellet without knocking the pellet from its resting space; dropping the pellet once picked up, or in any other way losing control of the pellet, or if the pellet was dropped or knocked before the animal consumed it, the attempt was recorded as a miss. If the animal did not touch the pellet, then the reach did not count as an attempt; thus, the percent of successful grasps was based upon the total number pellets provided. Initially, paw preference was determined in a series of preliminary blocks that were not scored as part of the animals’ prehension success. Mice were then trained until they hit a 30% percent success rate. Once this level was achieved, the space between the opening of the cage and the bar loaded with food pellets was gradually increased to a maximum distance of 1 cm to increase the difficulty of the task. A training block consisted of 30 pellets with each pellet presented 1 at a time; the percentage of successful grasps was chosen as the behavioral measure of motor function. After familiarization and paw determination, the animals underwent 2 blocks of 30 attempts per training day. The animals had 1 training day off per week (including 1 day off after stroke induction). During training, mice were also fed at the end of each day with additional food pellets, which were placed in their cage, to maintain their weight at 85% of baseline. Untrained mice were treated just like the trained mice (allowed to run free in their home cage, were food restricted, fed the same pellets, and maintained on the same light/dark cycle) but never had to reach for their pellets.
Stroke Induction
The location of motor areas was identified based on previous anatomic32 and functional29 mapping, which has suggested that these areas are geographically consistent within a given strain. Focal cortical ischemia was induced by photothrombosis of the cortical microvessels with some modification to previously described protocols.33 Each mouse was anesthetized with 4.5 mL/kg of a Ketamine (21 mg/mL) plus Xylazine (3.2 mg/mL) mixture and placed in a stereotaxic frame (Stoelting, Wood Dale, IL). Temperature was monitored and maintained at 36.5 to 37.5°C with the help of a heating pad. At the dorsal aspect of the head, the skull was exposed by a median incision of the skin, the periostium was removed and the bregma point identified. The skull was thinned using a fine dremmel. A fiber optic bundle of a cold light source (Zeiss 1500 electronic, Jena, Germany) with a 20 gauge aperture was centered at 2 mm lateral and 0.5 mm anterior from bregma (caudal forelimb area [CFA]);29 or 0 mm laterally and 0.5 mm anteriorly from bregma (AGm) and placed against the skull. The brains were then illuminated through the intact skull for 15 minutes starting 5 minutes after the intraperitoneal injection of 150 μl of a 10 mg/mL rose Bengal solution in sterile normal saline. The scalp was then sutured and mice were allowed to awaken while still on the heating pad. Animals undergoing sham had an identical procedure performed, except that no illumination occurred.
Tissue Preparation, Immunofluorescence, and Histology
On the day of sacrifice, mice were deeply anesthetized with 2.5% avertin and transcardially perfused with 4% paraformaldehyde in 0.1 mol/L sodium phosphate, pH7.4. The brains were dissected out and placed in 4% paraformaldehyde for 24 hours. Brains were subsequently coronally sliced at 50 μm on a vibrating microtome. Free floating sections for immunofluorescence were washed 3×5 minutes with phosphate buffered saline, blocked for 2 to 4 hours in block solution (10% normal goat serum and 0.04% triton X-100, in tris buffered saline and incubated overnight at 4°C with primary antibody diluted in block solution. Sigma primary antibodies diluted at 1:1000 included anticalbindin, anticalretinin, and antiparvalbumin. Sections were subsequently washed 3×5 minutes in tris buffered saline with 0.04% triton and incubated at room temperature for 3 to 4 hours with secondary antibodies (Alexa goat antirabbit 633 dilution, 1:500). Sections were washed 2×5minutes in tris buffered saline + triton x100, 1×5 min tris buffered saline, and mounted in Invitrogen ProLong Gold reagent. Coronal sections were also obtained for Cresyl violet staining from experimental and control brains.
Counting
In pilot data we noted a reduction in inhibitory interneuron expression in what appeared to be medial premotor cortex. To define a counting area anatomically (and not based on visually perceived differences of marker expression) we identified AGm on coronal sections between 0.6 mm to 1 mm from Bregma using anatomic localizations previously defined.29,32 We utilized the more strict definition proposed by Tennant et al29 which defines a medial-lateral boundary, in which we obtained a 0.375 mm2 area slice extending from the medial and dorsal pial boundaries of a coronal slice. This area represents a subtotal of the AGm boundaries, so that we did not confound our counts with cells from neighboring areas. The entire extent of a 50 μm slice was then imaged at 1.5 μm intervals and reconstructed in 3 dimensions using Imaris (Bitplane) imaging software. This 1.8×107 μm3 volume was taken from each animal both ipsilateral and contralateral to the indicated condition. An investigator blinded to the experimental condition counted the number of cells immunofluorescently labeled with PV, CR, or CB within this volume. A cell was counted as positive if it had any immunofluorescent label for the indicated marker. Statistics were performed using GraphPad Prism 1-way ANOVA with Fisher posttest.
Results
Infarction in Primary Motor Cortex Led to Loss of Prehension, Which Recovered With Training
To study motor recovery after stroke, we followed the experimental paradigm outlined in Figure 1A. We trained wild-type mice to perform a skilled prehension task whereupon they reached a maximum accuracy after ≈7 to 9 training days (Figure 2C). Further training after 9 days did not lead to any further increase in successful attempts (data not shown). Using published criteria (Figure 2A),29,32 we induced an ≈0.5 mm3 focal stroke involving the CFA (the primary motor cortical control for the mouse forelimb) contralateral to the paw used for prehension after the mouse had experienced at least 9 and as many as 11 training days (Figure 2B). This infarction led to a significant decrement in skilled prehension accuracy that recovered after 5 to 6 training days (Figure 2C) after which time mice were euthanized. We saw no decrement in skilled prehension accuracy after sham procedures (Figure 2D) or with isolated focal strokes in AGm (Figure 4B), indicating that the decrement in skilled prehension is related to the area infarcted and not to a nonspecific effect of the procedure.
Figure 1.
Schematic of time course for mouse familiarization, training, and stroke induction. A, Mice underwent skilled prehension training, photocoagulation-induced stroke in caudal forelimb area (CFA), retraining, and subsequent sacrifice on the indicated training days. B, Mice underwent skilled prehension training, photocoagulation-induced stroke in CFA, retraining, subsequent photocoagulation-induced stroke in medial agranular cortex (AGm), retraining, and subsequent sacrifice on the indicated training days.
Figure 2.
Focal motor infarction impairs skilled prehension with subsequent recovery. A, Schematic of mouse brain showing relative locations of caudal forelimb area (CFA), medial agranular cortex (AGm), and primary somatosensory cortex (S1). B, Representative Cresyl violet stained coronal slice showing focal CFA infarction. Scale bar, 500 μm. C and D, prehension success (mean±SEM) for mice undergoing CFA stroke (C; n=5) or sham procedure (D; n=3) on the indicated training days.
Figure 4.
Recovered skilled prehension success is ablated via infarct in medial agranular cortex (AGm). A, Representative Cresyl violet stained coronal slice exemplifying focal AGm cortical infarction. Scale bar, 500 μm. B, Prehension success (mean±SEM) for mice experiencing stroke in AGm (n=4). C and D, Prehension success (mean±SEM) for mice undergoing caudal forelimb area (CFA) stroke followed by ipsilesional (C; n=5) or contralesional (D; n=3) AGm stroke on the indicated training days.
Recovery of Prehension After Stroke Was Associated With Decreased Inhibitory Markers in a Medial PreMotor Area
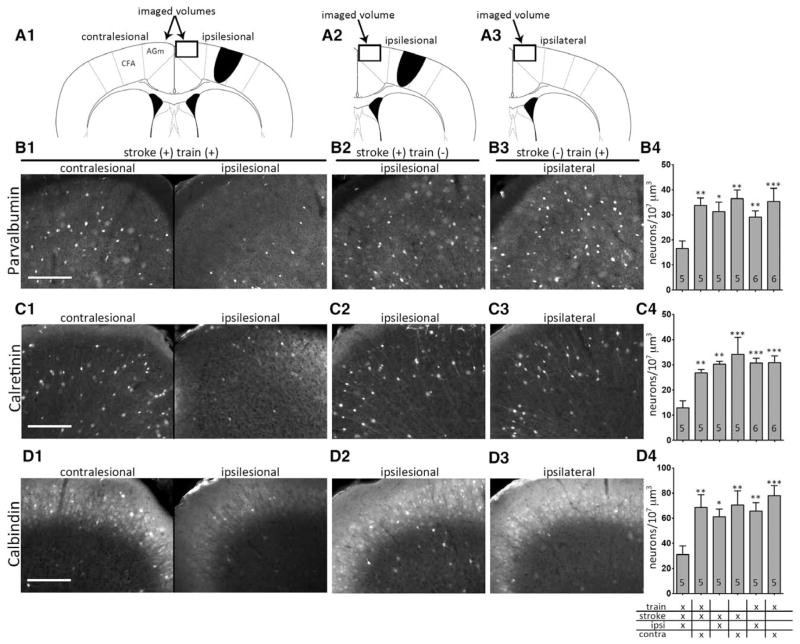
Using immunofluorescence, we examined the interneuron markers PV, CR, and CB in motor cortices after different stroke and training conditions. There was a visually apparent decrease in AGm inhibitory interneuron markers in mice that experienced training, CFA stroke, and retraining to a plateaued level of skilled prehension success (Figure 3B–3D) with sacrifice 6 to 7 training days after infarction. We did not observe similar decreases in these markers in other areas including motor cortex adjacent to the CFA infarct and rostral forelimb area (RFA; data not shown). We assume that decreased PV, CR, and CB expression was directly related to the degree of inhibition in AGm24–27; however, we cannot rule out 2 other alternatives. First, reduced PV, CR, and CB expression may represent a loss of inhibitory neurons rather than decreased inhibitory signaling. Second, it is possible that changes in inhibition can occur in AGm and in other cortical areas in the absence of visually apparent changes in PV, CR, and CB expression.
Figure 3.
Recovery of skilled prehension after stroke is associated with decreased inhibitory markers. A1–A3, Schematics of caudal forelimb area (CFA) stroke and the imaged medial agranular cortex (AGm) volumes (box) of animals undergoing stroke with retraining (A1), stroke without training (A2), and training without stroke (A3). Representative immunofluorescent images labeled with PV (B), CR (C), or CB (D) from the indicated conditions: (1) Training, CFA stroke, and retraining; (2) CFA stroke without retraining; (3) Training without CFA stroke. B4–D4, Number of PV (B4), CR (C4), and CB (D4) positive neurons per 107 μm3 in the AGm. The noted conditions include animals undergoing training, CFA stroke, and subsequent retraining; animals undergoing stroke without retraining; and animals undergoing training without stroke. Ipsi refers to cortex ipsilesional to stroke (in stroked animals) and ipsilateral to cortex responsible for the reaching paw (in nonstroked animals); contra refers to cortex contralesional to stroke (in stroked animals) and contralateral to cortex responsible for the reaching paw (in nonstroked animals). The number in each bar represents the number of animals for each condition. Asterisks denote the level of statistical significant (*P<0.05, **P<0.01, ***P<0.001) for ipsilateral AGm in animals with stroke and retraining compared with all other conditions; no other comparison was statistically significant. Scale bars, 200 μm.
Blinded quantification of cells expressing these markers showed a statistically significant decrease in ipsilesional AGm as compared with contralesional AGm (Figure 3B4–3D4). To assess whether our observed reduction in inhibitory interneuron markers was secondary to the motor infarction itself, a result of motor training, or both, we assessed inhibitory interneuron markers in animals sacrificed 6 to 7 days after stroke without retraining or 6 to 7 days after reaching a plateau level of skilled prehension without stroke. In neither condition did we see a statistically significant change in PV, CR, or CB levels in AGm (Figure 3B4–3D4), suggesting that the combination of training and stroke is responsible for the decline of these markers.
Recovery of Prehension Was Reversed by a Second Infarct in AGm
Because AGm is known to make corticospinal projections29,34 and displayed a focal reduction in inhibitory interneuron markers, we hypothesized that AGm plays a role in the recovery of skilled prehension after focal motor infarction. To test this, we induced 2 temporally and spatially separate strokes in the same animal (Figure 1B). Specifically, we trained mice to perform the skilled prehension task, induced a focal motor stroke in CFA, and observed their return to baseline prehension by 6 days; these mice then underwent a second focal stroke in the AGm (Figure 4A). This second stroke led to a decline in prehension accuracy only if the infarct was ipsilesional to the initial CFA stroke (Figure 4C) and not when it was contralesional (Figure 4D). Critically, focal infarction of AGm in isolation, that is, without a first infarct in CFA, had no discernible effect on skilled prehension (Figure 4B). These data indicate that AGm becomes necessary, if not sufficient, for prehension after CFA stroke but not before.
Discussion
We introduce a mouse model of poststroke reorganization that allows both anatomic and histological identification of reorganized cortical regions that mediate recovery of skilled prehension. Our data suggest that AGm can reorganize after a focal CFA stroke to recover control of prehension. This mouse model, with all of the attendant genetic benefits, offers a convenient and robust model of poststroke recovery. It is important to note that good arguments have been made to support the continued use of rodent models to gain insight into recovery from stroke in humans.35,36
AGm is defined histologically (as opposed to the electrophysiologically-defined CFA and RFA) by its medial location and lack of a readily identifiable neocortical layer 4. Although the lateral borders of AGm have been shown to overlap with CFA and contain vibrissa and head motor representations, AGm elicits fewer movements with cortical microstimulation as compared with CFA.28–30,32 AGm fits the definition of a pre-motor area because AGm makes connections with the spinal cord and with CFA34, 37. That a premotor region can undergo behaviorally relevant reorganization has been demonstrated for RFA in rats.17,38,39 Of particular relevance to our current study, subsequent damage of the reorganized RFA can reinstate the initial postinjury symptoms.17 These rodent premotor data are consistent with primate data showing reorganization in premotor areas after primary motor damage.15,16,18 Thus, our findings are consistent with AGm acting as a premotor area that can reorganize after CFA stroke and affect behavioral outcome similarly to other premotor areas. Interestingly, the mice also recovered after the second stroke in AGm, which suggests that other areas (perhaps RFA) play a role in recovery.
We sought to demonstrate reorganization in AGm with a double-lesion approach and to confirm what was observed in pilot data, namely a consistent reduction in PV, CR, and CB in AGm after stroke in CFA. Our experiments were not designed to reveal synaptic, axonal, or cellular changes. Furthermore, we cannot discount the possibility that reorganization took place in RFA in the absence of visualized changes in PV, CR, and CB. Nevertheless, it is clear that behaviorally relevant reorganization took place within AGm. Although RFA overlaps with the anterior-lateral portions of AGm, the posterior-medial portions of AGm (assayed in the current study) differ from RFA in their contributions to baseline motor control in a way that could explain the fact that both can mediate recovery but diverge with respect to inhibitory marker effects. In healthy rodents, AGm stimulation elicits less movement than RFA stimulation28,29 and is less associated with limb control.28,30 Thus, the degree and mechanism of reorganization may be premotor-region specific and depend on its prestroke contribution to motor control.
We became interested in the AGm when we observed significant reductions in inhibitory interneuron markers in medial motor areas after poststroke training in pilot data. In nonischemic tissue, PV, CR, and CB serve as markers for the identity of 3 largely nonoverlapping subpopulations of inhibitory interneurons.22,23 CB and CR neurons preferentially control dendritic synaptic inputs22,23; PV neurons determine cortical excitability as well as the synchronization of pyramidal cell output activity via perisomatic synapses.23,40–43 PV interneurons in particular have been implicated in a diverse number of functions including both motor output44 and memory formation.45,46 In stroke tissue, others have shown a time-dependent decrease in PV expression in an acutely ischemic core47–49; however, we are unaware of reports describing PV, CR, or CB expression changes in medial or other premotor areas. Finally, there is strong evidence that PV, CR, and CB expression indicates that a cell is an active inhibitory interneuron.24–27 Taken in relation with reports that modulation of cortical excitatory/inhibitory balance is important for neuronal plasticity in healthy cortex50,51 as well as reorganization in poststroke cortex,21 our results suggest that decreased inhibition in AGm is causally related to poststroke recovery. Although we favor a model in which there is a decrement of inhibitory neurotransmission, our data cannot discriminate this from a loss of inhibitory interneurons, perhaps through use-driven neuronal death. One potential explanation for our result is that ischemia causes a reduction in inhibition that serves as a background for subsequent reorganization and unmasking of silent synapses.52–54 Interestingly, however, we did not see a reduction in inhibitory markers poststroke in the absence of training. Thus, it appears that decreased expression of PV, CR, and CB is a marker for reorganization caused by an interaction between endogenous plasticity and motor learning after a focal stroke in primary motor cortex. Although previous reports have shown that rehabilitation training improves motor outcome in rodents and can alter measures of synaptic activity,7,55–57 we are unaware of specific studies looking at a training effect on inhibitory interneurons. Our results are consistent with a growing body of data suggesting a correlation between decreased inhibition and the capacity of a motor cortical area to mediate recovery.20,21
Acknowledgments
The authors acknowledge the help of Xiaopei Tang for her assistance with these experiments.
Source of Funding
This work was supported by a startup fund from the Johns Hopkins department of Neurology to Dr Zeiler. Dr Krakauer was supported by R01 NS052804-05. Dr Worley was supported by RO1 NS39156. Imaris imaging and Johns Hopkins University imaging core were supported by MH084020.
Footnotes
Disclosures
None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke. Neurologic and functional recovery the Copenhagen Stroke Study. Phys Med Rehabil Clin N Am. 1999;10:887–906. [PubMed] [Google Scholar]
- 4.Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992;23:1084–1089. doi: 10.1161/01.str.23.8.1084. [DOI] [PubMed] [Google Scholar]
- 5.Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 6.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012;26:923–931. doi: 10.1177/1545968312440745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 8.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004;24:1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gharbawie OA, Gonzalez CL, Williams PT, Kleim JA, Whishaw IQ. Middle cerebral artery (MCA) stroke produces dysfunction in adjacent motor cortex as detected by intracortical microstimulation in rats. Neuroscience. 2005;130:601–610. doi: 10.1016/j.neuroscience.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Castro-Alamancos MA, Borrel J. Functional recovery of forelimb response capacity after forelimb primary motor cortex damage in the rat is due to the reorganization of adjacent areas of cortex. Neuroscience. 1995;68:793–805. doi: 10.1016/0306-4522(95)00178-l. [DOI] [PubMed] [Google Scholar]
- 11.Winship IR, Murphy TH. Remapping the somatosensory cortex after stroke: insight from imaging the synapse to network. Neuroscientist. 2009;15:507–524. doi: 10.1177/1073858409333076. [DOI] [PubMed] [Google Scholar]
- 12.Brown JA. Recovery of motor function after stroke. Prog Brain Res. 2006;157:223–228. doi: 10.1016/S0079-6123(06)57015-3. [DOI] [PubMed] [Google Scholar]
- 13.Dijkhuizen RM, Singhal AB, Mandeville JB, Wu O, Halpern EF, Finklestein SP, et al. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003;23:510–517. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav. 2002;77:677–682. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- 15.Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38(2 suppl):840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- 16.Dancause N. Vicarious function of remote cortex following stroke: recent evidence from human and animal studies. Neuroscientist. 2006;12:489–499. doi: 10.1177/1073858406292782. [DOI] [PubMed] [Google Scholar]
- 17.Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Dancause N, Barbay S, Frost SB, Zoubina EV, Plautz EJ, Mahnken JD, et al. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J Neurophysiol. 2006;96:3506–3511. doi: 10.1152/jn.00792.2006. [DOI] [PubMed] [Google Scholar]
- 19.Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol. 2003;54:464–472. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- 20.Carmichael ST. Brain excitability in stroke: the yin and yang of stroke progression. Arch Neurol. 2012;69:161–167. doi: 10.1001/archneurol.2011.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Druga R. Neocortical inhibitory system. Folia Biol (Praha) 2009;55:201–217. [PubMed] [Google Scholar]
- 23.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 24.Mainardi M, Landi S, Berardi N, Maffei L, Pizzorusso T. Reduced responsiveness to long-term monocular deprivation of parvalbumin neurons assessed by c-Fos staining in rat visual cortex. PLoS ONE. 2009;4:e4342. doi: 10.1371/journal.pone.0004342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bender R, Hoffmann MC, Frotscher M, Nitsch C. Species-specific expression of parvalbumin in the entorhinal cortex of the Mongolian gerbil: dependence on local activity but not extrinsic afferents. Neuroscience. 2000;99:423–431. doi: 10.1016/s0306-4522(00)00208-6. [DOI] [PubMed] [Google Scholar]
- 26.Tropea D, Kreiman G, Lyckman A, Mukherjee S, Yu H, Horng S, et al. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat Neurosci. 2006;9:660–668. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]
- 27.Schwaller B. Cytosolic Ca2+ buffers. Cold Spring Harb Perspect Biol. 2010;2:a004051. doi: 10.1101/cshperspect.a004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donoghue JP, Wise SP. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol. 1982;212:76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- 29.Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA, et al. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb Cortex. 2011;21:865–876. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brecht M, Krauss A, Muhammad S, Sinai-Esfahani L, Bellanca S, Margrie TW. Organization of rat vibrissa motor cortex and adjacent areas according to cytoarchitectonics, microstimulation, and intracellular stimulation of identified cells. J Comp Neurol. 2004;479:360–373. doi: 10.1002/cne.20306. [DOI] [PubMed] [Google Scholar]
- 31.Farr TD, Whishaw IQ. Quantitative and qualitative impairments in skilled reaching in the mouse (Mus musculus) after a focal motor cortex stroke. Stroke. 2002;33:1869–1875. doi: 10.1161/01.str.0000020714.48349.4e. [DOI] [PubMed] [Google Scholar]
- 32.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press; 2001. [Google Scholar]
- 33.Lee JK, Park MS, Kim YS, Moon KS, Joo SP, Kim TS, et al. Photochemically induced cerebral ischemia in a mouse model. Surg Neurol. 2007;67:620–625. doi: 10.1016/j.surneu.2006.08.077. discussion 625. [DOI] [PubMed] [Google Scholar]
- 34.Reep RL, Corwin JV, Hashimoto A, Watson RT. Efferent connections of the rostral portion of medial agranular cortex in rats. Brain Res Bull. 1987;19:203–221. doi: 10.1016/0361-9230(87)90086-4. [DOI] [PubMed] [Google Scholar]
- 35.Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- 36.Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci. 2009;10:519–529. doi: 10.1038/nrn2652. [DOI] [PubMed] [Google Scholar]
- 37.Reep RL, Corwin JV. Topographic organization of the striatal and thalamic connections of rat medial agranular cortex. Brain Res. 1999;841:43–52. doi: 10.1016/s0006-8993(99)01779-5. [DOI] [PubMed] [Google Scholar]
- 38.Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc Natl Acad Sci USA. 2006;103:11370–11375. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rouiller EM, Moret V, Liang F. Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens Mot Res. 1993;10:269–289. doi: 10.3109/08990229309028837. [DOI] [PubMed] [Google Scholar]
- 40.Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85:677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- 41.Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, et al. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- 42.Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 43.Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28:310–316. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Leppä E, Linden AM, Vekovischeva OY, Swinny JD, Rantanen V, Toppila E, et al. Removal of GABA(A) receptor 2 subunits from parvalbumin neurons causes wide-ranging behavioral alterations. PLoS ONE. 2011;6:e24159. doi: 10.1371/journal.pone.0024159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gogolla N, Caroni P, Lüthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 46.Murray AJ, Sauer JF, Riedel G, McClure C, Ansel L, Cheyne L, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang IK, Yoo KY, Kim DS, Jung JY, Kim KS, Park JK, et al. Changes in parvalbumin immunoreactivity in the parietofrontal cortex after transient forebrain ischemia in the Mongolian gerbil. Mol Cells. 2004;17:304–308. [PubMed] [Google Scholar]
- 48.Tortosa A, Ferrer I. Parvalbumin immunoreactivity in the hippocampus of the gerbil after transient forebrain ischaemia: a qualitative and quantitative sequential study. Neuroscience. 1993;55:33–43. doi: 10.1016/0306-4522(93)90452-l. [DOI] [PubMed] [Google Scholar]
- 49.Ferrer I, Soriano MA, Vidal A, Planas AM. Survival of parvalbumin-immunoreactive neurons in the gerbil hippocampus following transient forebrain ischemia does not depend on HSP-70 protein induction. Brain Res. 1995;692:41–46. doi: 10.1016/0006-8993(95)00527-w. [DOI] [PubMed] [Google Scholar]
- 50.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 51.Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 53.Donoghue JP, Suner S, Sanes JN. Dynamic organization of primary motor cortex output to target muscles in adult rats. II. Rapid reorganization following motor nerve lesions. Exp Brain Res. 1990;79:492–503. doi: 10.1007/BF00229319. [DOI] [PubMed] [Google Scholar]
- 54.Sanes JN, Suner S, Lando JF, Donoghue JP. Rapid reorganization of adult rat motor cortex somatic representation patterns after motor nerve injury. Proc Natl Acad Sci USA. 1988;85:2003–2007. doi: 10.1073/pnas.85.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones TA, Allred RP, Adkins DL, Hsu JE, O’Bryant A, Maldonado MA. Remodeling the brain with behavioral experience after stroke. Stroke. 2009;40(3 suppl):S136–S138. doi: 10.1161/STROKEAHA.108.533653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Higo N. Training-induced recovery of manual dexterity after a lesion in the motor cortex. Keio J Med. 2010;59:4–9. doi: 10.2302/kjm.59.4. [DOI] [PubMed] [Google Scholar]
- 57.Johansson BB. Environmental influence on recovery after brain lesions–experimental and clinical data. J Reprod Med. 2003:11–16. doi: 10.1080/16501960310010089. [DOI] [PubMed] [Google Scholar]