Abstract
Background
Autologous bone marrow-derived mononuclear cells (AMNC) have shown therapeutic promise for central nervous system insults such as stroke and traumatic brain injury (TBI). We hypothesized that intravenous injection of AMNC provides neuroprotection which leads to cognitive improvement after TBI.
Methods
A controlled cortical impact (CCI) rodent traumatic brain injury (TBI) model was used to examine blood-brain barrier permeability (BBB), neuronal and glial apoptosis and cognitive behavior. Two groups of rats underwent CCI with (CCI-Autologous) or without AMNC treatment (CCI-Alone), consisting of 2 million AMNC/kilogram body weight harvested from the tibia and intravenously injected 72 hr after injury. CCI-Alone animals underwent sham harvests and received vehicle injections.
Results
96 hr after injury, AMNC significantly reduced the BBB permeability in injured animals, and there was an increase in apoptosis of pro-inflammatory activated microglia in the ipsilateral hippocampus. At 4 weeks after injury, we examined changes in spatial memory after TBI due to AMNC treatment. There was a significant improvement in probe testing of CCI-Autologous group in comparison to CCI-Alone in the Morris Water Maze paradigm.
Conclusions
Our data demonstrate that the intravenous injection of AMNC after TBI leads to neuroprotection by preserving early BBB integrity and increasing activated microglial apoptosis. In addition, AMNC also improves cognitive function.
Keywords: Behavior (rodent), blood-brain barrier, brain trauma, Inflammation, Microglia
Introduction
Traumatic brain injury (TBI) affects nearly 1.5 million people each year in the United States, with nearly 50,000 patients dying as a result (1). There are long-term deficits associated with TBI which lead to an annual economic impact of 60 billion dollars (2). The initial injury is followed by secondary changes that include edema and cell death. In order to minimize secondary changes, maintaining optimal cerebral perfusion pressure and limiting elevations of intracranial pressure have been utilized. Long-term treatments for TBI include, motor, cognitive and behavioral rehabilitation. Recently, cellular therapy has been studied in preclinical: early phase clinical trials to attenuate the long-term effects of central nervous system (CNS) injuries. Specifically, AMNC have been used as therapy for stroke and TBI in both clinical and pre-clinical models (3–5). The use of AMNC is facilitated by the ability to extract, isolate, and prepare for injection the mononuclear fraction for injection quickly after bone marrow harvest. The most important benefit of utilizing AMNC treatment is that it circumvents any host immunological reactivity.
TBI is characterized by enhanced vascular permeability due to the breakdown of the BBB and subsequent edema. There is accumulation of a variety of effector cells from the peripheral blood to the injured regions of the brain. There is also activation of microglia, the resident macrophages of the brain. While a microglial response is necessary to remove necrotic tissue and induce myelin repair, prolonged microglial activation can potentially modulate neurons and astrocytes and subsequently affect behavior. Recent evidence suggests that activated microglia modulate neuronal changes that can result in the development of neuropathic pain (6).
Resident microglia, in the presence of anti-inflammatory cytokines such as interleukin-4 (IL-4) and interleukin-10 (IL-10) are generally in a resting/ramified state (7, 8). In an unperturbed environment, microglia have a distinct morphology, consisting of a small, static cell body with dynamic and branched processes. After a CNS injury, microglia are activated and possess a distinct ameboid-like morphology. They become phagocytotic (9), and are responsible for continued production of pro-inflammatory and potentially cyto-destructive molecules. Due to the breakdown of the BBB, there is also influx of infiltrating macrophages (10) and release of pro-inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor (TNF) (11). In stroke models, adult progenitor cell therapy has led to decreased tissue levels of pro-inflammatory cytokines and increased levels of IL-10, (5, 12). We hypothesized that the intravenous injection of AMNC after TBI attenuates the inflammatory response which leads to improved cognitive function. To test our hypothesis, a series of experiments were completed to investigate the effect of AMNC therapy on the BBB permeability, apoptosis, and cognitive functions after TBI.
Methods
All protocols involving the use of animals were in compliance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Texas Health Science Center Institutional Animal Care and Use Committee (AWC-07-055). Adult male Sprague Dawley rats (250–275 grams) were used for all experiments.
Controlled cortical impact injury
Rats were anesthetized with 4% isoflurane and subsequently placed in the supine position with their heads in a stereotaxic device. A CCI device (eCCI Model 6.3; VCU, Richmond, VA) was used to administer a unilateral brain injury (13). A single impact of 3.1-mm depth (6-mm tip), with a velocity of 5.6 m/sec and a dwell time of 150 msec was used to make an impact onto the right parietal association cortex. In all experiments, there were 3 groups: CCI-Alone and CCI-Autologous underwent CCI injury while a sham group received a craniectomy only.
Bone marrow harvest
Seventy two hours after CCI injury, on the anterior surface of the bilateral lower legs, a 2.5 cm incision was made. The surface of the tibia was exposed and a small burr hole was drilled 1 cm below the tibial plateau. 1ml of bone marrow per tibia was aspirated from the bilateral tibias and the skin incisions were then closed. For all experiments, AMNC was harvested at 72 hrs and injected 74 hrs after injury.
Bone Marrow Mononuclear Cell Isolation
The bone marrow was filtered to remove any residual bone fragments or adipose tissue, followed by a dilution of 2:1 with Hank's balanced salt solution (HBSS). The diluted solution was then layered over an equal volume of Ficoll-Hypaque (GE Healthcare, NJ) solution. After centrifuging, AMNC were carefully aspirated and washed and re-suspended in PBS. Cells were adjusted to a concentration of 2 million cells / kg body weight in 1ml.
Cell injection
Two groups of rats (CCI-Alone and CCI-Autologous) were anesthetized and placed in the supine position. A right internal jugular vein cut-down and cannulation was completed followed by injection of the cell suspension for the treatment group and PBS vehicle alone for the uninjured and CCI injury alone groups.
Experimental Design
Short-term
Evan's blue blood brain barrier permeability analysis
Twenty four hours after AMNC treatment, 4ml/kg of 3% Evan's blue dye (EB) in PBS was injected via tail vein. 60 minutes after injection, the animals were euthanized via right atrial puncture and perfused with 4% paraformaldehyde. The brain (without cerebellum) was isolated and divided through the midline and the mass of each hemisphere measured (ipsilateral and contralateral to injury). It was then homogenized in 3 mL of formamide (Sigma Aldrich, St. Louis, MO, USA). Overnight incubation was done at 50 C° to allow for dye extravasation. 100 µL of solution from each sample was transferred to a 96 well plate (in triplicate) and absorbance was measured at 620 nm. All values were normalized to hemisphere weight.
Immunohistochemistry
Twenty four hours after AMNC treatment, the rats were anesthetized and brains harvested. The brains were sectioned at 50 µm and stained using a standard free floating staining protocol. They were washed in PBS with 0.01% Triton X-100 [(PBST) T-8787, (Sigma Aldrich, St. Louis, MO, USA)] for 1 min, and then incubated for 30 min in PBS with 0.2% Triton X-100. The sections were then blocked for 1 hr at room temperature (RT) in 3% goat serum (# 005-000-121, Jackson Immuno Research, PA) in PBST. Primary antibodies were prepared in PBTB [PBS with 0.01% Triton X-100, 2 % Bovine Serum Albumin (A9647, Sigma Aldrich, St. Louis, MO, USA)] and 1% goat serum, and incubated at 4° C overnight: Neuronal Nuclei [(NeuN) 1:100, MAB377, Millipore, Billerica, MA, USA], Cleaved Caspase 3 [(CC3)1:1500, # D175, Cell Signaling Technology, Danvers MA, USA], Glial Fibrillary Acidic Protein [(GFAP), 1:250, #IFO3L, Calbiochem, Danvers, MA, USA] and CD11b/c (1:250, ab1211, Abcam, Cambridge, MA, USA). Subsequently, the sections were rinsed briefly then washed with PBST, and incubated with secondary antibodies (1:500; green/488:A11029 and 1:500; red/568:A11011, Invitrogen) in PBTB for 2 hours at RT. The sections were again rinsed briefly and mounted, and cover-slipped with Fluoromount-G (Southern Biotechnology Associates, Birmingham, AL).
Quantification of Immunohistochemistry
Six photomicrographs from a single slice were taken of the hippocampus at 20X using a Nikon fluorescent microcope (TE2000-U). Ipsilateral hippocampi were quantified (dentate gyrus, medial blade- polymorph layer; CA3 and the CA1 region). We counted all the cells, and the data shown in the graphs concerns cells dually labeled cells with CD11b/c and CC3. Microglia (CD11b/c+) cells were further classified based on morphology: small, static cell body with dynamic and branched processes were classified as inactivated, distinct ameboid morphology as activated and cell bodies that were ameboid with some branched processes were classified as slightly activated. The data shown consists only of activated microglia.
Long-Term
Cognitive function testing with the Morris Water Maze
Cognitive function was tested using the Morris Water Maze at 2 weeks (Days 14–19) and 4 weeks (days 28–32) after injury to assess spatial memory and spatial learning. All groups were tested by a blinded investigator in a hidden platform, learning paradigm version (14) version of the Morris Water Maze task (15). Animals were tested using 4 trials per day/5 days. Latency to platform was measured as the time to find the platform (Chromotrack, San Diego Instruments, San Diego, CA, USA). To test memory retrieval, probe trials (removal of platform) were administered 6 hrs after completion of the platform testing. We measured the time the animal spent in the same quadrant as the platform, in an area with a diameter 3 times the size of the platform (3×) and the number of times the animal crossed over the previous location of the platform.
Data Analysis
Unless otherwise indicated, all values are represented as mean ± SEM. Values were compared using analysis of variance (ANOVA) with a post-hoc Tukey analysis or Dunnet’s Test. A p value of ≤ 0.05 was used to denote statistical significance. * indicates statistical significance p <0.05, ** indicates statistical significance p <0.01 and *** indicates statistical significance p <0.005.
Results
AMNC treatment preserves the blood brain barrier
There was a significant difference between Sham (0.10 ± 0.03) and CCI-Alone (0.48 ± 0.09) in the right (injured) hemisphere (Fig 1. p < 0.001) as measured by EB extravasation. In addition, there was a significant reduction in CCI-Autologous (0.20 ± 0.05) when compared to CCI-Alone. The decrease in permeability after AMNC treatment left no differences between Sham and CCI-Autologous.
Figure 1. Autologous cell therapy preserves the blood brain barrier.
Blood brain barrier (BBB) permeability measured via Evan's blue extravasation. BBB permeability measurement (mean absorbance/weight of tissue (mg) from homogenized cortical tissue (Y axis) derived from both hemispheres. There was a significant decrease in BBB permeability after autologous treatment (CCI-Autologous) when compared to CCI-Alone in the injured right hemisphere. There was also a significant increase in BBB permeability in the CCI-Alone when compared to Sham in the injured right hemisphere. There were no significant differences between Sham and CCI-Autologous in the right hemisphere. Additionally there were no differences between all three groups in the left hemispheres.
AMNC treatment increases activated microglial/macrophages apoptosis in the hippocampus
There was a lack of apoptotic activated microglia/macrophages observed in shams (Fig. 2A). AMNC treatment significantly increased the population of ameboid CD11b/c+ cells that co-stained for CC3 (70 ± 14) when compared to CCI-Alone (38 ± 5, p <0.05, Fig. 2A–D), suggesting that apoptosis of activated microglia/macrophages occurs in these regions in the treated animals. Numbers of ameboid CD11b/c+/ CC3+ cells in CCI-Alone were also significantly increased when compared to shams (12 ± 2, p <0.05). There were no differences in the number of inactivated or slightly activated microglia/macrophages among the groups (data not shown).
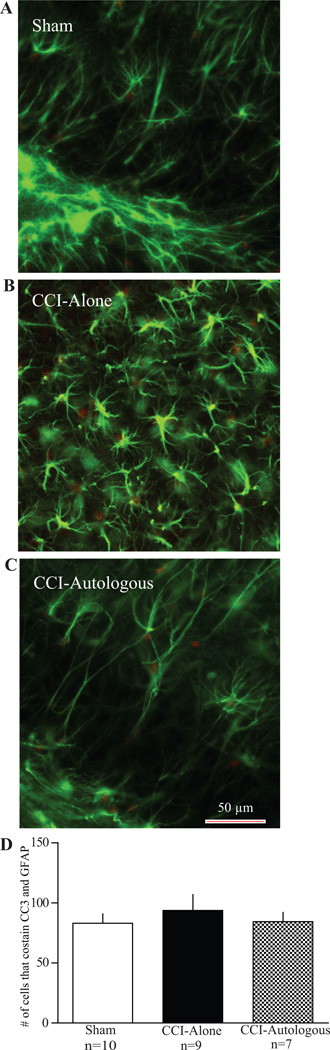
Figure 2. Autologous cell therapy results in apoptosis of activated pro-inflammatory microglia mediated by cleaved caspase 3 (CC3).
Photomicrographs of microglia from hippocampal brain slices stained for cleaved caspase 3 (red) and microglia (green). A: Example of a sham brain slice. There is modest staining of CC3. In addition there are non-activated microglia (morphology). B: Example of a CCI-Alone brain slice. There are activated ameboid microglia but little overlap between the activated microglia and CC3. C: Example of CCI- Autologous brain slice. There is co-staining of CC3 and activated microglia. Scale bar 50 µm. D: Graph comparing the number of cells that co-stained with CC3 and microglia antibody. There is a significant increase in apoptotic (CC3+) and activated microglia in brain slices that received autologous cell treatment when compared to CCI-Alone. In addition, there was an increase in apoptotic, activated microglia between CCI-Alone and Sham.
AMNC treatment does not affect early neuronal apoptosis in the hippocampus
AMNC treatment did not affect neuronal apoptosis among all groups (p > 0.05, Fig. 3A–D) when ipsilateral hippocampal regions were stained with NeuN and CC3. Overall, a modest number of neurons were CC3+ in all three groups [sham (15 ± 2); CCI-Alone (18 ± 4) and CCI-Autologous (17 ± 4).
Figure 3. Autologous cell therapy does not affect neuronal apoptosis.
Photomicrographs of neurons from hippocampal brain slices stained for cleaved caspase 3 (red) and NeuN (green). A: Example of a sham brain slice. There is modest staining of CC3 with no overlap with NeuN. B: Example of a CCI-Alone brain slice. Like sham animals, there is very little overlap between CC3 and NeuN. C: Example of CCI - Autologous brain slice. There is very little overlap between CC3 and NeuN. Scale bar 50 µm D: Graph comparing the number of cells that co-stained with CC3 and NeuN. There is a modest, but not significant decrease in overlap between CC3 and NeuN in brain slices that received autologous cell treatment when compared to CCI-Alone.
AMNC treatment does not affect early astrocytic apoptosis in the hippocampus
There was an attenuation of co-staining in the AMNC treated group (84 ± 8) when compared to CCI-Alone (94 ± 13; p > 0.05, Fig. 4A–D), but this changes was not statistically significant, although CCI-Autologous apoptosis were similar to sham levels (83 ± 8).
Figure 4. Autologous cell therapy does not affect astrocytic apoptosis.
Photomicrographs of astrocytes from hippocampal brain slices stained for cleaved caspase 3 (red) and GFAP (green). A: Example of a sham brain slice. There is modest staining of CC3 with no overlap with GFAP. B: Example of a CCI-Alone brain slice. Like sham animals, there is very little overlap between CC3 and GFAP. C: Example of CCI - Autologous brain slice. There is very little overlap between CC3 and GFAP. Scale bar 50 µm D: Graph comparing the number of cells that co-stained with CC3 and GFAP. There is a modest, but not significant decrease in overlap between CC3 and GFAP in hippocampal brain slices that received autologous cell treatment when compared to CCI-Alone.
Cognitive testing: Morris Water Maze
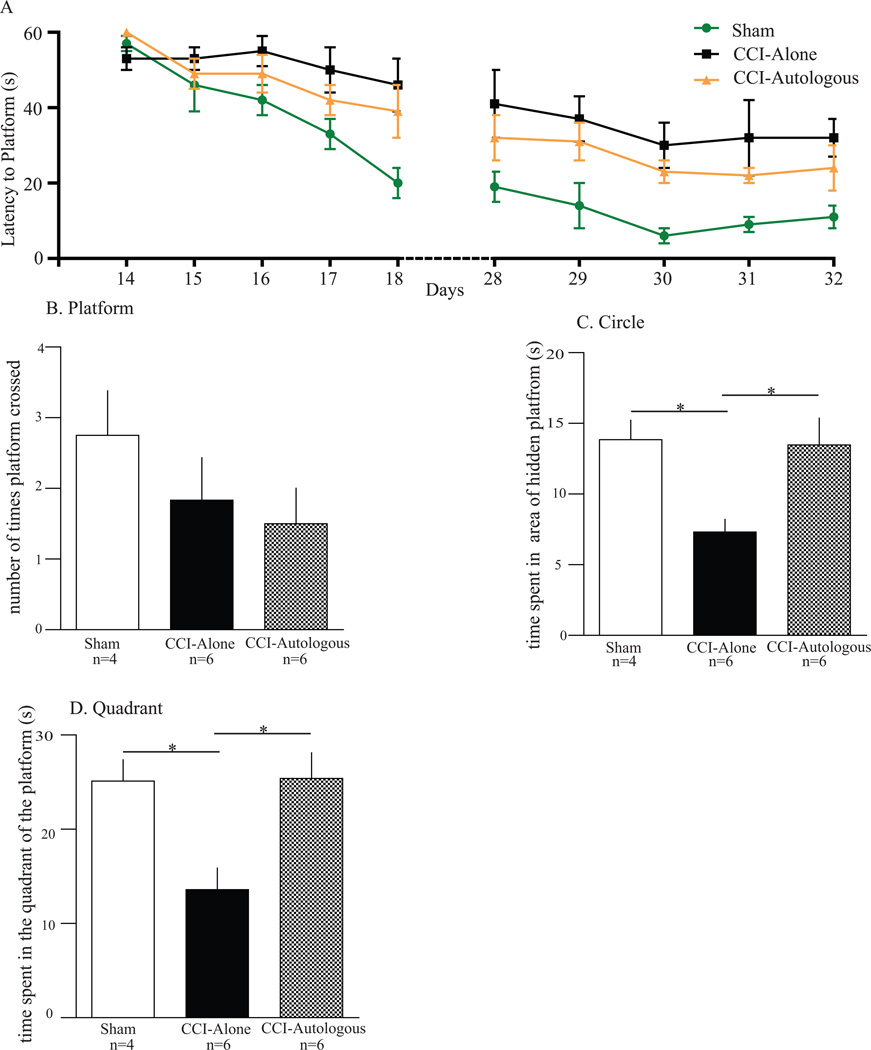
There was a trend towards decreased latency to platform for the CCI-Autologous when compared to the CCI-Alone, the values fail to reach significance for both 2 weeks and 4 weeks (Figure 5A). After 5 days of training, we did probe trials to estimate the strength and accuracy of acquired memory after removal of the platform. At 2 weeks (probe trials), we did not observe any statistical differences between CCI-Autologous and CCI-Alone (data not shown). However, at 4 weeks, analysis of the time spent in the quadrant (Fig. 5D) show that the CCI-Alone (14 ± 2.3) animals spent significantly less time in close proximity to the location of the platform when compared to Sham animals (25 ± 2.3; p < 0.05). Furthermore, CCI-Autologous animals significantly increased the quadrant times (25 ± 2.7) when compared to CCI-Alone (p < 0.05). Similar significant differences were observed when calculating time spent in an area with 3 times the diameter of the platform centered in the same location (circle time; Fig 5C). CCI-Alone (7.3 ± 0.89) animals spent significantly less time in the 3X location of the platform when compared to Sham animals (14 ± 1.4; p < 0.05). Additionally, CCI-Autologous animals significantly increased the circle time (14 ± 1.9) when compared to CCI-Alone (p < 0.05; Fig. 5C). There was no difference in swim speeds across all groups (data not shown). The total number of times crossing the previous location of the platform was also recorded, and there were no significant differences among the three groups (Sham 2.8 ± 0.63, CCI-Alone 1.8 ± 0.60, and CCI-Autologous 1.5 ± 0.50, Fig. 5B).
Figure 5. Autologous cell therapy improves circle and quadrant time in the Morris Water Maze during probe trial.
Probe trials completed on 3 groups of animals (Sham, CCI-Alone, and CCI-Autologous) after 5 days of Morris Water Maze training (4 weeks following cortical injury). A. 2 weeks and 4 weeks post-injury training of the MWM. There was no significant difference between CCI-Autologous and CCI-Alone after the training. B. There were no significant differences in the swim speed (velocity) of the animals among all three groups. C. There were no differences in the number the times the animal crossed the area where the platform use to be. D. There was a significant increase in the circle times for the CCI-Autologous group as compared to the CCI-Alone. E. There was a significant increase in the time spent in the quadrant where the platform was located previously for the CCI-Autologous group as compared to the CCI-Alone.
Discussion
AMNC treatment after TBI preserves the BBB (Fig. 1), protects the brain by increasing apoptosis of activated microglia (Fig. 2) and improves cognitive function (Fig. 5). Our model was specifically developed to allow for the injection of such non-expanded cells that could bypass the potential immunogenic rejection of the heterologous implanted cells. The brain is isolated from the bloodstream and its contents due to BBB. TBI results in a disruption of this barrier, and allows for subsequent influx of infiltrating macrophages (11) and release of pro-inflammatory cytokines (10). This serves to propagate the cycle of infiltrating macrophages, release of pro-inflammatory cytokines and activation of microglia. The BBB is also an important therapeutic target for intracranial pressure management. An intact osmotic BBB allows for more effective intravenous hyperosmolar therapy for the management of post-traumatic cerebral edema and the resultant elevations in intracranial pressure. Furthermore, BBB permeability is a clinically relevant marker of the end-result of the neuro-inflammatory response at the level of the neurovascular niche. TBI increases BBB permeability significantly ipsilateral to the injury when compared to sham, and AMNC treatment after TBI results in preservation of the BBB as examined by EB extravasation (Fig. 1). Circulating AMNC, alone, does not affect the uninjured side among the groups (Fig. 1). This suggests that presence alone of AMNC does not alter BBB permeability. Case analysis of patients that suffered TBI displayed activated microglia with white matter degeneration up to 47 years (16). Additional evidence from patients after TBI suggests that activated microglia can be present up to 17 years after injury (17). Sustained, uncontrolled and over-activated microglia can propagate the secondary effects of TBI. Preserving the BBB has early therapeutic benefit and is a marker for mitigation of the neuroinflammatory response to injury.
AMNC treatment 72 hrs after TBI selectively increased apoptosis of activated microglia/macrophages as measured by CC3 (Fig. 2). Inflammatory markers are elevated beginning as early as 6 hours and lasting to 72 hours after injury (4, 18–20). For this reason we selected a time point that has been universally accepted as being a time of elevated edema and inflammation in both rodent models and humans. Microglia are classically activated by pro-inflammatory stimuli, and are accepted as participants in the early phase of traumatic injury (21). At rest, microglia display distinct morphology: small, static cell body with dynamic and branched processes (see Fig. 2A). In response to injury and/or pro-inflammatory signals, microglia shorten and widen their processes (slighty activated) before transitioning to the phagocytotic ameboid morphology [(22) activated: Fig. 2B and C]. In this activated state, microglia/macrophages phagocytose necrotic and cellular debris. The fate of activated microglia after they have phagocytosed necrotic tissue is yet unknown. CC3, a part of the cysteinyl-aspartate specific proteases, is triggered by cell surface receptors (Toll-like receptors) as well as other caspases (23). CC3 is generally responsible for apoptotic cell death (20) and its activation leads to inappropriate disablement of structural protein and repair enzymes and eventual cell death (24). We observed a significant increase in microglia that displayed an ameboid morphology (activated form) and that co-stained for CC3/CD11b/c after AMNC treatment when compared to CCI Alone (Fig 2), indicating an increase in the apoptosis of activated microglia due to AMNC treatment. Similar results have been reported spinal cord injury models with transplanted precursor cells (25). Further cellular signals need to be examined in the CC3 apoptotic pathway. In addition, we did not observe any significant changes in co-staining of CC3/NeuN (neuronal) and CC3/GFAP (reactive astrocytes) due to AMNC treatment when compared to CCI-Alone. This suggests that AMNC is specifically targeting activated microglia. Our data suggest that AMNC treatment likely provides short-term cerebral protection by preserving the BBB (Fig. 1) and causing activated microglia to go through a CC3-dependent apoptosis (Fig. 2).
AMNC treatment significantly improved spatial learning in our rodent model. Spatial learning as measured by the Morris Water Maze is critically dependent upon the hippocampus (26). Spatial learning encompasses acquisition and spatial localization of relevant visual cue that are consolidated and retained. The memory of the location of the platform is retrieved over the training and probe trials (27). We did not observe any changes in the acquisition at 2 and 4 weeks after injury (Fig. 5A), but, during the probe trials (4 weeks only), we observed significant differences in time spent in the quadrant where the platform was and circle time around the platform. AMNC treated animals displayed times that were similar to sham animals (Fig. 5C and D). It is possible that AMNC affects the retrieval process more than the consolidation and retention process. A potential mechanism could be that AMNC treatment attenuates the prolonged microglial/macrophage activation by increasing the apoptosis of activated microglia/macrophages, thereby preserving neuronal and astrocytic functions. Alternative mechanisms may involve AMNC affecting neural plasticity phenomena such as long-term potentiation and long-term depression in the hippocampus after injury.
The development of a model for the intravenous injection of AMNC represents a clearly translatable pre-clinical model. It negates any potential immune rejection thereby decreasing the possibility of propagating the already pro inflammatory environment with graft rejection. Our data demonstrates that AMNC treatment after TBI is neuroprotective by preserving the BBB, increasing microglial/macrophages apoptosis and improving spatial memory. The mechanism of action of AMNC, like other cellular therapies is likely via systemic modulation of the inflammatory response, and not through direct replacement of lost cells (28, 29). This study provides evidence for association, but not causation between apoptosis of activated microglia due to AMNC treatment and improvement in cognitive behavior. Limitations of this study include examination of a single time point of treatment; later time points of treatment need to be examined, along with behavior that includes spatial learning and fear conditioning. Further elucidation of the regulation of microglia by AMNC treatment after TBI may offer insight into optimal dosing strategies. While this preliminary investigation into the potential role of AMNC for TBI has shown promise, further definition of potential cell-cell signaling between AMNC and microglia/macrophages may delineate the mechanisms of this therapy.
Acknowledgments
Supported by grants: NIH T32 GM 08 79201; M01 RR 02558; Brown Foundation, Inc. Children’s Memorial Hermann Hospital Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contribution
Supinder S. Bedi was involved in the planning of experiments, completion of experiments, data analysis and manuscript preparation.
Peter A. Walker was involved in the planning of experiments, completion of experiments, data analysis and manuscript preparation.
Shinil K. Shah was involved in the planning of experiments, completion of experiments and data analysis.
Fernando Jimenez was involved in planning of experiments and completion of experiments.
Chelsea P. Thomas was involved in planning of experiments and completion of experiments.
Philippa Smith was involved in planning of experiments and completion of experiments.
Robert A. Hetz was involved in literature search and manuscript preparation
Hasen Xue was involved in planning of experiments and completion of experiments.
Shibani Pati was involved in planning of experiments.
Pramod K. Dash was involved in planning of experiments.
Charles S. Cox, Jr. was involved in the planning of experiments, data analysis and manuscript preparation.
Disclosure/Conflict of Interest
There are no known conflicts between the authors and the information presented in this paper.
Contributor Information
Supinder S. Bedi, Email: Supinder.bedi@uth.tmc.edu.
Peter A. Walker, Email: Peter.A.Walker@uth.tmc.edu.
Shinil K. Shah, Email: Shinil.K.Shah@uth.tmc.edu.
Fernando Jimenez, Email: Fernando.Jimenez@uth.tmc.edu.
Chelsea P. Thomas, Email: Chelsea.P.Thomas@uth.tmc.edu.
Philippa Smith, Email: Philippa.Smith@uth.tmc.edu.
Robert A. Hetz, Email: Robert.A.Hetz@uth.tmc.edu.
Hasen Xue, Email: Hasen.Xue@uth.tmc.edu.
Shibani Pati, Email: SPati@bloodsystems.org.
Pramod K. Dash, Email: P.Dash@uth.tmc.edu.
Charles S. Cox, Jr., Email: Charles.S.Cox@uth.tmc.edu.
References
- 1.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999 Dec;14(6):602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Faul M, Wald MM, Rutland-Brown W, Sullivent EE, Sattin RW. Using a cost-benefit analysis to estimate outcomes of a clinical treatment guideline: testing the Brain Trauma Foundation guidelines for the treatment of severe traumatic brain injury. J Trauma. 2007 Dec;63(6):1271–1278. doi: 10.1097/TA.0b013e3181493080. [DOI] [PubMed] [Google Scholar]
- 3.Savitz SI, Misra V, Kasam M, Juneja H, Cox CS, Jr, Alderman S, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011 Jul;70(1):59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 4.Cox CS, Jr, Baumgartner JE, Harting MT, Worth LL, Walker PA, Shah SK, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery. 2011 Mar;68(3):588–600. doi: 10.1227/NEU.0b013e318207734c. [DOI] [PubMed] [Google Scholar]
- 5.Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr, Aronowski J, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010 Jan;30(1):140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, et al. P2×4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003 Aug 14;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 7.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003 Jan;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 8.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005 May 27;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 9.Smith HS. Activated microglia in nociception. Pain Physician. 2010 May-Jun;13(3):295–304. [PubMed] [Google Scholar]
- 10.Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007 Jan 10;500(2):267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 11.Pineau I, Sun L, Bastien D, Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in an IL-1 receptor/MyD88-dependent fashion. Brain Behav Immun. 2010 May;24(4):540–553. doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Liu N, Chen R, Du H, Wang J, Zhang Y, Wen J. Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol. 2009 Jun;6(3):207–213. doi: 10.1038/cmi.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lighthall JW. Controlled cortical impact: a new experimental brain injury model. J Neurotrauma. 1988;5(1):1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto ST, Longhi L, Saatman KE, Conte V, Stocchetti N, McIntosh TK. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev. 2004 Jul;28(4):365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci U S A. 1997 Mar 18;94(6):2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2012 Jan;136(Pt 1):28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011 Sep;70(3):374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 18.Harting MT, Jimenez F, Adams SD, Mercer DW, Cox CS., Jr Acute, regional inflammatory response after traumatic brain injury: Implications for cellular therapy. Surgery. 2008 Nov;144(5):803–813. doi: 10.1016/j.surg.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bareyre F, Wahl F, McIntosh TK, Stutzmann JM. Time course of cerebral edema after traumatic brain injury in rats: effects of riluzole and mannitol. J Neurotrauma. 1997 Nov;14(11):839–849. doi: 10.1089/neu.1997.14.839. [DOI] [PubMed] [Google Scholar]
- 20.Stocchetti N, Colombo A, Ortolano F, Videtta W, Marchesi R, Longhi L, et al. Time course of intracranial hypertension after traumatic brain injury. J Neurotrauma. 2007 Aug;24(8):1339–1346. doi: 10.1089/neu.2007.0300. [DOI] [PubMed] [Google Scholar]
- 21.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010 Oct;7(4):366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graeber MB. Changing face of microglia. Science. 2010 Nov 5;330(6005):783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 23.Porter AG. Protein translocation in apoptosis. Trends Cell Biol. 1999 Oct;9(10):394–401. doi: 10.1016/s0962-8924(99)01624-4. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997 Aug;22(8):299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 25.Cusimano M, Biziato D, Brambilla E, Donega M, Alfaro-Cervello C, Snider S, et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain. 2012 Feb;135(Pt 2):447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982 Jun 24;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 27.Terry AV., Jr Spatial Navigation (Water Maze) Tasks. 2009 [PubMed] [Google Scholar]
- 28.Walker PA, Letourneau PA, Bedi S, Shah SK, Jimenez F, CS Progenitor cells as remote "bioreactors": neuroprotection via modulation of the systemic inflammatory response. World J Stem Cells. 2011 Feb 26;3(2):9–18. doi: 10.4252/wjsc.v3.i2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker PA, Shah SK, Jimenez F, Aroom KR, Harting MT, Cox CS., Jr Bone marrow-derived stromal cell therapy for traumatic brain injury is neuroprotective via stimulation of non-neurologic organ systems. Surgery. 2012 Nov;152(5):790–793. doi: 10.1016/j.surg.2012.06.006. [DOI] [PubMed] [Google Scholar]