Abstract
Vitiligo is an autoimmune disease of the skin that results in disfiguring white spots. There are no FDA-approved treatments for vitiligo, and most off-label treatments yield unsatisfactory results. Vitiligo patients have increased numbers of autoreactive, melanocyte-specific CD8+ T cells in the skin and blood, which are directly responsible for melanocyte destruction. Here we report that gene expression in lesional skin from vitiligo patients reveals an IFN-γ-specific signature, including the chemokine CXCL10. CXCL10 is elevated in both vitiligo patient skin and serum and CXCR3, its receptor, is expressed on pathogenic T cells. To address the function of CXCL10 in vitiligo, we employed a mouse model of disease that also exhibits an IFN-γ-specific gene signature, expression of CXCL10 in the skin, and upregulation of CXCR3 on antigen-specific T cells. Mice that receive Cxcr3−/− T cells develop minimal depigmentation, as do mice lacking Cxcl10 or treated with CXCL10 neutralizing antibody. CXCL9 promotes autoreactive T cell global recruitment to the skin but not effector function while, in contrast, CXCL10 is required for effector function and localization within the skin. Surprisingly, CXCL10 neutralization in mice with established, widespread depigmentation induces reversal of disease, evidenced by repigmentation. These data identify a critical role for CXCL10 in both the progression and maintenance of vitiligo, and thereby support inhibiting CXCL10 as a targeted treatment strategy.
Introduction
Vitiligo is a disease of the skin that afflicts ~0.5–2% of the population and results in prominent, disfiguring white spots that may become widespread (1). Vitiligo pathogenesis incorporates both intrinsic defects within melanocytes that activate the cellular stress response, as well as autoimmune mechanisms that target these cells (2–12). Patients with vitiligo have increased numbers of autoreactive, melanocyte-specific CD8+ T cells in the skin and blood (13, 14). T cells infiltrate the skin during vitiligo and localize to the epidermis where melanocytes, their target cells, reside. In lesional skin, CD8+ T cells are found in close proximity to dying melanocytes (15, 16), and one study reported that melanocyte-specific, CD8+ T cells isolated from lesional skin migrated into nonlesional skin ex vivo, found their melanocyte targets in situ, and killed them (13). The melanocyte niche in the basal layer of the epidermis is not vascularized, so T cells that migrate into the skin in vitiligo must efficiently navigate through the dermis to find their targets and mediate depigmentation. The signals that promote melanocyte-specific T cell migration into and through the skin during vitiligo are unknown.
There are currently no FDA-approved medical treatments for vitiligo, and available off-label treatments are problematic and often ineffective. Narrow-band UVB (nbUVB) light is only provided in select facilities and requires time away from work and school multiple times per week, resulting in lost productivity. Topical steroids are impractical when large surface areas are affected, and carry risk of systemic absorption with suppression of the adrenal axis, in addition to striae and skin atrophy (1). Topical calcineurin inhibitors appear to have fewer risks but are costly, less effective, and treat only focal disease (17). Systemic immunosuppression has been reported to halt the progression of vitiligo and reverse disease through repigmentation (18), however the risks associated with a non-targeted systemic approach are substantial, and usually deemed unacceptable when compared to the benefits. Therefore, the identification of targeted therapies with an improved safety profile would be a substantial advance. Recent progress in understanding the key cytokines that promote psoriasis and related autoimmune diseases (i.e. rheumatoid arthritis, inflammatory bowel disease, and others) have resulted in biologic treatments with excellent efficacy and safety profiles, substantially improving patients’ quality of life (19). The cytokines that drive vitiligo pathogenesis have not been well defined, although they are not likely shared with these diseases, as biologic treatments for psoriasis have been ineffective for vitiligo (20–22).
The dynamics at play within established vitiligo lesions are unknown, although depigmented skin is widely thought to be inactive, such that T cells have left the tissue and melanocytes are unable to regenerate. However the ability of even long-standing lesions to repigment following treatment suggests that this is not always the case, but rather an active immune process is at work to maintain depigmentation. Further support for this hypothesis is found in the rapid repigmentation of lesional human vitiligo skin following transplantation onto nude mice (23), suggesting that host factors play an important role in maintaining depigmentation.
Here, we report that both human vitiligo patients and a mouse model of vitiligo reflect a uniquely IFNγ-specific TH1 cytokine signature in the skin that includes the IFNγ-dependent chemokines CXCL9, 10, and 11. CXCL10 is highly expressed in the skin and serum of patients with vitiligo, and it promotes autoreactive T cell localization to the epidermis to initiate vitiligo in our mouse model. Targeting CXCL10 therapeutically in mice not only prevents disease development, but also induces repigmentation in established lesions, implicating this chemokine as a critical player in both the progression and maintenance of vitiligo. Our results support further exploration of CXCL10 neutralization as a targeted systemic therapeutic approach in the treatment of vitiligo.
Results
Gene expression in vitiligo reflects a TH1-mediated immune response
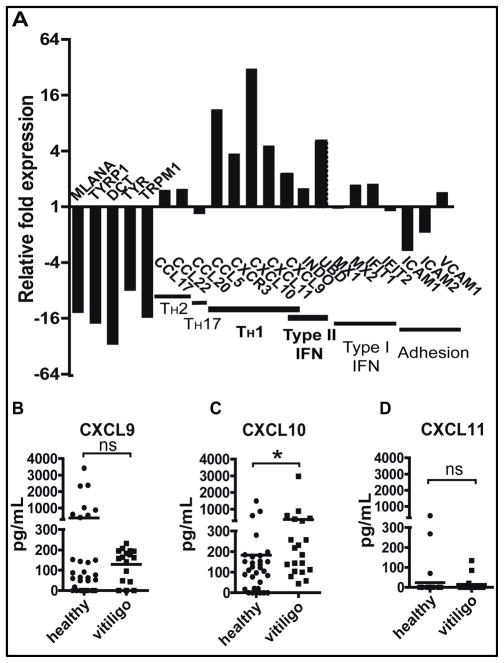
To identify key cytokine pathways in vitiligo, we measured the gene expression profile in lesional skin of vitiligo patients. Our criteria for selecting skin specimens relied on the presence of T cell infiltrates to determine inflammatory gene signatures in active lesions. Lesional biopsies frequently miss the T cells, as visible depigmentation of the skin does not become apparent until long after melanocyte death, up to 48 days in humans (24). Consequently, we screened a clinical archive of formalin fixed, paraffin-embedded (FFPE) biopsies for those that contained a T cell infiltrate, selected samples from 5 vitiligo patients and 5 age- and site-matched controls, and analyzed their RNA expression profile using the Illumina DASL assay. We observed significant loss of melanocyte-specific transcripts in lesional skin compared to healthy controls, validating this approach. Chemokine expression revealed a predominantly TH1-mediated immune response (Figure 1A). Based on a small panel of genes whose expression is more dependent on either Type I IFN (IFNα/β) or Type II IFN (IFNγ), we identified a distinctly IFNG-specific signature (Figure 1A).
Fig. 1. Vitiligo patients express IFNγ-dependent chemokines in skin and blood.
(A) We analyzed the gene expression profiles within skin biopsies from vitiligo patients compared to age- and site-matched controls using quantile normalization (n=5 per group). Melanocyte-specific transcripts were significantly reduced, consistent with melanocyte destruction in vitiligo. Chemokine expression reflected a TH1 profile, and the expression of specific gene targets of either Type I or Type II IFNs revealed an IFNγ-dominant response. The expression of genes capable of T cell recruitment to the skin reflected expression of chemokines, rather than adhesion molecules. (B–D) Serum samples from vitiligo donors or healthy controls were analyzed by ELISA to determine levels of CXCL9 (B), CXCL10 (C), and CXCL11 (D). CXCL10 was significantly elevated, whereas CXCL9 and CXCL11 were not (n=16–32 donors assayed in duplicate; two-tailed Mann-Whitney U test, *p=0.0148).
IFNγ has previously been shown to be induced in human vitiligo (13, 25), and is required for the development of depigmentation in a mouse model of vitiligo (26). IFNγ signaling induces the expression of over 200 different gene targets (27), however our earlier results suggested that IFNγ was specifically required for T cell accumulation within the skin (26). Therefore, IFNγ-dependent chemokines, which induce T cell homing into peripheral tissues, and endothelial adhesion molecules, which promote transmigration into tissues, were top candidates for this role (28, 29). We found that the chemokines CXCL9, CXCL10, CXCL11, and CCL5 were highly induced in vitiligo, while adhesion molecules ICAM1, ICAM2, and VCAM1 were not (Figure 1A). We confirmed these expression data in an additional 11 samples (8 vitiligo samples, 3 controls) using NanoString nCounter profiling (Figure S1A).
Previous studies reported elevated levels of CXCL9 and CXCL10 in the serum of patients with autoimmune thyroiditis and primary adrenal insufficiency (30, 31). To determine whether these chemokines could be detected in vitiligo patients as well, serum from vitiligo patients and healthy controls was measured via ELISA. We found that CXCL10 was indeed significantly elevated in vitiligo patients, but CXCL9 and CXCL11 were not significantly different from healthy controls (Figure 1B–D).
CXCR3 is expressed on autoreactive T cells
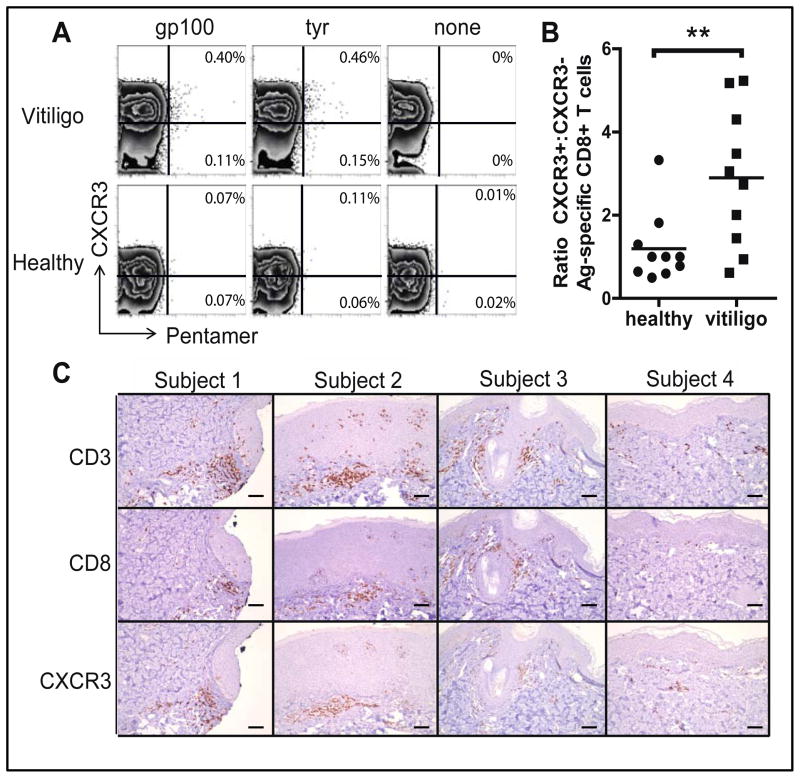
To examine the potential for autoreactive T cells to respond to IFNγ-associated chemokines expressed in the skin of patients with vitiligo, we measured the expression of CXCR3, the common receptor for CXCL9, CXCL10 and CXCL11, on melanocyte-specific CD8+ T cells. We analyzed the blood of 5 HLA-A2+ vitiligo patients and 5 HLA-A2+ healthy controls using melanocyte antigen-specific HLA pentamers (gp100 and tyrosinase) to identify autoreactive CD8+ T cells (Figure 2A). Consistent with previous studies (13, 14), ~0.5 – 1% of CD8+ T cells were positive for each pentamer in vitiligo patients, a significant difference from healthy controls (Figure S2). We found that the majority of pentamer+ cells in vitiligo patients expressed CXCR3, unlike in healthy controls (Figure 2B). To determine whether CXCR3 is expressed in the lesional skin of patients with vitiligo, we used immunohistochemistry on skin biopsies from 4 patients with vitiligo, which revealed CXCR3 expression within each sample (Figure 2C, Figure S3).
Fig. 2. CXCR3 is expressed on antigen-specific cells in the blood and CD8+ T cells in the skin lesions of vitiligo patients.
(A) Expression of CXCR3 on melanocyte antigen-specific pentamer+ CD8+ T cells in the blood of HLA-A2+ patients with vitiligo, pre-gated on CD8+ T cells. Flow plots representative of 5 vitiligo patients and 5 healthy controls are shown; gp100-specific pentamer (gp100), tyrosinase-specific pentamer (Tyr), and control, no pentamer (None). (B) The ratio of CXCR3+:CXCR3− antigen-specific T cells is significantly higher in vitiligo patients than in healthy controls (n=5 donors with 2 pentamers each; two-tailed unpaired t test, **p=0.0098). (C) Immunohistochemical staining of CD3, CD8, and CXCR3 in the skin of 4 patients with active vitiligo, identified from a FFPE clinical tissue bank. All showed positive staining for CD3, CD8, and CXCR3.
A mouse model of vitiligo reflects the IFNγ signature observed in patient samples
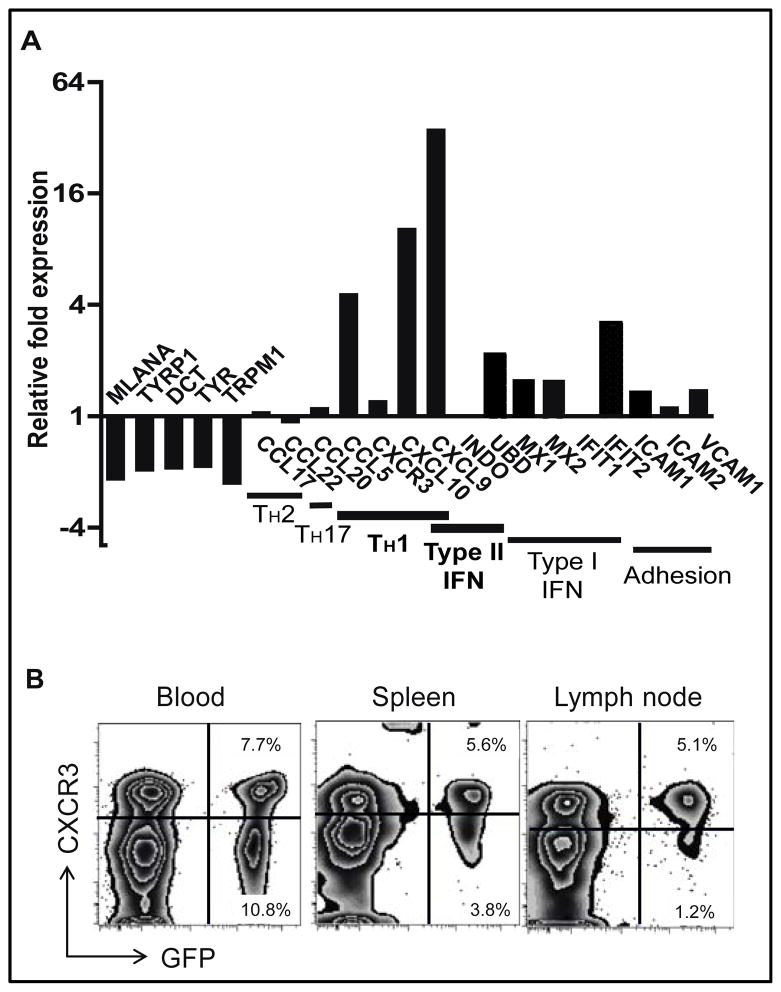
To interrogate the role of CXCR3 chemokines in vitiligo, we used a mouse model that develops depigmentation of the epidermis, similar to human disease (26). Unlike wild-type mice, Krt14-Kitl* mice possess increased numbers of epidermal melanocytes, similar to human skin (32). Following adoptive transfer of premelanosome protein-specific CD8+ T cells (PMELs) and in vivo activation recombinant vaccinia virus expressing their cognate antigen (PMEL), Krt14-Kitl* host mice develop patchy epidermal depigmentation on their ears, tails, noses, and footpads (26). Gene expression profiling of lesional skin from mice with vitiligo revealed a similar chemokine signature to human vitiligo, with a minimal type I IFN gene signature (Figure 3A). Cxcl11, often co-expressed with Cxcl9 and Cxcl10, is not expressed in the C57BL/6 mouse strain due to a spontaneous null mutation (28), and so further studies in our mouse model focused on the contributions of Cxcl9 and Cxcl10. Both chemokines were expressed in mouse skin following vitiligo induction, and were dependent on IFNγ for their expression (Figure 3A, Figure S1B & C). Flow cytometry analysis of cells from the blood, spleen, and skin-draining lymph nodes of mice with vitiligo revealed that a substantial proportion of autoreactive T cells express CXCR3 (Figure 3B, Figure S4, Table S1), similar to humans with disease. Thus, expression of CXCL9, CXCL10, and CXCR3 strongly associated with the depigmentation in vitiligo in both humans and mice, and so we tested their functional significance in our mouse model.
Fig. 3. Expression of CXCL9, CXCL10, and CXCR3 in a mouse model of vitiligo.
(A) Gene expression in fresh ear skin from mice 5 weeks after vitiligo induction and controls were analyzed via microarray using quantile normalization (n=3 per group). As in human disease, mice displayed TH1-specific response in the skin, and expressed the IFNγ-dependent chemokines CXCL9, CXCL10, and CCL5. (B) CXCR3 was expressed on melanocyte-specific CD8+ T cells (GFP+ PMEL) in the blood, spleen, and skin-draining lymph nodes from mice with vitiligo, gated on CD8+ T cells (representative flow plots, n=4 mice from 3 experiments).
CXCR3 expression on T cells is required for the development of vitiligo
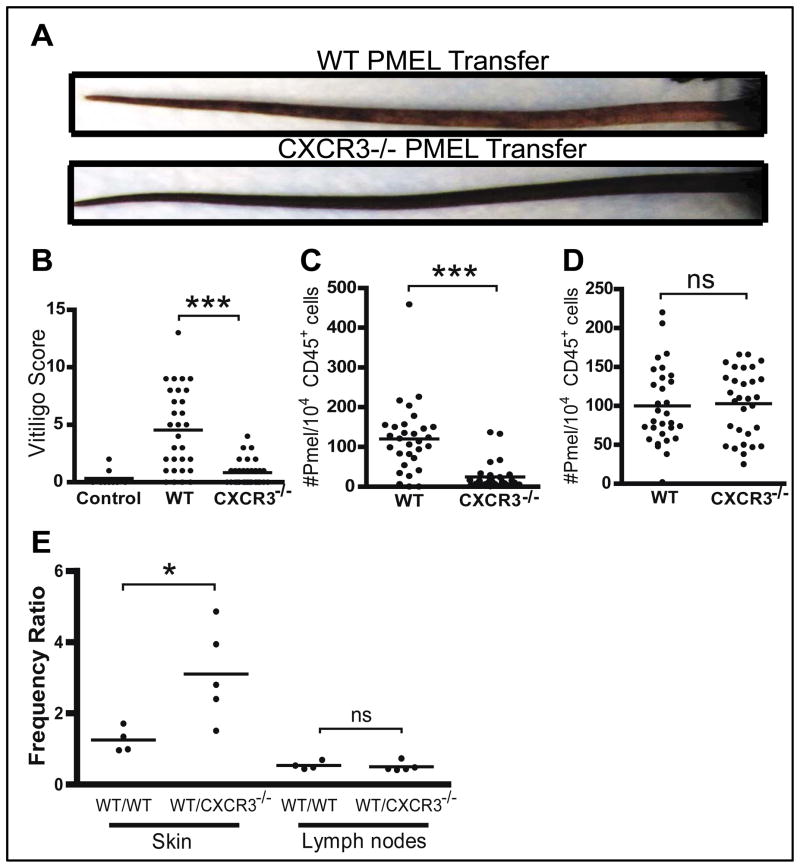
To test whether CXCR3 is required for the development of depigmentation, we adoptively transferred either wild-type (WT) or Cxcr3−/− PMEL T cells to induce vitiligo. Cxcr3−/− T cells were impaired in their ability to induce depigmentation (Figure 4A & B) and failed to accumulate in the skin (Figure 4C), despite normal numbers within the skin-draining lymph nodes (Figure 4D). This pattern is similar to that following treatment with IFNγ neutralizing antibody (26), and supports the observation that Cxcr3−/− TCR transgenic host mice are protected from vitiligo in a separate mouse model of hair depigmentation (33). In order to quantify the decreased efficiency of Cxcr3−/− T cell migration to the skin within the same host, we adoptively transferred equal numbers of CFP+ WT PMEL T cells and GFP+ WT or Cxcr3−/− PMEL T cells into hosts and measured their total numbers in the skin and skin-draining lymph nodes 5 weeks after transfer using flow cytometry. On average, WT T cells were 3 times more prevalent than Cxcr3−/− T cells in the skin, despite similar numbers in skin-draining lymph nodes (Figure 4E, Figure S5).
Fig. 4. CXCR3 expression on T cells is required for the development of vitiligo in mice.
(A) Vitiligo was induced through adoptive transfer of WT or Cxcr3−/− PMELs. Hosts receiving Cxcr3−/− PMELs developed less depigmentation than those that received WT PMELs (representative images of tail depigmentation shown). (B) The degree of depigmentation was scored blindly by one observer 5 weeks after vitiligo induction. Disease scores were significantly lower in mice that received Cxcr3−/− PMELs compared to those that received WT PMELs (one way ANOVA with Tukey’s post-tests: control vs. WT p<0.0001, WT vs. Cxcr3−/− p<0.0001). (C) There was impaired accumulation of Cxcr3−/− PMELs in the ear skin 5–6 weeks after vitiligo induction (two-tailed unpaired t test p<0.0001), however there was (D) no significant difference in the skin-draining lymph nodes. ((A–D) data pooled from 3 independent experiments, n=30 mice per group). (E) WT CFP+ PMELs and WT or Cxcr3−/− GFP+ PMELs were co-transferred in equal proportions into hosts to induce vitiligo. The ratio of WT:WT or WT:Cxcr3−/− PMELs (frequency ratio) in the skin and skin-draining lymph nodes was analyzed by flow cytometry. The frequency ratio for WT:Cxcr3−/− was significantly higher than WT:WT in the skin, but equal frequency ratios were found skin-draining lymph nodes, indicating impaired skin homing of Cxcr3−/− PMELs (n=4–5 mice per group, 3 experiments; representative experiment shown; two-tailed unpaired t test, skin p=0.0303).
CXCL9, CXCL10 and CXCL11 do not act redundantly in vitiligo
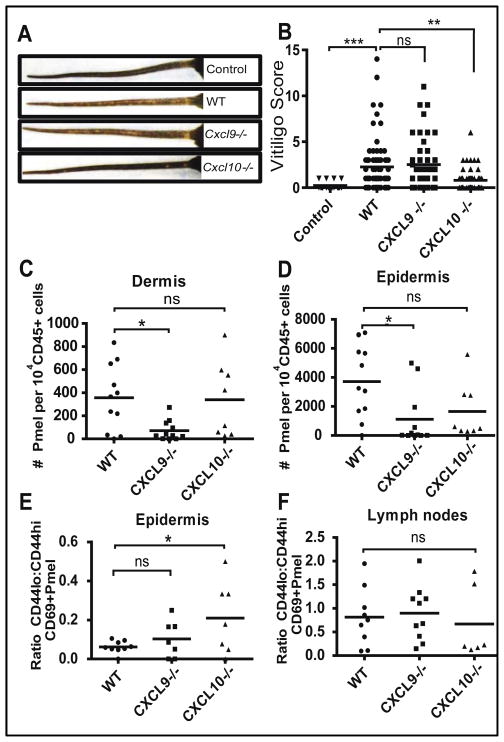
Since CXCL9, CXCL10, and CXCL11 all signal through the CXCR3 receptor, we sought to determine whether individual chemokines were required for depigmentation, or whether they act redundantly, by testing chemokine-deficient hosts in our model. Both Cxcl9−/− and Cxcl10−/− mice were originally created on the 129 mouse strain, and then subsequently backcrossed to C57BL/6. Because of close genetic linkage of Cxcl9, Cxcl10, and Cxcl11, this approach results in Cxcl9−/− and Cxcl10−/− C57BL/6 mice with an intact Cxcl11 gene, unlike the parent strain (28). Therefore, when either knockout mouse is tested on the C57BL/6 background, Cxcl11 expression in the knockout strain could potentially compensate for the deficient chemokine. In our mouse model of vitiligo, Cxcl10−/− hosts developed minimal depigmentation (Figure 5A & B) similar to when Cxcr3−/− T cells were tested, suggesting that vitiligo depends on this single chemokine, and that CXCL11 cannot substitute for its function. In contrast, Cxcl9−/− hosts developed depigmentation comparable to WT controls.
Fig. 5. CXCL9 and CXCL10 play non-redundant roles in vitiligo: CXCL9 induces global recruitment, while CXCL10 promotes epidermal positioning and activation status.
Vitiligo was induced in WT, Cxcl9−/− or Cxcl10−/− hosts. (A) Representative pictures of tail depigmentation. (B) The degree of depigmentation was scored blindly 5–6 weeks after vitiligo induction. Cxcl10−/− mice had significantly lower disease scores than WT mice (n=27 control, 80 WT, 44 Cxcl9−/−, 59 Cxcl10−/− mice from 9 separate experiments; one-way ANOVA p<0.0001 with Tukey’s post-tests: control vs. WT p=0.0003, control vs. Cxcl9−/− p=0.0002, WT vs. Cxcl10−/− p=0.0009, Cxcl9−/− vs. Cxcl10−/− p=0.0007). PMEL accumulation in the skin was analyzed by flow cytometry of the dermis and epidermis. (C) Reduced number of PMELs in the dermis of Cxcl9−/−, but not Cxcl10−/− mice (one-way ANOVA p=0.0219 with Tukey’s post-tests: WT vs. Cxcl9−/− p=0.0304). (D) Reduced number of PMELs in the epidermis of Cxcl9−/−, and a trend toward reduced PMELs in Cxcl10−/− mice compared to WT mice (one-way ANOVA p=0.022 with Tukey’s post-tests: WT vs. Cxcl9−/− p=0.0223). ((C–D) data pooled from 2 separate experiments, n=11 WT, 11 Cxcl9−/−, and 8 Cxcl10−/− age-matched male mice). (E) The ratio of antigen-experienced (CD69+) CD44lo to CD44hi PMELs was significantly higher in the epidermis of Cxcl10-deficient mice compared to WT mice (n=9 WT, 7 Cxcl9−/−, and 7 Cxcl10−/− pooled from 2 separate experiments, zero ratios not reported; one way ANOVA with Tukey’s post-tests: WT vs. Cxcl10−/− p=0.0478). (F) However this ratio was unchanged in skin-draining lymph nodes, indicating an epidermis-specific defect.
CXCL10 promotes epidermal positioning and effector function of autoreactive T cells
To assess the functional contributions of CXCL10 to depigmentation, we analyzed the positioning of PMEL T cells in the layers of the skin and their activation status 5–6 weeks post-transfer. Since melanocytes are located in the epidermis, we first addressed whether or not PMELs could migrate into the epidermis in chemokine knockout mice using flow cytometry to separately analyze the dermal and epidermal layers of the skin. Cxcl9−/− hosts exhibited lower numbers of PMELs in both the dermis (Figure 5C) and epidermis (Figure 5D) as compared to WT hosts, supporting its role as a global positioning and recruiting chemokine (34). However, despite the decreased recruitment efficiency of PMELs in Cxcl9−/− hosts, the PMELs were still highly functional, as they were capable of inducing depigmentation comparable to WT hosts. In contrast, Cxcl10−/− hosts had similar numbers of PMELs in the dermis as compared to WT hosts (Figure 5C), and a trend toward a lower number of cells in the epidermis (Figure 5D), suggesting that CXCL10 is required for T cell function within the skin beyond simple recruitment, and may play a role in directed migration within the skin.
CXCL10 has also been reported to play an important role in T cell tethering and activation (34). We therefore assessed whether the cells responded to antigen and target cells in the same capacity in our WT and Cxcl10−/− mice. Using CD69 as a marker of skin memory T cells that had been previously activated in vivo (35), we addressed whether or not epidermal PMELs maintained high CD44 expression (Figure 5E & F). We found that the ratio of CD44lo to CD44hi epidermal CD69+ PMELs was significantly higher in Cxcl10−/− hosts, suggesting that they have an altered activation status compared to PMELs in WT mice (Figure 5E). This ratio was not elevated in the lymph nodes of host mice, indicating a skin-specific effect (Figure 5F).
Neutralization of CXCL10 both prevents and reverses depigmentation in vitiligo
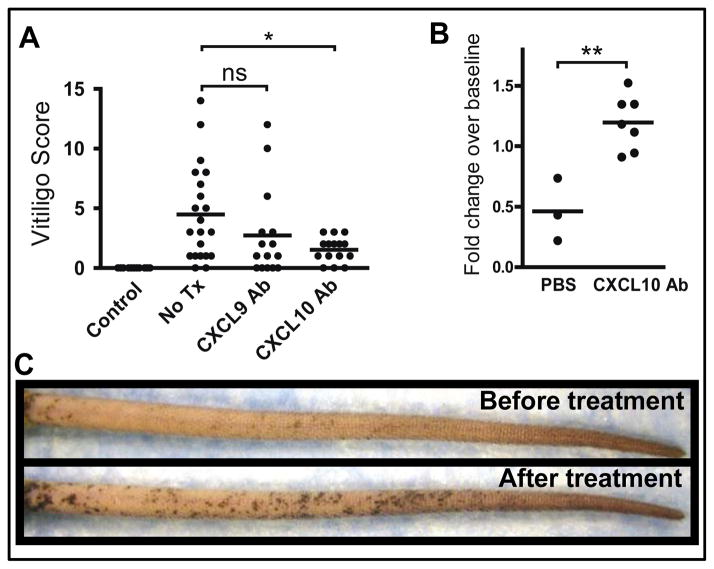
Cxcl9−/− and Cxcl10−/− hosts have previously been shown to exhibit defects in T cell priming against viruses (34, 36, 37). Therefore, in order to avoid defective priming of PMELs in our model and to assess the roles of these chemokines during the effector phase of disease in WT hosts, we treated mice with either CXCL9 or CXCL10 neutralizing antibody or PBS control beginning 2 weeks after adoptive transfer. This timeframe thereby allowed for normal activation of the PMEL T cells and clearance of the virus before chemokine neutralization. Consistent with our observations in chemokine-deficient hosts, neutralization of CXCL10 significantly reduced depigmentation in our model, while neutralization of CXCL9 did not (Figure 6A).
Fig. 6. CXCL10 neutralizing antibody both prevents and reverses vitiligo.
(A) Vitiligo was induced and mice were treated with either no treatment or PBS (No Tx, n=21), CXCL9 neutralizing antibody (CXCL9 Ab, n=15), or CXCL10 neutralizing antibody (CXCL10 Ab, n=15). Antibodies were administered 3x weekly beginning 2 weeks after vitiligo induction. The degree of depigmentation was scored blindly by one observer 5 weeks after vitiligo induction (after 3 weeks of treatment). CXCL10 Ab-treated mice had significantly lower disease scores than control mice (pooled from 3 independent experiments; one-way ANOVA p=0.0012, with Tukey’s post-tests: control vs. no treatment p=0.0011, no tx vs. CXCL10 Ab p=0.0273). (B–C) Mice with extensive vitiligo (>50% depigmentation on the tail) were treated with CXCL10 neutralizing antibody (CXCL10 Ab, n=7) or vehicle control (PBS, n=3) for 8 weeks. (B) Degree of repigmentation in the tails. Photographs of mouse tails before and 8 weeks after treatment were analyzed using ImageJ software to quantify the extent of repigmentation. Fold change over baseline reflects the total pigmentation following treatment divided by total pigmentation prior to treatment, therefore a score of 1.0 reflects no change. CXCL10 Ab treatment resulted in repigmentation of the tails, whereas control mice experienced disease progression (one-tailed unpaired t test, **p=0.0019). (C) Repigmentation in a mouse with vitiligo following 8 weeks of treatment with CXCL10 Ab (representative example shown).
In order to test the efficacy of CXCL10 neutralization as a treatment of established disease, vitiligo was induced as above. Eight to ten weeks later, approximately 10% of mice exhibited >50% depigmentation of the tails, and we randomized them into two groups, one treated with CXCL10 neutralizing antibody, and a control group treated with PBS (Figure 6B) or isotype control antibody (Figure S6). Approximately 4 weeks after initiation of treatment, mice receiving CXCL10 neutralizing antibody began to develop repigmentation, which continued for an additional 4 weeks, whereas control mice primarily exhibited minimal improvement or progression. Repigmentation occurred from the hair follicles on the tail (Figure 6C).
Discussion
The recent success of systemic biologic therapies that target cytokines in psoriasis and other inflammatory diseases suggest that a similar approach might be effective for vitiligo. Our observation that the immune response in vitiligo is TH1-specific is consistent with a previous report (38), and may explain why targeted treatments for psoriasis, a prototypic TH17-mediated disease, are ineffective for vitiligo (20–22). The identification of an IFNG-specific signature in vitiligo is in sharp contrast to other inflammatory skin diseases, including dermatomyositis, cutaneous lupus erythematosus, and lichen planus, which express an IFN signature that reflects both Type I (IFNA/B) and Type II (IFNG) interferons, with a predominance of Type I over Type II (39, 40). Consistent with our findings, other studies report increased expression of IFNG in vitiligo skin, however they did not measure the expression of downstream gene targets or their functional/biological significance (13, 25, 38). We previously reported that neutralizing IFNγ in a mouse model of vitiligo prevented depigmentation (26). Together, these data suggest that targeting IFNγ-dependent events may be a particularly effective treatment strategy for vitiligo.
The expression of CXCR3 on autoreactive T cells in both human vitiligo patients and in our mouse model, as well as the requirement of CXCR3 for autoreactive T cell accumulation in the skin and subsequent depigmentation, implicates this chemokine pathway as functionally required for vitiligo. Therefore, small molecule inhibitors of CXCR3, currently in development by multiple sources (41), may be effective therapies. The clear dominance of CXCL10 over CXCL9 in our mouse model of vitiligo is intriguing, providing an opportunity to target CXCL10 alone for developing new treatments. Depending on the model of inflammation studied, the CXCR3 ligands can have redundant, dominant, cooperative, or even antagonistic functions (28). CXCL10 dominance has been reported primarily during viral infections of peripheral tissues (28), but also in the IL-10null model of inflammatory bowel disease (42). In the MRL-lpr lupus mouse model, Cxcl9−/− mice were protected from disease while Cxcl10−/− mice were not, suggesting CXCL9 dominance in that system (43). Other models, including brain inflammation following malarial infection (44) and HSV-2 infection of the spinal cord (45), required both CXCL9 and CXCL10 for T cell recruitment, suggesting that spatiotemporal differences in chemokine expression may be responsible for a cooperative, coordinated role of these chemokines (28). The fact that mice on the C57BL/6 background do not express CXCL11 suggests that vitiligo in our mouse model does not depend on this chemokine. The presence of intact Cxcl11 in Cxcl9−/− mice did not exacerbate the disease, and in Cxcl10−/− mice Cxcl11 was unable to compensate for its absence. These data suggest that CXCL11 does not play a major role in our mouse model of vitiligo, however we cannot yet rule out a role in human disease. Additional studies will be required to determine which cell types produce CXCL9 and CXCL10 in vitiligo, how they interact to promote vitiligo pathogenesis, and the relative importance of each chemokine in humans.
Our observations suggest that CXCL10 is required for more than T cell recruitment to the target tissue. This is consistent with recently described functional roles for CXCL10 within infected tissues and lymph nodes beyond simple recruitment, including proper localization within the lymph node microenvironment (36, 46), increased migration velocity in the brain (47), promoting T cell interaction with dendritic cells (46), and lymph node retention (48). Any or all of these CXCL10-dependent functions may also be important in vitiligo. The decreased localization of autoreactive T cells to the epidermis in Cxcl10−/− mice with relatively normal numbers in the dermis suggest that proper localization within the skin is one of these important functions, in contrast to CXCL9-deficiency, which reveals a more global defect in skin-homing despite normal effector function. The appearance of CD69+CD44lo autoreactive T cells within the skin of CXCL10−/− hosts suggests that CXCL10 may be required for maintained expression of this memory marker within the skin. Alternatively, CXCL10 may antagonize the development of a CD44lo population of CD8+ T cells, which was shown to be capable of suppressing autoimmune responses in the gut (49). A global defect in priming in our model is unlikely, since this population is not present within skin draining lymph nodes. CD44 is important for the survival, activation, directed migration (particularly through basement membranes) and general “fitness” of memory CD8+ T cells within peripheral tissues (50). Whether our observations represent a direct effect of CXCL10 on CD44 expression in memory cells, or a secondary effect following mislocalization within the skin, is unknown. The specific roles CXCL10 plays within the skin in vitiligo are important but probably complex, and will require further study.
Previous studies, including ours, reported that interfering with IFNγ prevents the onset of depigmentation in various mouse models of vitiligo, including genetic deficiency or antibody neutralization (26, 33). However successful treatment strategies should be able to reverse established disease through repigmentation, rather than simply prevent it. Depigmentation in patients with vitiligo can be reversed upon successful treatment with topical steroids, topical calcineurin inhibitors, or nbUVB light therapy (1), and this reversal is primarily due to melanocyte regeneration from reservoirs in hair follicles within the lesion (10). CXCL10 antibody treatment induced epidermal repigmentation in mice with established vitiligo, and therefore may offer a targeted systemic therapeutic option for patients with disease. A therapeutic strategy based on these observations may incorporate existing neutralizing antibodies or chemical inhibitors of IFNγ (51), CXCL10 (52), or CXCR3 (41, 53), or inhibitors of IFNγ or CXCL10 signaling through chemical inhibition or RNAi-induced knockdown. Completed clinical trials have tested either CXCL10 neutralization or CXCR3 blockade for psoriasis, Crohn’s disease, and ulcerative colitis, with disappointing results (41). However unlike vitiligo, these diseases are characterized by the expression of additional cytokines and chemokines that likely reflect a more robust inflammatory response with multiple redundant pathways mediating T cell recruitment to sites of inflammation (54). Therefore, vitiligo may be the optimal autoimmune disease in which to test compounds that target the IFNγ-CXCL10-CXCR3 cytokine axis.
In addition to its therapeutic implications, the ability of CXCL10 neutralization to promote repigmentation in our mouse model reveals that 1) mouse melanocytes are capable of regeneration as in human vitiligo, and 2) CXCL10 is required to prevent regeneration. The ability of melanocytes to quickly regenerate following treatment suggests that there is active autoimmunity in depigmented skin that can be disrupted with treatment, including neutralization of CXCL10. Exactly how CXCL10 maintains depigmentation in vitiligo is unknown, although any of the functional roles for CXCL10 described above (localization, migration velocity, dendritic cell interaction, or tissue retention) may be important. A recent study found that antigen-specific CD8+ resident memory T cells (Trm cells) that remain within the vaginal mucosa following viral infection act primarily as sentinels to detect reinfection (55). In this role, they produce large amounts of IFNγ upon antigen reencounter, which stimulates production of chemokines to recruit additional central memory effectors to help control the infection. Therefore, a population of PMELs may remain within depigmented skin and act as sentinels for melanocyte regeneration, producing IFNγ after melanocyte encounter, which induces chemokines and recruits additional central memory PMELs to target these cells and prevent repigmentation.
Our studies provide the basis for further exploration of CXCL10 as a treatment target for vitiligo, however, they have potential limitations. There are reports describing elevated IL-17 in vitiligo patient serum as well as IL-17+ T cells in lesional skin (56–59), although the functional significance of these observations is currently unclear. We did not see increased expression of IL-17 or the IL-17-dependent chemokine CCL20 in any of our 13 vitiligo patients. It is possible that our patient population reflects one subset of vitiligo with an IFNγ-specific profile, and that our mouse model reflects this particular subset. There may be multiple subtypes of vitiligo based on cytokine expression, though this has yet to be determined. Nevertheless, our results identify CXCL10 as an important mediator of vitiligo pathogenesis in a mouse model of disease. In addition, they reveal that the maintenance of depigmentation in vitiligo is an active process of immunity that requires CXCL10. Ultimately, clinical trials will be required to test the therapeutic implications of our observations to patients with this devastating disease.
Materials and Methods
Study design
The overall study design was based on controlled laboratory experimentation using ex vivo human tissue samples and a mouse model for in vivo mechanistic studies. The research objectives at the outset of the study were to test the hypothesis that IFNγ-inducible chemokines were responsible for the recruitment of autoreactive T cells to the skin. This hypothesis was formed on the basis of previously reported observations in our mouse model (26). Sample size was determined using the approach described by Dell, et al. (60). Briefly, each experiment was powered to detect a difference between group means of twice the observed standard deviation, with a power of 0.8 and a significance level of 0.05. Replicate experiments were performed two or three times. Inclusion/exclusion criteria for human patient samples were as follows: Vitiligo lesional skin was obtained from clinical biopsies that revealed characteristic features of vitiligo, including a prominent T cell infiltrate. Control skin was acquired from tumor excision tips without notable pathology from donors without vitiligo. Human blood and serum was collected from patients examined by a dermatologist (JEH) and diagnosed with vitiligo, as well as healthy volunteers with no known autoimmune diseases. Mice used for these studies were on the C57BL/6J (B6) background, or a mixed 129 x C57BL/6 background that had been backcrossed to B6 for more than 10 generations. Mice were randomly selected and assigned to treatment groups as described below. Scoring of vitiligo progression in mice was done by a single investigator who was blinded to treatments. For repigmentation experiments, scores were quantified objectively using Image J without blinding.
Study subjects
The collection of serum and blood from healthy donors and patients with vitiligo was approved by the Institutional Review Board (IRB) at the University of Massachusetts Medical School (UMMS) and the University of British Columbia. All participants gave written informed consent before all procedures. Identification and acquisition of FFPE clinical tissue samples was IRB-approved at the respective institutions, and all samples were deidentified prior to use in experiments.
ELISAs
Human CXCL9, CXCL10 and CXCL11 duo set ELISA kits were used to analyze patient serum according to the manufacturer’s instructions (R&D Systems). Optical densities were measured using a Perkin Elmer EnVision 2102 multilabel reader, and 450nm–595nm values were used to calculate concentrations using a 4 parameter logarithmic standard curve.
Mice
KRT14-Kitl*4XTG2Bjl (Krt14-Kitl*) mice were a gift from BJ Longley, University of Wisconsin. PMEL TCR transgenic mice are Thy1.1+, and were obtained from The Jackson Laboratory, stock no. 005023, B6.Cg Thy1a/CyTg(TcraTcrb)8Rest/J. GFP-PMEL and CFP-PMEL TCR transgenic mice were produced by crossing PMEL transgenic mice with DPEGFP mice, which express GFP in T cells (provided by U. von Andrian, Harvard Medical School, Boston, MA), or with CAG-ECFP [The Jackson Laboratory, stock no. 004218, B6.129(ICR)-Tg(CAG-ECFP)CK6Nagy/J], respectively. CFP-PMEL TCR transgenic mice were backcrossed to B6 for a total of 10 generations. Cxcr3−/− PMEL TCR transgenic mice were produced by crossing GFP-PMEL mice with the B6.129P2-Cxcr3tm1Dgen/J strain (The Jackson Laboratory, stock no. 005796). To develop Cxcl9- and Cxcl10-deficient recipients, B6.129S4-Cxcl9tm1Jmf (provided by Joshua M. Farber, NIAID, Bethesda, MD) and B6.129S4-Cxcl10tm1Adl/J (The Jackson Laboratory, stock no. 006087) mice were crossed with Krt14-Kitl*. For consistency, the Krt14-Kitl* allele was heterozygous on all mice used in experiments. All mice were on a C57BL/6J background, were maintained in pathogen-free facilities at UMMS, and procedures were approved by the UMMS Institutional Animal Care and Use Committee and in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Vitiligo induction & chemokine neutralization
Vitiligo was induced through adoptive transfer of PMEL CD8+ T cells as described previously (26). Briefly, PMEL CD8+ T cells were isolated from the spleens of PMEL TCR transgenic mice through negative selection on microbeads (Miltenyi Biotech) according to the manufacturer’s instructions. Purified CD8+ T cells (1×106) were injected intravenously into sublethally irradiated (500 rads 1 day before transfer) Krt14-Kitl* hosts (12–16 weeks of age). Recipient mice also received i.p. injection of 1×106 pfu rVV-hPMEL (N Restifo, NCI, NIH) on the same day of transfer. Mice used as controls for vitiligo were sublethally irradiated but did not receive rVV-hPMEL or PMEL CD8+ T cell transfer. In order to measure the migration efficiency of Cxcr3−/− T cell to the skin within the same host, equal numbers (0.5 ×106) of CFP+ WT T cells and GFP+ WT or Cxcr3−/− T cells were injected intravenously.
CXCL9 and CXCL10 blockade was performed by i.p. injection of 100 μg of either CXCL9 (clone 2A6) or CXCL10 (clone 1F11) neutralizing antibodies three times weekly for the duration of the observation period (3–5 weeks). Mice with vitiligo used as treatment controls for chemokine blockade were treated with either no antibody or with an equal volume of PBS. Vitiligo score was objectively quantified by an observer blinded to the experimental groups, using a point scale based on the extent of depigmentation at four easily visible locations, including the ears, nose, rear footpads, and tails as described previously (26). Each location was examined and the extent of depigmentation estimated as a percentage of the anatomic site; both left and right ears, and left and right rear footpads were estimated together and therefore evaluated as single sites. Points were awarded as follows: no evidence of depigmentation (0%) received a score of 0, > 0–10% = 1 point, > 10–25% = 2 points, > 25–75% = 3 points, > 75–<100% = 4 points, 100% = 5 points. The “vitiligo score” was the sum of the scores at all four sites, with a maximum score of 20 points.
To induce repigmentation, 7–8 weeks after induction of vitiligo, mice with at least 50% tail depigmentation were randomly assigned to receive either CXCL10 neutralizing antibody as before, or control treatment (PBS or isotype antibody as indicated) for a total of 8 weeks. Treatment efficacy was objectively quantified by comparison of the tail photographs before and after treatment using ImageJ software (NIH). All images were converted to 8-bit black and white and the brightness threshold adjusted so that all pigmented areas of the tails were highlighted. Highlighted pixels were quantified and the mean level of pigmentation determined. Fold change over baseline reflects the mean pigmentation following treatment divided by mean pigmentation prior to treatment, and therefore a score of 1.0 reflects no change.
Flow cytometry
Ears, tails, spleens and skin-draining lymph nodes were harvested at the indicated times. Spleens were disrupted, and the red blood cells lysed using RBC Lysis buffer. Ears and tail skin were incubated in skin digest media [RPMI containing 0.5% DNase I (Sigma-Aldrich) and 0.5 mg ml−1 of liberase TL enzyme blend (Roche)] and processed using a medimachine (BD Biosciences) as described previously (26). For separation of the dermis and epidermis, tail skin samples were incubated with 2.4U mL−1 dispase (Roche) for 1h at 37°C. Epidermis was removed and mechanically disrupted using 70μm cell strainers, and dermis samples were incubated with 1mg mL−1 collagenase IV with 0.5mg mL−1 DNAse I (Sigma-Aldrich) for 1h at 37°C on a shaker. Human peripheral blood mononuclear cells (PBMCs) were isolated using Histopaque®-1077(Sigma-Aldrich) and washed in PBS. All cells were filtered through a 70 μm mesh prior to analysis. The following antibodies were obtained from Biolegend: Mouse (CD4, CD8b, CD45.2, CD90.1, CXCR3, CD44, CD69, Fc block), human (CD3, CD4, CD8, CD19, CD45, CXCR3). The following fluorescent-conjugated pentamers were obtained from ProImmune Ltd: Tyrosinase 369–377 (371D) YMDGTMSQV, gp100 (PMEL) 209–217 ITDQVPFSV. 10 × 106 PBMCs were allocated per staining condition. To improve the sensitivity of detection, the cells were incubated first at 37°C for 30 minutes in 50μl Dasatinib solution (50nM) (Axon Medchem BV) as previously described (61). Then 10μl labeled pentamers were added and incubated at room temperature for 10 minutes, followed by washing and the addition of secondary antibodies. Pentamer-positive cells were identified by gating on CD45+, CD19−, CD3+, CD4−, CD8+ lymphoid cells (Fig S2). The data were collected and analyzed using BD LSR II flow cytometer (BD Biosciences) and FlowJo (Tree Star, Inc.).
Gene expression profiling and validation
Mouse Whole-Genome 6 version 2.0 (Illumina, Inc.) was used for expression profiling of fresh mouse ear skin, and Whole-Genome DASL HT version 4.0 Assay (Illumina, Inc.) was used for expression profiling of formalin-fixed, paraffin-embedded (FFPE) human samples, according to the manufacturer’s instructions. Ear skin from mice with vitiligo was compared to control mice that were irradiated but did not receive PMEL TCR transgenic T cells or VV-gp100. Lesional skin with characteristic features of vitiligo and a notable T cell infiltrate was identified from 5 vitiligo patients in a clinical biopsy database and compared to 5 age- and site-matched controls, which were obtained from tumor excision tips (University of Pennsylvania, Philadelphia, PA). Fold changes were calculated using quantile normalized data (Matlab 2012b) by dividing the average intensity of vitiligo samples by the average intensity of controls. Only genes significantly detected over background (p<0.05) in at least one sample were considered.
Expression of genes of interest in mouse tissues was confirmed using quantitative real-time reverse transcriptase-PCR on ear skin from an additional 5 control and 6 vitiligo mice. Briefly, ears were minced into <5mm strips, stored in RNAlater (Ambion) overnight at 4°C and then transferred to −20°C until RNA was extracted using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s instructions. Complimentary DNA (cDNA) was generated with iScript cDNA synthesis kit (Bio-Rad). Real-time PCR was conducted with cDNA and iQ SYBR Green (Bio-Rad) in a Bio-Rad iCycler iQ, according to manufacturer recommendations. Mouse primer sequences are as follows: Mx1 5′-CGATCAAGACGGCCATGAGTC-3′ (sense), 5′-CTCGTCGGTGTCATCTTCTGC-3′ (antisense); Cxcl9 5′-ATCTCCGTTCTTCAGTGTAGCAATG-3′ (sense), 5′-ACAAATCCCTCAAAGACCTCAAACAG-3′ (antisense); Cxcl10 5′-AGGGGAGTGATGGAGAGAGG-3′ (sense), 5′-TGAAAGCGTTTAGCCAAAAAAGG-3′ (antisense); actin-beta (Actb): 5′-GGCTGTATTCCCCTCCATCG-3′ (sense), 5′-CCAGTTGGTAACAATGCCATGT-3′ (antisense). Gene expression is reported relative to the average expression in control mice after normalization to expression of beta-actin.
Human microarray data were validated using nCounter® Analysis System (NanoString Technologies, Inc.) in a separate series of FFPE samples from an additional 3 healthy and 8 vitiligo skin biopsies distinct from those used for the Illumina DASL assay. Samples were selected as before, RNA isolated by High Pure RNA Paraffin Kit (Roche), and analyzed by Illumina Bioanalyzer. Based on manufacturer recommendations, RNA samples were adjusted to input ~100 ng of RNA fragments greater than 300 nucleotides, which was then hybridized to the cartridge. Expression for each sample was normalized to 10 internal reference genes, and all data analysis was performed according to manufacturer recommendations.
Immunohistochemistry
Immunohistochemical studies were performed on 5-μm sections of 4 FFPE clinical biopsy specimens with characteristic features of vitiligo and a notable mononuclear infiltrate. Samples were distinct from those used for gene expression analyses. Slides were first deparaffinized and then rehydrated. The sections were blocked for endogenous peroxidase activity with an application of Dual Endogenous Block (Dako) and then incubated with one of the following antibodies: anti-CD3 mouse monoclonal antibody, clone F7.2.38, (Dako) at a dilution of 1:200, anti-CD8 mouse monoclonal antibody, clone C8/144B, (Dako) at a dilution of 1:80, or anti-CXCR3 mouse monoclonal antibody, clone 1C6/CXR3, (BD Pharmingen) at a dilution of 1:100. Following a rinse in wash buffer (1 X TBS containing 0.05% Tween 20), sections were incubated with the Dako Envision+ HRP detection reagent, washed, and treated with a solution of diaminobenzidine and hydrogen peroxide (Dako) for 10 minutes to produce the visible brown pigment.
Statistical analyses
All statistical analyses were performed using GraphPad Prism software. Dual comparisons were made using the unpaired Student’s t test (parametric data, mouse studies) or Mann-Whitney U test (nonparametric data, human ELISAs). Groups of 3 or more were analyzed via ANOVA with Tukey’s post-tests for experiments comparing genotypes of mice, or with Dunnett’s post-tests for experiments comparing treatments to controls. P values less than 0.05 were considered significant.
Supplementary Material
Fig. S1. Validation of selected transcripts in humans and mice.
Fig. S2. Frequencies of melanocyte antigen-specific T cells in peripheral blood of healthy or vitiligo patients.
Fig. S3. Staining controls for vitiligo biopsy immunohistochemistry.
Fig. S4. Gating strategy for examining CXCR3+ mouse T cells.
Fig. S5. Cotransfers of WT and CXCR3-deficient PMELs.
Fig. S6. Repigmentation following treatment with CXCL10 neutralizing antibody but not isotype control antibody.
Table S1. Percentages of CXCR3+ and CXCR3− Pmels in vitiligo mice; gated on total CD8+ cells.
Acknowledgments
We thank clinic patients (of JEH and YZ) for donating blood, B.J. Longley for Krt14-Kitl* mice, U. von Andrian for DPEGFP mice, J.M. Farber for Cxcl9-deficient mice, N. Restifo for recombinant vaccinia virus, M. Frisoli and K. Essien for technical assistance, and M. Brehm, D. Greiner, and C. Hartigan for critical support in collecting blood for ELISA and flow analysis.
Funding: Supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number AR061437 and research grants from the Charles H. Hood Foundation, Vitiligo Research Foundation, and Dermatology Foundation (to JEH). The University of Massachusetts Center for Clinical Research was responsible for blood and serum collection, and is supported by National Institutes of Health Clinical & Translational Sciences Award UL1TR000161. The University of Pennsylvania Skin Disease Research Center Core was responsible for identification and processing of human tissue samples for gene expression analysis, and is supported by National Institutes of Health grant 5-P30-AR057217-03. The Wistar Institute Genomics Core performed Illumina gene expression analyses and is supported by National Institutes of Health grant P30 CA010815. Flow cytometry equipment used for this study is maintained by the University of Massachusetts Medical School and the University of Pennsylvania Flow Cytometry Core Facility.
Footnotes
Author contributions: J.E.H., A.D.L., C.A.H., and A.D. designed the study. M.R., P.A., J.M.R., T.H.H., and K.D. performed experiments. M.S., Y.Z., A.D., and J.E.H. provided patient and patient sample management. M.R., J.M.R., K.D., and J.E.H. drafted the manuscript, and A.D.L., C.A.H., J.E.H., J.M.R., and T.H.H. critically revised the manuscript.
Competing interests: The authors declare they have no competing interests.
Data and Materials Availability: The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (62) and are accessible through GEO Series accession number GSE53148 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE53148).
References and Notes
- 1.Taieb A, Picardo M. Clinical practice. Vitiligo. N Engl J Med. 2009;360:160–169. doi: 10.1056/NEJMcp0804388. [DOI] [PubMed] [Google Scholar]
- 2.Passeron T, Ortonne JP. Activation of the unfolded protein response in vitiligo: the missing link? J Invest Dermatol. 2012;132:2502–2504. doi: 10.1038/jid.2012.328. [DOI] [PubMed] [Google Scholar]
- 3.Shah AA, Sinha AA. Oxidative stress and autoimmune skin disease. Eur J Dermatol. 2013;23:5–13. doi: 10.1684/ejd.2012.1884. [DOI] [PubMed] [Google Scholar]
- 4.Laddha NC, Dwivedi M, Mansuri MS, Gani AR, Ansarullah M, Ramachandran AV, Dalai S, Begum R. Vitiligo: interplay between oxidative stress and immune system. Exp Dermatol. 2013;22:245–250. doi: 10.1111/exd.12103. [DOI] [PubMed] [Google Scholar]
- 5.van den Boorn JG, Melief CJ, Luiten RM. Monobenzone-induced depigmentation: from enzymatic blockade to autoimmunity. Pigment cell & melanoma research. 2011;24:673–679. doi: 10.1111/j.1755-148X.2011.00878.x. [DOI] [PubMed] [Google Scholar]
- 6.Toosi S, Orlow SJ, Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol. 2012;132:2601–2609. doi: 10.1038/jid.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosenson JA, Zloza A, Klarquist J, Barfuss AJ, Guevara-Patino JA, Poole IC. HSP70i is a critical component of the immune response leading to vitiligo. Pigment cell & melanoma research. 2012;25:88–98. doi: 10.1111/j.1755-148X.2011.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosenson JA, Zloza A, Nieland JD, Garrett-Mayer E, Eby JM, Huelsmann EJ, Kumar P, Denman CJ, Lacek AT, Kohlhapp FJ, Alamiri A, Hughes T, Bines SD, Kaufman HL, Overbeck A, Mehrotra S, Hernandez C, Nishimura MI, Guevara-Patino JA, Le Poole IC. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Science translational medicine. 2013;5:174ra128. doi: 10.1126/scitranslmed.3005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. Journal of the American Academy of Dermatology. 2011;65:473–491. doi: 10.1016/j.jaad.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 10.Glassman SJ. Vitiligo, reactive oxygen species and T-cells. Clin Sci (Lond) 2011;120:99–120. doi: 10.1042/CS20090603. [DOI] [PubMed] [Google Scholar]
- 11.Schallreuter KU, Bahadoran P, Picardo M, Slominski A, Elassiuty YE, Kemp EH, Giachino C, Liu JB, Luiten RM, Lambe T, Le Poole IC, Dammak I, Onay H, Zmijewski MA, Dell’Anna ML, Zeegers MP, Cornall RJ, Paus R, Ortonne JP, Westerhof W. Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp Dermatol. 2008;17:139–140. doi: 10.1111/j.1600-0625.2007.00666_1.x. discussion 141–160. [DOI] [PubMed] [Google Scholar]
- 12.Spritz RA. Six decades of vitiligo genetics: genome-wide studies provide insights into autoimmune pathogenesis. J Invest Dermatol. 2012;132:268–273. doi: 10.1038/jid.2011.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, Melief CJ, Vyth-Dreese FA, Luiten RM. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129:2220–2232. doi: 10.1038/jid.2009.32. [DOI] [PubMed] [Google Scholar]
- 14.Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. The Journal of experimental medicine. 1998;188:1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Wijngaard R, Wankowicz-Kalinska A, Le Poole C, Tigges B, Westerhof W, Das P. Local immune response in skin of generalized vitiligo patients. Destruction of melanocytes is associated with the prominent presence of CLA+ T cells at the perilesional site. Lab Invest. 2000;80:1299–1309. doi: 10.1038/labinvest.3780138. [DOI] [PubMed] [Google Scholar]
- 16.Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. The American journal of pathology. 1996;148:1219–1228. [PMC free article] [PubMed] [Google Scholar]
- 17.Felsten LM, Alikhan A, Petronic-Rosic V. Vitiligo: a comprehensive overview Part II: treatment options and approach to treatment. Journal of the American Academy of Dermatology. 2011;65:493–514. doi: 10.1016/j.jaad.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 18.Taieb A, Alomar A, Bohm M, Dell’anna ML, De Pase A, Eleftheriadou V, Ezzedine K, Gauthier Y, Gawkrodger DJ, Jouary T, Leone G, Moretti S, Nieuweboer-Krobotova L, Olsson MJ, Parsad D, Passeron T, Tanew A, van der Veen W, van Geel N, Whitton M, Wolkerstorfer A, Picardo M. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5–19. doi: 10.1111/j.1365-2133.2012.11197.x. [DOI] [PubMed] [Google Scholar]
- 19.Baker EL, Coleman CI, Reinhart KM, Phung OJ, Kugelman L, Chen W, White CM, Mamolo CM, Cappelleri JC, Baker WL. Effect of Biologic Agents on Non-PASI Outcomes in Moderate-to-Severe Plaque Psoriasis: Systematic Review and Meta-Analyses. Dermatology and therapy. 2012;2:9. doi: 10.1007/s13555-012-0009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alghamdi KM, Khurrum H, Taieb A, Ezzedine K. Treatment of generalized vitiligo with anti-TNF-alpha Agents. J Drugs Dermatol. 2012;11:534–539. [PubMed] [Google Scholar]
- 21.Alghamdi KM, Khurrum H, Rikabi A. Worsening of vitiligo and onset of new psoriasiform dermatitis following treatment with infliximab. Journal of cutaneous medicine and surgery. 2011;15:280–284. doi: 10.2310/7750.2011.10068. [DOI] [PubMed] [Google Scholar]
- 22.Dayel SB, Alghamdi K. Failure of alefacept in the treatment of vitiligo. J Drugs Dermatol. 2013;12:159–161. [PubMed] [Google Scholar]
- 23.Gilhar A, Pillar T, Eidelman S, Etzioni A. Vitiligo and idiopathic guttate hypomelanosis. Repigmentation of skin following engraftment onto nude mice. Arch Dermatol. 1989;125:1363–1366. doi: 10.1001/archderm.125.10.1363. [DOI] [PubMed] [Google Scholar]
- 24.Iizuka H. Epidermal turnover time. J Dermatol Sci. 1994;8:215–217. doi: 10.1016/0923-1811(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 25.Grimes PE, Morris R, Avaniss-Aghajani E, Soriano T, Meraz M, Metzger A. Topical tacrolimus therapy for vitiligo: therapeutic responses and skin messenger RNA expression of proinflammatory cytokines. J Am Acad Dermatol. 2004;51:52–61. doi: 10.1016/j.jaad.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869–1876. doi: 10.1038/jid.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanda C, Weitzel P, Tsukahara T, Schaley J, Edenberg HJ, Stephens MA, McClintick JN, Blatt LM, Li L, Brodsky L, Taylor MW. Differential gene induction by type I and type II interferons and their combination. J Interferon Cytokine Res. 2006;26:462–472. doi: 10.1089/jir.2006.26.462. [DOI] [PubMed] [Google Scholar]
- 28.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMurray RW. Adhesion molecules in autoimmune disease. Semin Arthritis Rheum. 1996;25:215–233. doi: 10.1016/s0049-0172(96)80034-5. [DOI] [PubMed] [Google Scholar]
- 30.Rotondi M, Falorni A, De Bellis A, Laureti S, Ferruzzi P, Romagnani P, Buonamano A, Lazzeri E, Crescioli C, Mannelli M, Santeusanio F, Bellastella A, Serio M. Elevated serum interferon-gamma-inducible chemokine-10/CXC chemokine ligand-10 in autoimmune primary adrenal insufficiency and in vitro expression in human adrenal cells primary cultures after stimulation with proinflammatory cytokines. J Clin Endocrinol Metab. 2005;90:2357–2363. doi: 10.1210/jc.2004-1062. [DOI] [PubMed] [Google Scholar]
- 31.Antonelli A, Ferrari SM, Frascerra S, Galetta F, Franzoni F, Corrado A, Miccoli M, Benvenga S, Paolicchi A, Ferrannini E, Fallahi P. Circulating chemokine (CXC motif) ligand (CXCL)9 is increased in aggressive chronic autoimmune thyroiditis, in association with CXCL10. Cytokine. 2011;55:288–293. doi: 10.1016/j.cyto.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 32.Kunisada T, Lu SZ, Yoshida H, Nishikawa S, Mizoguchi M, Hayashi S, Tyrrell L, Williams DA, Wang X, Longley BJ. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J Exp Med. 1998;187:1565–1573. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregg RK, Nichols L, Chen Y, Lu B, Engelhard VH. Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J Immunol. 2010;184:1909–1917. doi: 10.4049/jimmunol.0902778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, von Andrian UH, Moon JJ, Mempel TR, Luster AD. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity. 2012;37:1091–1103. doi: 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 36.Sung JH, Zhang H, Moseman EA, Alvarez D, Iannacone M, Henrickson SE, de la Torre JC, Groom JR, Luster AD, von Andrian UH. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. 2012;150:1249–1263. doi: 10.1016/j.cell.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kastenmuller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity. 2013;38:502–513. doi: 10.1016/j.immuni.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wankowicz-Kalinska A, van den Wijngaard RM, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, Storkus WJ, Das PK. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Laboratory investigation; a journal of technical methods and pathology. 2003;83:683–695. doi: 10.1097/01.lab.0000069521.42488.1b. [DOI] [PubMed] [Google Scholar]
- 39.Wong D, Kea B, Pesich R, Higgs BW, Zhu W, Brown P, Yao Y, Fiorentino D. Interferon and biologic signatures in dermatomyositis skin: specificity and heterogeneity across diseases. PLoS One. 2012;7:e29161. doi: 10.1371/journal.pone.0029161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wenzel J, Tuting T. An IFN-associated cytotoxic cellular immune response against viral, self-, or tumor antigens is a common pathogenetic feature in “interface dermatitis”. J Invest Dermatol. 2008;128:2392–2402. doi: 10.1038/jid.2008.96. [DOI] [PubMed] [Google Scholar]
- 41.Wijtmans M, Verzijl D, Leurs R, de Esch IJ, Smit MJ. Towards small-molecule CXCR3 ligands with clinical potential. ChemMedChem. 2008;3:861–872. doi: 10.1002/cmdc.200700365. [DOI] [PubMed] [Google Scholar]
- 42.Hyun JG, Lee G, Brown JB, Grimm GR, Tang Y, Mittal N, Dirisina R, Zhang Z, Fryer JP, Weinstock JV, Luster AD, Barrett TA. Anti-interferon-inducible chemokine, CXCL10, reduces colitis by impairing T helper-1 induction and recruitment in mice. Inflamm Bowel Dis. 2005;11:799–805. doi: 10.1097/01.mib.0000178263.34099.89. [DOI] [PubMed] [Google Scholar]
- 43.Menke J, Zeller GC, Kikawada E, Means TK, Huang XR, Lan HY, Lu B, Farber J, Luster AD, Kelley VR. CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. Journal of the American Society of Nephrology: JASN. 2008;19:1177–1189. doi: 10.1681/ASN.2007111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campanella GS, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA, Colvin RA, Luster AD. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci U S A. 2008;105:4814–4819. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thapa M, Welner RS, Pelayo R, Carr DJ. CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J Immunol. 2008;180:1098–1106. doi: 10.4049/jimmunol.180.2.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, von Andrian UH, Moon JJ, Mempel TR, Luster AD. CXCR3 Chemokine Receptor-Ligand Interactions in the Lymph Node Optimize CD4(+) T Helper 1 Cell Differentiation. Immunity. 2012;37:1091–1103. doi: 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris TH, Banigan EJ, Christian DA, Konradt C, Tait Wojno ED, Norose K, Wilson EH, John B, Weninger W, Luster AD, Liu AJ, Hunter CA. Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Nature. 2012;486:545–548. doi: 10.1038/nature11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoneyama H, Narumi S, Zhang Y, Murai M, Baggiolini M, Lanzavecchia A, Ichida T, Asakura H, Matsushima K. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J Exp Med. 2002;195:1257–1266. doi: 10.1084/jem.20011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho J, Kurtz CC, Naganuma M, Ernst PB, Cominelli F, Rivera-Nieves J. A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol. 2008;180:2573–2580. doi: 10.4049/jimmunol.180.4.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baaten BJ, Tinoco R, Chen AT, Bradley LM. Regulation of Antigen-Experienced T Cells: Lessons from the Quintessential Memory Marker CD44. Frontiers in immunology. 2012;3:23. doi: 10.3389/fimmu.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hommes DW, Mikhajlova TL, Stoinov S, Stimac D, Vucelic B, Lonovics J, Zakuciova M, D’Haens G, Van Assche G, Ba S, Lee S, Pearce T. Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn’s disease. Gut. 2006;55:1131–1137. doi: 10.1136/gut.2005.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayer L, Sandborn WJ, Stepanov Y, Geboes K, Hardi R, Yellin M, Tao X, Xu LA, Salter-Cid L, Gujrathi S, Aranda R, Luo AY. Anti-IP-10 antibody (BMS-936557) for ulcerative colitis: a phase II randomised study. Gut. 2013 doi: 10.1136/gutjnl-2012-303424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jenh CH, Cox MA, Cui L, Reich EP, Sullivan L, Chen SC, Kinsley D, Qian S, Kim SH, Rosenblum S, Kozlowski J, Fine JS, Zavodny PJ, Lundell D. A selective and potent CXCR3 antagonist SCH 546738 attenuates the development of autoimmune diseases and delays graft rejection. BMC immunology. 2012;13:2. doi: 10.1186/10.1186/1471-2172-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christophi GP, Rong R, Holtzapple PG, Massa PT, Landas SK. Immune markers and differential signaling networks in ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2012;18:2342–2356. doi: 10.1002/ibd.22957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang CQ, Cruz-Inigo AE, Fuentes-Duculan J, Moussai D, Gulati N, Sullivan-Whalen M, Gilleaudeau P, Cohen JA, Krueger JG. Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS One. 2011;6:e18907. doi: 10.1371/journal.pone.0018907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elela MA, Hegazy RA, Fawzy MM, Rashed LA, Rasheed H. Interleukin 17, Interleukin 22 and FoxP3 expression in tissue and serum of non-segmental vitiligo: A case- controlled study on eighty-four patients. Eur J Dermatol. 2013 doi: 10.1684/ejd.2013.2023. [DOI] [PubMed] [Google Scholar]
- 58.Tembhre MK, Sharma VK, Sharma A, Chattopadhyay P, Gupta S. T helper and regulatory T cell cytokine profile in active, stable and narrow band ultraviolet B treated generalized vitiligo. Clinica chimica acta; international journal of clinical chemistry. 2013;424:27–32. doi: 10.1016/j.cca.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Khan R, Gupta S, Sharma A. Circulatory levels of T-cell cytokines (interleukin [IL]-2, IL-4, IL-17, and transforming growth factor-beta) in patients with vitiligo. J Am Acad Dermatol. 2012;66:510–511. doi: 10.1016/j.jaad.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 60.Dell RB, Holleran S, Ramakrishnan R. Sample size determination. ILAR J. 2002;43:207–213. doi: 10.1093/ilar.43.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lissina A, Ladell K, Skowera A, Clement M, Edwards E, Seggewiss R, van den Berg HA, Gostick E, Gallagher K, Jones E, Melenhorst JJ, Godkin AJ, Peakman M, Price DA, Sewell AK, Wooldridge L. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. Journal of immunological methods. 2009;340:11–24. doi: 10.1016/j.jim.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic acids research. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Validation of selected transcripts in humans and mice.
Fig. S2. Frequencies of melanocyte antigen-specific T cells in peripheral blood of healthy or vitiligo patients.
Fig. S3. Staining controls for vitiligo biopsy immunohistochemistry.
Fig. S4. Gating strategy for examining CXCR3+ mouse T cells.
Fig. S5. Cotransfers of WT and CXCR3-deficient PMELs.
Fig. S6. Repigmentation following treatment with CXCL10 neutralizing antibody but not isotype control antibody.
Table S1. Percentages of CXCR3+ and CXCR3− Pmels in vitiligo mice; gated on total CD8+ cells.