Abstract
Understanding how the immune system facilitates or controls HIV-1 disease progression has important implications for the design of effective interventions. We report that although B-cell dysregulations associated with HIV-1 disease progression are accompanied by an overall decrease in the percentage of total blood B-cells, we observe an increase in relative frequencies of cells presenting characteristics of both transitional immature and first-line marginal zone (MZ) B-cell populations, we designated as precursor MZ-like B-cells. B-cells with similar attributes have been associated with IL-10 expression and “regulatory” potential. As such, the relative frequencies of precursor MZ-like B-cells expressing IL-10 are increased in the blood of viremic HIV-1-infected individuals when compared to HIV-negative subjects. Importantly, in aviremic HIV-1 Elite-Controllers (EC), we found unaltered relative percentages of precursor MZ-like B-cells which presented normal IL-10 expression patterns. Furthermore, EC had increased relative frequencies of blood MZ-like B-cells expressing LT-α. Thus in contrast to viremic HIV-1-infected individuals, EC present MZ-like B-cell populations which IL-10 and LT-α expression profiles may favour homeostasis of immune responses and lymphoid microenvironments.
Introduction
It is well known that the contribution of the B-cell compartment to effective viral control is impeded in the vast majority of HIV-1-infected individuals. Indeed, B-cell dysregulations are observed early, persist throughout the course of infection, and are not fully restored by therapy. These B-cell alterations favour the overall inflammatory burden and often lead to autoimmune manifestations and malignancies [1]–[2].
We have previously shown that HIV-transgenic (Tg)-mice, which develop a negative factor (Nef)-dependant AIDS-like disease [3], present B-cell dysregulations that are similar to those reported for HIV-infected individuals [4]. Strikingly, these animals present an enlarged splenic marginal zone (MZ), in which accumulated myeloid dendritic cells (mDCs) likely contribute to MZ expansion, polyclonal B-cell activation and breakage of tolerance through delivery of excessive signals such as B lymphocyte stimulator (BLyS/BAFF) [4]–[5]. A similar B-cell profile was reported for BLyS/BAFF-Tg [6] and autoimmune-regulatory-(AIRE)-deficient mice, in which BLyS/BAFF is elevated in serum and over-expressed by DCs [7]–[8]. Accordingly, DCs play a pivotal role in regulating B-cell development, activation and survival mainly through production of growth factors such as BLyS/BAFF [9]–[11], known to highly influence the transitional immature (TI) and MZ B-cell pools [12]–[13].
MZ B-cells constitute early first-line defence against invading pathogens. In humans, they likely constitute a heterogeneous niche that is not restricted to the spleen, as they have been found in blood, lymphoid organs and mucosal-associated structures [12]–[13]. Reminiscent of what had been observed in HIV-Tg mice [4], we found that B-cell dysregulations in HIV-1-infected rapid and classic progressors were accompanied by increased relative frequencies within total blood B-cells of a population presenting features shared by both TI and MZ B-cells, which we designated “precursor” MZ-like B-cells. Concomitantly, these individuals presented increased BLyS/BAFF levels in blood and on surface of blood mDCs [14]. In contrast, relative frequencies of precursor MZ-like B-cells as well as BLyS/BAFF expression status were unaltered in HIV-1 Elite-Controllers (EC) [14]. Instead, decreased frequencies of more “mature” MZ-like B-cells were observed in the blood B-cell compartment of EC.
B-cells are involved in regulating the development, proliferation and maintenance of CD4+ T-cell populations, through both contact and/or cytokine mediated effector and regulatory functions [15]. Regulatory “Breg” potential has not yet been attributed to a particular B-cell population and relies rather on IL-10 expression/production and function. Both precursor and mature B-cells with MZ attributes as well as TI and memory populations have been ascribed such Breg potential [16]–[17]. Recently, increased percentages of Breg producing IL-10 were observed in chronically HIV-infected subjects [18]. This has prompted us to assess whether IL-10 expression profiles by precursor and mature MZ-like as well as TI and memory B-cell populations are modulated during HIV-1 infection. In addition, the fact that high levels of Lymphotoxin (LT)-α have been associated with autoimmune and inflammatory contexts [19], and that increased LT-α to IL-10 B-cell expression ratios have been observed in patients with multiple sclerosis [20], prompted us to also assess B-cell LT-α expression profiles. We show increased frequencies of total B-cells expressing IL-10 in the blood of HIV-infected rapid and classic progressors as compared to those observed in HIV-uninfected donors. The most significant increase in cells expressing IL-10 is within the precursor MZ-like B-cell population. Furthermore, in contrast to viremic HIV-1-infected individuals, EC present MZ-like B-cell populations which IL-10 and LT-α expression profiles may favour homeostasis of immune responses and lymphoid microenvironments.
Methods
Ethics Statement
Written informed consent was obtained from all subjects, and the research conformed to ethical guidelines and was approved by the Ethics Review board of the Centre de Recherche du CHUM. The reference number for the project is: SL 05.028.
Subjects
Thirty male HIV-1-infected subjects were selected from the Montreal Primary HIV Infection (PHI) cohort: 13 were classified as rapid and 17 as classic progressors. The date of infection was estimated using criteria established by the Acute HIV Infection and Early Disease Research Program (NIAID, Bethesda, MD). Rapid progressors had blood CD4+ T-cell counts below 250 cells/mm3 within 2 years of infection. Blood samples were taken in acute (0–3 months) and/or early (5–8 months) phases of infection, and 3–6 and 9–12 months after initiation of antiretroviral therapy (ART). Classic progressors were ART-naive individuals whose blood CD4+ T-cell counts remained above 500 cells/mm3 for the 2 year follow-up. Blood samples were obtained in the acute, early and chronic (24 months) phases of infection. Moreover, blood samples from 13 slow progressors were obtained from the Slow Progressors cohort, where patients were infected for 8 years or more with blood CD4+ T-cell counts above 500 cells/mm3 and presented low (7 viremic patients) to undetectable (6 aviremic patients/EC) viral loads in absence of ART. Blood samples from 20 age- and sex-matched HIV-negative healthy volunteers were also obtained. HIV viral loads were determined in plasma using Versant HIV-1 RNA 3.0 Assay (Siemens Medical Solutions Diagnostics, Tarrytown, NY). Blood CD4+ T-cell counts were determined as reported [21]. None of the subjects had syphilis, hepatitis B or C.
Evaluation of plasma IL-10 and LT-α concentrations
Plasma levels of IL–10 were determined using the Cytometric Bead Array Human Inflammation Kit (BD-Biosciences, San Jose, CA, USA) according to the manufacturer's instructions. Data were acquired on a FACSAria and analysed with the FCAP software (BD-Biosciences). Plasma levels of LT-α were measured with the Milliplex Map magnetic bead kit (Millipore, Billerica, MA, USA) according to the manufacturer's protocol using the Luminex 200 Total System (Luminex Corporation, Austin, TX, USA).
Evaluation of blood B-cell populations by flow-cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated on Ficoll gradient, resuspended in heat-inactivated fetal bovine serum (hi-FBS) containing 10% dimethyl sulfoxyde and stored in liquid nitrogen. One million PBMCs per sample were used for staining. Live/dead exclusion was performed using Aqua-LIVE/DEAD Fixable Stain (Invitrogen Life technologies, Eugene, OR, USA). Non-specific binding sites were blocked using fluorescence-activated cell sorting (FACS) buffer (1× PBS, 2% hi-FBS, and 0.1% sodium azide) supplemented with 20% hi-FBS and 10 µg mouse IgG (Sigma-Aldrich, St-Louis, MO, USA). The following conjugated mouse anti-human monoclonal antibodies were used: PacificBlue-anti-CD19, APC-Cy7-anti-CD10 (BioLegend, San Diego, CA, USA), AlexaFluor700-anti-CD27, FITC-anti-IgM, PE-anti-CD21 (BD-Biosciences), PerCP-eFluor710-anti-CD1c (eBioscience, San Diego, CA, USA), APC-anti-LT-α (LifeSpan BioSciences, Seattle, WA, USA), and rat anti-human PE-Cy7-anti-IL-10 (BioLegend). Intracellular labelling was performed using the Cytofix/Cytoperm Fixation/Permeabilization kit and perm/wash buffer (BD-Biosciences). Intracellular non-specific binding sites were blocked using perm/wash buffer containing 20% hi-FBS, 50% rat serum and 20 µg mouse IgG. Cells were kept at 4°C in 1.25% paraformaldehyde for 18 hours prior to analysis. Data acquisition of 105 events per sample was performed with LSRFortessa (BD-Biosciences), and analysis was done with FlowJo7.6.3 software (TreeStar, Ashland, OR, USA). All stainings were compared to that of fluorescence minus one (FMO) values and isotype controls (see Figure S1 for details). Anti-mouse Ig(κ) Compbeads and CS&T Beads (BD-Biosciences) were used to optimize fluorescence compensation settings and calibrate the LSRFortessa, respectively.
Statistical analyses
The statistical significance of differences between groups was assessed with Fisher exact test for categorical variables and unpaired Student t test or one-way analysis of variance when continuous variables were normally distributed or with Mann-Whitney U test otherwise. Wilcoxon signed rank test was used for pairwise comparisons of different phases of infection within each group. Spearman rank test was used to determine correlations between continuous variables. Outliers' samples were withdrawn from statistical analyses when the values were greater than the mean plus three times standard deviation. Analyses were performed using GraphPad Prism 5.03 for Windows (GraphPad Software Inc, La Jolla, CA, USA).
Results
Socio-demographic and clinical characteristics of HIV-1-infected individuals
The socio-demographic and clinical characteristics of the HIV-1-infected individuals are shown in Table 1, and the longitudinal assessment of blood CD4+ T-cell counts and viral loads are depicted in Figure S2A-B. There were no significant correlations between blood CD4+ T-cell counts or viral loads and B-cell populations, LT-α and IL-10 plasma levels studied here either within groups or among all subjects during early or chronic infection (data not shown).
Table 1. Sociodemographic and clinical characteristics of HIV-infected individuals.
| Rapid progressors | Classic progressors | Viremic slow progressors | Aviremic slow progressors | P | |
| Characteristics | (n = 13) | (n = 17) | (n = 7) | (n = 6) | |
| Age at first visit (years) | 34±7 | 38±8 | 46±9 | 44±9 | 0.005a |
| Sex (male/female) | 12/1 | 17/0 | 7/0 | 3/3 | 0.005 |
| Race (white/other) | 12/1 | 16/1 | 6/1 | 5/1 | NS |
| CD4+ T cells/mm3 | |||||
| Acute phase | 533±140 | 781±213 | na | na | 0.002 |
| Early phase | 446±40 | 714±46 | na | na | 0.0008 |
| Chronic phase | 443±193 | 629±244 | 562±122 | 888±122 | 0.011b |
| Nadir | 254±118 | 432±140 | 506±129 | 506±175 | 0.0005c |
| Viremia, ×103 copies/ml d | |||||
| Acute phase | 366±705 | 76±126 | na | na | NS |
| Early phase | 121±186 | 71±190 | na | na | NS |
| Chronic phase | 7.65±15.3 | 37.7±62.3 | 3.01±1.86 | <0.05d | 0.003e |
| Peak | 570±808 | 202±236 | 8.5±6.5 | 0.065±0.027 | 0.0001f |
Age, CD4 and viremia are expressed as mean ±SD. Sex and race were compared using Fisher exact test. Pairwise comparisons of CD4 and viremia for early phases were performed using unpaired Student's t tests. Comparisons among all groups (age at first visit, CD4, viremia in the chronic phase, nadir CD4) were performed with the one-way analysis of variance test. n, numbers; NS, not significant; na, not available.
P = 0.004 and 0.050 for the comparison of age between rapid and viremic slow progressors, and classic and viremic slow progressors, respectively, as determined by the Mann-Whitney test.
P = 0.008 for the comparison of CD4+ T cells/mm3 in chronic phase between rapid progressors and aviremic slow progressors, as determined by the Mann-Whitney test.
P = 0.0008 and 0.001 and 0.020 nadir CD4 for the comparison between rapid and classic progressors, rapid and viremic slow progressors, and rapid and aviremic slow progressors, respectively, as determined by the Mann-Whitney test.
Fifty copies/ml corresponds to the detection threshold of the viral load test.
P = 0.002 and 0.002 for the comparison of viremia in chronic phase between classic progressors and aviremic slow progressors, and viremic and aviremic slow progressors, respectively, as determined by the Mann-Whitney test.
P = 0.006, 0.0007, 0.0005, 0.0004 and 0.001 for the comparison of peak viremia between rapid progressors and viremic slow progressors, rapid progressors and aviremic slow progressors, classic progressors and viremic slow progressors, classic progressors and aviremic slow progressors, and viremic and aviremic slow progressors, respectively, as determined by the Mann-Whitney test.
Longitudinal monitoring of blood B-cell populations in HIV-1-infected individuals with different rates of disease progression
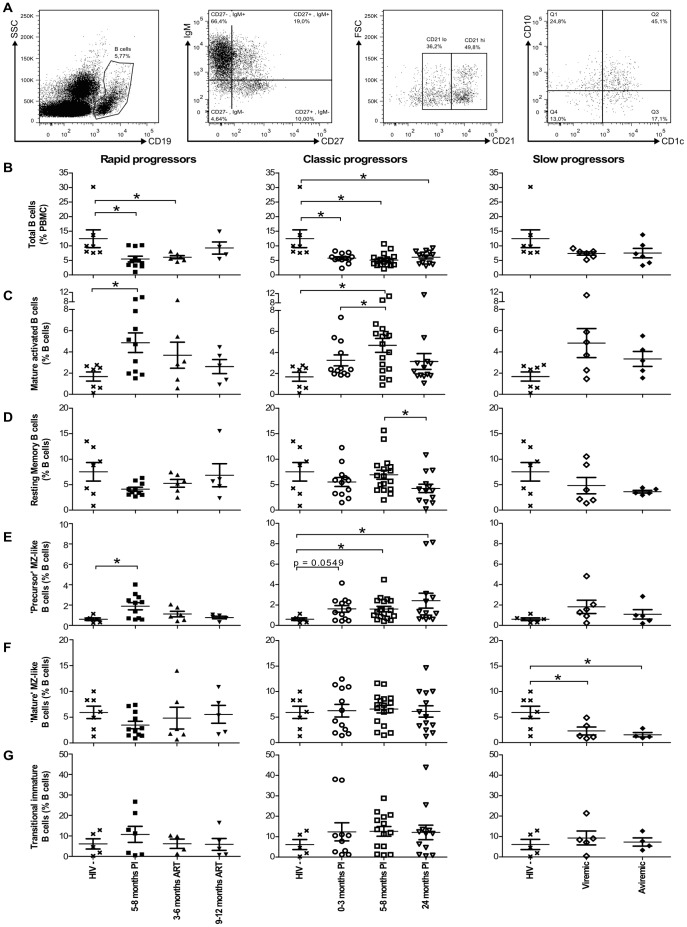
We have previously reported that the frequencies of blood precursor and mature MZ-like B-cell populations are different in the context of HIV disease control or progression [14]. To further the characterization of these B-cells, we have adapted our staining cocktail for the detection of intracellular expression of IL-10 and LT-α (see below). The analyses of B-cell populations using this modified staining strategy confirm previous observations and are depicted in Figure 1. The mean percentages of total live blood CD19+ B-cells in all HIV-1-infected individuals were lower throughout the course of infection than those observed in HIV-negative donors, reaching statistical significance in both rapid and classic progressors (Figure 1B). Upon analysis for variations in the relative frequencies of different B-cell populations, we observed that the mean relative percentages of mature activated (CD19+CD27+IgM−CD21l°CD10−) and precursor MZ-like B-cells (CD19+CD27+IgMhiCD21l°CD1c+CD10+), were significantly elevated in rapid and classic progressors (Figure 1C, E). There was a trend for increased relative frequencies of TI B-cells (CD19+CD27−IgM+CD21+CD10+) in chronically HIV-1-infected classic progressors (Figure 1G). In contrast, the relative frequency of the more mature MZ-like B-cell population (CD19+CD27+IgMhiCD21hiCD1c+CD10−) within total blood B-cells remained unaltered in both rapid and classic progressors (Figure 1F, left and middle panels), whereas the percentages were significantly decreased in both viremic and aviremic slow progressors, the latter also referred herein as EC (Figure 1F, right panel). The relative frequencies of resting switched memory B-cells (CD19+CD27+IgM−CD21hiCD10−) were decreased in chronically infected classic progressors (Figure 1D, middle panel). Naïve resting B-cells (CD19+CD27−IgM+CD21hiCD10−), were slightly lower in all HIV-1-infected individuals (data not shown).
Figure 1. Longitudinal analysis of blood B-cell populations of HIV-1 infected individuals.
(A) Representative plot showing gating strategy on live PBMCs. Total CD19+ B-cells were selected based on expression of CD27 and/or IgM, and levels of CD21. CD1c and CD10 expression were used for further characterisation of blood MZ and TI B-cell populations respectively, as reported [13]. Quadrants were set based on the expression values obtained with fluorescence minus one (FMO) and isotype controls. Mature activated B-cells are defined as CD19+CD27+IgM-CD21loCD1c-CD10-, resting switched memory B-cells are CD19+CD27+IgM-CD21hiCD10-, precursor marginal-zone (MZ)-like B-cells are CD19+CD27+IgM+CD21loCD1c+ CD10+, mature MZ-like B-cells are CD19+CD27+IgM+CD21hiCD1c+CD10- and transitional immature (TI) B-cells are CD19+CD27-IgM+CD21hiCD1c-CD10+. The graphs present (B) percentages of total B-cells (mean events gated: 9320±1750), and the relative frequencies of (C) mature activated (mean events gated: 360±67), (D) resting switched memory (mean events gated: 632±301), (E) precursor MZ-like (mean events gated: 145±36), (F) mature MZ-like (mean events gated: 327±233) and (G) TI (mean events gated: 944±174) B-cell populations in the blood of rapid progressors (left panels; 5–8 months PI (n = 11), 3–6 months ART (n = 6) and 9–12 months ART (n = 5)), classic progressors (middle panels; 0–3 months PI (n = 12), 5–8 months PI (n = 17), and 24 months PI (n = 13)), and viremic and aviremic slow progressors (EC) (right panels; viremic (n = 6); aviremic (n = 5)). The same values for HIV-negative donors in the left, middle and right panels are used as a control group (n = 7). Cell population frequencies were compared using the Wilcoxon signed rank test and the Mann-Whitney U test for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. Data shown are mean ±SEM. * p<0.05. PI, post-infection; ART, antiretroviral therapy.
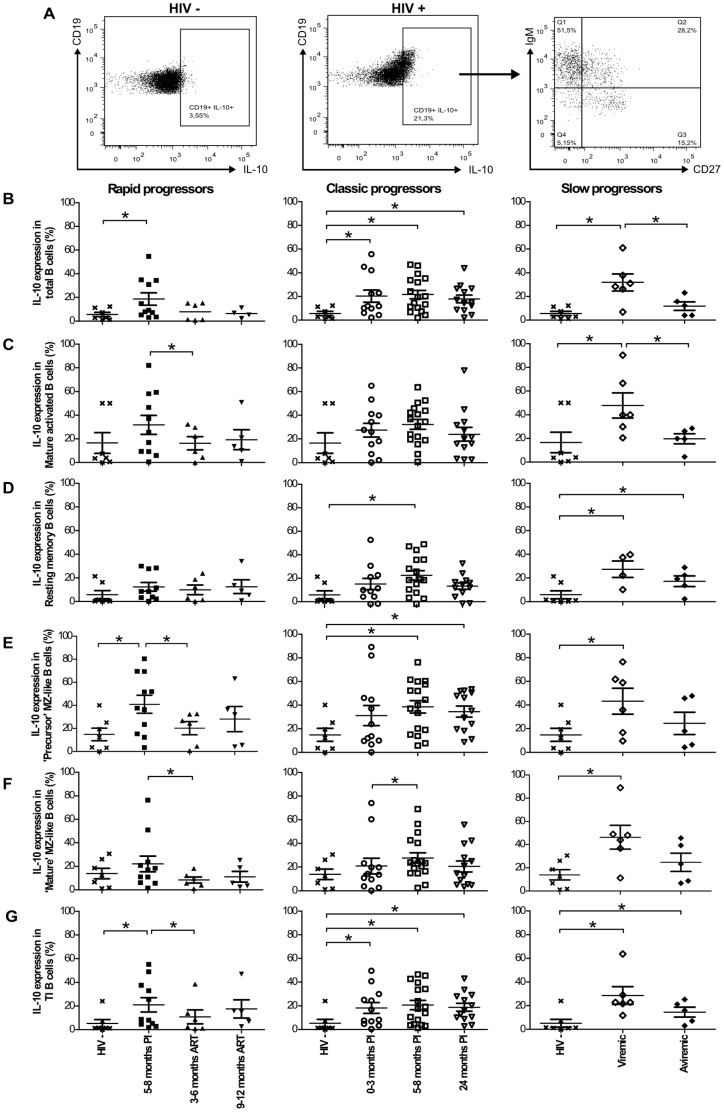
Longitudinal monitoring of IL-10 expression by blood B-cell populations in HIV-1-infected individuals with different rates of disease progression
Blood B-cell populations presented in Figure 1 were assessed ex vivo for intracellular IL-10 expression based on the same strategy of flow-cytometry analysis depicted in Figure 1A. Figure 2A shows a representative profile of IL-10 expression on total live B-cells from HIV- and HIV+ donors, and distribution within IgM and CD27 quadrants of expression. Note that the majority of IL-10 expressing B-cells are IgM+ (Figure 2A). Because IL-10 expression might be influenced by the fluctuations in B-cell population frequencies shown in Figure 1, data are expressed as percentages of IL-10 expressing cells within each B-cell population. Frequencies of total B-cells expressing IL-10 in all viremic HIV-1-infected individuals were significantly higher than those observed in HIV-negative donors (Figure 2B). The percentages of total B-cells expressing IL-10 were restored to normal levels following therapy in rapid progressors (Figure 2B, left panel). Similar patterns of IL-10 expression were observed for precursor MZ-like and TI B-cell populations in all viremic HIV-1-infected individuals (Figure 2E, G), as well as for mature activated and resting switched memory B-cells in viremic slow progressors (Figure 2C-D). Relative frequencies of resting switched memory B-cells expressing IL-10 were increased in classic progressors during the early phase of infection (Figure 2D, middle panel), whereas those of mature MZ-like B-cells expressing IL-10 were elevated in viremic slow progressors (Figure 2F, right panel). Importantly, percentages of IL-10 expression in all B-cell populations of aviremic slow progressors/EC were similar to those observed in HIV-negative donors, except for resting switched memory and TI B-cells, in which the relative frequencies of IL-10 expressing cells were significantly increased (Figure 2D, G, right panels). The frequencies of naïve resting B-cells expressing IL-10 remained unaltered in all HIV-infected individuals (data not shown). We did not detect changes in IL-10 expression levels following analysis of geometric mean fluorescence intensities (geoMFI) (data not shown). Figure S3 shows a recapitulative analysis of B-cell IL-10 expression profiles. The heterogeneity of CD19 expression may be explained by the presence of blasts or other B-cell populations that were not specifically analyzed in the present study. The contribution to IL-10 expression from B-cell populations not analyzed herein will require further investigation.
Figure 2. IL-10 expression by blood B-cell populations.
(A) Representative plot showing gating strategy on live total CD19+ B-cells from HIV- and HIV+ donors, expressing IL-10. Frequencies of cells expressing IL-10 within (B) total, (C) mature activated, (D) resting switched memory, (E) precursor marginal zone (MZ)-like, (F) mature MZ-like and (G) transitional immature (TI) B-cell populations in the blood of rapid progressors (left panels; 5–8 months PI (n = 11), 3–6 months ART (n = 6) and 9–12 months ART (n = 5)), classic progressors (middle panels; 0–3 months PI (n = 12), 5–8 months PI (n = 17), and 24 months PI (n = 13)), and viremic and aviremic slow progressors (EC) (right panels; viremic (n = 6); aviremic (n = 5)). The same values for HIV-negative donors in the left, middle and right panels are used as a control group (n = 7). Data are expressed as percentages of IL-10 expression within each B-cell population. Cell population frequencies were compared using the Wilcoxon signed rank test and the Mann-Whitney U test for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. Data shown are mean ±SEM. * p<0.05. PI, postinfection; ART, antiretroviral therapy.
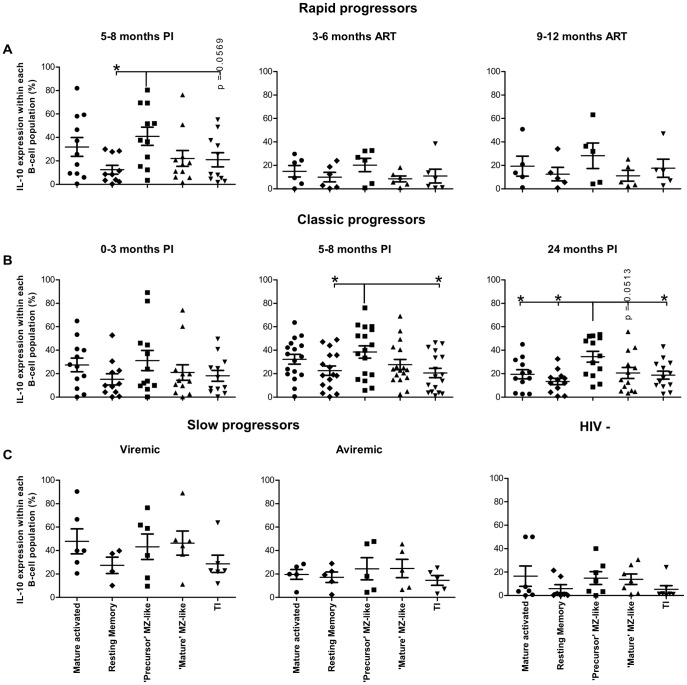
Data were plotted in order to compare the percentage of IL-10 expressing cells between each B-cell population, within each patient group at various time points (Figure 3). Amongst the B-cell populations analyzed, the precursor MZ-like B-cells were the main expressors of IL-10 during the early phase of infection in both the rapid and classic progressor groups (Figure 3A, left panel; B, middle panel). This was also true for classic progressors in the chronic phase of infection (Figure 3B, right panel). All B-cell populations contributed equally to IL-10 expression in the slow progressor and HIV-negative donor groups (Figure 3C). Of note, plasma levels of IL-10 were found to be elevated throughout follow-up in all HIV-1-infected individuals, including ART-treated rapid progressors and aviremic slow progressors/EC (Figure S2C).
Figure 3. Percentage of IL-10 expressing cells for each B-cell population, within each patient group.
Percentage of IL-10 expressing cells within mature activated, resting switched memory, precursor marginal zone (MZ)-like, mature MZ-like and transitional immature (TI) B-cells within (A) rapid progressors (5–8 months PI (n = 11), 3–6 months ART (n = 6) and 9–12 months ART (n = 5), (B) classic progressors (0–3 months PI (n = 12), 5–8 months PI (n = 17), and 24 months PI (n = 13)), (C) slow progressors (viremic (n = 6); aviremic (n = 5)), and HIV-negative individuals (n = 7). Percentages were compared using the Mann-Whitney U test between the B-cell populations. Data shown are mean ±SEM. *p<0.05. PI, postinfection; ART, antiretroviral therapy.
Overall, frequencies of total blood B-cells expressing IL-10 in all viremic HIV-1-infected individuals were significantly higher than those observed in HIV-negative donors. Amongst the B-cell populations analyzed, the relative percentage of IL-10 expressing cells is significantly increased within the precursor MZ-like, TI and memory populations. The most significant increase in cells expressing IL-10 was within the precursor MZ-like population. Importantly, we found unaltered percentages of total and of MZ-like B-cells expressing IL-10 in aviremic slow progressors/EC.
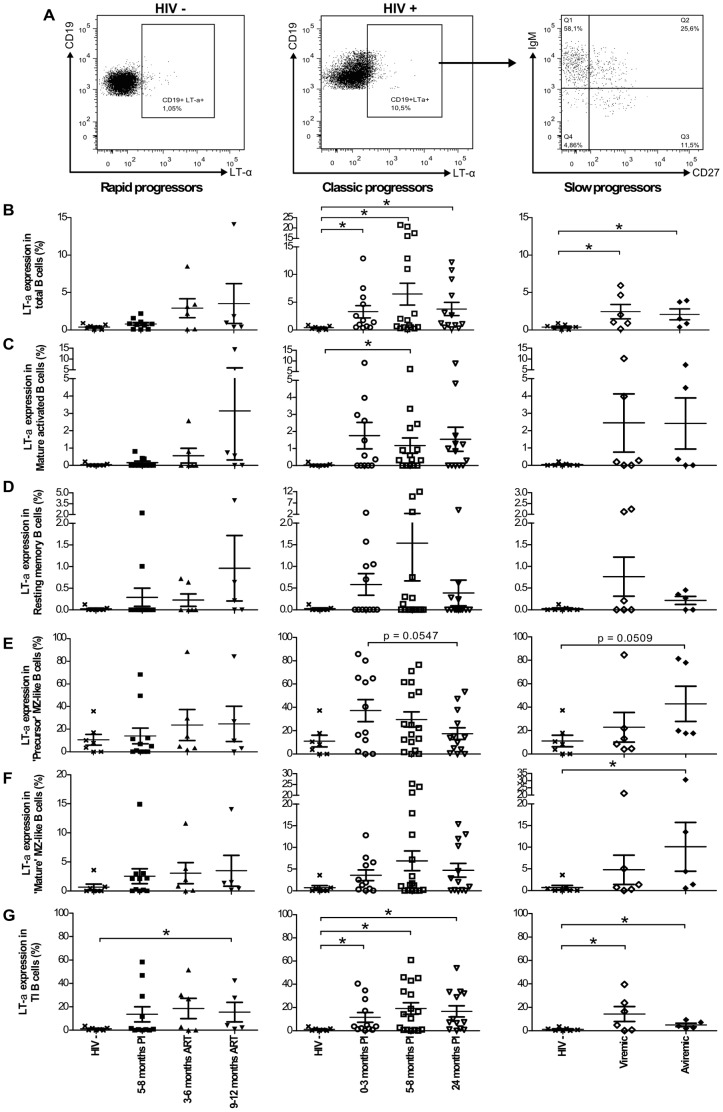
Longitudinal monitoring of LT-α expression by blood B-cell populations in HIV-1-infected individuals with different rates of disease progression
Blood B-cell populations presented in Figure 1 were assessed ex vivo for intracellular LT-α based on the same strategy of flow-cytometry analysis depicted in Figure 1A. Figure 4A shows a representative profile of total live B-cells expressing LT-α in HIV- and HIV+ donors, and distribution within IgM and CD27 quadrants of expression. The data are presented as percentage of LT-α expressing cells within each B-cell population. There was a significant increase in frequencies of total B-cells expressing LT-α in the blood of both classic and slow progressors when compared to HIV-negative donors (Figure 4B). There was a trend for increased frequencies of total B-cells expressing LT-α following therapy in rapid progressors (Figure 4B, left panel). Similar patterns of LT-α expression were observed in mature activated, precursor MZ-like, mature MZ-like and TI B-cell populations (Figure 4C, E–G). The relative frequencies of resting switched memory B-cells expressing LT-α were not significantly affected (Figure 4D), as were those of naïve resting B-cells (data not shown). We did not detect changes in LT-α expression levels following analysis of geoMFI (data not shown). A recapitulative analysis of B-cell LT-α expression profiles is provided in Figure S4.
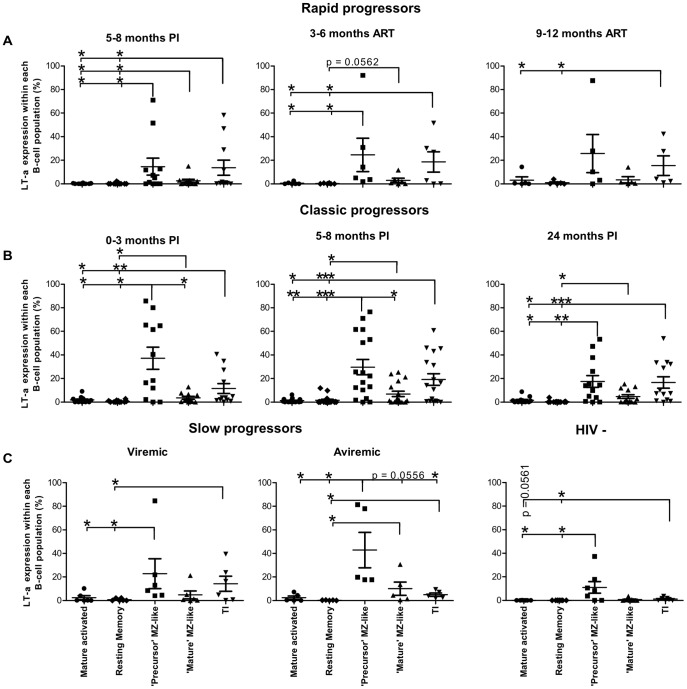
Figure 4. LT-α expression by blood B-cell populations.
(A) Representative plot showing gating strategy on live total CD19+ B-cells from HIV- and HIV+ donors, expressing LT-α. Frequencies of cells expressing LT-α within (B) total, (C) mature activated, (D) resting switched memory, (E) precursor marginal zone (MZ)-like, (F) mature MZ-like and (G) transitional immature (TI) B-cells expressing LT-α in the blood of rapid progressors (left panels; 5–8 months PI (n = 11), 3–6 months ART (n = 6) and 9–12 months ART (n = 5)), classic progressors (middle panels; 0–3 months PI (n = 12), 5–8 months PI (n = 17), and 24 months PI (n = 13)), and viremic and aviremic slow progressors (EC) (right panels; viremic (n = 6); aviremic (n = 5)). The same values for HIV-negative donors in the left, middle and right panels are used as a control group (n = 7). Data are expressed as percentages of LT-α expression within each B-cell population. Cell population frequencies were compared using the Wilcoxon signed rank test and the Mann-Whitney U test for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. Data shown are mean ±SEM. * p<0.05. PI, postinfection; ART, antiretroviral therapy.
Data were plotted in order to compare the percentage of LT-α expressing cells between each B-cell population, within each patient group at various time points (Figure 5). Amongst the B-cell populations analyzed, the precursor MZ-like and TI B-cells were the main contributors of LT-α expression during the acute, early and chronic phases of infection in all viremic HIV-1-infected individuals (Figure 5A-B; C, left panel). The precursor MZ-like B-cells were the main contributors of LT-α expression in the aviremic slow progressor/EC and HIV-negative groups (Figure 5C). Plasma levels of LT-α in all HIV-infected individuals were similar to those observed in HIV-negative donors (Figure S2D). Overall, increased relative frequencies of precursor MZ-like B-cells expressing LT-α appear associated with a better control of disease progression.
Figure 5. Percentage of LT-α expressing cells for each B-cell population, within each patient group.
Percentage of LT-α expressing cells within mature activated, resting switched memory, precursor marginal zone (MZ)-like, mature MZ-like and transitional immature (TI) B-cells within (A) rapid progressors (5–8 months PI (n = 11), 3–6 months ART (n = 6) and 9–12 months ART (n = 5), (B) classic progressors (0–3 months PI (n = 12), 5–8 months PI (n = 17), and 24 months PI (n = 13)), (C) slow progressors (viremic (n = 6); aviremic (n = 5)), and HIV-negative individuals (n = 7). Percentages were compared using the Mann-Whitney U test between the B-cell populations. Data shown are mean ±SEM. * p<0.05, ** p<0.001, *** p<0.0001. PI, postinfection; ART, antiretroviral therapy.
Discussion
The percentages of total live blood CD19+ B-cells were lower in all HIV-1-infected individuals when compared to those observed in HIV-negative donors. However, despite the fact that precursor MZ-like B-cells may represent only a small fraction of circulating B-cells, their increased relative frequencies in the blood of HIV-1-infected rapid and classic progressors, may significantly influence the ability to mount efficient B-cell responses and disease progression. Indeed, given the frequent auto-reactive and cross-reactive repertoires of MZ type populations [12]–[13], the increased relative frequency of these cells may be associated with polyclonal B-cell activation and hyperglobulinemia previously reported for these individuals [14], leading to breakage of tolerance and autoimmune manifestations. Moreover, their increased relative proportions may reflect the host response to lymphopenia observed in rapid progressors [22] and the overall inflammation and high levels of BLyS/BAFF [23]–[29] previously found in the plasma and on surface of blood mDCs in these individuals [14]. In support of our findings, it has been shown that human blood and tonsil MZ-like B-cells naturally bind to gp120 through C-type lectins, and that this is increased by BLyS/BAFF in vitro, subsequently leading to polyclonal IgG and IgA responses, of which a fraction is gp120-specific [12]. Furthermore, the fact that the individuals presented herein experience microbial translocation [14] is in line with human MZ B-cells being particularly solicited in response to encapsulated bacteria [30]. In contrast, relative blood levels of precursor MZ-like B-cells in EC were similar to those observed in HIV-negative donors [14]. Moreover, EC had low-inflammatory profiles, unaltered levels of BLyS/BAFF and undetectable elements of microbial translocation [14], suggesting they are able to maintain a certain degree of immune integrity.
Interestingly, both viremic and aviremic slow progressors/EC had decreased relative frequencies of more mature MZ-like B-cells within their blood B-cell compartment when compared to both rapid and classic progressors, and HIV-negative donors. This finding could suggest that the capacity to recruit this population to peripheral sites may be beneficial to the “control” of disease progression. Intriguingly, a similar decrease in blood CD27+IgM+IgD+ MZ type B-cells has been recently reported in patients with MyD88, IRAK4, and TIRAP deficiencies [30]. Whether there is a relationship with the reduced frequencies of mature MZ-like B-cells we observe in slow progressors warrants further investigation.
Although our sample size was limited, we were able to accurately and reproducibly measure ex vivo IL-10 expression by blood B-cells. We found that in the context of detectable viremia, the percentages of total blood B-cells expressing IL-10 were increased when compared to HIV-negative individuals (Figure 2B). Amongst the B-cell populations analysed, the percentage of IL-10 expressing cells was significantly increased within the precursor MZ-like, TI and switched memory populations. Strikingly, the most significant increase in cells expressing IL-10 was within the precursor MZ-like population. Importantly, the relative increase in precursor MZ-like B-cells expressing IL-10 correlated with high levels of serum IgG1 and IgG3 in the early phase of HIV-1-infection in classic progressors (p = 0.0184; p = 0.0499, respectively), and with high levels of serum IgA in chronic classic progressors (p = 0.0479). These are likely to reflect polyclonal B-cell activation associated with the overall inflammatory condition we observed in HIV-1 progressors [14], [31], as B-cell IL-10 production has been shown to be modulated by BLyS/BAFF [32] and various signals, such as delivered via TLR, CD40 and the B-cell receptor (BCR) [16]–[17].
Our findings could also suggest an attempt from the host to dampen the overwhelming inflammatory burden by soliciting regulatory functions from various B-cell populations, such as switched memory, TI and MZ-like B-cells, which have been variably associated with Breg capacity [16]–[17]. Of course, we cannot rule out that precursor MZ-like B-cells are more sensitive to the overall HIV disease process, but it may be that they represent a population more prone to Breg potential. Frequencies of populations with similar characteristics and Breg attributes have been shown to be increased in the blood of systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) patients, albeit these cells were refractory to in vitro stimulation, produced less IL-10 and lacked suppressive activity [16]. Likewise, it is possible that in the context of HIV disease progression, populations ascribed to Breg potential may have exhausted and/or dysregulated regulatory capacities.
Nevertheless, the overall outcome of increased frequencies of B-cells expressing IL-10 may well be to sustain chronic B-cell activation and dysregulation, and may lead to imbalanced Treg/Teffector ratios [15] associated with HIV disease progression [33]. Furthermore, high level of Breg activity was recently shown to be involved in suppression of antiviral T effector functions in vitro during HIV [18] and chronic hepatitis B [34] infections, thus impending viral eradication.
In the context of HIV disease control, such as encountered in EC, the frequencies of total and MZ-like B-cells expressing IL-10, remained unaltered. Although increased frequencies of resting switched memory and TI blood B-cells expressing IL-10 were found in EC, these were lower than in the MZ-like populations. These findings suggest that EC are capable, to a certain extent, to regulate their IL-10 expression status and possibly “Breg” activity, and this may help in maintaining homeostasis of immune responses associated with HIV control.
Although high levels of LT-α have been associated with autoimmune and inflammatory conditions [19], plasma levels of LT-α in all HIV-1-infected subjects were similar to those found in HIV-negative donors. Amongst the B-cell populations analyzed, the precursor MZ-like B-cells were the main contributors to LT-α expression in all subjects regardless of HIV-1 infection and rate of disease progression. The percentages of IL-10 expressing cells were significantly greater than those of LT-α within most B-cell populations except for precursor MZ-like and TI B-cells, which frequencies of IL-10 and LT-α expression were similar. Importantly, EC had increased frequencies of MZ-like B-cells expressing LT-α resulting in higher LT-α to IL-10 expression ratios. Studies in the murine system have elegantly shown the importance of LT-α in the organization and maintenance of lymphoid structures, as well as in the modulation of immune responses [19], through a process involving a positive CXCL13 feedback loop [35]. Interestingly, plasma levels of CXCL13 were higher in viremic individuals compared to HIV-negative donors [36] and EC (Chagnon-Choquet J unpublished data), suggesting that the higher relative percentages of MZ-like B-cells expressing LT-α in EC may help to maintain CXCL13 levels and a certain lymphoid homeostasis.
The first-line MZ-like B-cells described herein likely contribute directly to the generation of HIV-specific Abs. Indeed, human MZ B-cells express IGHV1-2*02 [37], which has been repeatedly found to encode mutated HIV broadly neutralizing Abs (bNAbs), such as VRC01 [38]. Interestingly, the recent characterization of transient Gp41-specific IgA in mucosal genital fluids from patients within the first weeks after HIV transmission, suggest these Abs might have originated from first-line B-cell populations [39]. Of note, BLyS/BAFF was elevated immediately preceding the appearance of these Abs. Interestingly, repeated treatment of mice with BLyS/BAFF increased the MZ compartment, and generated an increased response to Env immunization and bNAbs from these animals [40]. Understanding the dynamics of BLyS/BAFF and its role in homeostasis of immune responsiveness appears pivotal to the design of vaccine strategies soliciting protective B-cell responses. Based on our observations, the capacity to contain BLyS/BAFF expression levels seems associated with control of disease progression, whereas excessive BLyS/BAFF may promote immune dysregulation and disease progression [41].
The findings reported herein are in line with growing evidence suggesting that first-line B-cell responses are involved in the battle against HIV [42], and with the importance of MZ type B-cells in health and disease [12]-[13]. Although further characterization is required to identify the exact nature of the MZ-like B-cells described herein, they certainly deserve further interest.
Supporting Information
Flow-cytometry gating strategy based on fluorescence minus one and isotype controls. Dot plots showing gating strategy based on fluorescence minus one and isotype control for (A) CD19, (B) CD21, (C) CD27 and IgM, (D) CD1c and CD10, (E) IL-10 and (F) LT-α.
(TIF)
Longitudinal variations of blood CD4+ T-cell counts, viral loads and IL-10 and LT-α concentrations of HIV-1 infected individuals. (A) Blood CD4+ T-cell counts (cell/mm3) were determined by flow-cytometry in rapid progressors (left panel; 0–3 months PI (n = 13), 5–8 months PI (n = 13), 3–6 months ART (n = 7), 9–12 months ART(n = 5)), classic progressors (middle panel; 0–3 months PI (n = 16), 5–8 months PI (n = 16), 24 months PI (n = 11)), and slow progressors (right panel; viremic (n = 6), aviremic (n = 5)). (B) Viral loads (log copies/ml) were quantified by in vitro signal amplification nucleic acid probe assay of HIV-1 RNA (bDNA) in the plasma of rapid progressors (left panel; 0–3 months PI (n = 13), 5–8 months PI (n = 13), 3–6 months ART (n = 7), 9–12 months ART (n = 6)), classic progressors (middle panel; 0–3 months PI (n = 17), 5–8 months PI (n = 17), 24 months PI (n = 11)), and slow progressors (right panel; viremic (n = 6), aviremic (n = 6)). (C) Concentrations of IL-10 measured longitudinally in the plasma of rapid progressors (left panel; 0–3 months PI (n = 12), 5–8 months PI (n = 13), 3–6 months ART (n = 9), 9–12 months ART (n = 7)), classic progressors (middle panel; 0–3 months PI (n = 17), 5–8 months PI (n = 17), 24 months PI (n = 11)) and slow progressors (right panel; viremic (n = 7), aviremic (n = 5)). The same values for HIV-negative donors (n = 20) in the left, middle and right panels are used as a control group. (D) Concentrations of LT-α measured longitudinally in the plasma of rapid progressors (left panel; 0–3 months PI (n = 10), 5–8 months PI (n = 12), 3–6 months ART (n = 6), 9–12 months ART (n = 4)), classic progressors (middle panel; 0–3 months PI (n = 14), 5–8 months PI (n = 10), 24 months PI (n = 9)) and slow progressors (right panel; viremic (n = 4), aviremic (n = 4)). The same values for HIV-negative donors (n = 18) in the left, middle and right panels are used as a control group. Cell populations, viral loads and plasma concentrations were compared using the Wilcoxon signed-rank test and Mann-Whitney U test for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. Data shown are mean ±SEM. Significance levels are shown as * p<0.05, ** p<0.001, *** p<0.0001. PI, post-infection; ART, antiretroviral therapy.
(TIF)
Contribution of each blood B-cell population to IL-10 expression. Percentages of IL-10 expression within each B-cell population; ‘mature’ marginal zone (MZ)-like (purple), ‘precursor’ MZ-like (cherry red), mature activated (yellow), transitional immature (TI) (blue) and resting switched memory (orange) B-cells, for rapid progressors (left panel; 5–8 months PI (n = 11), 3–6 months ART (n = 6), 9–12 months ART (n = 5)), classic progressors (middle panel; 0–3 months PI (n = 12), 5–8 months PI (n = 17), 24 months PI (n = 13)), and slow progressors (right panel; viremic (n = 6), aviremic (n = 5)). The same value for HIV-negative donors in the left, middle and right panels are used as a control group (n = 7). Cell population frequencies were compared using the Mann-Whitney U test between the study groups. Data shown are mean ± SEM. * p<0.05. PI, post-infection; ART, antiretroviral therapy.
(TIF)
Contribution of each blood B-cell population to LT-α expression. Percentages of LT-α expression within each B-cell population; ‘mature’ marginal zone (MZ)-like (purple), ‘precursor’ MZ-like (cherry red), mature activated (yellow), transitional immature (TI) (blue) and resting switched memory (orange) B-cells, for rapid progressors (left panel; 5–8 months PI (n = 11), 3–6 months ART (n = 6), 9–12 months ART (n = 5)), classic progressors (middle panel; 0–3 months PI (n = 12), 5–8 months PI (n = 17), 24 months PI (n = 13)), and slow progressors (right panel; viremic (n = 6), aviremic (n = 5)). The same value for HIV-negative donors in the left, middle and right panels are used as a control group (n = 7). Cell population frequencies were compared using the Mann-Whitney U test between the study groups. Data shown are mean ±SEM. * p<0.05. PI, post-infection; ART, antiretroviral therapy.
(TIF)
Acknowledgments
Part of this work was presented at Keystone symposium on B cell Development and Function joint with HIV vaccines, February 10th-15th 2013, CO, USA.
We are grateful to the participants of the Montreal PHI and Slow Progressors study groups. We thank Dr Pavel Chrobak for his valuable comments. We also thank Dr Dominique Gauchat for invaluable flow-cytometry expertise, and Christian Charbonneau and Jean-François Trudel for bio-imaging support. Marie-Pierre Boisvert, Maryse Lainesse, Rebecca Bordi, Véronique Lafontaine, Bader Yassine-Diab and Younes Chouick for processing the blood samples.
The members of the Montreal Primary HIV Infection and Slow Progressor Study Groups are Anne Vassal and Mario Legault (CRCHUM, coordinators); Jean Pierre Routy (CUSM, principal investigator Primary HIV Study), Cécile Tremblay (CRCHUM, principal investigator Slow Progressor Study), Réjean Thomas, Benoit Trottier, Sylvie Vézina, Louise Charest, Catherine Milne, Jason Friedman, Emmanuelle Huchet (Clinique médicale l'Actuel), Jean-Guy Baril, Pierre Côté, Bernard Lessard, Serge Dufresne, Marc-André Charron (Clinique médicale du Quartier Latin). Roger LeBlanc (Clinique médicale urbaine OPUS), Louise Labrecque, Danielle Rouleau, Claude Fortin, Marie Munoz, Julie Bruneau (UHRESS Hôtel-Dieu, Notre-Dame, St-Luc), Norbert Gilmore, Richard lalonde, Martin Potter, Marina Klein, ALexandra de Pokomandy, Jason Szabo, Nicole Bernard (UHRESS thoracique & Hôpital général de Montréal).
Funding Statement
This work was supported by grant # CHR-126633 from the Canadian Institutes of Health Research (CIHR) and by the Réseau SIDA from the Fonds de Recherche du Québec en Santé (FRQS). Josiane Chagnon-Choquet holds a Studentship from the Fondation Gabriel Marquis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Moir S, Fauci AS (2009) B cells in HIV infection and disease. Nat Rev Immunol 9: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poudrier J, Chagnon-Choquet J, Roger M (2012) Influence of Dendritic cells on B-cell Responses during HIV Infection. Clin Dev Immunol doi:10.1155/2012/592187. [DOI] [PMC free article] [PubMed]
- 3. Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, et al. (1998) Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95: 163–175. [DOI] [PubMed] [Google Scholar]
- 4. Poudrier J, Weng X, Kay DG, Paré G, Calvo EL, et al. (2001) The AIDS disease of CD4C/HIV transgenic mice shows impaired germinal centers and autoantibodies and develops in the absence of IFN-gamma and IL-6. Immunity 15: 173–185. [DOI] [PubMed] [Google Scholar]
- 5. Poudrier J, Weng X, Kay DG, Hanna Z, Jolicoeur P (2003) The AIDS-like disease of CD4C/human immunodeficiency virus transgenic mice is associated with accumulation of immature CD11bHi dendritic cells. J Virol 77: 11733–11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML (2010) B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev 237: 205–225. [DOI] [PubMed] [Google Scholar]
- 7. Hässler S, Ramsey C, Karlsson MC, Larsson D, Herrmann B, et al. (2006) AIRE-deficient mice develop haematopoietic irregularities and marginal zone B-cell lymphoma. Blood 108: 1941–1948. [DOI] [PubMed] [Google Scholar]
- 8. Lindh E, Lind SM, Lindmark E, Hässler S, Perheentupa J, et al. (2008) AIRE regulates T-cell-independent B-cell responses through BAFF. Proc Natl Acad Sci U S A 105: 18466–18471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Batista FD, Harwood NE (2009) The who, how and where of antigen presentation to B cells. Nat Rev Immunol 9: 15–27. [DOI] [PubMed] [Google Scholar]
- 10. Macpherson G, Kushnir N, Wykes M (1999) Dendritic cells, B cells and the regulation of antibody synthesis. Immunol Rev 172: 325–34. [DOI] [PubMed] [Google Scholar]
- 11. Cerutti A, Puga I, Cols M (2011) Innate control of B cell responses. Trends in Immunol 32(5): 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cerutti A, Cols M, Puga I (2013) Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol 13: 118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weill JC, Weller S, Reynaud CA (2009) Human Marginal Zone B cells. Annu Rev Immunol 27: 267–85. [DOI] [PubMed] [Google Scholar]
- 14. Fontaine J, Chagon-Choquet J, Valcke HS, Poudrier J, Roger M (2011) High expression levels of B Lymphocyte Stimulator (BLyS) by dendritic cells correlate with HIV-related B cell disease progression in humans. Blood 117: 145–155. [DOI] [PubMed] [Google Scholar]
- 15. Lund FE, Randall TD (2010) Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol 10: 236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mauri C, Bosma A (2012) Immune Regulatory function of B cells. Annu Rev Immunol 30: 221–241. [DOI] [PubMed] [Google Scholar]
- 17. Gray D, Gray M (2010) What are Regulatory B cells? Eur J Immunol 40: 2677–2679. [DOI] [PubMed] [Google Scholar]
- 18.Siewe B, Stapleton JT, Martinson J, Keshavarzian A, Kazmi N, et al. (2013) Regulatory B cell frequency correlates with markers of HIV disease progression and attenuates anti-HIV CD8-T cell function in vitro. J Leuk Biol Epub ahead of print February 22: , 2013 - doi:10.1189/jlb.0912436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calmon-Hamaty F, Combe B, Hahne M, Morel J (2011) Lymphotoxin α revisited: general features and implications in rheumatoid arthritis. Arthritis Research & Therapy 13: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bar-Or A, Fawaz L, Fan B, Ghorayeb C, Calabresi PA, et al. (2010) Abnormal B cell cytokine responses a trigger of T cell-mediated disease in MS? Annals of Neurology 67(4): 452–61. [DOI] [PubMed] [Google Scholar]
- 21. Mercier F, Boulassel MR, Yassine-Diab B, Tremblay C, Bernard NF, et al. (2008) Persistent human immunodeficiency virus-1 antigenaemia affects the expression of interleukin-7Ralpha on central and effector memory CD4+ and CD8+ T cell subsets. Clin Exp Immunol 152: 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malaspina A, Moir S, Chaitt DG, Rehm CA, Kottilil S, et al. (2007) Idiopathic CD4+ T lymphocytopenia is associated with increases in immature/transitional B cells and serum levels of IL-7. Blood 109: 2086–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thien M, Phan TG, Gardam S, Amesbury M, Basten A, et al. (2004) Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20: 785–798. [DOI] [PubMed] [Google Scholar]
- 24. Varin MM, Le Pottier L, Youinou P, Saulep D, Mackay F, et al. (2010) B-cell tolerance breakdown in Sjögren's syndrome: focus on BAFF. Autoimmunity Rev 9(9): 604–8. [DOI] [PubMed] [Google Scholar]
- 25. Cancro MP, D'Cruz DP, Khamashta MA (2009) The role of B Lymphocyte Stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest 119: 1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tangye SG, Bryant VL, Cuss AK, Good KL (2006) BAFF, APRIL and human B cell disorders. Seminars in Immunol 18: 305–317. [DOI] [PubMed] [Google Scholar]
- 27. Liu Z, Davidson A (2011) BAFF and selection of autoreactive B cells. Trends Immunol 32: 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stohl W, Cheema GS, Briggs WS, Xu D, Sosnovtseva S, et al. (2002) B lymphocyte stimulator protein-associated increase in circulating autoantibody levels may require CD4+ T cells: lessons from HIV-infected patients. Clin Immunol 104: 115–122. [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez B, Valdez H, Freimuth W, Butler T, Asaad R, et al. (2003) Plasma levels of B-lymphocyte stimulator increase with HIV disease progression. AIDS 17: 1983–2000. [DOI] [PubMed] [Google Scholar]
- 30. Weller S, Bonnet M, Delagreverie H, Israel L, Chrabieh M, et al. (2012) IgM+IgD+CD27+ B cells are markedly reduced in IRAK-4-, MyD88-, and TIRAP- but not UNC-93B-deficient patients. Blood 120(25): 4992–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fontaine J, Coutlée F, Tremblay C, Routy JP, Poudrier J, et al. (2009) HIV infection affects blood myeloid dendritic cells after successful therapy and despite nonprogressing clinical disease. J Infect Dis 199: 1007–1018. [DOI] [PubMed] [Google Scholar]
- 32. Yehudai D, Snir A, Peri R, Halasz K, Haj T, et al. (2012) B cell-activating factor enhances interleukin-6 and interleukin-10 production by ODN-activated human B cells. Scand J Immunol 76(4): 371–7. [DOI] [PubMed] [Google Scholar]
- 33. Klatt NR, Funderburg NT, Brenchley JM (2013) Microbial translocation, immune activation, and HIV disease. Trends Microbiol 21 (1): 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Das JI, Ellis G, Pallant C, Ross Lopes A, Khanna P, et al. (2012) IL-10–Producing Regulatory B Cells in the Pathogenesis of Chronic Hepatitis B Virus Infection. J Immunol 189: 3925–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mark Ansel K, Ngo VN, Hyman PL, Luther SA, Förster R, et al. (2000) A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406: 309–314. [DOI] [PubMed] [Google Scholar]
- 36. Cagigi A, Mowafi F, Phuong Dang LV, Tenner-Racz K, Atlas A, et al. (2008) Altered expression of the receptor-ligand pair CXCR5/CXCL13 in B cells during chronic HIV-1 infection. Blood 112: 4401–4410. [DOI] [PubMed] [Google Scholar]
- 37. Pujanauski LM, Edward N. Janoff EN, Pelanda R, Torres RM (2013) Mouse marginal zone B cells harbor specificities similar to human broadly neutralizing HIV antibodies. Proc Natl Acad Sci USA 110(4): 1422–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, et al. (2010) Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329(5993): 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yates NL, Stacey AR, Nolen TL, Vandergrift NA, Moody MA, et al. (2013) HIV-1 gp41 envelope IgA is frequently elicited after transmission but has an initial short response half-life. Mucosal Immunol doi:10.1038/mi.2012.107. [DOI] [PMC free article] [PubMed]
- 40. Dosenovic P, Soldemo M, Scholz JL, O'Dell S, Grasset EK, et al. (2012) BLyS-Mediated Modulation of Naive B Cell Subsets Impacts HIV Env-Induced Antibody Responses. J Immunol 188: 6018–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poudrier J, Roger M (2011) Dendritic Cell Status Modulates the Outcome of HIV-Related B Cell Disease Progression. PLoS Pathog 7(8): e1002154 doi:10.1371/journal.ppat.1002154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Borrow P (2011) Innate immunity in acute HIV-1 infection. Curr Opin HIV AIDS 6(5): 353–363 doi:10.1097/COH.0b013e3283495996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow-cytometry gating strategy based on fluorescence minus one and isotype controls. Dot plots showing gating strategy based on fluorescence minus one and isotype control for (A) CD19, (B) CD21, (C) CD27 and IgM, (D) CD1c and CD10, (E) IL-10 and (F) LT-α.
(TIF)
Longitudinal variations of blood CD4+ T-cell counts, viral loads and IL-10 and LT-α concentrations of HIV-1 infected individuals. (A) Blood CD4+ T-cell counts (cell/mm3) were determined by flow-cytometry in rapid progressors (left panel; 0–3 months PI (n = 13), 5–8 months PI (n = 13), 3–6 months ART (n = 7), 9–12 months ART(n = 5)), classic progressors (middle panel; 0–3 months PI (n = 16), 5–8 months PI (n = 16), 24 months PI (n = 11)), and slow progressors (right panel; viremic (n = 6), aviremic (n = 5)). (B) Viral loads (log copies/ml) were quantified by in vitro signal amplification nucleic acid probe assay of HIV-1 RNA (bDNA) in the plasma of rapid progressors (left panel; 0–3 months PI (n = 13), 5–8 months PI (n = 13), 3–6 months ART (n = 7), 9–12 months ART (n = 6)), classic progressors (middle panel; 0–3 months PI (n = 17), 5–8 months PI (n = 17), 24 months PI (n = 11)), and slow progressors (right panel; viremic (n = 6), aviremic (n = 6)). (C) Concentrations of IL-10 measured longitudinally in the plasma of rapid progressors (left panel; 0–3 months PI (n = 12), 5–8 months PI (n = 13), 3–6 months ART (n = 9), 9–12 months ART (n = 7)), classic progressors (middle panel; 0–3 months PI (n = 17), 5–8 months PI (n = 17), 24 months PI (n = 11)) and slow progressors (right panel; viremic (n = 7), aviremic (n = 5)). The same values for HIV-negative donors (n = 20) in the left, middle and right panels are used as a control group. (D) Concentrations of LT-α measured longitudinally in the plasma of rapid progressors (left panel; 0–3 months PI (n = 10), 5–8 months PI (n = 12), 3–6 months ART (n = 6), 9–12 months ART (n = 4)), classic progressors (middle panel; 0–3 months PI (n = 14), 5–8 months PI (n = 10), 24 months PI (n = 9)) and slow progressors (right panel; viremic (n = 4), aviremic (n = 4)). The same values for HIV-negative donors (n = 18) in the left, middle and right panels are used as a control group. Cell populations, viral loads and plasma concentrations were compared using the Wilcoxon signed-rank test and Mann-Whitney U test for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. Data shown are mean ±SEM. Significance levels are shown as * p<0.05, ** p<0.001, *** p<0.0001. PI, post-infection; ART, antiretroviral therapy.
(TIF)
Contribution of each blood B-cell population to IL-10 expression. Percentages of IL-10 expression within each B-cell population; ‘mature’ marginal zone (MZ)-like (purple), ‘precursor’ MZ-like (cherry red), mature activated (yellow), transitional immature (TI) (blue) and resting switched memory (orange) B-cells, for rapid progressors (left panel; 5–8 months PI (n = 11), 3–6 months ART (n = 6), 9–12 months ART (n = 5)), classic progressors (middle panel; 0–3 months PI (n = 12), 5–8 months PI (n = 17), 24 months PI (n = 13)), and slow progressors (right panel; viremic (n = 6), aviremic (n = 5)). The same value for HIV-negative donors in the left, middle and right panels are used as a control group (n = 7). Cell population frequencies were compared using the Mann-Whitney U test between the study groups. Data shown are mean ± SEM. * p<0.05. PI, post-infection; ART, antiretroviral therapy.
(TIF)
Contribution of each blood B-cell population to LT-α expression. Percentages of LT-α expression within each B-cell population; ‘mature’ marginal zone (MZ)-like (purple), ‘precursor’ MZ-like (cherry red), mature activated (yellow), transitional immature (TI) (blue) and resting switched memory (orange) B-cells, for rapid progressors (left panel; 5–8 months PI (n = 11), 3–6 months ART (n = 6), 9–12 months ART (n = 5)), classic progressors (middle panel; 0–3 months PI (n = 12), 5–8 months PI (n = 17), 24 months PI (n = 13)), and slow progressors (right panel; viremic (n = 6), aviremic (n = 5)). The same value for HIV-negative donors in the left, middle and right panels are used as a control group (n = 7). Cell population frequencies were compared using the Mann-Whitney U test between the study groups. Data shown are mean ±SEM. * p<0.05. PI, post-infection; ART, antiretroviral therapy.
(TIF)