Abstract
Frontotemporal dementia and amyotrophic lateral sclerosis are the main syndromes of the chromosome 9 ORF72 (C9ORF72) hexanucleotide repeat expansion, but studies have shown a substantial phenotypic diversity that includes psychiatric presentations. This study describes hippocampal sclerosis dementia (HSD) in carriers of the C9ORF72 mutation. We compared clinical and neuropathological features of HSD in carriers and non-carries autopsied at Johns Hopkins. Carriers presented with amnesia, agitation, dissocial behavior and impaired self-care, whereas non-carriers showed little agitation. The groups were not dissimilar in cognitive or motor dysfunction. Neuropathological examination of carriers showed cerebellar neuronal inclusions positive for ubiquitin, p62, and ubiquilin-2, and negative for TAR DNA-binding protein 43. Non-carriers did not have cerebellar inclusions. C9ORF72 repeat-associated non-ATG (RAN) translation was confirmed by immunohistochemistry.
These observations broaden the C9ORF72 phenotype and place HSD in the FTD spectrum. The amnesic phenotype of HSD, which is consistent with the focal hippocampal atrophy, should be included in clinical categorizations of FTD.
Keywords: C9ORF72 hexanucleotide repeat expansion, hippocampal sclerosis, dementia, frontotemporal dementia
1. INTRODUCTION
Hippocampal sclerosis is defined by marked loss of pyramidal neurons and gliosis in CA1 and subiculum of the hippocampus (Dickson et al., 1994). It has been found in up to 5% of brain bank cases of dementia (Zarow et al., 2008), and in 12% of autopsy cases from a community series of elders who fit clinical criteria for Alzheimer disease (AD) (Leverenz et al., 2002). Hippocampal sclerosis has been observed amid various neurodegenerative states (Probst et al., 2007; Zarow et al., 2008), whereas focal hippocampal sclerosis with dementia has not. Our group has proposed that hippocampal sclerosis dementia (HSD) may fit into the frontotemporal dementia (FTD) classification, based on clinical (Blass et al., 2004) and neuropathological (Hatanpaa et al., 2004) similarities; this is consistent with observations that hippocampal sclerosis is found in >75% of tau-negative FTD with ubiquitin-positive inclusions (Amador-Ortiz et al., 2006; Attems and Jellinger, 2006; Josephs and Dickson, 2007), and that TAR-DNA binding protein 43 (TDP-43) inclusions are found in nearly 90% of dementia cases showing hippocampal sclerosis (Pao et al., 2011). Cases of ‘pure’ HSD with tauopathy have also been reported (Beach et al., 2006; Probst et al., 2007), but it is not clear if these fit in the neuropathological classification of FTD. Interestingly FTD carrying the chromosome 9 open reading frame 72 (C9ORF72) hexanucleotide (GGGGCC) repeat expansion mutation (DeJesus-Hernandez et al., 2011; Renton et al., 2011) have hippocampal sclerosis amid widespread cortical and subcortical pathology in some series (Bigio et al., 2012; Murray et al., 2011). This report describes clinical and neuropathological characteristics in our TDP-43 positive ‘pure’ HSD series, comparing features in carriers and non-carriers of the C9ORF72 mutation.
2. METHODS
Ten cases of TDP-43 positive and tau-negative hippocampal sclerosis with dementia were identified from the Johns Hopkins University Brain Resource Center. None had progranulin mutation. Demographic variables and clinical variables were abstracted from medical records. The Johns Hopkins IRB approved this study.
The brains were fixed in 10% buffered formaldehyde. Tissue sections were stained with hematoxylin-eosin and with silver (Hirano method) and assessed for histopathologic features of AD using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) score for neuritic plaques (Mirra et al., 1991) and the Braak’s method for staging neurofibrillary change (H. Braak and E. Braak, 1995). Tissue sections underwent immunohistochemical (IHC) staining with antibodies for ubiquitin (DakoCytomation, rabbit polyclonal, 1:500), ubiquilin-2 (Novus Biologicals, hnRNP F (5F5) mouse monoclonal, 1:500), TDP-43 (ProteinTech Group, TARDBP rabbit polyclonal, 1:500), nucleoporin p62 (BD Transduction Laboratories, mouse anti-p62 lck ligand, 1:100), phosphorylated tau (gift of Dr. Peter Davies, Albert Einstein College of Medicine, PHF-1 monoclonal, 1:100), and α-synuclein (BD Transduction Laboratories, Syn-1 mouse monoclonal, 1:500). Immunoreactivity was visualized using the diaminobenzidine reaction.
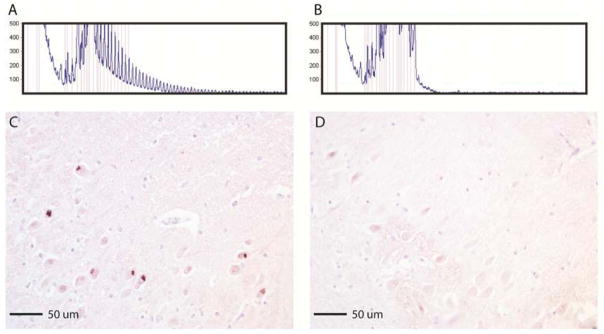
Repeat-primed PCR was performed (Renton et al., 2011) on brain tissues; 100 ng of genomic DNA was amplified using a reverse primer consisting of ~four GGGGCC repeats with an anchor tail, a 6FAM-fluorescent labeled forward primer located 280 bp telomeric to the repeat sequence, and an anchor primer corresponding to the anchor tail of the reverse primer. Fragment length analysis was undertaken on an ABI 3730xl genetic analyzer (Applied Biosystems, Foster City, CA, USA), and data analyzed using GeneMapper software (version 4, ABI). Repeat expansion were identified by the characteristic saw tooth pattern showing a 6 bp periodicity (Figure 2A). Cases were classified as having pathogenic repeat expansions (defined as >30 repeats) or wild-type alleles (< 20 repeats). Isolation of genomic DNA (gDNA) from post-mortem brain tissue was performed using the DNeasy kit (Qiagen) and 8–10 ug of DNA was digested with XbaI (New England Biolabs). Samples run on a 0.7% agarose gel were transferred to an N+ Hybond membrane (Amersham) for Southern analysis. Blots were probed with an α-32P-dCTP labeled fragment corresponding to a region just upstream of the C9ORF72 repeat region. IHC staining was performed on tissue sections using antibody 3154 (Center for NeuroGenetics, University of Florida, rabbit polyclonal), which recognizes sense and antisense repeat-associated non-ATG (RAN) translated GP RAN proteins expressed across the C9ORF72 mutation (Zu et al., 2013). Brain tissues screened negative for progranulin mutations.
Figure 2.
Repeat-primed PCR analysis of genomic DNA from the brain tissue of a C9ORF72 mutation carrier demonstrates the characteristic saw tooth pattern associated with the GGGGCC hexanucleotide repeat expansion (A), which the control sample does not show (B). Immunohistochemical staining of the hippocampus CA1 region of a carrier (C) with polyclonal antibody 3154 (GP), which recognizes the GP repeat motif that is expressed in both the sense and the antisense directions. Multiple pyramidal neurons display cytoplasmic inclusions containing RAN proteins, with no similar staining seen in controls (D).
3. RESULTS
Three of the 10 cases (see Table) were carriers of the C9ORF72 mutation. One carrier had a relative with neuropsychiatric disorder, whereas non-carriers had no relatives with neuropsychiatric disorder or dementia. Median age (years) at onset (66.9 IQR 66.5–71.1 vs. 69.9 interquartile range (IQR) 66.5–78.6, p=1.00 (Fisher’s exact test) and median duration (years) of illness were similar (11.2 IQR 7.6–12.8 vs. 9.2 IQR 7.3–13.7, p=0.524 (Fisher’s exact test).
Table 1.
Table Characteristics of TDP-43 positive Hippocampal Sclerosis Dementia
| C9ORF72 mutation | ID | Sex | Age at Onset | Age at death | Years of illness | Initial diagnosis | Final diagnosis | CERAD stage | Braak stage | TDP43 type | UBQ2* | p62* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | 1 | F | 67.0 | 74.6 | 7.6 | AD | AD | 0 | II | A | H+ C+ | H+ C+ |
| + | 2 | F | 66.6 | 79.4 | 12.8 | AD | FTD | 0 | II | A | H+ C+ | H+ C+ |
| + | 3 | F | 71.0 | 82.2 | 11.2 | Other | AD | 0 | II | A | H+ C+ | H+ C+ |
| − | 4 | M | 47.7 | 55.0 | 7.3 | AD | AD | 0 | 0 | A | H+ C− | H+ C− |
| − | 5 | M | -- | 69.0 | -- | AD | AD | 0 | II | C | H+ C− | H+ C− |
| − | 6 | M | 65.5 | 73.9 | 8.4 | AD | AD | 0 | 0 | Ind. | H+ C− | H+ C− |
| − | 7 | M | 67.6 | 81.8 | 14.3 | AD | FTD | 0 | II | B | H+ C− | H+ C− |
| − | 8 | F | 83.3 | 96.9 | 13.7 | AD | AD | 0 | II | C | H+ C− | H+ C− |
| − | 9 | F | 72.2 | 82.2 | 10.0 | AD | AD | A | 0 | Ind. | H+ C− | H+ C− |
| − | 10 | M | 78.6 | 83.3 | 4.7 | AD | FTD | 0 | III | Ind. | H+ C− | H+ C− |
Initial and final clinical diagnoses are shown; case #1 had a family history of neuropsychiatric disorder.
Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) amyloid plaque staging, A = low likelihood of Alzheimer disease (AD), B = intermediate, C = high. Braak neurofibrillary tangle staging, I/II = prodromal, III/IV early-moderate AD, V/VI = late stage.
TDP43 type = indeterminate (Ind.) in cases where neocortical inclusions are absent or sparse and assignment to a type (A, B, C or D) cannot be made.
Cytoplasmic ubiquilin-2 (UBQ) and p62 inclusion bodies in hippocampal (H) and cerebellar neurons (C).
Symptoms appearing in the first three years of illness defined the presentation. Carriers presented with amnesia, and two showed irritability/agitation, dissocial behavior and/or neglect of self-care. This compares with 4/7 of non-carriers presenting with amnesia, 1/7 with irritability/agitation, 5/7 with dissocial behavior, and 2/7 with neglect of self-care. Disorientation, executive dysfunction, aphasia, agnosia and apraxia were uncommon in both groups at onset, but present in all later. All cases – carriers and non-carriers – developed depression, irritability or agitation during the illness. None showed motor dysfunction at illness onset; tremor, rigidity and abnormal gait developed beyond the fifth year of illness. Cerebellar signs were not seen in any case. Carriers did not show hyperphagia at onset, and developed anorexia, hypophagia and dysphagia later. Incontinence also developed late in all cases. The initial clinical diagnosis in 9 of the 10 HSD cases was AD; in three cases FTD became the final clinical diagnosis. None of the cases had amyotrophic lateral sclerosis (ALS).
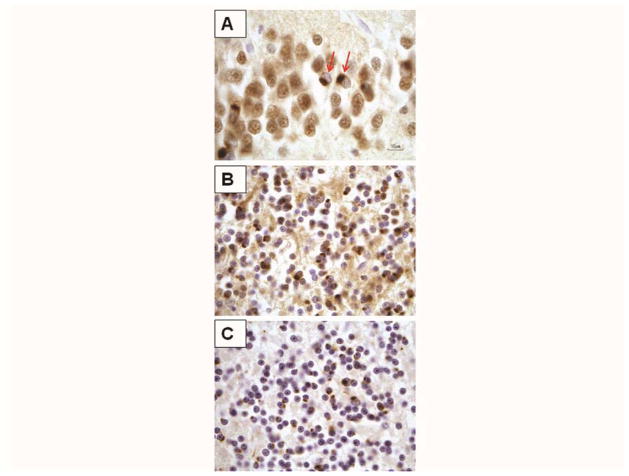
Mean brain weight was 946 grams (range 830–1,050) in carriers and 1196 grams (range 970–1,390) in non-carriers (p=0.055, two-tailed T test). All cases showed atrophy and degenerative changes predominantly in hippocampus, amygdala and entorhinal cortex, and relatively modest changes in the frontal cortex. Carriers had mild to severe atrophy of the caudate and mild neuronal loss in the substantia nigra, whereas half the non-carriers showed caudate atrophy and nigral degeneration. Gross atrophy of cerebellum or brainstem was not observed in either group. Ubiquitin-, TDP-43-, p62-positive and tau-negative cytoplasmic inclusions were present in granule cells of the hippocampal dentate gyrus in both groups (Figure 1A). Neocortical TDP-43 inclusions were also observed in all of the carriers and in 4/7 non-carriers. The TDP-43 inclusions corresponded to type A in the harmonized classification (Mackenzie et al., 2011) in all carriers, whereas the morphology was more varied in non-carriers. The main difference between the two groups were the cytoplasmic ubiquitin, P62, and ubiquilin-2 positive inclusions seen in the granule cells of the cerebellum in all carriers (Figure 1B and C), but absent in non-carriers. Cerebellar neurons did not have cytoplasmic TDP-43 inclusions in either group, and showed normal nuclear TDP-43 staining in all cases. We did not observe loss of cerebellar neurons in either group.
Figure 1.
A: Hippocampus immunostained with TDP-43 antibody. Paranuclear TDP-43-positive inclusions are seen in granule cells of the fascia dentata (arrows). The cells lack nuclear TDP-43 immunoreactivity. B and C: Cerebellum granule cell layer, Cytoplasmic inclusions are abundant and immunoreactive for ubiquitin (B) and p62 (C). These inclusions are not TDP-43 immunoreactive (not shown).
Our Southern blot analyses were successful for C9ORF72 mutation positive and negative control lymphoblastoid cell lines and peripheral blood lymphocyte samples, but not in our cases – the limited quantity and poor quality of genomic DNA extracted from the brain tissues of the carriers prevented estimation of the sizes of these repeat expansions. On the other hand, IHC staining identified repeat-associated non-ATG (RAN) proteins in the CA1 field of the hippocampus in carriers (Figure 2C), and not in control samples (Figure 2D), confirming that the C9ORF72 mutation is expressed.
4. DISCUSSION
Notwithstanding the small sample and retrospective design, this study links the C9ORF72 mutation with focal hippocampal sclerosis and an amnesic dementia and confirms translation of C9ORF72 RAN proteins in the carriers. Thus it broadens the C9ORF72 phenotype beyond the FTD, ALS and psychiatric states (psychosis (Floris et al., 2012; Snowden et al., 2012) and affective disorder (Floris et al., 2013; Synofzik et al., 2012)) with which this mutation is already associated. During peer review of this paper, another case of amnesic dementia with positive C9ORF72 RAN IHC was published (Murray et al., 2013). Two earlier clinical-pathologic studies of C9ORF72 carriers (Bigio et al., 2012; Murray et al., 2011) described hippocampal sclerosis amid widespread cortical and subcortical pathology. One (Murray et al., 2011) noted amnesic presentation, pre-mortem AD diagnosis and older age at death in some cases, in contrast to cases who had behavioral presentation and FTD diagnosis. It is possible some of those cases had focal hippocampal sclerosis and amnesic dementia. Hippocampal sclerosis has also been associated with rs5848;c.*78C>T (Dickson et al., 2010; Pao et al., 2011), the T-allele of a common genetic variant in the 3′ region of the progranulin gene linked to TDP-43 positive FTD (Rademakers et al., 2008).
Amnesic presentation is also seen in patients who do not carry the C9ORF72 mutation; this phenomenon matches previous descriptions of HSD as an amnesic condition that typically is presumed to be AD (Ala et al., 2000; Leverenz et al., 2002; Probst et al., 2007). A community study (Leverenz et al., 2002) showed that 12% of elders whose symptoms fit diagnostic criteria for AD were found at autopsy to have HSD. On the other hand, clinical differences between HSD and AD have been described: an earlier report from our group showed higher cumulative prevalence during the illness (than in AD) of behavioral features reminiscent of FTD (Blass et al., 2004), suggesting differences facilitate differential diagnosis. Also HSD cases have also shown less executive dysfunction than AD in the early phase of illness (Corey-Bloom et al., 1997; Leverenz et al., 2002). However the clinical reliability and utility of these behavioral and cognitive differences are unclear. Therefore, it is not yet possible to identify a clinical phenotype that reliably distinguishes HSD from AD. Hence the recent suggestion for screening for the C9ORF72 mutation in clinical and research populations of AD (Majounie et al., 2012).
The function of C9ORF72 is still unknown, but recent studies point to a few non-exclusive pathological mechanisms for the expansion mutation: 1) RNA gain-of-function via sequestration of RNA-binding proteins (Almeida et al., 2013; Mori et al., 2013a; Xu et al., 2013; Zu et al., 2013); 2) loss of function of the C9ORF72 protein, which is possibly involved in vesicular trafficking (Lashley et al., 2013; Levine et al., 2013); 3) abnormal proteasome processing has been inferred from observations that ubiquilin-2 IHC correlates more tightly with the C9ORF72 expansion (Brettschneider et al., 2012) and cortical degeneration (Irwin et al., 2013) than p62 and TDP-43; and 4) a recently described mechanism, RAN translation of antisense RNA transcripts (Ash et al., 2013; Mori et al., 2013b; Zu et al., 2013; 2011), gives rise to novel aggregating proteins. The discovery of RAN translation in C9ORF72, which mirrors other repeat expansion diseases, suggests sense and antisense proteins as targets for treatment development in FTD (Cleary and Ranum, 2013).
HSD in C9ORF72 carriers and non-carriers shows histopathologic characteristics consistent with FTD. Since histopathologic diversity in FTD was described some 40 years ago (Constantinidis et al., 1974), it has become widely recognized that variation in the morphology and distribution of pathology undergirds the protean nature of FTD phenotypes (Boeve, 2007). The amnesic phenotype of HSD, which is consistent with the focal hippocampal atrophy and sometimes behaviorally congruent with FTD, can be understood in this sense – and requires accommodation in our clinical categorizations. An amnesic presentation makes HSD difficult to distinguish from AD on clinical grounds, so it will be important to determine which technologies – from psychometry, bioassays, genetics, and radiology (such as amyloid PET) – can facilitate its detection in the clinic.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging (Z01-AG000949-02); the Johns Hopkins Alzheimer’s Disease Research Center (National Institutes on Aging grant P50AG05146); the Samuel I. Newhouse Foundation; the Robert Hall family; the Richman Family professorship; the Jane Tanger Black Fund for Young-Onset Dementia Research; the W.F. Keck Foundation; the ALS Association; and Target ALS.
Footnotes
Disclosure: Dr. Traynor has a patent pending on clinical testing and therapeutic intervention for the C9ORF72 mutation. Drs. Ranum and Zu have patents pending for RAN translation, and for RAN translation in C9ORF72 ALS/FTD. Dr. Onyike has received support for clinical trials sponsored by Forest Inc. (Lancet Neurol 2013; 12: 149–56) and Tau Therapeutics (ClinicalTrials.gov ID: NCT01626378).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Ala TA, Beh GO, William H, Frey I. Pure hippocampal sclerosis: a rare cause of dementia mimicking Alzheimer’s disease. Neurology. 2000;54:843–848. doi: 10.1212/wnl.54.4.843. [DOI] [PubMed] [Google Scholar]
- Almeida S, Gascon E, Tran H, Chou HJ, Gendron TF, DeGroot S, Tapper AR, Sellier C, Charlet-Berguerand N, Karydas A, Seeley WW, Boxer AL, Petrucelli L, Miller BL, Gao FB. Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathol. 2013;126:385–399. doi: 10.1007/s00401-013-1149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW. Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol. 2006;113:245–252. doi: 10.1007/s00401-006-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash PEA, Bieniek KF, Gendron TF, Caulfield T, Lin WL, DeJesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW, Rademakers R, Boylan KB, Dickson DW, Petrucelli L. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J, Jellinger KA. Hippocampal sclerosis in Alzheimer disease and other dementias. Neurology. 2006;66:775. doi: 10.1212/01.wnl.0000200959.50898.26. [DOI] [PubMed] [Google Scholar]
- Beach TG, Sue L, Scott S, Layne K, Newell A, Walker D, Baker MC, Sahara N, Yen SH, Hutton M, Caselli RJ, Adler C, Connor D, Sabbagh M. Hippocampal Sclerosis Dementia with Tauopathy. Brain Pathology. 2006;13:263–278. doi: 10.1111/j.1750-3639.2003.tb00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigio EH, Weintraub S, Rademakers R, Baker MC, Ahmadian SS, Rademaker A, Weitner BB, Mao Q, Lee K-H, Mishra M, Ganti RA, Mesulam M-M. Frontotemporal lobar degeneration with TDP-43 proteinopathy and chromosome 9p repeat expansion in C9ORF72: clinicopathologic correlation. Neuropathology. 2012:no–no. doi: 10.1111/j.1440-1789.2012.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass DM, Hatanpaa KJ, Brandt J, Rao V, Steinberg M, Troncoso JC, Rabins PV. Dementia in hippocampal sclerosis resembles frontotemporal dementia more than Alzheimer disease. Neurology. 2004;63:492–497. doi: 10.1212/01.wnl.0000133008.89613.82. [DOI] [PubMed] [Google Scholar]
- Boeve BF. Links between frontotemporal lobar degeneration, corticobasal degeneration, progressive supranuclear palsy, and amyotrophic lateral sclerosis. Alzheimer Dis Assoc Disord. 2007;21:S31–8. doi: 10.1097/WAD.0b013e31815bf454. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Brettschneider J, Deerlin VM, Robinson JL, Kwong L, Lee EB, Ali YO, Safren N, Monteiro MJ, Toledo JB, Elman L, McCluskey L, Irwin DJ, Grossman M, Molina-Porcel L, Lee VMY, Trojanowski JQ. Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion. Acta Neuropathol. 2012;123:825–839. doi: 10.1007/s00401-012-0970-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JD, Ranum LPW. Repeat-associated non-ATG (RAN) translation in neurological disease. Hum Mol Genet. 2013;22:R45–R51. doi: 10.1093/hmg/ddt371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis J, Richard J, Tissot R. Pick’s disease. Histological and clinical correlations. Eur Neurol. 1974;11:208–217. doi: 10.1159/000114320. [DOI] [PubMed] [Google Scholar]
- Corey-Bloom J, Sabbagh MN, Bondi MW, Hansen L, Alford MF, Masliah E, Thal LJ. Hippocampal Sclerosis Contributes to Dementia in the Elderly. Neurology. 1997;48:154–160. doi: 10.1212/wnl.48.1.154. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker MC, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GYYR, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman HH, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Baker MC, Rademakers R. Common Variant in GRNIs a Genetic Risk Factor for Hippocampal Sclerosis in the Elderly. Neurodegenerative Dis. 2010;7:170–174. doi: 10.1159/000289231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Davies P, Bevona C, Hoeven KH, Factor SM, Grober E, Aronson MK, Crystal HA. Hippocampal sclerosis: a common pathological feature of dementia in very old (?80 years of age) humans. Acta Neuropathol. 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- Floris G, Borghero G, Cannas A, Stefano F, Costantino E, Murru MR, Brunetti M, Restagno G, Traynor BJ, Marrosu MG, Chiò A, Marrosu F. Frontotemporal dementia with psychosis, parkinsonism, visuo-spatial dysfunction, upper motor neuron involvement associated to expansion of C9ORF72: a peculiar phenotype? J Neurol. 2012;259:1749–1751. doi: 10.1007/s00415-012-6444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris G, Borghero G, Cannas A, Stefano FD, Murru MR, Corongiu D, Cuccu S, Tranquilli S, Marrosu MG, Chiò A, Marrosu F. Bipolar affective disorder preceding frontotemporal dementia in a patient with C9ORF72 mutation: is there a genetic link between these two disorders? J Neurol. 2013 doi: 10.1007/s00415-013-6833-2. [DOI] [PubMed] [Google Scholar]
- Hatanpaa K, Blass DM, Pletnikova O, Crain B, Bigio EH, Hedreen J, White CL, Troncoso JC. Most cases of dementia with hippocampal sclerosis may represent frontotemporal dementia. Neurology. 2004;63:538–542. doi: 10.1212/01.wnl.0000129543.46734.c0. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, McMillan CT, Brettschneider J, Libon DJ, Powers J, Rascovsky K, Toledo JB, Boller A, Bekisz J, Chandrasekaran K, Wood EM, Shaw LM, Woo JH, Cook PA, Wolk DA, Arnold SE, van Deerlin VM, McCluskey LF, Elman L, Lee VMY, Trojanowski JQ, Grossman M. Cognitive decline and reduced survival in C9orf72 expansion frontotemporal degeneration and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2013;84:163–169. doi: 10.1136/jnnp-2012-303507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Dickson DW. Hippocampal sclerosis in tau-negative frontotemporal lobar degeneration. Neurobiol Aging. 2007;28:1718–1722. doi: 10.1016/j.neurobiolaging.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Lashley T, Hardy J, Isaacs AM. RANTing about C9orf72. Neuron. 2013;77:597–598. doi: 10.1016/j.neuron.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Agustin CM, Tsuang D, Peskind ER, Edland SD, Nochlin D, DiGiacomo L, Bowen JD, McCormick WC, Teri L, Raskind MA, Kukull WA, Larson EB. Clinical and neuropathological characteristics of hippocampal sclerosis: a community-based study. Arch Neurol. 2002;59:1099–1106. doi: 10.1001/archneur.59.7.1099. [DOI] [PubMed] [Google Scholar]
- Levine TP, Daniels RD, Gatta AT, Wong LH, Hayes MJ. The product of C9orf72, a gene strongly implicated in neurodegeneration, is structurally related to DENN Rab-GEFs. Bioinformatics. 2013;29:499–503. doi: 10.1093/bioinformatics/bts725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IRA, Neumann M, Baborie A, Sampathu DM, du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DMA, Lee VMY. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majounie E, Abramzon Y, Renton AE, Perry R, Bassett SS, Pletnikova O, Troncoso JC, Hardy J, Singleton AB, Traynor BJ. Repeat expansion in C9ORF72 in Alzheimer’s disease. N Engl J Med. 2012;366:283–284. doi: 10.1056/NEJMc1113592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer”s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer”s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mori K, Lammich S, Mackenzie IRA, Forné I, Zilow S, Kretzschmar H, Edbauer D, Janssens J, Kleinberger G, Cruts M, Herms J, Neumann M, Van Broeckhoven C, Arzberger T, Haass C. hnRNP A3 binds to GGGGCC repeats and is a constituent of p62-positive/TDP43-negative inclusions in the hippocampus of patients with C9orf72 mutations. Acta Neuropathol. 2013a;125:413–423. doi: 10.1007/s00401-013-1088-7. [DOI] [PubMed] [Google Scholar]
- Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C, Haass C, Edbauer D. The C9orf72 GGGGCC Repeat Is Translated into Aggregating Dipeptide-Repeat Proteins in FTLD/ALS. Science. 2013b;339:1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- Murray ME, Bieniek KF, Banks Greenberg M, DeJesus-Hernandez M, Rutherford NJ, van Blitterswijk M, Niemantsverdriet E, Ash PE, Gendron TF, Kouri N, Baker M, Goodman IJ, Petrucelli L, Rademakers R, Dickson DW. Progressive amnestic dementia, hippocampal sclerosis, and mutation in C9ORF72. Acta Neuropathol. 2013;126:545–554. doi: 10.1007/s00401-013-1161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker MC, Duara R, Graff-Radford NR, Wszolek ZK, Ferman TJ, Josephs KA, Boylan KB, Rademakers R, Dickson DW. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122:673–690. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao WC, Dickson DW, Crook JE, Finch NA, Rademakers R, Graff-Radford NR. Hippocampal Sclerosis in the Elderly: Genetic and Pathologic Findings, Some Mimicking AlzheimerDisease Clinically. Alzheimer Dis Assoc Disord. 2011;25:364–368. doi: 10.1097/WAD.0b013e31820f8f50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst A, Taylor KI, Tolnay M. Hippocampal sclerosis dementia: a reappraisal. Acta Neuropathol. 2007;114:335–345. doi: 10.1007/s00401-007-0262-1. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Eriksen JL, Baker MC, Robinson T, Ahmed Z, Lincoln SJ, Finch N, Rutherford NJ, Crook RJ, Josephs KA, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Caselli RJ, Wszolek ZK, Uitti RJ, Feldman HH, Hutton ML, Mackenzie IR, Graff-Radford NR, Dickson DW. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet. 2008;17:3631–3642. doi: 10.1093/hmg/ddn257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Hölttä-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chiò A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ The ITALSGEN Consortium28. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AMT, Jones M, Gerhard A, Davidson YS, Robinson A, Gibbons L, Hu Q, Duplessis D, Neary D, Mann DMA, Pickering-Brown SM. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135:693–708. doi: 10.1093/brain/awr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synofzik M, Biskup S, Leyhe T, Reimold M, Fallgatter AJ, Metzger F. Suicide attempt as the presenting symptom of c9orf72 dementia. Am J Psychiatry. 2012;169:1211–1213. doi: 10.1176/appi.ajp.2012.12060733. [DOI] [PubMed] [Google Scholar]
- Xu Z, Poidevin M, Li X, Li Y, Shu L, Nelson DL, Li H, Hales CM, Gearing M, Wingo TS, Jin P. Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc Natl Acad Sci. 2013;110:7778–7783. doi: 10.1073/pnas.1219643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarow C, Sitzer TE, Chui HC. Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep. 2008;8:363–370. doi: 10.1007/s11910-008-0057-3. [DOI] [PubMed] [Google Scholar]
- Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, Margolis J, Peterson M, Markowski TW, Ingram MAC, Nan Z, Forster C, Low WC, Schoser B, Somia NV, Clark HB, Schmechel S, Bitterman PB, Gourdon G, Swanson MS, Moseley M, Ranum LPW. Non-ATG–initiated translation directed by microsatellite expansions. Proc Natl Acad Sci. 2011;108:260–265. doi: 10.1073/pnas.1013343108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Liu Y, Bañez-Coronel M, Reid T, Pletnikova O, Lewis J, Miller TM, Harms MB, Falchook AE, Subramony SH, Ostrow LW, Rothstein JD, Troncoso JC, Ranum LPW. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci. 2013;110:E4968–77. doi: 10.1073/pnas.1315438110. [DOI] [PMC free article] [PubMed] [Google Scholar]