Abstract
Studies have shown differences in specific cognitive ability domains and risk of Alzheimer’s disease between the men and women at later age. However it is important to know that sex differences in cognitive function during adulthood may have their basis in both organizational effects, i.e., occurring as early as during the neuronal development period, as well as in activational effects, where the influence of the sex steroids influence brain function in adulthood. Further, the rate of cognitive decline with aging is also different between the sexes. Understanding the biology of sex differences in cognitive function will not only provide insight into Alzheimer’s disease prevention, but also is integral to the development of personalized, gender-specific medicine. This review draws on epidemiological, translational, clinical, and basic science studies to assess the impact of sex differences in cognitive function from young to old, and examines the effects of sex hormone treatments on Alzheimer’s disease in men and women.
1. Introduction
The popular quote, “Men are from Mars and women are from Venus”, has been commonly applied to many different situations including physiology, sociology and pathology; the gender differences in cognitive functioning are no exception. The differences of learning and memory between male and female brains are confirmed by both human and animal studies from early development stages throughout their life spans. In addition, many neurological diseases exhibit gender biases, such that one sex has a greater prevalence or severity of the disease than the other. Neurological diseases in the young and the elderly also demonstrate gender-specific responses to therapies. However, the question is how much, rather than whether or not, the biology of sex contributes to normal cognitive function. Accordingly, such understanding may provide better insight into the factors that contribute to the risk of cognitive impairment. Here, we will be focusing on sex hormones - especially the role of estrogens, progesterone and testosterone – on mechanisms that relate to neuronal function and associated cognitive ability in the adult and aged individual.
2. Sex differences in cognition
Gender differences in cognitive function in adulthood and ageing have been well demonstrated. For example, men perform better on spatial memory while women excel at verbal and object location (Table 1). The sex differences in cognitive function and brain structures in later life have been demonstrated by Magnetic resonance imaging (MRI) in human studies. For instance, studies found that men demonstrated larger amygdala and thalamus volumes compared to women [1; 2; 3], whereas the size of hippocampus is larger in females compared to males [1; 4]. It is also worth noticing that there are a relatively higher number of androgen receptors in the amygdala [5] and a relatively higher number of estrogen receptors in the hippocampus [6].
Table 1.
Human studies of spatial rotation, navigation, object location and verbal memory Spatial rotation
| authors | Year | Case number | Age (years) | advantage | P value |
|---|---|---|---|---|---|
| Sharps et al. [311] | 1993 | 60 | 18–36 | Male | <0.001 |
| Epting and Overman [312] | 1998 | 47 | 19–41 | Male | <0.01 |
| Moffat et al [313] | 1998 | 74 | 20s | Male | <0.001 |
| Levine et al. [314] | 1999 | 288 | 4–7 | Male | <0.005 |
| Silverman et al. [315] | 2000 | 111 | 20s | Male | <0.001 |
| Peters [316] | 2005 | 212 | 20s | Male | <0.0001 |
| Silverman et al. [31] | 2007 | 95742 | 20s–30s | Male | <0.05 |
| Kaufman [317] | 2007 | 100 | 16–18 | Male | <0.0001 |
| Maylor et al [318] | 2007 | 198121 | 20–65 | Male | <0.001 |
| Jansen and Heil [319] | 2010 | 150 | 20–70 | Male | <0.01 |
| Tzuriel and Egozi [320] | 2010 | 116 | 6–7 | Male | <0.01 |
| Puts et al [14] | 2010 | 337 | 20s | Male | <0.0001 |
| Lange-Kuttner & Ebersbach [321] | 2013 | 97 | 6–9 | Male | <0.05 |
| Mantyla [13] | 2013 | 72 | 19–40 | Male | <0.01 |
| Christie et al [322] | 2013 | 60 | 20s | Male | <0.05 |
| Jansen and Kaltner [323] | 2013 | 60 | 60–71 | Male | <??? |
| Navigation | |||||
| Astur et al. [324] | 1998 | 48 | 20s | Male | <0.05 visual water maze |
| Moffat et al. [313] | 1998 | 74 | 20s | Male | <0.001 |
| Map view Silverman et al. [315] | 2000 | 186 | 20s | Male | <0.001 in 3D test |
| Malinowski and Gillespie [325] | 2001 | 1042 | unknown | Male | <0.001 |
| Beatty [326] | 2002 | 98 | 16–60 | Male | <0.05 |
| Driscoll et al [21] | 2005 | 70 | 20–60+ | Male | <0.005 visual water maze |
| Postman et al. [327] | 2004 | 64 | 20s | Male | <0.05 only in 3D test |
| Tippett et al [328] | 2009 | 24 | 60–80 | Male | <0.01 2D and AD tests |
| Chai and Jacobs [329] | 2009 | 84 | 18–25 | Male | <0.001 visual water maze |
| Vestergren et al [330] | 2012 | 1115 | 25–85 | Male | <0.05 |
| Persson et al. [331] | 2013 | 24 | 18–35 | Male | <0.05 |
| Object location | |||||
| Portin et al. [332] | 1995 | 389 | 62 | Female | <0.001 |
| McGivern et al [29] | 1997 | 483 | 10–20 | Female | <0.0001 |
| Epting and Overman [312] | 1998 | 47 | 19–41 | NS | NS |
| Postman et al. [327] | 2004 | 64 | 20s | NS | NS |
| Herrera-Guzman et al [333] | 2004 | 90 | 50–80 | Female | <0.05 in cube analysis |
| Herrera-Guzman et al [333] | 2004 | 90 | 50–80 | Male | <0.05 in incomplete letters |
| Silverman et al. [31] | 2007 | 95742 | 20s–30s | Female | <0.05 |
| Ardila et al [334] | 2011 | 788 | 5–16 | Female | <0.05 |
| Bracco et al [335] | 2011 | 83 | 21–60 | NS | NS |
| McGivern et al. [336] | 2012 | 141 | 18–26 | Female | <0.001 in accuracy |
| McGugin et al. [337] | 2012 | 227 | 20s | Female | <0.001 |
| Verbal memory | |||||
| Trahan and Quintana [338] | 1990 | 140 | unknown | Female | <0.05 in recall |
| Mann et al. [339] | 1990 | 175 | teens | Female | <0.001 in fluency, recall |
| Youngjohn et al. [340] | 1991 | 1492 | 20–70 | Female | <0.005 in recall |
| Savage and Gouvier [341] | 1992 | 134 | 15–76 | NS in delay recall | |
| Portin et al. [332] | 1995 | 389 | 62 | Female | <0.005 in WAIS |
| Berenbaum et al. [342] | 1997 | 57 | 20–40 | Female | <0.05 in CVLT |
| Kimura and Clarke [343] | 2002 | 81 | 20s | Female | <0.01 in CVLT |
| Yonker et al. [344] | 2003 | 36 | 35–85 | Female | <0.05 in recall |
| Kimura and Seal [345] | 2003 | 53 | unknown | Female | <0.05 in recall |
| Neri et al [346] | 2012 | 900 | 65+ | NS in fluency | |
| Munro et al [347] | 2012 | 957 | 67–89 | Female | <0.001 in verbal learning |
| Murre et al [348] | 2013 | 28116 | 11–80 | Female | <0.001 |
| Heinzel et al [349] | 2013 | 523 | 51–82 | Female | <0.001 in semantic fluency |
CVLT=California Verbal Learning Test, WAIS=Wechsler Adult Intelligence Scale
2.1. Sex-type cognitive behavioral tests
Differing performances between the sexes have been observed on a number of common learning tasks in both human and animal literature. There are four classes of memory tasks for which sex differences have been frequently reported: spatial, verbal, autobiographical, and emotional memory. Typically, it has been commonly believed that males show an advantage on spatial tasks, and females on verbal tasks. However, evidence now shows that the male spatial advantage does not apply to certain spatial tasks, and that the female advantage in verbal processing extends into many memory tasks which are not explicitly verbal [7]. For example, spatial tests can be further divided into three components: spatial perception, spatial rotation (spatial working memory) and spatial visualization (navigation) [8].). In Table 1, we include a review of human spatial ability and verbal performance with sex-favored components.
Human studies
Spatial rotation memory test
There are simple (two-dimensional stimuli) and complex (three dimensional stimuli) tasks. The rotation of simple two-dimensional stimuli can lead to greater activation of the left parietal area rather than the right parietal area, while the complex three-dimensional rotations are associated with more right parietal activation than left parietal activation [9]. Interestingly, brain imaging studies have identified distinctly different networks activating during mental rotation tasks for men and women, such as increased activation in the parietal lobules in men, and increased activity in frontal areas in women [10; 11; 12]. The unique frontal activity in women has been interpreted as evidence of a different cognitive strategy from men to solving mental rotation problems. Studies also showed that if sex hormone variation across the menstrual cycle in women was taken into account, the different activation areas during mental rotation tasks were no longer observed [12; 13], suggests that activity in the parietal lobule region may be sensitive to ovarian hormones in women. A study on fluctuations in salivary testosterone and performance of male-biased spatial rotation task in 160 women and 177 men showed that circulating testosterone does not contribute substantially to sex differences in spatial rotation memory in young men and women [14]. A recent study of 308 twins showed that puberty testosterone levels are significantly related to poor performance in mental rotation test in male young adults [15]. The negative relationship between testosterone levels and mental rotation performance is also reported in older male as the higher the testosterone levels, the poorer performance the males showed [16]. Another study showed that female users using hormone contraception that had lower levels of 17b-estradiol and progesterone had poorer spatial rotation as well as reduced verbal (What are you trying to say here?) compared to non-users [17]. Furthermore, a longitudinal study of 10 male and 7 female for 8 weeks showed that both genders showed significant sex hormone related cycle effect in mental rotation performance at the beginning of the study. However, at the end of the study, none of the hormones were significantly related to performance. Thus, the relationship between hormones and mental rotation performance disappeared with repeated testing [18].
Spatial navigation test
Navigation tests, also called a way-finding, are commonly conducted by having subjects reconstruct a path through a map or real space. There are two different approaches that may be involved: egocentric and allocentric strategies (figure 1). An egocentric strategy involves more local landmarks as and directional cues as personal directions. An allocentric strategy uses the absolute position of general landmarks, such as distance as absolute directions [19]. Individuals with hippocampal sclerosis were more impaired in navigating through a virtual maze in which learning was associated with egocentric memory. The allocentric memory impairment is found in patients with extensive hippocampal sclerosis plus subcortical deterioration, suggesting a combination of hippocampal and cortical damage is associated with negative changes in allocentric memory [20]. Patients with temporal lobe epilepsy without hippocampal sclerosis do not display cognitive deficits of allocentric or egocentric memory. Indeed, men tend to favor a more allocentric strategy (accurate judgments of distance), while women are more frequently egocentric (able to recall more street names and building shapes as landmarks) navigators. Although males perform better than female in the navigation strategy, the relationship between navigation and one’s level of testosterone has not been consistently demonstrated [21; 22]. It is worth pointing out that the outcome of sex-specific navigation test is closely related to the experimental conditions. For example, when test was performed within a single room or within an indoor environment without absolute directional cues, men and women perform the same [23]. On the other hand, men significantly outperform women in navigating through a large outdoor space [24].
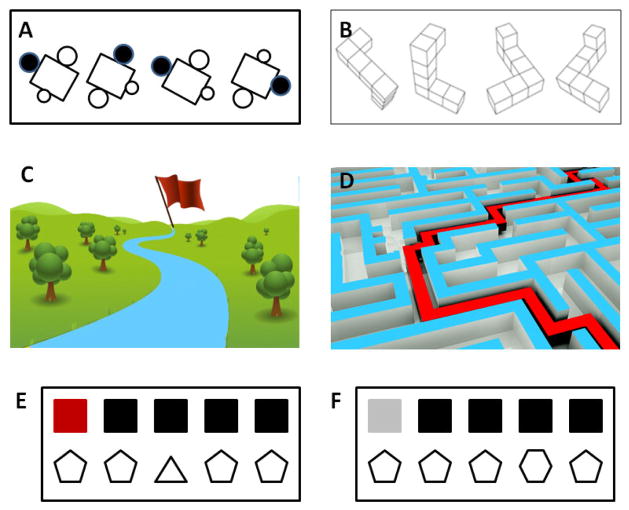
Figure 1.
Spatial rotation tasks with simple 2-dimensianl stimuli (A) or complex 3-dimensianl stimuli (B). In tests, two or more objects were either identical or mirror images of each other were placed at different orientation in space with varying degrees of angular disparity. Spatial navigation tests through virtual park (C) or virtual maze (D) as a determinant of capability in allocentric and egocentric memory, respectively. Object location test at a easy (E) or a difficulty (F) trials are shown for feature stimuli.
Recent human studies using a computerized water maze to mirror rodent tests of object recognition and spatial navigation test showed a faster and more efficient performance by college-aged males compared to females of the same age. Studies also reported that older adults’ spatial navigation learning were preferentially related to processing of landmark information, whereas processing of boundary information played a more prominent role in younger adults [25]. Efficient spatial navigation requires not only accurate spatial knowledge but also the selection of appropriate strategies. Successful performance using an allocentric place strategy was observed in young participants, while older participants were able to recall the route when approaching intersections from the same direction as during encoding and failed to use the correct strategy when approaching intersections from new directions [26]. Aging specifically impairs switching navigational strategy to an allocentric navigational strategy.
A traditional object location memory task is designed by presenting different arrays of common objects between the training phases. The test requires participants to identify the difference between the 2 selections. In human studies, the medial temporal lobe and perirhinal cortex are impaired in various types of object location tasks, but only when the objects have a high number of overlapping features. Meanwhile, patients with medial temporal lesions that are confined to the hippocampus showed normal performance on object location tasks regardless of the level of feature ambiguity [27; 28]. Significant female advantages have been observed in several studies of object location memory [29; 30; 31]. This is opposed to mental rotation and navigation tasks, suggesting that object location differs from other spatial tasks in terms of its cognitive demands. Similarly, females relying on an array of local objects for navigational cues has been reported in several well-studied phenomenon in the laboratory through animals using the radial arm maze, such as female rats showing impaired performance if local landmarks are removed [32].
Verbal memory
Two kinds of general measures of verbal memory have been used in most studies to identify sex differences. One is the Controlled Oral Word Association Test (COWAT) to test verbal fluency and another is the Rey Auditory Verbal Learning Test (RAVLT), also known as the California Verbal Learning Test (CVLT) which has participants recall a list of words. Women outperform men in both measures. Interestingly, the female advantage in verbal memory is consistent throughout the life span [33; 34], suggesting circulating sex hormone independency. Women generally score higher than men on verbal memory tasks, possibly because women tend to use semantic clustering in recall. Studies showed that the sex differences in recall and semantic clustering in the verbal learning test diminished with a shorter word list in a relative small sample study [35]. A 10-year longitudinal study of over 600 non-demented adults, aged 35–80 years, found stable sex differences across five age groups—women outperformed men on verbal memory, verbal recognition, and semantic fluency tasks, while men demonstrated better visuospatial ability [36]. Some studies showed that healthy elderly women have better immediate word learning [37], verbal memory, and episodic memory compared to age-matched men [38]. However, a recent meta-analysis of neurocognitive data from 15 studies (n = 828 men; 1,238 women) showed that men modestly but significantly outperformed women in all of the cognitive domains been examined, including verbal and visuospatial tasks and tests of episodic and semantic memory, while age and MMSE were not associated with the male-advance in memory [39]. Some also reported better visual memory [40], working memory [41], and episodic memory [41] in elderly men than women. Furthermore, others have also reported no sex differences in the elderly for verbal memory. So, there exists no clear pattern of sex advantages for memory in the healthy elderly, and any sex differences appear to be task dependent. A cross-sectional analysis of the association between sex hormones, metabolic parameters, and psychiatric diagnoses with verbal memory in healthy aged men showed that higher levels of serum sex hormone binding globulin (SHBG) were associated with a worse verbal memory [42], suggesting levels of free testosterone influence male verbal memory. However, findings of sex-differences in verbal memory in young adults or early adolescents are contradictory. Studies showed no association between the sex-dependent verbal memory and age, level of sex hormone, or puberty development in teenage boys and girls [43]. Furthermore, a recent study including 366 women and 330 men aged between 16 and 69 years of age, showed that women outperformed men on auditory memory tasks due to female advancement in verbal memory, whereas male adolescents and older male adults showed higher level performances on visual episodic and visual working memory measures [44].
Animal studies
A traditional object location memory task
Indeed, it has been shown that rodents are able to discriminate a novel from a familiar object (one-trial object recognition), detect a mismatch between the past and present location of a familiar object (one-trial object-place recognition), and are also able to discriminate between different objects in terms of their relative recency (temporal order memory [45]. Studies of object-in-place task with combination of object recognition and object location showed that rats were able to discriminate moved from unmoved objects after a brief 5-min delay, regardless of biological sex or hormone status. The ovariectomized female rats treated with estradiol and progesterone discriminated moved from unmoved objects while ovariectomized vehicle-treated females and gonadally intact males did not, suggesting that female rats outperform males only when circulating levels of estrogens and progesterone are elevated [46].
Spatial working memory
A male advantage in spatial working memory has been identified in rodents using the Morris water maze. Similar as reported in human, studies of rats showed that male rats outperform female rats in geometric reference and female rats preferentially use landmark cues in the snow cone water maze (SWM) [47; 48]. A recent study further reported that the sex preferences in SWM spatial learning in rats (geometric cues vs. landmark cues) are associated with entorhinal cortex thickness, not the size of hippocampus [49]. It is known that hippocampal neurogenesis is linked to spatial learning and memory. Ovarian hormones not only modulate neurogenesis in the hippocampus but also modulate hippocampus-dependent learning and memory across a variety of species from rodents to prim [50]. Interestingly, spatial strategy is associated with hippocampal cell proliferation. Interestingly, hippocampal cell proliferation is increased in female rats but decreased in male [51; 52], suggesting that navigational strategies in the Morris water maze (MWM) task are related differentially to changes in cell proliferation in the hippocampus in males and females. In agreement with sex differences in neurogenesis, a recent study also showed that spatial training in MWM increased neurogenesis (increase in BrdU cells) only in males while performance during spatial training was positively correlated with new cell activation (BrdU co-labeled with zif268) in females but not males [53]. Moreover, a new study reported that 17β-estradiol increased neurogenesis in the hippocampus and increased activation of these new neurons enhanced spatial memory retrieval, while estrone decreased cell survival and had no significant effect on activation of these new neurons[54]. The levels of circulating testosterone also altered water maze performance in male rats. Male rats castrated at pre-puberty period had lower levels of testosterone than sham-castrated rats and showed better spatial learning (shorter escape latency and traveled distance) than the sham-castrated males at the same age in MWM [55]. A low testosterone dose (0.125 mg) in castrated adult male rats caused a significant increase in the use of a cued-response strategy relative to control males [56]. While it is unclear whether testosterone directly regulates spatial learning, evidence suggested that optimal testosterone level affects spatial learning in males.
The radial arm maze (RAM) is also commonly used to measure rodent spatial learning and memory. In the RAM, between three and 24 arms extend from a central, round arena. Each arm has a food receptacle, which may or may not have food. Rodents are trained on food locations relative to distal cues in the room. RAM testing includes recording of error rates and the number of trials to criterion as measures of spatial memory and learning performance, respectively. In RAM, male rodents outperform females by using distal cues such as the shape of the room as an orientation strategy [57; 58].
The Barnes maze (BM) is another measurement for spatial learning using visual cues. BM has several advantages over the MWM as it is less physically taxing and may be less stressful to rodents, especially for mice as measured by corticosterone levels [59]. Sex differences in BM have been reported in mice with strain specificity. For instance, male mice showed better learning performance than females in the DBA/2J and C3H/HeJ strains, but there were no sex differences in the other 12 strains, including C57BL/6J [60].
2.2 The Biological Basis for Sex-Differences in adult/aging-associated cognitive function
The major biological basis of the gender-dependent cognitive activity is sex-hormone dependent. These sex hormone-driven differences in cognitive function start from the stage of neuronal development and lasts until the end of their lifespan. For example, prenatal androgen facilitates spatial ability and social behavior. In addition, a reduced sensitivity to sex hormones in cognitive function has been noticed during puberty in human and animal studies. The sections below outline the impact of sex and/or sex-hormones at different stages of development on later life cognitive/behavioral function.
Influence of sex/sex-hormones during the perinatal period on later life behavior
The perinatal period is critical for neurological and psychiatric disorders. Accordingly, it is not surprising that most adulthood mental disorders begin in childhood and adolescence [61]. However, it is unknown whether the impact of early neurological development on cognitive function is different in males and females. Epidemiological studies have shown that prenatal stress increases the development of neurodevelopmental disorders, such as schizophrenia and autism [62; 63]. Studies have also showed that perinatal exposure to air pollution increases autism disorder more so for boys than for girls [64]. Postnatal hypoxia ischemia injury causes a greater deficit in rapid auditory processing and spatial learning in male rats than in female rats [65]. A recent gene expression microarray study showed that developmental exposure to lead (Pb) can have significant effects on the hippocampal transcriptome with a clear sex difference in rats, with the different panels of gene expression in the hippocampus observed from Pb exposure during early development (gestation and lactation) or during a later (postnatal) developmental period [66]. The impact of brain development in the perinatal period on adulthood cognition is also evidenced by studies showing that developmental ethanol exposure can affect cognitive function in humans, while animal studies showed that adolescent female rats appeared more susceptible than males to ethanol-induced spatial learning impairments [67; 68; 69].
While it is still unclear why the sex differences in brain development plays critical roles in cognition in adulthood, there are two major theories which both contribute to the biology of sex-dependent development at early age: sex hormone dependent and sex hormone independent. In early fetal development, the level of sex hormones does not differ between males and females and thus, the undeveloped gonads have therefore been called bi-potential gonads. Differentiation of the male fetal gonads into testes is controlled by the sex-determining region of the Y chromosome (SRY) protein. The female fetus lacks the protein and instead develops ovaries from the bi-potential gonads [70; 71]. Studies in rats showed that the sex differences in circulating levels of sex hormones (mainly testosterone) only have a short critical period (between embryonic day 18 and 10 days after birth) to cause permanent sex-dependent changes in brain morphology and functions [72]. Interestingly, the testosterone roles in brain development of sex differences might be mediated by its metabolite estradiol, a conversion process facilitated by neuronal aromatase [73]. Studies showed that perinatal estradiol treatment can reproduce the masculinizing effects of testosterone on brain organization and behavior in gonadectomized animals [74]. Furthermore, masculinizing effects of testosterone on brain development and function were also effectively blocked by estrogen antagonists and aromatase inhibitors [75; 76]. The sex hormone-dependent effect on brain development was demonstrated by studies that showed that neonatal female rats injected with testosterone showed a male-sized medial preoptic nucleus (MPN) in adulthood whereas perinatal castration of male rat pups resulted in a female-sized MPN [77], suggesting that the vertebrate brain is organized in a sex-dependent fashion under the control of perinatal gonadal steroid hormones, such as estrogens or testosterone [78].
While exposure to circulating sex steroids is a chief contributor to the sex-dependent neuronal development, sexual dimorphism is also exhibited in many brain diseases afflicting children and the elderly. Studies showed that embryonic neurons (before sexual differentiation) isolated from male and female rats have different cellular responses to stress and female cells show greater sensitivity to ethanol than male cells, suggesting that they are independent of hormonal effects [79]. Moreover, female (XX) neurons are more sensitive to agents that induce apoptosis, while male (XY) neurons react more to oxygen deprivation, ischemia and other forms of stress [80]. Further, recent studies showed that there are significant differences in the location and expression of the genes controlling key enzymes between females and males [81]. All together, the data suggest that genetic mechanisms may be equally important to the organizational effects of sex-steroids in controlling sexual-specific neuronal characteristics.
Influence of sex/sex-hormones during puberty on later life sex-specific behavior
While sex hormones present during early development are well known to have permanent effects on sex-related behaviors in human and various animals (as outlined above), recent work has renewed interest in puberty as another organizational period for the effects of sex hormones. For example, animal studies showed that testosterone administration at during puberty induced an increase in mating behavior in adult gonadectomized male hamsters, but not through testosterone administration simulating late puberty [82], suggesting a decreasing sensitivity to the organizational effects of sex hormones across age. Similarly, female mice exposed to stress in early adolescence showed more disturbed sexual behaviors than those exposed to stress in late adolescence, emphasizing the importance of timing of exposure even within adolescence [83]. While it is difficult to prove that puberty is another organizational period for human behavior due to human hormones not being able to be manipulated experimentally, one study showed greater depression in early maturing adolescent girls than that of on-time and late-maturing girls [84], suggesting that some behavioral effects of pubertal timing persist into adulthood. In addition, variations in pubertal timing have been examined in relation to cognition. For example, late maturing boys and girls had higher spatial abilities than their early maturing counterparts. While the neural mechanism underlying this effect was originally considered to be hemispheric specialization (lateralization), confirming evidence was not straightforward and later studies suggested that unspecified brain reorganization occurred during puberty that was related to sexual differentiation [85]. Furthermore, studies reported that a longer reproductive period is associated with better cognition in later life, including verbal fluency [86], consistent with hypotheses of declining sensitivity to hormones with age [82]. One recent study showed that among men, there was a small-to-moderate effect of pubertal timing on three-dimensional mental rotations test scores, with early maturers performing better than late maturers; no significant link between pubertal timing and verbal fluency scores was found [87]. Possible reasons for these include that men who experienced early puberty may have greater amount circulating testosterone levels than men with late puberty and thus, circulating hormones could be driving the effect of pubertal timing on spatial ability and that pubertal timing is a reflection of testosterone exposure during early development with early androgen exposure facilitating spatial abilities [88; 89]. However, there were no significant relationships between timing and either cognitive measure among women [87], suggesting the pubertal timing effects on male-typed cognition were not observed in women.
The brain regional maturation is dependent on sex hormone, as the circulating testosterone levels positively correlate with amygdala volume (higher testosterone = larger volume) but negatively impact hippocampal volume. Thus, this remains consistent with sex-specific differences in amygdala and hippocampal volume [1]. The sex hormone-dependent of brain regional development has been further supported by reduced amygdale, cerebral and lobar volume, and enlarged ventricles in young males with androgen insufficiency compared to normal male controls [90; 91]. The reduction of in brain volumes of specific regions could explain the deficits in executive function of boys with insufficient androgen as reported [92]. Although estrogens play significant roles in androgens during brain development, it also plays critical roles in feminization. Girls with partial or complete loss of an X-chromosome showed disproportionately reduced hippocampal volumes and increased amygdala volume relative to age-matched controls [93]. This estrogen-related brain regional volume reduction found in females with endogenous estrogen deficiency is further evidenced by the impairment of executive performance on the task as reported by other groups of researchers [94].
Influence of sex/sex hormones during adulthood
While sex differences in cognition may be attributed to organizational effects of sex steroid that result in sexually dimorphic regions of the brain relevant to learning/memory functions, such differences may also be attributed to “activational” effects of sex-steroid during adulthood. For example, recent animal studies showed that endogenous sex hormone levels changed by reproductive experience in females are associated with enhanced hippocampus-dependent memory [95], supporting the theory that parity may result in a higher level of exposure to estrogens, and as a consequence, changes the way the brain responds to estrogens in later life [50; 96].
Furthermore, sex steroids have been linked to many of the mechanisms thought to be associated with cognitive decline and several types of dementia, which has high risks in both genders with age. A recent review by Hogervorst found that women who undergo surgical menopause or had menopause before 47 years old without hormone treatments have an increased risk for global cognitive impairment and dementia in later life [97], suggesting that the earlier the age of hormone loss (such as through surgical menopause), the higher the risk for cognitive impairment. The ageing-related gender differences in cognition have been reported in healthy populations. For instance, older male subjects were not only slower but were also less accurate at matching objects across 3D rotation compared to younger subjects, whereas there was no significant age difference for female subjects. In addition, older males performed even worse than older females, suggesting a general male disadvantage in mental rotations tasks is associated with ageing [98]. One study worth to point out shows that women using oral contraceptives containing androgenic progestins, have better spatial abilities than women using other types of oral contraceptives and women not using oral contraceptives [99], indicating that estradiol might be less important than testosterone for cognitive sexual differentiation, especially organizational effects on male-type cognition such as spatial ability. On the other hand, women in high estradiol phases of the menstrual cycle have better verbal fluency (female-type cognition) than those in low estradiol phases, and naturally cycling women have better verbal fluency than women using oral contraceptives [17; 100].
Cognitive impairment in AD
Memory problems are the most frequent complaint by patients presenting for clinical assessment when dementia is suspected. Whether AD and other dementia patients can be clearly distinguished on the basis of memory test performance is a question we ask. Several studies showed that AD patients face more problems in anterograde episodic memory than frontotemporal lobar degeneration (FTLD) patients [101; 102; 103; 104]. Others reported that both AD and FTLD dementia groups did not differ in their verbal and forgetting rates, besides a potential worse visual memory in the AD group compared to the FTLD group [105]. Mild cognitive impairment (MCI) is a clinical condition often associated with early stage of AD. A study of 425 MCI patients showed that early onset MCI patients suffer more visuospatial memory impairment, while a late onset of MCI showed more verbal memory problems; however, both are individual conditions at an increased risk of conversion to AD [106]. Moreover, other studies also reported no significant differences in the demographic characteristics of patients with mixed dementia and AD, where patients with mixed dementia were significantly more impaired than AD patients in global cognitive composite, attention and visuoconstruction [107]. Other cognitive measurements have also been used in MCI and AD. For example, an anti-saccade task is a measurement for executive function and correlated with AD-sensitive cortical thickness in MCI subjects, but not in normal elderly patients as reported by Heuer [108]. A study using a new cognitive test, hard test your memory (H-TYM), in 97 mild AD/MCI and 200 control subjects demonstrated a significant detection of mild AD/MCI who “pass” the MMSE from controls [109].
3. Sex differences in Prevalence of Alzheimer’s disease
AD is the most common cause of dementia in the elderly and is characterized by two major pathological lesions: intracellular inclusions of tau protein in the form of neurofibrillary tangles, and extracellular plaque formation by accumulations of amyloid beta peptide (Aβ) derived from the β-amyloid precursor protein (APP). Accumulating Aβ, a proteolytic byproduct of amyloid precursor protein (APP) metabolism, is an important aspect of AD pathology. APP is processed by two competing pathways, the amyloidogenic pathway through β-secretase (BACE1) and γ-secretase, which produce β-APPs and Aβ40/Aβ42 peptides, and the predominant non-amyloidogenic pathway via α-secretase, which produces neuroprotective α-APPs and several non-amyloidogenic peptides [110].
3.1. Women have a higher prevalence of AD
In both genders, epidemiological studies show an increased risk of AD with the age-related loss of sex steroid hormones. Sex differences in prevalence and severity of AD have been found. Clinical and pre-clinical studies have shown that females carry an increased risk of developing AD pathology compared to males, even after controlling for increased life span [111; 112]. Women not only have a higher risk of developing AD than age-matched men such as higher numbers of female AD patients than male patient with AD [113; 114; 115; 116], but also showed significantly age-related faster decline and greater deterioration of cognition than elderly male [40; 41; 117]. Furthermore, both neuroimaging and postmortem human studies showed difference AD pathology in men and women, such as men with AD had more pronounced pathology in right hemisphere where women with AD often had more manifest pathology than that in male AD patients [118; 119].
Although clear gender differences have been reported in the incidence and prevalence of dementia [120], the reasons for these differences are still awaiting a clear understanding. There are several major biological hypotheses on the sex differences in AD, such as differences in age-related sex hormone reduction (estrogens, progesterone, testosterone), various of genetic risks (ApoE, etc.), impact from risks of other diseases (diabetes, depression, cardiovascular disease) and sex differences in brain anatomy, age-related declines in brain volume, and brain glucose metabolism in the brain of men and women [121; 122; 123; 124; 125; 126].
3.2. Differences in changes of sex hormones between men and women
Compared to age-matched controls, reduced circulating levels of 17β-estradiol and testosterone are observed in female and male patients with AD, respectively [112; 127], suggesting an undeniable effect of sex hormones in the epidemiology and pathology of AD. In women, estrogens are produced from cholesterol primarily in the ovaries and placenta, although small but significant amounts can be produced by non-reproductive organs, such as the liver, heart, skin, and brain. Three major forms of physiological estrogens exist in females: estrone (E1), estradiol (E2, or 17β-estradiol), and estriol (E3). E1 is usually a major product in the serum except for the ovarian follicle stage of premenopausal women. E2 is the major product of the entire biosynthesis process and is the most potent estrogens during the premenopausal period of a woman’s life (see review by Cui et al. [121]). With the increase in longevity, women spend, on average, one third of life without sufficient endogenous estrogens. Where testosterone levels decline slowly in advanced age in men, the sharp decline of estrogens in postmenopausal women may contribute the female prevalence in AD. Estrogens are known to have protective effect on the brain, and loss of estrogens during menopause could, in part, lead to the deficits seen in brain metabolism (mitochondrial impairment) in AD [128]. Estrogens also play critical roles in neurogenesis. As reviewed by Galea [50], E2 can increase neurogenesis in various brain regions such as dentate gyrus of hippocampus, and these newly generated neurons in the hippocampus contribute to region-specific learning and memory. Studies showed hippocampal atrophy in females with mild cognitive impairment (MCI), suggested an important role of estrogens in the association between cognitive function and hippocampus [129]. The brain regional effect of estrogens have also been confirmed by recent studies which further highlighted the potent effects of estrogens on neuronal morphology and plasticity in area CA1 of hippocampus [130; 131; 132; 133; 134; 135]. Another important neuroprotective role of estrogens in AD is supported by the observation that estrogens can reduce Aβ levels, or prevent them from rising, in the presence of pathological triggers [136]. Estrogens can also reduce Aβ production by favoring the non-amyloidogenic pathway through MAPK/ERK activation, reducing β-secretase (BACE1) levels, and promoting Aβ clearance by stimulating microglial phagocytosis and degradation and regulating the levels of major enzymes involved in Aβ degradation [137]. Estrogens can also be protective by regulating the Bcl-2 protein family, increasing the expression of antiapoptotic Bcl-xL and Bcl-w and suppressing expression of proapoptotic Bim to prevent neuronal loss from Aβ-mediated toxicity [138]. A reduction in the levels of hyperphosphorylated tau (a major component of neurofibrillary tangles) can also be induced by estrogens through kinases and phosphatases, such as the GSK-3β, Wnt, or PKA pathways [139].
During menopause or after ovariectomy, there is a significant reduction in both estradiol and progesterone. Depletion of estradiol and progesterone result in elevated Aβ levels in transgenic mouse models of AD [95; 122; 140]. Like estrogens, progesterone plays neuroprotective roles in against AD, such as modulating gamma-secretase, another set of enzyme involve Aβ production [141] and increasing Aβ clearance medicated via insulin degradation enzyme (IDE) [142]. Furthermore, studies from human, transgenic mice and cells showed that progesterone also modulates Tau, another pathological molecule in AD and frontotemporal dementia [143]. Unlike estradiol playing a role as an activator of alpha-secretase (an enzyme protecting Aβ production) and an inhibitor of beta-secretase (a key enzyme for Aβ production), progesterone showed no effect on alpha-secretase [141; 144]. Moreover, bioinformatics analysis revealed that progesterone administration in ovariectomized rats was associated with a down-regulation of beta-secretase gene expression [145]. Progesterone administration in mid-aged wild-type and transgenic AD mice showed an improved performance in the object recognition and T-maze task, which are mediated by the cortex and hippocampus [146]. Interestingly, a cyclic delivery of progesterone in female transgenic AD mouse model not only significantly reduced Aβ levels by itself, but also enhanced the neuroprotective roles of estrogens in AD pathology when in combination with estradiol treatment. In contrast, the continuous delivery of progesterone not only failed to alter Aβ levels, but also inhibited the protective effects of estrogens against AD [147].
While the precipitous loss of ovarian estrogens and progestagens at menopause has been presumed to account for the increased female susceptibility to AD, recent studies in both animals and humans suggest that depletion of brain-derived, rather than circulating, estrogens act as more direct and significant risk factors [122]. Studies showed that non-reproductive organs, such as adipose tissue and brain are affected by age related losses of estrogens [95; 121; 122]. Analysis of brain estrogens, particularly E2, in the postmortem brain samples from female AD patients and age-matched healthy subjects, studies demonstrated a great reduction of E2 levels and estrogen biosynthesis in AD brains while same serum E2 levels were found in both AD and controls, suggesting that brain estrogen deficiency, not circulating E2, is associated with AD pathology [122]. The differences in brain estrogens level between female AD and health individuals provide some level of explanation on why only 13–15% aged female developed AD although every woman lost sufficient endogenous estrogens after menopause in advanced age. The risk of brain estrogen deficiency in female developing AD has also been supported by animal studies. Animals with genetic depletion of estrogen synthase, aromatase, express very low levels of estrogens in the brain compared to the mice with ovariectomy. Studies reported that APP transgenic mice with low brain estrogens developed earlier and more severe AD pathology than ovariectomized APP mice [122]. Furthermore, a greater and better neuroprotective response from estrogen treatment was found in the APP mice with brain estrogen deficiency compared to in the ovariectomized APP mice [95]. Together, evidences suggest an important role of brain estrogens in female risk of developing AD.
Both estrogens and androgens regulate key processes implicated in AD pathogenesis, in particular the accumulation of β-amyloid protein. While the prevalence and incidence of AD are higher in women, in men, men also experience a robust age-related increase in the risk of AD which is significantly affected by normal age-related depletion of testosterone. However, compared to the precipitous decline of estrogens and progesterone in women, men exhibit a much more slow and gradual decrease in bioavailable testosterone at a rate of ~2% per year during normal aging [148; 149]. The brain is not only affected by age related loss of estrogens, but of the loss of androgen during aging. In human studies, age-related androgen loss has also been associated with increased AD risk. Men with AD exhibit lower circulating [150; 151] and brain [152; 153] levels of testosterone than age-matched, non-demented men without AD. Testosterone levels in brain have also been found to be inversely related to soluble brain Aβ in men that show early development of AD-related neuropathology, suggesting a possible mechanism linking brain testosterone and AD risk [153]. In addition, the age-related testosterone loss appears to precede clinical [151] and neuropathological [152; 153] diagnoses of AD, suggesting that androgen depletion is a precursor event that likely contributes to rather than results from the disease process. Moreover, there is no linkage between the brain estrogens levels with AD diagnosis in men, suggesting sex differences in the relationships between sex steroid hormones and AD risk (see review [154]). The roles of testosterone in risk of AD have been also supported by animal studies. In male 3x-Tg-AD transgenic mice, orchiectomy (ORX)-induced depletion of endogenous androgens significantly accelerates AD-like pathology and impairment of hippocampal-dependent behavioral performance, where treatment of ORX 3xTg-AD mice with testosterone [155]. In male APP23 mice, another AD mouse model, increasing endogenous testosterone by genetic disrupting aromatase showed significant reduction of AD pathology and improved cognitive function [126].
3.3. Genetic influences on the effect of sex/sex hormones on AD-related impairment
A common polymorphism that confers risk, the APOE-4 genotype or allele, increases the chance of developing the disease over the other forms (APOE-2 or APOE-3). The APOE-4 allele contributes to 40%–50% of the genetic basis for late onset AD [156]. ApoE4 has sex-dependent effects, whereby the risk of developing AD is higher in apoE4-expressing females than males. Recent study showed that women with ApoE4 showed sharp decline of cognitive function than men with ApoE4 [157]. Female ApoE4 carriers with MCI have higher risk of cardiovascular mortality than male APoE4 carriers with MCI [158]. The sex differences in ApoE4 related cognitive impairment is unlikely due to the volume change of hippocampus, since there were no significant differences among the ApoE4+/+, ApoE4+/− and ApoE−/− groups of participants in their hippocampal volumes or hippocampal glucose metabolism measurements [159]. In the animal studies, a significant impairment of spatial learning along with age-related depletion of hilar GABA neurons was found in female ApoE-knockin mice, not in ApoE male mice [160].
It was also suggested that the gene encoding for neuronal sortilin receptor 1 (SORL1) may affect late-onset Alzheimer’s disease (LOAD) through a female-specific mechanism. A recent study investigated the SNP variants of SORL1 with cognitive function and gender and found that a possible protective effect of the SNPs, such as the rs2070045, influence cognitive function in a sex-specific manner, such that men with this SNP have better performance on verbal, episodic and spatial memory at least before age 75, but detrimental effects for women’s performance [161]. However, a recent study of 96 LOAD and 120 controls failed to repeat the association between SORL1 and LOAD which might be related to the small sample size [162]. Thrombospondins-4 (THBS-4) is 1 of 5 members of the THBS family of multidomain extracellular matrix proteins detected in various tissue including vascular, muscle and nervous system. While multiple studies reported a sex difference between THBS4 polymorphism and myocardial infarction [163; 164], experiments indicated that THBS4 expression increases with age in human brain regardless of gender, but the interactions between THBS4 genotype and reduced volume of gray matter were only found in female AD patients [165].
3.4. Sex differences in risk of other diseases
Many other diseases are also contributing the sex differences in dementias and AD. For example, as we get older, the risk for type 2 diabetes, heart disease, and stroke increases.
Heart Diseases
Generally, compared to men, women have smaller and stiffer hearts and cardiac vessels, suffering more atherosclerosis. As cardiovascular fitness is negatively associated with dementia mortality for both men and women, studies reported that women benefit less from cardiovascular fitness than men [166; 167]. While amnestic MCI (aMCI) is hypothesized to preferentially progress to dementia and AD, an investigation of a total of 2719 participants with following up cognitive evaluation found that cardiac disease is an independent risk factor for nonamnestic MCI (naMCI) in women, preferentially progress to vascular and other non-AD dementias [168]. The sex differences in cardiovascular risk of AD is also supported by studies showed that men with high triglyceride (TG) and low HDL-cholesterol levels have an increased incidence of all-cause dementia but not AD, where women with low TG levels were associated with a decreased risk of AD [169].
Obesity and Diabetes
Approximately 16 million Americans have diabetes. Approximately 55% of these are women [170]. Recent studies showed that type I diabetes has no gender differences while type II diabetes showed a pronounced female bias in the first half of the last century [171]. While type 2 diabetes may increase the risk of AD in both sexes, studies showed that diabetes also increases the risk of MCI [172; 173; 174]. A recent study showed that diabetes was associated with a stronger risk of aMCI in men and a strong association of single-domain naMCI in women [175]. Data from most animal studies found that insulin deficiency may result in AD pathology, including increased tau phosphorylation at multiple sites, increased tau cleavage and greater neuronal and synaptic damage, even with increased amyloid-β peptide production [176; 177].
Depression
Depression is one of the most common psychiatric disorders in older adults and is highly associated with an increased risk for AD, although it is unclear whether it represents an actual risk factor or is a prodromal state [178]. It is well recognized that women are more likely to suffer from depression than men throughout life [179; 180; 181]. The depression is prevalent in 35–50% of patients already diagnosed with AD and 3–63% in individual with MCI [182; 183]. A new longitudinal analysis of 2160 subjects at age of 65 or older found that depression was related to a higher risk of prevalent MCI, and progression from prevalent MCI to dementia [184], suggesting a possible role of depression in promoting the conversion of MCI to AD. Although women have higher rates of depression and anxiety, and these are emerging as risk factors for AD [185; 186], one longitudinal study evaluating more than 1300 subjects found the depression is a risk factor for AD only in men, and not in women [182]. The linkage between depression and AD was further documented by a recent study on postmortem brain tissue from individuals afflicted with major depression disorders and AD. This study found that the myelin/myelination abnormalities represent an important neurobiological link between the pathophysiologies underlying major depression and AD [187]. The sex differences in myelination disorders is also well documented in previous studies that less white matter and higher risk of multiple sclerosis have well found in women [188; 189]. Although depression in general is associated with risk of AD, whether the prevalence of depression in women contributes to the prevalence of AD remains further investigation.
4. The potential protective role of estrogens, progestins and androgens in AD – evidence from pre-clinical and clinical studies
While studies that reported impairments (or increased vulnerability) of brain function resulting from surgical removal of the ovaries supported the potential benefit of estrogens in maintaining a healthy brain, it is important to recognize that ovariectomy (like the menopause) leads to a significant reduction in not only circulating estradiol, but also in circulating progesterone. As such, the structural and functional impairments that are reported to occur following ovariectomy may result from the loss of not only circulating estrogens, but of progesterone as well. Accordingly, it should come as no surprise, that both estrogens and progesterone are effective neuroprotectants in females, with some evidence pointing to the protective effects of these hormones in males. In point of fact, both estrogens and progesterone have well-described neuroprotective effects against numerous insults in a variety of cell culture models, animal models and in humans. Further, with regard to the sex-difference reported in the prevalence of Alzheimer’s disease and other neurodegenerative diseases, there is an interest in how androgens, such as testosterone and its 5-alpha reduced metabolite, dihydrotestosterone (DHT), influence brain cell viability/vulnerability. In the sections below, we provide an overview how estrogens, progesterone and testosterone alter brain cell viability, and relate these observations to the reports of the efficacy of these hormones in neurodegenerative disease.
4.1. An overview of the brain-protective effects of estrogens
The potential for estrogens to exert their protective effects were gleaned from such studies as that showing gonadally-intact adult female rodents sustain less neuronal damage from global cerebral ischemia-reperfusion injury compared to age-matched males [190]. The pioneering work from the Simpkins laboratory, for example, clearly demonstrated that 17β-estradiol is a potent neuroprotectant in vitro [190] and is very effective against such insults as ischemia-induced brain damage [191].
Protective effects of estrogens have been widely reported in a variety of neuronal models and against different toxic insults [192]. Among the mechanisms thought to mediate these protective effects include its antioxidant effects [193] and attenuation of excitotoxicity, through inhibition of N-methyl-D-aspartate (NMDA) receptor activation [194]. Also, two major signaling pathways, ERK and PI-3K-Akt, have been well-characterized as being part of the pathway leading to inhibition of apoptosis and facilitation of neuronal survival [195; 196].
17β-estradiol has also been shown to enhance spine density and cognitive function (for review, see [130; 197]), in addition to reducing the formation/accumulation of β-amyloid [140; 198; 199; 200]. Mechanistically, the protective effects of estrogens against amyloid beta may be attributed to its influence on amyloid precursor processing [198; 201; 202], on clearance of amyloid beta [203] and/or on the degradation of amyloid beta [204; 205].
Further, the neuroprotective effects of estrogens have also been demonstrated in a variety of models of acute cerebral ischemia. These include transient and permanent middle cerebral artery occlusion models [191; 206; 207], global forebrain ischemia models [208], photothrombotic focal ischemia models [209], and glutamate-induced focal cerebral ischemia models [210]. The protective effects of estrogens have been described in multiple species, including rats, mice and gerbils [191; 211; 212]. And while the majority of studies have investigated the neuroprotective effects of estrogens in females, estrogens protection have also been noted in males [213; 214]. Collectively, these results indicate that estrogens could be valuable candidates for brain protection against a variety of insults relevant to age-associated diseases such as stroke and Alzheimer’s disease.
4.2. Overview of the brain-protective effects of progesterone
Progesterone has been reported to exert protective effects in a variety of experimental models that mimic certain pathogenic aspects of brain dysfunction seen with advanced age- or age-related neurodegenerative diseases such as Alzheimer’s disease. For example, physiologically relevant concentrations of progesterone have been shown to significantly attenuate oxidative injury resulting from glutamate [215; 216; 217; 218] and glucose deprivation–induced toxicity [219], and also protects against FeSO4- and amyloid βeta-peptide – induced toxicity in primary hippocampal cultures [219].
Progesterone is also an effective neuroprotectant in animal models of stroke. For example, Jiang et al. illustrated that the administration of progesterone before middle cerebral artery occlusion (MCAO) resulted in a marked reduction in cerebral infarction and reduced impairments that resulted from the occlusion [220]. Interestingly, post-ischemic administration of progesterone was also found to be protective [221; 222], and resulted in improvements in various functional measures, including the rotarod test, and adhesive-backed somatosensory and neurological scores [223]. The ability of progesterone to protect even when administered after the insult (albeit within a relatively narrow window) may suggest that both rapid/immediate and long-term mechanisms of progesterone action are involved in the protective effects of progesterone. Progesterone has also been shown to reduce the amount of cell death following an acute episode of global ischemia [224], and is thought to be related to the ability of progesterone to reduce lipid peroxidation, the generation of isoprostanes [225] and the expression of pro-inflammatory genes [226]. It is worth pointing out that some of the protective effects of progesterone may also be mediated by allopregnanolone, the major progesterone metabolite.
Another model in which progesterone has been shown to exert protective effects is in the traumatic brain injury (TBI) model. The administration of progesterone reduces cerebral edema for up to 24 hours after injury [227]. In a rodent model of medial frontal cortex impact injury, progesterone reduced complement factor C3, glial fibrillary acidic protein (GFAP), and nuclear factor kappa beta (NFκB) [226], all of which can be interpreted as protective mechanisms. Progesterone also decreased the levels of lipid peroxidation in male rats when administered after TBI [228].
Interestingly, there appears to be a sex difference in terms of the severity of impairment following TBI. Females appeared to have less spatial learning impairments when compared to their male counterparts. And though the lesion size was similar, females exhibited less ventricular dilation indicating lower edema and water retention [229]. In fact, direct assessments of edema reveal that progesterone treatment significantly attenuates the level of edema seen in injured animals in contrast to non-progesterone treated animals that had undergone experimental TBI [227].
The protective effects of progesterone are also evident in other regions of the central nervous system in addition to the hippocampus and cerebral cortex. For example, progesterone has also been shown to have a beneficial effect on spinal cord contusion injuries as supported by the work of Thomas et al. who found that there was a marked reduction in the size of the lesion and a prevention of secondary neuronal loss with progesterone treatment [230]. Further support for progesterone’s protective actions in the spinal cord comes from the observation that progesterone has been shown to promote morphological and functional recovery in the Wobbler mouse, an animal model of spinal cord degeneration [231; 232]. Progesterone can also induce re-myelination as supported by the increased expression of myelin proteins in the damaged sciatic nerves of both young adult rats and in 22–24-month-old males [233]. Thus, progesterone may be of potential therapeutic benefit in diseases where demyelination is an important component of its pathogenesis.
4.3. Overview of the brain-protective effects of androgens
Androgens have been shown to protect the brain against a variety of AD-related insults. For example, testosterone can protect against β-amyloid toxicity [234; 235; 236], oxidative stress [237; 238], heat shock induced hyperphosphorylation of tau [239], kainic acid toxicity [240] and stroke [241]. While some of the protective effects of testosterone may be mediated through prior aromatization to estradiol, other studies clearly implicate the androgen receptor [238; 240; 242]. Conversely, castration-induced androgen deficiency has been shown to result in such deficits as compromised synaptic ultrastructure [243].
While there is clearly support for the protective effects of androgens, adverse events associated with testosterone have also been noted. For example, testosterone exacerbated AMPA or kainic acid mediated toxicity of oligodendrocytes [244] and enhanced glutamate-mediated toxicity in hippocampal HT-22 cells [245]. Testosterone also increased the lesion size resulting from middle cerebral artery occlusion (a model of stroke) in male rats [213], and was shown to exacerbate motor deficits in a rat model of Parkinson’s disease [246]. While it is not completely clear what predicts the beneficial versus deleterious consequences, factors that include age, the cellular context (neurons versus glia), and the mechanisms that are in place to mediate the actions of androgens (such as genomic versus non-genomic mechanisms), have been proposed to play a role.
4.4. Clinical Studies of Estrogens in Alzheimer’s disease
The prevalence of AD is significantly greater in women than in men [247; 248; 249]. The incidence of AD may also be higher in postmenopausal women [115; 250], although this observation may be complicated by the relatively longer lifespan in women. The increase in risk/prevalence of AD in women may be related to the more precipitous decline in circulating estrogens and progesterone levels, and as such, has served as the basis for the hypothesis that interventions with estrogens may help protect against neurodegenerative diseases like AD.
In fact, several observational studies suggested that estrogens may help maintain cognitive function in women with AD who are current users of estrogen/hormone therapy [251; 252; 253]. Further, estrogens loss from natural or surgical menopause is associated with a decline in cognitive function that is reversed by ET [254; 255; 256]. Estrogens therapy (ET) affects cognitive function during “non pathological” brain aging as well [257; 258; 259]. Interestingly, the efficacy of cholinesterase inhibitor treatment appeared to be made more effective in women who were on hormone therapy [260], suggesting that estrogens may not only have protective effects on its own, but may also enhance the therapeutic efficacy of other AD-related drugs. Epidemiological evidence also suggests that post-menopausal ET reduces the risk or delays the onset of AD [248]. Estrogens were also found to ameliorate symptoms of disease in Parkinson’s disease (PD) [261; 262] and facilitated recovery from neurotrauma such as stroke [263; 264] in the vast majority but not all cases [265]. Further, mortality from stroke was found to be reduced in post-menopausal subjects who were taking ET at the time of stroke [266; 267; 268], and that estrogens status plays role in recovery from brain injury [269; 270].
While numerous observational/epidemiological studies have identified estrogens to be of benefit, results from randomized clinical trials have been much more equivocal. Most notably, results from the Women’s Health Initiative suggested that estrogens did not have a beneficial effect on dementia or cognitive function in older women [271; 272; 273; 274] and failed to improve symptoms in women with mild to moderate AD [275]. The WHI studies reported that Premarin® (and PremPro®) not only failed to positively affect conditions associated with neuroprotection, but that ET or combined hormone therapy (HT) may worsen cognition [271; 272; 273; 274; 275] and increase strokes [276; 277; 278] and cardiac arrests [276; 277]. Reanalysis of the WHI data, however, indicates that early postmenopausal treatment with estrogens can provide benefits [278; 279], as was reported previously in observational trials [252; 253; 254; 255; 256; 257; 258] and a large number of animal model data as indicated above. This observation prompted the consideration of the timing of estrogen therapy relative to the onset of the menopause. In fact, a growing number of studies [280; 281; 282] support the existence of the “window of opportunity hypothesis”, which states that there is a limited period after the menopause during which ET or HT is effective. In view of these new studies and others, it would appear that ET may not have universal utility in treating/preventing dementia. However, it is also clear that there are a growing number of “factors” that may influence the effect of estrogens (such as formulation of estrogens, route of delivery, etc.), or help predict the therapeutic efficacy in individuals (e.g., age). Such factors should be considered in the decision to consider use ET to help maintain a healthy brain, consistent with the recent recommendations of the North American Menopause Society [283].
4.5. Clinical studies addressing the protective effects of progesterone and related progestins
To date, there are no clinical trials that have specifically assessed the effects of progesterone or a related progestin when administered alone on cognitive outcomes in subjects with AD [284]. Rather, most studies that have addressed the protective effects of progestins (typically, medroxyprogesterone acetate) when administered in conjunction with an estrogen. However, there have been some studies addressing the role of progesterone alone on traumatic brain injury, showing in a phase II, randomized, double-blind, placebo-controlled clinical trial, that progesterone can improve functional recovery, at least when administered to those who experienced moderate, but not severe, traumatic brain injury [285; 286; 287; 288; 289].
As stated above, recent results from the Women’s Health Initiative-Memory Study (WHIMS) failed to reveal beneficial effects in reducing the risk of AD or “all-cause” dementia. As a consequence, these reports left the field unsettled as to the future of hormone therapy. Since the publication of these studies, it became apparent that there were important caveats to the data that needed to be considered. Among these included consideration of the type of hormone used. Indeed there are important differences in the neurobiology of two major progestins, the “natural” progestin, progesterone, and the synthetic medroxyprogesterone acetate (MPA), the most commonly used progestin in hormone therapy regimens.
MPA, a synthetic progestin derived from 17αahydroxyprogesterone, is often used in conjunction with estrogens to reduce the risk of certain cancers (cervical cancer, for example) resulting from unopposed estrogen therapy [290; 291]. First, though both progesterone and MPA can bind to the classical PR, it is important to recognize that there are important pharmacological and pharmacokinetic differences between MPA and progesterone. For example, orally administered MPA does not undergo any first pass effects [292], unlike progesterone. Furthermore, MPA has little binding affinity for sex hormone binding globulin [292]. In addition to differences in bioavailability and half-life, MPA also displays many non-progestagenic effects [292], including the ability to bind to the androgen receptor (AR) where it acts as a partial agonist [293] with a binding affinity (Kd) of approximately 2.1 nM [294]. Progesterone, in contrast, does not bind to the AR [292]. MPA can also bind to, and activate, glucocorticoid receptors [292; 295] with an effective concentration (EC50) that is nearly 300-fold lower than that for progesterone [295].
While progesterone and MPA may be equally effective at reducing the uterotrophic effects of un-opposed estrogen treatment, their effects on the brain are far from identical. In fact, it has become increasingly clear that while progesterone is neuroprotective, MPA is not. For example, our laboratory described that in cerebral cortical explants, the difference in neuroprotective efficacy between progesterone and MPA may have been attributed to their differential regulation of BDNF. Specifically, while progesterone increased both the mRNA and protein levels of BDNF in the cerebral cortex, MPA treatment resulted in a substantial inhibition [296]. Combined with the observation that progesterone’s protective effects may be dependent on neurotrophin signaling [218], this inhibition of BDNF expression by MPA may actually suggest that it have adverse consequences to brain function. Similarly, the Brinton laboratory has shown in hippocampal cultures that while progesterone is protective, MPA is not. In this model, the protective effects of progesterone appeared to be mediated, in part, by attenuating the glutamate-induced increase in intracellular Ca2+ levels. MPA, in contrast, failed to alter the glutamate-induced influx of Ca2+. Of significance was that MPA not only failed to elicit protective effects, but also blocked the beneficial effect of estradiol. In sharp contrast, progesterone did not inhibit the effect of estradiol [216]. Furthermore, while some of the neuroprotective effects of progesterone are mediated by its neuroactive metabolite, allopregnanolone (see discussion above), it is unclear if MPA is a substrate for the progesterone metabolizing enzymes, 5alpha-reductase and 3alpha-hydroxysteroid dehydrogenase. If anything, MPA has been shown to inhibit the biosynthetic enzymes associated with the conversion of progesterone to allopregnanolone. Thus, both the inability of MPA to be converted to neuroactive steroid metabolites in conjunction with its effect in reducing potential conversion of progesterone to allopregnanolone may contribute to its lack of neuroprotection.
As stated above, progesterone’s protective effects, in at least two neuronal models (cerebral cortical neurons and hippocampal neurons), was dependent on activation of the ERK/MAPK pathway [216; 217; 218]. While both progesterone and MPA can elicit ERK phosphorylation, only progesterone treatment resulted in nuclear translocation of ERK [217], the consequence of which is likely to regulate key genes, whose protein products may enable more long term/sustainable protection. In fact, progesterone, but not MPA, increased the expression of the anti-apoptotic Bcl-2 protein [215]. And as observed in the model of glutamate-induced Ca2+ influx, MPA not only failed to increase expression of Bcl-2, but actually inhibited that elicited by estradiol [216].
The disparity between the effects of progesterone and MPA has also been observed in vivo. For example, a study using rhesus monkeys illustrated that combined treatment with estradiol and progesterone protects against coronary vasospasm, whereas estradiol + MPA treatment did not [297]. And once again, in contrast to the antagonistic effects of MPA on estrogen’s effects, progesterone enhanced the protective effects of estrogens against exercise-induced myocardial ischemia in post-menopausal women to, whereas MPA did not [298]. Moreover, in a model of stroke (reversible focal stroke using the intraluminal filament model followed by 22 hours of reperfusion), MPA diminished the protective effects of conjugated equine estrogens (CEE) and MPA diminished estrogen’s ability to reduce stroke damage [299]. The functional antagonistic effects of MPA were also noted in the cholinergic system of monkeys, where MPA administered in conjunction with CEE reduced choline acetyl transferase (ChAT) in such cognition-relevant areas of the brain as the medial septum [300]. Similar consequences of MPA were seen in the cardiovascular system of cynomolgus monkeys. Adams et al., demonstrated that monkeys treated with CEE showed a 72% reduction in coronary artery atherosclerosis whereas there were no benefits observed in CEE plus MPA group [301]. Interestingly, with regards to the traumatic brain injury model, MPA required a larger dose than progesterone to accomplish a comparable reduction in cerebral edema. However regardless of the dose of MPA, MPA did not favor a better behavioral recovery than progesterone (reviewed in [302]).
4.6. Clinical studies addressing the potential beneficial effects of androgens
Unfortunately, there have been relatively few studies that have addressed the effect of testosterone treatment in men with cognitive impairment and AD. Further, these studies have generally had modest sample sizes. Nevertheless, some studies have shown that testosterone treatment improved ADAS Cog and MMSE scores [303], while others have found beneficial effects of testosterone in specific domains of cognitive function in MCI and AD patients [304]. Clinically, low levels of testosterone have been associated with increased amyloid burden [305], poorer cognitive performance [151], reduced brain metabolism [306] and a higher prevalence of Alzheimer’s Disease [151; 307] and supports the potential use of androgen supplementation to help restore these deficits.
However, not all studies show beneficial effects of testosterone. For example, Kenny et al., [308] and Lu et al., [309] failed to note beneficial effects of testosterone. And as described above, pre-clinical studies have shown that testosterone can exacerbate the effects of stroke in young adult male rodents [245]. Such controversy surrounding the potential benefit for androgen therapy in the elderly prompted the National Institute on Aging (NIA) and the National Cancer Institute (NCI) to request that the Institute of Medicine (IOM) conduct a 12-month study to evaluate the current understanding of the potential benefits and adverse effects of androgen therapy in older men. A major theme in the IOM analysis and recommendation was that additional work must be done before clear decisions can be made about the future of androgen therapy, and that future research should focus on the population most likely to benefit [310]. Only through such analysis will we be able to gauge for whom, and for which specific demographic, androgen therapy in the elderly is beneficial.
Summary
The differences in learning and memory between male and female brains are confirmed by both human and animal studies from early development stage throughout their life spans. In addition, many neurological diseases exhibit sex differences, exemplified by one sex having a greater prevalence or severity of the disease than the other sex. Women not only have a higher prevalence of AD than age-matched men, but also showed significantly age-related faster decline and greater deterioration of cognition than elderly male. While it is still unknown why females have higher risk of AD than men, this review draws on studies of sex differences in cognitive function from human to animal, clinical to basic science, early brain development to neurodegeneration at later age, normal aging to AD and highlighted the important impact of sex hormones with other genetic influences on the risk of AD. We believe that better understanding the biology of sex differences in cognitive function will not only provide insight into AD prevention, but also is integral to the development of personalized, gender-specific medicine.
Highlights.
Neurobiology of sex-type spatial memory from young to old
Sex differences in prevalence of Alzheimer’s disease
Potential protective role of sex hormones in Alzheimer’s disease
Acknowledgments
This work was supported by the American Health Assistance Foundation (G2006-118), and the National Institutes of Health (R01AG032441-01). We also thank Juliet Shen for editing and proofreading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19:464–73. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- 2.Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21:636–46. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koolschijn PC, Crone EA. Sex differences and structural brain maturation from childhood to early adulthood. Dev Cogn Neurosci. 2013;5:106–18. doi: 10.1016/j.dcn.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 5.Clark AS, MacLusky NJ, Goldman-Rakic PS. Androgen binding and metabolism in the cerebral cortex of the developing rhesus monkey. Endocrinology. 1988;123:932–40. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- 6.Morse JK, Scheff SW, DeKosky ST. Gonadal steroids influence axon sprouting in the hippocampal dentate gyrus: a sexually dimorphic response. Exp Neurol. 1986;94:649–58. doi: 10.1016/0014-4886(86)90244-x. [DOI] [PubMed] [Google Scholar]
- 7.Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–66. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- 8.Linn MC, Petersen AC. Emergence and characterization of sex differences in spatial ability: a meta-analysis. Child Dev. 1985;56:1479–98. [PubMed] [Google Scholar]
- 9.Roberts JE, Bell MA. Two- and three-dimensional mental rotation tasks lead to different parietal laterality for men and women. Int J Psychophysiol. 2003;50:235–46. doi: 10.1016/s0167-8760(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 10.Hugdahl K, Thomsen T, Ersland L. Sex differences in visuo-spatial processing: an fMRI study of mental rotation. Neuropsychologia. 2006;44:1575–83. doi: 10.1016/j.neuropsychologia.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Seurinck R, Vingerhoets G, de Lange FP, Achten E. Does egocentric mental rotation elicit sex differences? Neuroimage. 2004;23:1440–9. doi: 10.1016/j.neuroimage.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Gizewski ER, Krause E, Wanke I, Forsting M, Senf W. Gender-specific cerebral activation during cognitive tasks using functional MRI: comparison of women in mid-luteal phase and men. Neuroradiology. 2006;48:14–20. doi: 10.1007/s00234-005-0004-9. [DOI] [PubMed] [Google Scholar]
- 13.Mantyla T. Gender differences in multitasking reflect spatial ability. Psychol Sci. 2013;24:514–20. doi: 10.1177/0956797612459660. [DOI] [PubMed] [Google Scholar]
- 14.Puts DA, Cardenas RA, Bailey DH, Burriss RP, Jordan CL, Breedlove SM. Salivary testosterone does not predict mental rotation performance in men or women. Horm Behav. 2010;58:282–9. doi: 10.1016/j.yhbeh.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Vuoksimaa E, Kaprio J, Eriksson CJ, Rose RJ. Pubertal testosterone predicts mental rotation performance of young adult males. Psychoneuroendocrinology. 2012;37:1791–800. doi: 10.1016/j.psyneuen.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin DM, Wittert G, Burns NR, McPherson J. Endogenous testosterone levels, mental rotation performance, and constituent abilities in middle-to-older aged men. Horm Behav. 2008;53:431–41. doi: 10.1016/j.yhbeh.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Griksiene R, Ruksenas O. Effects of hormonal contraceptives on mental rotation and verbal fluency. Psychoneuroendocrinology. 2011;36:1239–48. doi: 10.1016/j.psyneuen.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Courvoisier DS, Renaud O, Geiser C, Paschke K, Gaudy K, Jordan K. Sex hormones and mental rotation: an intensive longitudinal investigation. Horm Behav. 2013;63:345–51. doi: 10.1016/j.yhbeh.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Maguire EA, Burgess N, O’Keefe J. Human spatial navigation: cognitive maps, sexual dimorphism, and neural substrates. Curr Opin Neurobiol. 1999;9:171–7. doi: 10.1016/s0959-4388(99)80023-3. [DOI] [PubMed] [Google Scholar]
- 20.Weniger G, Ruhleder M, Lange C, Irle E. Impaired egocentric memory and reduced somatosensory cortex size in temporal lobe epilepsy with hippocampal sclerosis. Behav Brain Res. 2012;227:116–24. doi: 10.1016/j.bbr.2011.10.043. [DOI] [PubMed] [Google Scholar]
- 21.Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm Behav. 2005;47:326–35. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Burkitt J, Widman D, Saucier DM. Evidence for the influence of testosterone in the performance of spatial navigation in a virtual water maze in women but not in men. Horm Behav. 2007;51:649–54. doi: 10.1016/j.yhbeh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Lewin C, Wolgers G, Herlitz A. Sex differences favoring women in verbal but not in visuospatial episodic memory. Neuropsychology. 2001;15:165–73. doi: 10.1037//0894-4105.15.2.165. [DOI] [PubMed] [Google Scholar]
- 24.Malinowski JC. Mental rotation and real-world wayfinding. Percept Mot Skills. 2001;92:19–30. doi: 10.2466/pms.2001.92.1.19. [DOI] [PubMed] [Google Scholar]
- 25.Schuck NW, Doeller CF, Schjeide BM, Schroder J, Frensch PA, Bertram L, Li SC. Aging and KIBRA/WWC1 genotype affect spatial memory processes in a virtual navigation task. Hippocampus. 2013 doi: 10.1002/hipo.22148. [DOI] [PubMed] [Google Scholar]
- 26.Wiener JM, de Condappa O, Harris MA, Wolbers T. Maladaptive bias for extrahippocampal navigation strategies in aging humans. J Neurosci. 2013;33:6012–7. doi: 10.1523/JNEUROSCI.0717-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barense MD, Bussey TJ, Lee AC, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS. Functional specialization in the human medial temporal lobe. J Neurosci. 2005;25:10239–46. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee AC, Rudebeck SR. Investigating the interaction between spatial perception and working memory in the human medial temporal lobe. J Cogn Neurosci. 2010;22:2823–35. doi: 10.1162/jocn.2009.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGivern RF, Huston JP, Byrd D, King T, Siegle GJ, Reilly J. Sex differences in visual recognition memory: support for a sex-related difference in attention in adults and children. Brain Cogn. 1997;34:323–36. doi: 10.1006/brcg.1997.0872. [DOI] [PubMed] [Google Scholar]
- 30.Levy LJ, Astur RS, Frick KM. Men and women differ in object memory but not performance of a virtual radial maze. Behav Neurosci. 2005;119:853–62. doi: 10.1037/0735-7044.119.4.853. [DOI] [PubMed] [Google Scholar]
- 31.Silverman I, Choi J, Peters M. The hunter-gatherer theory of sex differences in spatial abilities: data from 40 countries. Arch Sex Behav. 2007;36:261–8. doi: 10.1007/s10508-006-9168-6. [DOI] [PubMed] [Google Scholar]
- 32.Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology. 1991;16:155–76. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- 33.Gale SD, Baxter L, Connor DJ, Herring A, Comer J. Sex differences on the Rey Auditory Verbal Learning Test and the Brief Visuospatial Memory Test-Revised in the elderly: normative data in 172 participants. J Clin Exp Neuropsychol. 2007;29:561–7. doi: 10.1080/13803390600864760. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Aranda C, Martinussen M. Age-related differences in performance of phonemic verbal fluency measured by Controlled Oral Word Association Task (COWAT): a meta-analytic study. Dev Neuropsychol. 2006;30:697–717. doi: 10.1207/s15326942dn3002_3. [DOI] [PubMed] [Google Scholar]
- 35.Sunderaraman P, Blumen HM, DeMatteo D, Apa ZL, Cosentino S. Task demand influences relationships among sex, clustering strategy, and recall: 16-word versus 9-word list learning tests. Cogn Behav Neurol. 2013;26:78–84. doi: 10.1097/WNN.0b013e31829de450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Frias CM, Nilsson LG, Herlitz A. Sex differences in cognition are stable over a 10-year period in adulthood and old age. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006;13:574–87. doi: 10.1080/13825580600678418. [DOI] [PubMed] [Google Scholar]
- 37.van Hooren SA, Valentijn AM, Bosma H, Ponds RW, van Boxtel MP, Jolles J. Cognitive functioning in healthy older adults aged 64–81: a cohort study into the effects of age, sex, and education. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:40–54. doi: 10.1080/138255890969483. [DOI] [PubMed] [Google Scholar]
- 38.Gerstorf D, Herlitz A, Smith J. Stability of sex differences in cognition in advanced old age: the role of education and attrition. J Gerontol B Psychol Sci Soc Sci. 2006;61:245–9. doi: 10.1093/geronb/61.4.p245. [DOI] [PubMed] [Google Scholar]
- 39.Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer’s disease: a meta analysis. J Clin Exp Neuropsychol. 2012;34:989–98. doi: 10.1080/13803395.2012.712676. [DOI] [PubMed] [Google Scholar]
- 40.Proust-Lima C, Amieva H, Letenneur L, Orgogozo JM, Jacqmin-Gadda H, Dartigues JF. Gender and education impact on brain aging: a general cognitive factor approach. Psychol Aging. 2008;23:608–20. doi: 10.1037/a0012838. [DOI] [PubMed] [Google Scholar]
- 41.Read S, Pedersen NL, Gatz M, Berg S, Vuoksimaa E, Malmberg B, Johansson B, McClearn GE. Sex differences after all those years? Heritability of cognitive abilities in old age. J Gerontol B Psychol Sci Soc Sci. 2006;61:137–43. doi: 10.1093/geronb/61.3.p137. [DOI] [PubMed] [Google Scholar]
- 42.Takayanagi Y, Spira AP, McIntyre RS, Eaton WW. Sex Hormone Binding Globulin and Verbal Memory in Older Men. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herlitz A, Reuterskiold L, Loven J, Thilers PP, Rehnman J. Cognitive sex differences are not magnified as a function of age, sex hormones, or puberty development during early adolescence. Dev Neuropsychol. 2013;38:167–79. doi: 10.1080/87565641.2012.759580. [DOI] [PubMed] [Google Scholar]
- 44.Pauls F, Petermann F, Lepach AC. Gender differences in episodic memory and visual working memory including the effects of age. Memory. 2013;21:857–74. doi: 10.1080/09658211.2013.765892. [DOI] [PubMed] [Google Scholar]
- 45.Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Cost KT, Williams-Yee ZN, Fustok JN, Dohanich GP. Sex differences in object-in-place memory of adult rats. Behav Neurosci. 2012;126:457–64. doi: 10.1037/a0028363. [DOI] [PubMed] [Google Scholar]
- 47.Hausmann M, Slabbekoorn D, Van Goozen SH, Cohen-Kettenis PT, Gunturkun O. Sex hormones affect spatial abilities during the menstrual cycle. Behav Neurosci. 2000;114:1245–50. doi: 10.1037//0735-7044.114.6.1245. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez CA, Chamizo VD, Mackintosh NJ. Overshadowing and blocking between landmark learning and shape learning: the importance of sex differences. Learn Behav. 2011;39:324–35. doi: 10.3758/s13420-011-0027-5. [DOI] [PubMed] [Google Scholar]
- 49.Keeley RJ, Tyndall AV, Scott GA, Saucier DM. Sex difference in cue strategy in a modified version of the Morris water task: correlations between brain and behaviour. PLoS One. 2013;8:e69727. doi: 10.1371/journal.pone.0069727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones, and neurogenesis in the hippocampus: Hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013 doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- 51.Epp JR, Galea LA. Hippocampus-dependent strategy choice predicts low levels of cell proliferation in the dentate gyrus. Neurobiol Learn Mem. 2009;91:437–46. doi: 10.1016/j.nlm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Rummel J, Epp JR, Galea LA. Estradiol does not influence strategy choice but place strategy choice is associated with increased cell proliferation in the hippocampus of female rats. Horm Behav. 2010;58:582–90. doi: 10.1016/j.yhbeh.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Chow C, Epp JR, Lieblich SE, Barha CK, Galea LA. Sex differences in neurogenesis and activation of new neurons in response to spatial learning and memory. Psychoneuroendocrinology. 2013;38:1236–50. doi: 10.1016/j.psyneuen.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 54.McClure RE, Barha CK, Galea LA. 17beta-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Horm Behav. 2013;63:144–57. doi: 10.1016/j.yhbeh.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 55.Moradpour F, Naghdi N, Fathollahi Y, Javan M, Choopani S, Gharaylou Z. Pre-pubertal castration improves spatial learning during mid-adolescence in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:105–12. doi: 10.1016/j.pnpbp.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Spritzer MD, Fox EC, Larsen GD, Batson CG, Wagner BA, Maher J. Testosterone influences spatial strategy preferences among adult male rats. Horm Behav. 2013;63:800–12. doi: 10.1016/j.yhbeh.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LaBuda CJ, Mellgren RL, Hale RL. Sex differences in the acquisition of a radial maze task in the CD-1 mouse. Physiol Behav. 2002;76:213–7. doi: 10.1016/s0031-9384(02)00713-8. [DOI] [PubMed] [Google Scholar]
- 58.Lund TD, Lephart ED. Manipulation of prenatal hormones and dietary phytoestrogens during adulthood alter the sexually dimorphic expression of visual spatial memory. BMC Neurosci. 2001;2:21. doi: 10.1186/1471-2202-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–51. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Leary TP, Savoie V, Brown RE. Learning, memory and search strategies of inbred mouse strains with different visual abilities in the Barnes maze. Behav Brain Res. 2011;216:531–42. doi: 10.1016/j.bbr.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 61.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 62.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–52. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 63.Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord. 2008;38:481–8. doi: 10.1007/s10803-007-0414-0. [DOI] [PubMed] [Google Scholar]
- 64.Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, Koenen KC, Ascherio A, Weisskopf MG. Perinatal air pollutant exposures and autism spectrum disorder in the children of nurses’ health study II participants. Environ Health Perspect. 2013;121:978–84. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill CA, Threlkeld SW, Fitch RH. Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. Int J Dev Neurosci. 2011;29:381–8. doi: 10.1016/j.ijdevneu.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider JS, Anderson DW, Talsania K, Mettil W, Vadigepalli R. Effects of developmental lead exposure on the hippocampal transcriptome: influences of sex, developmental period, and lead exposure level. Toxicol Sci. 2012;129:108–25. doi: 10.1093/toxsci/kfs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol. 2000;18:331–54. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- 68.Mihalick SM, Crandall JE, Langlois JC, Krienke JD, Dube WV. Prenatal ethanol exposure, generalized learning impairment, and medial prefrontal cortical deficits in rats. Neurotoxicol Teratol. 2001;23:453–62. doi: 10.1016/s0892-0362(01)00168-4. [DOI] [PubMed] [Google Scholar]
- 69.Banuelos C, Gilbert RJ, Montgomery KS, Fincher AS, Wang H, Frye GD, Setlow B, Bizon JL. Altered spatial learning and delay discounting in a rat model of human third trimester binge ethanol exposure. Behav Pharmacol. 2012;23:54–65. doi: 10.1097/FBP.0b013e32834eb07d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990;348:448–50. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- 71.Koopman P. The genetics and biology of vertebrate sex determination. Cell. 2001;105:843–7. doi: 10.1016/s0092-8674(01)00408-1. [DOI] [PubMed] [Google Scholar]
- 72.Davis EC, Shryne JE, Gorski RA. A revised critical period for the sexual differentiation of the sexually dimorphic nucleus of the preoptic area in the rat. Neuroendocrinology. 1995;62:579–85. doi: 10.1159/000127053. [DOI] [PubMed] [Google Scholar]
- 73.Zuloaga DG, Puts DA, Jordan CL, Breedlove SM. The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Horm Behav. 2008;53:613–26. doi: 10.1016/j.yhbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bakker J, Brand T, van Ophemert J, Slob AK. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behav Neurosci. 1993;107:480–7. doi: 10.1037//0735-7044.107.3.480. [DOI] [PubMed] [Google Scholar]
- 76.Bakker J, van Ophemert J, Timmerman MA, de Jong FH, Slob AK. Endogenous reproductive hormones and nocturnal rhythms in partner preference and sexual behavior of ATD-treated male rats. Neuroendocrinology. 1995;62:396–405. doi: 10.1159/000127029. [DOI] [PubMed] [Google Scholar]
- 77.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–46. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 78.Bao AM, Swaab DF. Sexual differentiation of the human brain: relation to gender identity, sexual orientation and neuropsychiatric disorders. Front Neuroendocrinol. 2011;32:214–26. doi: 10.1016/j.yfrne.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 79.Penaloza C, Estevez B, Orlanski S, Sikorska M, Walker R, Smith C, Smith B, Lockshin RA, Zakeri Z. Sex of the cell dictates its response: differential gene expression and sensitivity to cell death inducing stress in male and female cells. FASEB J. 2009;23:1869–79. doi: 10.1096/fj.08-119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–70. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- 81.Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, Roemisch-Margl W, Polonikov A, Peters A, Theis FJ, Meitinger T, Kronenberg F, Weidinger S, Wichmann HE, Suhre K, Wang-Sattler R, Adamski J, Illig T. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011;7:e1002215. doi: 10.1371/journal.pgen.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ismail N, Garas P, Blaustein JD. Long-term effects of pubertal stressors on female sexual receptivity and estrogen receptor-alpha expression in CD-1 female mice. Horm Behav. 2011;59:565–71. doi: 10.1016/j.yhbeh.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? J Am Acad Child Adolesc Psychiatry. 1997;36:1768–76. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- 85.Waber DP. Neuropsychological aspects of Turner’s syndrome. Dev Med Child Neurol. 1979;21:58–70. doi: 10.1111/j.1469-8749.1979.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 86.Ryan J, Carriere I, Scali J, Ritchie K, Ancelin ML. Life-time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology. 2009;34:287–98. doi: 10.1016/j.psyneuen.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 87.Beltz AM, Berenbaum SA. Cognitive effects of variations in pubertal timing: is puberty a period of brain organization for human sex-typed cognition? Horm Behav. 2013;63:823–8. doi: 10.1016/j.yhbeh.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 88.Berenbaum SA, Bryk KL, Beltz AM. Early androgen effects on spatial and mechanical abilities: evidence from congenital adrenal hyperplasia. Behav Neurosci. 2012;126:86–96. doi: 10.1037/a0026652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Puts DA, McDaniel MA, Jordan CL, Breedlove SM. Spatial ability and prenatal androgens: meta-analyses of congenital adrenal hyperplasia and digit ratio (2D:4D) studies. Arch Sex Behav. 2008;37:100–11. doi: 10.1007/s10508-007-9271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giedd JN, Clasen LS, Wallace GL, Lenroot RK, Lerch JP, Wells EM, Blumenthal JD, Nelson JE, Tossell JW, Stayer C, Evans AC, Samango-Sprouse CA. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics. 2007;119:e232–40. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- 91.Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ. 2012;3:19. doi: 10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee NR, Wallace GL, Clasen LS, Lenroot RK, Blumenthal JD, White SL, Celano MJ, Giedd JN. Executive Function in Young Males with Klinefelter (XXY) Syndrome with and without Comorbid Attention-Deficit/Hyperactivity Disorder. J Int Neuropsychol Soc. 2011:1–9. doi: 10.1017/S1355617711000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kesler SR, Garrett A, Bender B, Yankowitz J, Zeng SM, Reiss AL. Amygdala and hippocampal volumes in Turner syndrome: a high-resolution MRI study of X-monosomy. Neuropsychologia. 2004;42:1971–8. doi: 10.1016/j.neuropsychologia.2004.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hart SJ, Davenport ML, Hooper SR, Belger A. Visuospatial executive function in Turner syndrome: functional MRI and neurocognitive findings. Brain. 2006;129:1125–36. doi: 10.1093/brain/awl046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li R, Cui J, Jothishankar B, Shen J, He P, Shen Y. Early reproductive experiences in females make differences in cognitive function later in life. J Alzheimers Dis. 2013;34:589–94. doi: 10.3233/JAD-122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barha CK, Galea LA. Motherhood alters the cellular response to estrogens in the hippocampus later in life. Neurobiol Aging. 2011;32:2091–5. doi: 10.1016/j.neurobiolaging.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 97.Hogervorst E. Effects Of Gonadal Hormones On Cognitive Behavior In Elderly Men And Women. J Neuroendocrinol. 2013 doi: 10.1111/jne.12080. [DOI] [PubMed] [Google Scholar]
- 98.Pilz KS, Konar Y, Vuong QC, Bennett PJ, Sekuler AB. Age-related changes in matching novel objects across viewpoints. Vision Res. 2011;51:1958–65. doi: 10.1016/j.visres.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wharton W, Hirshman E, Merritt P, Doyle L, Paris S, Gleason C. Oral contraceptives and androgenicity: influences on visuospatial task performance in younger individuals. Exp Clin Psychopharmacol. 2008;16:156–64. doi: 10.1037/1064-1297.16.2.156. [DOI] [PubMed] [Google Scholar]
- 100.Hampson E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain Cogn. 1990;14:26–43. doi: 10.1016/0278-2626(90)90058-v. [DOI] [PubMed] [Google Scholar]
- 101.Libon DJ, Xie SX, Moore P, Farmer J, Antani S, McCawley G, Cross K, Grossman M. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68:369–75. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- 102.Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, Hodges JR. A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology. 2000;55:1613–20. doi: 10.1212/01.wnl.0000434309.85312.19. [DOI] [PubMed] [Google Scholar]
- 103.Perri R, Koch G, Carlesimo GA, Serra L, Fadda L, Pasqualetti P, Pettenati C, Caltagirone C. Alzheimer’s disease and frontal variant of frontotemporal dementia-- a very brief battery for cognitive and behavioural distinction. J Neurol. 2005;252:1238–44. doi: 10.1007/s00415-005-0849-1. [DOI] [PubMed] [Google Scholar]
- 104.Giovagnoli AR, Erbetta A, Reati F, Bugiani O. Differential neuropsychological patterns of frontal variant frontotemporal dementia and Alzheimer’s disease in a study of diagnostic concordance. Neuropsychologia. 2008;46:1495–504. doi: 10.1016/j.neuropsychologia.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 105.Frisch S, Dukart J, Vogt B, Horstmann A, Becker G, Villringer A, Barthel H, Sabri O, Muller K, Schroeter ML. Dissociating memory networks in early Alzheimer’s disease and frontotemporal lobar degeneration - a combined study of hypometabolism and atrophy. PLoS One. 2013;8:e55251. doi: 10.1371/journal.pone.0055251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ye BS, Seo SW, Lee Y, Kim SY, Choi SH, Lee YM, Kim do H, Han HJ, Na DL, Kim EJ. Neuropsychological performance and conversion to Alzheimer’s disease in early- compared to late-onset amnestic mild cognitive impairment: CREDOS study. Dement Geriatr Cogn Disord. 2012;34:156–66. doi: 10.1159/000342973. [DOI] [PubMed] [Google Scholar]
- 107.Dong Y, Gan DZ, Tay SZ, Koay WI, Collinson SL, Hilal S, Venketasubramanian N, Chen C. Patterns of neuropsychological impairment in Alzheimer’s disease and mixed dementia. J Neurol Sci. 2013 doi: 10.1016/j.jns.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 108.Heuer HW, Mirsky JB, Kong EL, Dickerson BC, Miller BL, Kramer JH, Boxer AL. Antisaccade task reflects cortical involvement in mild cognitive impairment. Neurology. 2013 doi: 10.1212/WNL.0b013e3182a6cbfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brown JM, Wiggins J, Dong H, Harvey R, Richardson F, Hunter K, Dawson K, Parker RA. The hard Test Your Memory. Evaluation of a short cognitive test to detect mild Alzheimer’s disease and amnestic mild cognitive impairment. Int J Geriatr Psychiatry. 2013 doi: 10.1002/gps.4005. [DOI] [PubMed] [Google Scholar]
- 110.Selkoe DJ, Yamazaki T, Citron M, Podlisny MB, Koo EH, Teplow DB, Haass C. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann N Y Acad Sci. 1996;777:57–64. doi: 10.1111/j.1749-6632.1996.tb34401.x. [DOI] [PubMed] [Google Scholar]
- 111.Dye RV, Miller KJ, Singer EJ, Levine AJ. Hormone replacement therapy and risk for neurodegenerative diseases. Int J Alzheimers Dis. 2012;2012:258454. doi: 10.1155/2012/258454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC. Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am J Pathol. 2001;158:1173–7. doi: 10.1016/s0002-9440(10)64064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vina J, Lloret A. Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis. 2010;20(Suppl 2):S527–33. doi: 10.3233/JAD-2010-100501. [DOI] [PubMed] [Google Scholar]
- 114.Aronson MK, Ooi WL, Morgenstern H, Hafner A, Masur D, Crystal H, Frishman WH, Fisher D, Katzman R. Women, myocardial infarction, and dementia in the very old. Neurology. 1990;40:1102–6. doi: 10.1212/wnl.40.7.1102. [DOI] [PubMed] [Google Scholar]
- 115.Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55:809–15. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- 116.Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76:465–79. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 117.Henderson VW, Buckwalter JG. Cognitive deficits of men and women with Alzheimer’s disease. Neurology. 1994;44:90–6. doi: 10.1212/wnl.44.1.90. [DOI] [PubMed] [Google Scholar]
- 118.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–50. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 119.Phung TK, Waltoft BL, Laursen TM, Settnes A, Kessing LV, Mortensen PB, Waldemar G. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord. 2010;30:43–50. doi: 10.1159/000314681. [DOI] [PubMed] [Google Scholar]
- 120.De Deyn PP, Goeman J, Vervaet A, Dourcy-Belle-Rose B, Van Dam D, Geerts E. Prevalence and incidence of dementia among 75–80-year-old community-dwelling elderly in different districts of Antwerp, Belgium: the Antwerp Cognition (ANCOG) Study. Clin Neurol Neurosurg. 2011;113:736–45. doi: 10.1016/j.clineuro.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 121.Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013;19:197–209. doi: 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci U S A. 2005;102:19198–203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cahill L. His brain, her brain. Sci Am. 2005;292:40–7. [PubMed] [Google Scholar]
- 124.Carter CL, Resnick EM, Mallampalli M, Kalbarczyk A. Sex and gender differences in Alzheimer’s disease: recommendations for future research. J Womens Health (Larchmt) 2012;21:1018–23. doi: 10.1089/jwh.2012.3789. [DOI] [PubMed] [Google Scholar]
- 125.Swaab DF, Chung WC, Kruijver FP, Hofman MA, Ishunina TA. Structural and functional sex differences in the human hypothalamus. Horm Behav. 2001;40:93–8. doi: 10.1006/hbeh.2001.1682. [DOI] [PubMed] [Google Scholar]
- 126.McAllister C, Long J, Bowers A, Walker A, Cao P, Honda S, Harada N, Staufenbiel M, Shen Y, Li R. Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci. 2010;30:7326–34. doi: 10.1523/JNEUROSCI.1180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barron AM, Pike CJ. Sex hormones, aging, and Alzheimer’s disease. Front Biosci (Elite Ed) 2012;4:976–97. doi: 10.2741/e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Long J, He P, Shen Y, Li R. New evidence of mitochondria dysfunction in the female Alzheimer’s disease brain: deficiency of estrogen receptor-beta. J Alzheimers Dis. 2012;30:545–58. doi: 10.3233/JAD-2012-120283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fleisher A, Grundman M, Jack CR, Jr, Petersen RC, Taylor C, Kim HT, Schiller DH, Bagwell V, Sencakova D, Weiner MF, DeCarli C, DeKosky ST, van Dyck CH, Thal LJ. Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62:953–7. doi: 10.1001/archneur.62.6.953. [DOI] [PubMed] [Google Scholar]
- 130.Mukai H, Kimoto T, Hojo Y, Kawato S, Murakami G, Higo S, Hatanaka Y, Ogiue-Ikeda M. Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochim Biophys Acta. 2010;1800:1030–44. doi: 10.1016/j.bbagen.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 131.Hojo Y, Higo S, Kawato S, Hatanaka Y, Ooishi Y, Murakami G, Ishii H, Komatsuzaki Y, Ogiue-Ikeda M, Mukai H, Kimoto T. Hippocampal synthesis of sex steroids and corticosteroids: essential for modulation of synaptic plasticity. Front Endocrinol (Lausanne) 2011;2:43. doi: 10.3389/fendo.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fester L, Prange-Kiel J, Zhou L, Blittersdorf BV, Bohm J, Jarry H, Schumacher M, Rune GM. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J Steroid Biochem Mol Biol. 2012;131:24–9. doi: 10.1016/j.jsbmb.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 133.Kramar EA, Chen LY, Lauterborn JC, Simmons DA, Gall CM, Lynch G. BDNF upregulation rescues synaptic plasticity in middle-aged ovariectomized rats. Neurobiol Aging. 2012;33:708–19. doi: 10.1016/j.neurobiolaging.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ooishi Y, Kawato S, Hojo Y, Hatanaka Y, Higo S, Murakami G, Komatsuzaki Y, Ogiue-Ikeda M, Kimoto T, Mukai H. Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. J Steroid Biochem Mol Biol. 2012;131:37–51. doi: 10.1016/j.jsbmb.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 135.Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience. 2012;202:131–46. doi: 10.1016/j.neuroscience.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anastasio TJ. Exploring the contribution of estrogen to amyloid-Beta regulation: a novel multifactorial computational modeling approach. Front Pharmacol. 2013;4:16. doi: 10.3389/fphar.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–88. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pike CJ. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer’s disease. J Neurochem. 1999;72:1552–63. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- 139.Zhang QG, Wang R, Khan M, Mahesh V, Brann DW. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J Neurosci. 2008;28:8430–41. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Levin-Allerhand JA, Lominska CE, Wang J, Smith JD. 17Alpha-estradiol and 17beta-estradiol treatments are effective in lowering cerebral amyloid-beta levels in AbetaPPSWE transgenic mice. J Alzheimers Dis. 2002;4:449–57. doi: 10.3233/jad-2002-4601. [DOI] [PubMed] [Google Scholar]
- 141.Jung JI, Ladd TB, Kukar T, Price AR, Moore BD, Koo EH, Golde TE, Felsenstein KM. Steroids as gamma-secretase modulators. FASEB J. 2013;27:3775–85. doi: 10.1096/fj.12-225649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jayaraman A, Carroll JC, Morgan TE, Lin S, Zhao L, Arimoto JM, Murphy MP, Beckett TL, Finch CE, Brinton RD, Pike CJ. 17beta-estradiol and progesterone regulate expression of beta-amyloid clearance factors in primary neuron cultures and female rat brain. Endocrinology. 2012;153:5467–79. doi: 10.1210/en.2012-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dang TN, Dobson-Stone C, Glaros EN, Kim WS, Hallupp M, Bartley L, Piguet O, Hodges JR, Halliday GM, Double KL, Schofield PR, Crouch PJ, Kwok JB. Endogenous progesterone levels and frontotemporal dementia: modulation of TDP-43 and Tau levels in vitro and treatment of the A315T TARDBP mouse model. Dis Model Mech. 2013;6:1198–204. doi: 10.1242/dmm.011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Amtul Z, Wang L, Westaway D, Rozmahel RF. Neuroprotective mechanism conferred by 17beta-estradiol on the biochemical basis of Alzheimer’s disease. Neuroscience. 2010;169:781–6. doi: 10.1016/j.neuroscience.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 145.Zhao L, Morgan TE, Mao Z, Lin S, Cadenas E, Finch CE, Pike CJ, Mack WJ, Brinton RD. Continuous versus cyclic progesterone exposure differentially regulates hippocampal gene expression and functional profiles. PLoS One. 2012;7:e31267. doi: 10.1371/journal.pone.0031267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Frye CA, Walf AA. Effects of progesterone administration and APPswe+PSEN1Deltae9 mutation for cognitive performance of mid-aged mice. Neurobiol Learn Mem. 2008;89:17–26. doi: 10.1016/j.nlm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 147.Carroll JC, Rosario ER, Villamagna A, Pike CJ. Continuous and cyclic progesterone differentially interact with estradiol in the regulation of Alzheimer-like pathology in female 3xTransgenic-Alzheimer’s disease mice. Endocrinology. 2010;151:2713–22. doi: 10.1210/en.2009-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 149.Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149:583–9. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- 150.Hogervorst E, Williams J, Budge M, Barnetson L, Combrinck M, Smith AD. Serum total testosterone is lower in men with Alzheimer’s disease. Neuro Endocrinol Lett. 2001;22:163–8. [PubMed] [Google Scholar]
- 151.Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–93. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- 152.Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. JAMA. 2004;292:1431–2. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- 153.Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging. 2011;32:604–13. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vest RS, Pike CJ. Gender, sex steroid hormones, and Alzheimer’s disease. Horm Behav. 2013;63:301–7. doi: 10.1016/j.yhbeh.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rosario ER, Chang L, Beckett TL, Carroll JC, Paul Murphy M, Stanczyk FZ, Pike CJ. Age-related changes in serum and brain levels of androgens in male Brown Norway rats. Neuroreport. 2009;20:1534–7. doi: 10.1097/WNR.0b013e328331f968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bookheimer S, Burggren A. APOE-4 genotype and neurophysiological vulnerability to Alzheimer’s and cognitive aging. Annu Rev Clin Psychol. 2009;5:343–62. doi: 10.1146/annurev.clinpsy.032408.153625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Holland D, Desikan RS, Dale AM, McEvoy LK. Higher Rates of Decline for Women and Apolipoprotein E {varepsilon}4 Carriers. AJNR Am J Neuroradiol. 2013 doi: 10.3174/ajnr.A3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Beydoun MA, Beydoun HA, Kaufman JS, An Y, Resnick SM, O’Brien R, Ferrucci L, Zonderman AB. Apolipoprotein E epsilon4 allele interacts with sex and cognitive status to influence all-cause and cause-specific mortality in U.S. older adults. J Am Geriatr Soc. 2013;61:525–34. doi: 10.1111/jgs.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Protas HD, Chen K, Langbaum JB, Fleisher AS, Alexander GE, Lee W, Bandy D, de Leon MJ, Mosconi L, Buckley S, Truran-Sacrey D, Schuff N, Weiner MW, Caselli RJ, Reiman EM. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 2013;70:320–5. doi: 10.1001/2013.jamaneurol.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Leung L, Andrews-Zwilling Y, Yoon SY, Jain S, Ring K, Dai J, Wang MM, Tong L, Walker D, Huang Y. Apolipoprotein E4 causes age- and sex-dependent impairments of hilar GABAergic interneurons and learning and memory deficits in mice. PLoS One. 2012;7:e53569. doi: 10.1371/journal.pone.0053569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Reynolds CA, Zavala C, Gatz M, Vie L, Johansson B, Malmberg B, Ingelsson E, Prince JA, Pedersen NL. Sortilin receptor 1 predicts longitudinal cognitive change. Neurobiol Aging. 2013;34:1710, e11–8. doi: 10.1016/j.neurobiolaging.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Olgiati P, Politis A, Albani D, Rodilossi S, Polito L, Zisaki A, Piperi C, Liappas I, Stamouli E, Mailis A, Batelli S, Forloni G, Marsano A, Balestri M, Soldatos CR, De Ronchi D, Kalofoutis A, Serretti A. Effects of SORL1 gene on Alzheimer’s disease. Focus on gender, neuropsychiatric symptoms and pro-inflammatory cytokines. Curr Alzheimer Res. 2013;10:154–64. doi: 10.2174/1567205011310020005. [DOI] [PubMed] [Google Scholar]
- 163.Cui J, Randell E, Renouf J, Sun G, Han FY, Younghusband B, Xie YG. Gender dependent association of thrombospondin-4 A387P polymorphism with myocardial infarction. Arterioscler Thromb Vasc Biol. 2004;24:e183–4. doi: 10.1161/01.ATV.0000147304.67100.ee. [DOI] [PubMed] [Google Scholar]
- 164.Wessel J, Topol EJ, Ji M, Meyer J, McCarthy JJ. Replication of the association between the thrombospondin-4 A387P polymorphism and myocardial infarction. Am Heart J. 2004;147:905–9. doi: 10.1016/j.ahj.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 165.Cagliani R, Guerini FR, Rubio-Acero R, Baglio F, Forni D, Agliardi C, Griffanti L, Fumagalli M, Pozzoli U, Riva S, Calabrese E, Sikora M, Casals F, Comi GP, Bresolin N, Caceres M, Clerici M, Sironi M. Long-standing balancing selection in the THBS4 gene: influence on sex-specific brain expression and gray matter volumes in Alzheimer disease. Hum Mutat. 2013;34:743–53. doi: 10.1002/humu.22301. [DOI] [PubMed] [Google Scholar]
- 166.Liu R, Sui X, Laditka JN, Church TS, Colabianchi N, Hussey J, Blair SN. Cardiorespiratory fitness as a predictor of dementia mortality in men and women. Med Sci Sports Exerc. 2012;44:253–9. doi: 10.1249/MSS.0b013e31822cf717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fallah N, Mitnitski A, Middleton L, Rockwood K. Modeling the impact of sex on how exercise is associated with cognitive changes and death in older Canadians. Neuroepidemiology. 2009;33:47–54. doi: 10.1159/000211953. [DOI] [PubMed] [Google Scholar]
- 168.Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Baertlein L, Boeve BF, Tangalos EG, Ivnik RJ, Mielke MM, Petersen RC. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ancelin ML, Ripoche E, Dupuy AM, Barberger-Gateau P, Auriacombe S, Rouaud O, Berr C, Carriere I, Ritchie K. Sex differences in the associations between lipid levels and incident dementia. J Alzheimers Dis. 2013;34:519–28. doi: 10.3233/JAD-121228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cowie CC, Harris MI, Eberhardt MS. Frequency and determinants of screening for diabetes in the U.S. Diabetes Care. 1994;17:1158–63. doi: 10.2337/diacare.17.10.1158. [DOI] [PubMed] [Google Scholar]
- 171.Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44:3–15. doi: 10.1007/s001250051573. [DOI] [PubMed] [Google Scholar]
- 172.Raffaitin C, Feart C, Le Goff M, Amieva H, Helmer C, Akbaraly TN, Tzourio C, Gin H, Barberger-Gateau P. Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology. 2011;76:518–25. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- 173.Verdelho A, Madureira S, Moleiro C, Ferro JM, Santos CO, Erkinjuntti T, Pantoni L, Fazekas F, Visser M, Waldemar G, Wallin A, Hennerici M, Inzitari D. White matter changes and diabetes predict cognitive decline in the elderly: the LADIS study. Neurology. 2010;75:160–7. doi: 10.1212/WNL.0b013e3181e7ca05. [DOI] [PubMed] [Google Scholar]
- 174.van Elderen SG, de Roos A, de Craen AJ, Westendorp RG, Blauw GJ, Jukema JW, Bollen EL, Middelkoop HA, van Buchem MA, van der Grond J. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology. 2010;75:997–1002. doi: 10.1212/WNL.0b013e3181f25f06. [DOI] [PubMed] [Google Scholar]
- 175.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Tangalos EG, Ivnik RJ, Mielke MM, Petersen RC. Cardiac disease associated with increased risk of nonamnestic cognitive impairment: stronger effect on women. JAMA Neurol. 2013;70:374–82. doi: 10.1001/jamaneurol.2013.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer’s disease: review and hypothesis. Neurobiol Aging. 2006;27:190–8. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 177.Park SA. A common pathogenic mechanism linking type-2 diabetes and Alzheimer’s disease: evidence from animal models. J Clin Neurol. 2011;7:10–8. doi: 10.3988/jcn.2011.7.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., 3rd Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–35. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 180.Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- 181.Piccinelli M, Wilkinson G. Gender differences in depression. Critical review. Br J Psychiatry. 2000;177:486–92. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- 182.Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH. Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol. 2005;57:381–7. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 183.Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Imbimbo BP, Santamato A, Vendemiale G, Seripa D, Pilotto A, Capurso A, Solfrizzi V. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18:98–116. doi: 10.1097/JGP.0b013e3181b0fa13. [DOI] [PubMed] [Google Scholar]
- 184.Richard E, Reitz C, Honig LH, Schupf N, Tang MX, Manly JJ, Mayeux R, Devanand D, Luchsinger JA. Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol. 2013;70:374–82. doi: 10.1001/jamaneurol.2013.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 186.Thielscher C, Thielscher S, Kostev K. The risk of developing depression when suffering from neurological diseases. Ger Med Sci. 2013;11 doi: 10.3205/000170. Doc02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nihonmatsu-Kikuchi N, Hayashi Y, Yu XJ, Tatebayashi Y. Depression and Alzheimer’s disease: novel postmortem brain studies reveal a possible common mechanism. J Alzheimers Dis. 2013;37:611–21. doi: 10.3233/JAD-130752. [DOI] [PubMed] [Google Scholar]
- 188.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schwendimann RN, Alekseeva N. Gender issues in multiple sclerosis. Int Rev Neurobiol. 2007;79:377–92. doi: 10.1016/S0074-7742(07)79017-7. [DOI] [PubMed] [Google Scholar]
- 190.Bishop J, Simpkins JW. Estradiol treatment increases viability of glioma and neuroblastoma cells in vitro. Mol Cell Neurosci. 1994;5:303–8. doi: 10.1006/mcne.1994.1036. [DOI] [PubMed] [Google Scholar]
- 191.Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–30. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 192.Green PS, Simpkins JW. Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci. 2000;18:347–58. doi: 10.1016/s0736-5748(00)00017-4. [DOI] [PubMed] [Google Scholar]
- 193.Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol. 1997;51:535–41. [PubMed] [Google Scholar]
- 194.Weaver CE, Jr, Park-Chung M, Gibbs TT, Farb DH. 17beta-Estradiol protects against NMDAinduced excitotoxicity by direct inhibition of NMDA receptors. Brain Res. 1997;761:338–41. doi: 10.1016/s0006-8993(97)00449-6. [DOI] [PubMed] [Google Scholar]
- 195.Singh M, Setalo G, Jr, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J Neurosci. 2000;20:1694–700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J Neurosci Res. 2000;60:321–7. doi: 10.1002/(SICI)1097-4547(20000501)60:3<321::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 197.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–37. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jaffe AB, Toran-Allerand CD, Greengard P, Gandy SE. Estrogen regulates metabolism of Alzheimer amyloid beta precursor protein. J Biol Chem. 1994;269:13065–8. [PubMed] [Google Scholar]
- 199.Xu H, Wang R, Zhang YW, Zhang X. Estrogen, beta-amyloid metabolism/trafficking, and Alzheimer’s disease. Ann N Y Acad Sci. 2006;1089:324–42. doi: 10.1196/annals.1386.036. [DOI] [PubMed] [Google Scholar]
- 200.Yao M, Nguyen TV, Pike CJ. Estrogen regulates Bcl-w and Bim expression: role in protection against beta-amyloid peptide-induced neuronal death. J Neurosci. 2007;27:1422–33. doi: 10.1523/JNEUROSCI.2382-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Manthey D, Heck S, Engert S, Behl C. Estrogen induces a rapid secretion of amyloid beta precursor protein via the mitogen-activated protein kinase pathway. Eur J Biochem. 2001;268:4285–91. doi: 10.1046/j.1432-1327.2001.02346.x. [DOI] [PubMed] [Google Scholar]
- 202.Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, Fried G, Jovanovic JN, Seeger M, Relkin NR, Liao F, Checler F, Buxbaum JD, Chait BT, Thinakaran G, Sisodia SS, Wang R, Greengard P, Gandy S. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med. 1998;4:447–51. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 203.Li R, Shen Y, Yang LB, Lue LF, Finch C, Rogers J. Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. J Neurochem. 2000;75:1447–54. doi: 10.1046/j.1471-4159.2000.0751447.x. [DOI] [PubMed] [Google Scholar]
- 204.Zhao L, Yao J, Mao Z, Chen S, Wang Y, Brinton RD. 17beta-Estradiol regulates insulin-degrading enzyme expression via an ERbeta/PI3-K pathway in hippocampus: relevance to Alzheimer’s prevention. Neurobiol Aging. 2011;32:1949–63. doi: 10.1016/j.neurobiolaging.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liang K, Yang L, Yin C, Xiao Z, Zhang J, Liu Y, Huang J. Estrogen stimulates degradation of beta-amyloid peptide by up-regulating neprilysin. J Biol Chem. 2010;285:935–42. doi: 10.1074/jbc.M109.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–65. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- 207.Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–8. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 208.Sudo S, Wen TC, Desaki J, Matsuda S, Tanaka J, Arai T, Maeda N, Sakanaka M. Beta-estradiol protects hippocampal CA1 neurons against transient forebrain ischemia in gerbil. Neurosci Res. 1997;29:345–54. doi: 10.1016/s0168-0102(97)00106-5. [DOI] [PubMed] [Google Scholar]
- 209.Fukuda K, Yao H, Ibayashi S, Nakahara T, Uchimura H, Fujishima M, Hall ED. Ovariectomy exacerbates and estrogen replacement attenuates photothrombotic focal ischemic brain injury in rats. Stroke. 2000;31:155–60. doi: 10.1161/01.str.31.1.155. [DOI] [PubMed] [Google Scholar]
- 210.Mendelowitsch A, Ritz MF, Ros J, Langemann H, Gratzl O. 17beta-Estradiol reduces cortical lesion size in the glutamate excitotoxicity model by enhancing extracellular lactate: a new neuroprotective pathway. Brain Res. 2001;901:230–6. doi: 10.1016/s0006-8993(01)02359-9. [DOI] [PubMed] [Google Scholar]
- 211.Chen J, Xu W, Jiang H. 17 beta-estradiol protects neurons from ischemic damage and attenuates accumulation of extracellular excitatory amino acids. Anesth Analg. 2001;92:1520–3. doi: 10.1097/00000539-200106000-00033. [DOI] [PubMed] [Google Scholar]
- 212.Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B, Krieg JC, Krieglstein J. Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: evidence for a receptor-independent antioxidative mechanism. J Cereb Blood Flow Metab. 1999;19:1263–9. doi: 10.1097/00004647-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 213.Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796:296–8. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- 214.Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–70. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- 215.Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–12. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 216.Nilsen J, Brinton RD. Impact of progestins on estradiol potentiation of the glutamate calcium response. Neuroreport. 2002;13:825–30. doi: 10.1097/00001756-200205070-00018. [DOI] [PubMed] [Google Scholar]
- 217.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100:10506–11. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85:2441–9. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66:1836–44. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 220.Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735:101–7. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- 221.Kumon Y, Kim SC, Tompkins P, Stevens A, Sakaki S, Loftus CM. Neuroprotective effect of postischemic administration of progesterone in spontaneously hypertensive rats with focal cerebral ischemia. J Neurosurg. 2000;92:848–52. doi: 10.3171/jns.2000.92.5.0848. [DOI] [PubMed] [Google Scholar]
- 222.Morali G, Letechipia-Vallejo G, Lopez-Loeza E, Montes P, Hernandez-Morales L, Cervantes M. Post-ischemic administration of progesterone in rats exerts neuroprotective effects on the hippocampus. Neurosci Lett. 2005;382:286–90. doi: 10.1016/j.neulet.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 223.Chen J, Chopp M, Li Y. Neuroprotective effects of progesterone after transient middle cerebral artery occlusion in rat. J Neurol Sci. 1999;171:24–30. doi: 10.1016/s0022-510x(99)00247-6. [DOI] [PubMed] [Google Scholar]
- 224.Cervantes M, Gonzalez-Vidal MD, Ruelas R, Escobar A, Morali G. Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch Med Res. 2002;33:6–14. doi: 10.1016/s0188-4409(01)00347-2. [DOI] [PubMed] [Google Scholar]
- 225.Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol. 1997;31:1–11. doi: 10.1007/BF02815156. [DOI] [PubMed] [Google Scholar]
- 226.Pettus EH, Wright DW, Stein DG, Hoffman SW. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049:112–9. doi: 10.1016/j.brainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 227.Roof RL, Duvdevani R, Heyburn JW, Stein DG. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp Neurol. 1996;138:246–51. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- 228.Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–88. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- 229.Attella MJ, Nattinville A, Stein DG. Hormonal state affects recovery from frontal cortex lesions in adult female rats. Behav Neural Biol. 1987;48:352–67. doi: 10.1016/s0163-1047(87)90918-6. [DOI] [PubMed] [Google Scholar]
- 230.Thomas AJ, Nockels RP, Pan HQ, Shaffrey CI, Chopp M. Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine. 1999;24:2134–8. doi: 10.1097/00007632-199910150-00013. [DOI] [PubMed] [Google Scholar]
- 231.Gonzalez Deniselle MC, Lopez Costa JJ, Gonzalez SL, Labombarda F, Garay L, Guennoun R, Schumacher M, De Nicola AF. Basis of progesterone protection in spinal cord neurodegeneration. J Steroid Biochem Mol Biol. 2002;83:199–209. doi: 10.1016/s0960-0760(02)00262-5. [DOI] [PubMed] [Google Scholar]
- 232.Gonzalez Deniselle MC, Lopez-Costa JJ, Saavedra JP, Pietranera L, Gonzalez SL, Garay L, Guennoun R, Schumacher M, De Nicola AF. Progesterone neuroprotection in the Wobbler mouse, a genetic model of spinal cord motor neuron disease. Neurobiol Dis. 2002;11:457–68. doi: 10.1006/nbdi.2002.0564. [DOI] [PubMed] [Google Scholar]
- 233.Ibanez C, Shields SA, El-Etr M, Leonelli E, Magnaghi V, Li WW, Sim FJ, Baulieu EE, Melcangi RC, Schumacher M, Franklin RJ. Steroids and the reversal of age-associated changes in myelination and remyelination. Prog Neurobiol. 2003;71:49–56. doi: 10.1016/j.pneurobio.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 234.Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–5. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- 235.Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1–42 toxicity through heat shock protein 70. J Neurosci. 2004;24:5315–21. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gouras GK, Xu H, Gross RS, Greenfield JP, Hai B, Wang R, Greengard P. Testosterone reduces neuronal secretion of Alzheimer’s beta-amyloid peptides. Proc Natl Acad Sci U S A. 2000;97:1202–5. doi: 10.1073/pnas.97.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ahlbom E, Grandison L, Bonfoco E, Zhivotovsky B, Ceccatelli S. Androgen treatment of neonatal rats decreases susceptibility of cerebellar granule neurons to oxidative stress in vitro. Eur J Neurosci. 1999;11:1285–91. doi: 10.1046/j.1460-9568.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 238.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–62. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 239.Papasozomenos S, Shanavas A. Testosterone prevents the heat shock-induced overactivation of glycogen synthase kinase-3 beta but not of cyclin-dependent kinase 5 and c-Jun NH2-terminal kinase and concomitantly abolishes hyperphosphorylation of tau: implications for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2002;99:1140–5. doi: 10.1073/pnas.032646799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122:573–8. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 241.Pan Y, Zhang H, Acharya AB, Patrick PH, Oliver D, Morley JE. Effect of testosterone on functional recovery in a castrate male rat stroke model. Brain Res. 2005;1043:195–204. doi: 10.1016/j.brainres.2005.02.078. [DOI] [PubMed] [Google Scholar]
- 242.Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–26. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 243.Li S, Kang L, Zhang C, Xie G, Li N, Zhang Y, Du J, Cui H. Effects of dihydrotestosterone on synaptic plasticity of hippocampus in male SAMP8 mice. Exp Gerontol. 2013;48:778–85. doi: 10.1016/j.exger.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 244.Caruso A, Di Giorgi Gerevini V, Castiglione M, Marinelli F, Tomassini V, Pozzilli C, Caricasole A, Bruno V, Caciagli F, Moretti A, Nicoletti F, Melchiorri D. Testosterone amplifies excitotoxic damage of cultured oligodendrocytes. J Neurochem. 2004;88:1179–85. doi: 10.1046/j.1471-4159.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 245.Yang SH, Perez E, Cutright J, Liu R, He Z, Day AL, Simpkins JW. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J Appl Physiol (1985) 2002;92:195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- 246.Cunningham RL, Macheda T, Watts LT, Poteet E, Singh M, Roberts JL, Giuffrida A. Androgens exacerbate motor asymmetry in male rats with unilateral 6-hydroxydopamine lesion. Horm Behav. 2011;60:617–24. doi: 10.1016/j.yhbeh.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bachman DL, Wolf PA, Linn R, Knoefel JE, Cobb J, Belanger A, D’Agostino RB, White LR. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42:115–9. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- 248.Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–32. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 249.Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–9. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 250.Geerlings MI, Ruitenberg A, Witteman JC, van Swieten JC, Hofman A, van Duijn CM, Breteler MM, Launer LJ. Reproductive period and risk of dementia in postmenopausal women. JAMA. 2001;285:1475–81. doi: 10.1001/jama.285.11.1475. [DOI] [PubMed] [Google Scholar]
- 251.Doraiswamy PM, Bieber F, Kaiser L, Krishnan KR, Reuning-Scherer J, Gulanski B. The Alzheimer’s Disease Assessment Scale: patterns and predictors of baseline cognitive performance in multicenter Alzheimer’s disease trials. Neurology. 1997;48:1511–7. doi: 10.1212/wnl.48.6.1511. [DOI] [PubMed] [Google Scholar]
- 252.Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME, Buckwalter JG. Estrogen replacement therapy in older women. Comparisons between Alzheimer’s disease cases and nondemented control subjects. Arch Neurol. 1994;51:896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- 253.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol. 1994;140:256–61. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 254.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–95. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 255.Sherwin BB. Estrogen and cognitive functioning in women. Proc Soc Exp Biol Med. 1998;217:17–22. doi: 10.3181/00379727-217-44200. [DOI] [PubMed] [Google Scholar]
- 256.Sherwin BB. Estrogen effects on cognition in menopausal women. Neurology. 1997;48:S21–6. doi: 10.1212/wnl.48.5_suppl_7.21s. [DOI] [PubMed] [Google Scholar]
- 257.Maki PM, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psychiatry. 2001;158:227–33. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- 258.Resnick SM, Maki PM. Effects of hormone replacement therapy on cognitive and brain aging. Ann N Y Acad Sci. 2001;949:203–14. doi: 10.1111/j.1749-6632.2001.tb04023.x. [DOI] [PubMed] [Google Scholar]
- 259.Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34:171–82. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- 260.Schneider LS, Farlow MR, Henderson VW, Pogoda JM. Effects of estrogen replacement therapy on response to tacrine in patients with Alzheimer’s disease. Neurology. 1996;46:1580–4. doi: 10.1212/wnl.46.6.1580. [DOI] [PubMed] [Google Scholar]
- 261.Marder K, Tang MX, Alfaro B, Mejia H, Cote L, Jacobs D, Stern Y, Sano M, Mayeux R. Postmenopausal estrogen use and Parkinson’s disease with and without dementia. Neurology. 1998;50:1141–3. doi: 10.1212/wnl.50.4.1141. [DOI] [PubMed] [Google Scholar]
- 262.Horstink MW, Strijks E, Dluzen DE. Estrogen and Parkinson’s disease. Adv Neurol. 2003;91:107–14. [PubMed] [Google Scholar]
- 263.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 264.Paganini-Hill A. Estrogen replacement therapy and stroke. Prog Cardiovasc Dis. 1995;38:223–42. doi: 10.1016/s0033-0620(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 265.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–9. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 266.Finucane FF, Madans JH, Bush TL, Wolf PH, Kleinman JC. Decreased risk of stroke among postmenopausal hormone users. Results from a national cohort. Arch Intern Med. 1993;153:73–9. [PubMed] [Google Scholar]
- 267.Henderson BE, Paganini-Hill A, Ross RK. Decreased mortality in users of estrogen replacement therapy. Arch Intern Med. 1991;151:75–8. [PubMed] [Google Scholar]
- 268.Falkeborn M, Persson I, Terent A, Adami HO, Lithell H, Bergstrom R. Hormone replacement therapy and the risk of stroke. Follow-up of a population-based cohort in Sweden. Arch Intern Med. 1993;153:1201–9. [PubMed] [Google Scholar]
- 269.Groswasser Z, Cohen M, Keren O. Female TBI patients recover better than males. Brain Inj. 1998;12:805–8. doi: 10.1080/026990598122197. [DOI] [PubMed] [Google Scholar]
- 270.Bayir H, Marion DW, Puccio AM, Wisniewski SR, Janesko KL, Clark RS, Kochanek PM. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J Neurotrauma. 2004;21:1–8. doi: 10.1089/089771504772695896. [DOI] [PubMed] [Google Scholar]
- 271.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 272.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–72. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 273.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 274.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 275.Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. JAMA. 2000;283:1007–15. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 276.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 277.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 278.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 279.Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S, Eaton CB, Kostis JB, Caralis P, Prentice R. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–65. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 280.Zhang QG, Wang RM, Scott E, Han D, Dong Y, Tu JY, Yang F, Reddy Sareddy G, Vadlamudi RK, Brann DW. Hypersensitivity of the hippocampal CA3 region to stress-induced neurodegeneration and amyloidogenesis in a rat model of surgical menopause. Brain. 2013;136:1432–45. doi: 10.1093/brain/awt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Sohrabji F, Selvamani A, Balden R. Revisiting the timing hypothesis: biomarkers that define the therapeutic window of estrogen for stroke. Horm Behav. 2013;63:222–30. doi: 10.1016/j.yhbeh.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Scott E, Zhang QG, Wang R, Vadlamudi R, Brann D. Estrogen neuroprotection and the critical period hypothesis. Front Neuroendocrinol. 2012;33:85–104. doi: 10.1016/j.yfrne.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.S. North American Menopause. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19:257–71. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19:257–71. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. 402, e1–2. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- 286.Junpeng M, Huang S, Qin S. Progesterone for acute traumatic brain injury. Cochrane Database Syst Rev. 2011:CD008409. doi: 10.1002/14651858.CD008409.pub2. [DOI] [PubMed] [Google Scholar]
- 287.Vandromme M, Melton SM, Kerby JD. Progesterone in traumatic brain injury: time to move on to phase III trials. Crit Care. 2008;12:153. doi: 10.1186/cc6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wali B, Sayeed I, Stein DG. Improved behavioral outcomes after progesterone administration in aged male rats with traumatic brain injury. Restor Neurol Neurosci. 2011;29:61–71. doi: 10.3233/RNN-2011-0579. [DOI] [PubMed] [Google Scholar]
- 289.Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hirvonen E. Progestins. Maturitas. 1996;23(Suppl):S13–8. doi: 10.1016/0378-5122(96)01005-5. [DOI] [PubMed] [Google Scholar]
- 291.Gambrell RD., Jr The role of hormones in the etiology and prevention of endometrial cancer. Clin Obstet Gynaecol. 1986;13:695–723. [PubMed] [Google Scholar]
- 292.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH. Classification and pharmacology of progestins. Maturitas. 2003;46(Suppl 1):S7–S16. doi: 10.1016/j.maturitas.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 293.Winneker RC, Bitran D, Zhang Z. The preclinical biology of a new potent and selective progestin: trimegestone. Steroids. 2003;68:915–20. doi: 10.1016/s0039-128x(03)00142-9. [DOI] [PubMed] [Google Scholar]
- 294.Hackenberg R, Hofmann J, Wolff G, Holzel F, Schulz KD. Down-regulation of androgen receptor by progestins and interference with estrogenic or androgenic stimulation of mammary carcinoma cell growth. J Cancer Res Clin Oncol. 1990;116:492–8. doi: 10.1007/BF01613000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Koubovec D, Ronacher K, Stubsrud E, Louw A, Hapgood JP. Synthetic progestins used in HRT have different glucocorticoid agonist properties. Mol Cell Endocrinol. 2005;242:23–32. doi: 10.1016/j.mce.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 296.Jodhka PK, Kaur P, Underwood W, Lydon JP, Singh M. The differences in neuroprotective efficacy of progesterone and medroxyprogesterone acetate correlate with their effects on brain-derived neurotrophic factor expression. Endocrinology. 2009;150:3162–8. doi: 10.1210/en.2008-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Miyagawa K, Vidgoff J, Hermsmeyer K. Ca2+ release mechanism of primate drug-induced coronary vasospasm. Am J Physiol. 1997;272:H2645–54. doi: 10.1152/ajpheart.1997.272.6.H2645. [DOI] [PubMed] [Google Scholar]
- 298.Rosano GM, Webb CM, Chierchia S, Morgani GL, Gabraele M, Sarrel PM, de Ziegler D, Collins P. Natural progesterone, but not medroxyprogesterone acetate, enhances the beneficial effect of estrogen on exercise-induced myocardial ischemia in postmenopausal women. J Am Coll Cardiol. 2000;36:2154–9. doi: 10.1016/s0735-1097(00)01007-x. [DOI] [PubMed] [Google Scholar]
- 299.Littleton-Kearney MT, Klaus JA, Hurn PD. Effects of combined oral conjugated estrogens and medroxyprogesterone acetate on brain infarction size after experimental stroke in rat. J Cereb Blood Flow Metab. 2005;25:421–6. doi: 10.1038/sj.jcbfm.9600052. [DOI] [PubMed] [Google Scholar]
- 300.Gibbs RB, Nelson D, Anthony MS, Clarkson TB. Effects of long-term hormone replacement and of tibolone on choline acetyltransferase and acetylcholinesterase activities in the brains of ovariectomized, cynomologus monkeys. Neuroscience. 2002;113:907–14. doi: 10.1016/s0306-4522(02)00239-7. [DOI] [PubMed] [Google Scholar]
- 301.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:217–21. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- 302.Stein DG. The case for progesterone. Ann N Y Acad Sci. 2005;1052:152–69. doi: 10.1196/annals.1347.011. [DOI] [PubMed] [Google Scholar]
- 303.Tan RS, Pu SJ. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. The aging male: the official journal of the International Society for the Study of the Aging Male. 2003;6:13–7. [PubMed] [Google Scholar]
- 304.Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–8. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 305.Verdile G, Laws SM, Henley D, Ames D, Bush AI, Ellis KA, Faux NG, Gupta VB, Li QX, Masters CL, Pike KE, Rowe CC, Szoeke C, Taddei K, Villemagne VL, Martins RN, ARG for the, Associations between gonadotropins, testosterone and beta amyloid in men at risk of Alzheimer’s disease. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.147. [DOI] [PubMed] [Google Scholar]
- 306.Tan RS. Testosterone effect on brain metabolism in elderly patients with Alzheimer’s disease: comparing two cases at different disease stages. Aging clinical and experimental research. 2013;25:343–7. doi: 10.1007/s40520-013-0049-2. [DOI] [PubMed] [Google Scholar]
- 307.Butchart J, Birch B, Bassily R, Wolfe L, Holmes C. Male sex hormones and systemic inflammation in Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27:153–6. doi: 10.1097/WAD.0b013e318258cd63. [DOI] [PubMed] [Google Scholar]
- 308.Kenny AM, Fabregas G, Song C, Biskup B, Bellantonio S. Effects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive loss. J Gerontol A Biol Sci Med Sci. 2004;59:75–8. doi: 10.1093/gerona/59.1.m75. [DOI] [PubMed] [Google Scholar]
- 309.Lu PH, Masterman DA, Mulnard R, Cotman C, Miller B, Yaffe K, Reback E, Porter V, Swerdloff R, Cummings JL. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Arch Neurol. 2006;63:177–85. doi: 10.1001/archneur.63.2.nct50002. [DOI] [PubMed] [Google Scholar]
- 310.Liverman CT, Blazer DG. Testosterone and Aging: Clinical Research Directions. The National Academies Press; Washington D.C: 2004. [PubMed] [Google Scholar]
- 311.Sharps MJ, Welton AL, Price JL. Gender and task in the determination of spatial cognitive performance. Psychol Women Q. 1993;17:71–83. [Google Scholar]
- 312.Epting LK, Overman WH. Sex-sensitive tasks in men and women: a search for performance fluctuations across the menstrual cycle. Behav Neurosci. 1998;112:1304–17. doi: 10.1037//0735-7044.112.6.1304. [DOI] [PubMed] [Google Scholar]
- 313.Moffat SD, Hampson E, Hatzipantelis M. Navigation in a “virtual” maze: Sex differences and correlation with psychometric measures of spatial ability in humans. Evol Hum Behav. 1998;19:73–87. [Google Scholar]
- 314.Levine SC, Huttenlocher J, Taylor A, Langrock A. Early sex differences in spatial skill. Dev Psychol. 1999;35:940–9. doi: 10.1037//0012-1649.35.4.940. [DOI] [PubMed] [Google Scholar]
- 315.Silverman II, Choi J, Mackewn A, Fisher M, Moro J, Olshansky E. Evolved mechanisms underlying wayfinding. further studies on the hunter-gatherer theory of spatial sex differences. Evol Hum Behav. 2000;21:201–213. doi: 10.1016/s1090-5138(00)00036-2. [DOI] [PubMed] [Google Scholar]
- 316.Peters M. Sex differences and the factor of time in solving Vandenberg and Kuse mental rotation problems. Brain Cogn. 2005;57:176–84. doi: 10.1016/j.bandc.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 317.SBK Sex differences in mental rotation and spatial visualization ability: Can they be accounted for by differences in working memory capacity? Inteligence. 2007;35:211–223. [Google Scholar]
- 318.Maylor EA, Reimers S, Choi J, Collaer ML, Peters M, Silverman I. Gender and sexual orientation differences in cognition across adulthood: age is kinder to women than to men regardless of sexual orientation. Arch Sex Behav. 2007;36:235–49. doi: 10.1007/s10508-006-9155-y. [DOI] [PubMed] [Google Scholar]
- 319.Jansen P, Heil M. Gender differences in mental rotation across adulthood. Exp Aging Res. 2010;36:94–104. doi: 10.1080/03610730903422762. [DOI] [PubMed] [Google Scholar]
- 320.Tzuriel D, Egozi G. Gender differences in spatial ability of young children: the effects of training and processing strategies. Child Dev. 2010;81:1417–30. doi: 10.1111/j.1467-8624.2010.01482.x. [DOI] [PubMed] [Google Scholar]
- 321.Lange-Kuttner C, Ebersbach M. Girls in detail, boys in shape: gender differences when drawing cubes in depth. Br J Psychol. 2013;104:413–37. doi: 10.1111/bjop.12010. [DOI] [PubMed] [Google Scholar]
- 322.Christie GJ, Cook CM, Ward BJ, Tata MS, Sutherland J, Sutherland RJ, Saucier DM. Mental rotational ability is correlated with spatial but not verbal working memory performance and P300 amplitude in males. PLoS One. 2013;8:e57390. doi: 10.1371/journal.pone.0057390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Jansen P, Kaltner S. Object-based and egocentric mental rotation performance in older adults: The importance of gender differences and motor ability. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2013 doi: 10.1080/13825585.2013.805725. [DOI] [PubMed] [Google Scholar]
- 324.Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res. 1998;93:185–90. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 325.Malinowski JC, Gillespie WT. Individual differences in performance on a large-scale, real-world wayfinding task. J Environ Psychol. 2001;21:73–82. [Google Scholar]
- 326.Beatty WW. Sex difference in geographical knowledge: driving experience is not essential. J Int Neuropsychol Soc. 2002;8:804–10. doi: 10.1017/s1355617702860088. [DOI] [PubMed] [Google Scholar]
- 327.Postma A, Jager G, Kessels RP, Koppeschaar HP, van Honk J. Sex differences for selective forms of spatial memory. Brain Cogn. 2004;54:24–34. doi: 10.1016/s0278-2626(03)00238-0. [DOI] [PubMed] [Google Scholar]
- 328.Tippett WJ, Lee JH, Mraz R, Zakzanis KK, Snyder PJ, Black SE, Graham SJ. Convergent validity and sex differences in healthy elderly adults for performance on 3D virtual reality navigation learning and 2D hidden maze tasks. Cyberpsychol Behav. 2009;12:169–74. doi: 10.1089/cpb.2008.0218. [DOI] [PubMed] [Google Scholar]
- 329.Chai XJ, Jacobs LF. Sex differences in directional cue use in a virtual landscape. Behav Neurosci. 2009;123:276–83. doi: 10.1037/a0014722. [DOI] [PubMed] [Google Scholar]
- 330.Vestergren P, Ronnlund M, Nyberg L, Nilsson LG. Multigroup Confirmatory Factor Analysis of the Cognitive Dysfunction Questionnaire: instrument refinement and measurement invariance across age and sex. Scand J Psychol. 2012;53:390–400. doi: 10.1111/j.1467-9450.2012.00970.x. [DOI] [PubMed] [Google Scholar]
- 331.Persson J, Herlitz A, Engman J, Morell A, Sjolie D, Wikstrom J, Soderlund H. Remembering our origin: Gender differences in spatial memory are reflected in gender differences in hippocampal lateralization. Behav Brain Res. 2013;256C:219–228. doi: 10.1016/j.bbr.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 332.Portin R, Saarijarvi S, Joukamaa M, Salokangas RK. Education, gender and cognitive performance in a 62-year-old normal population: results from the Turva Project. Psychol Med. 1995;25:1295–8. doi: 10.1017/s0033291700033262. [DOI] [PubMed] [Google Scholar]
- 333.Herrera-Guzman I, Pena-Casanova J, Lara JP, Gudayol-Ferre E, Bohm P. Influence of age, sex, and education on the Visual Object and Space Perception Battery (VOSP) in a healthy normal elderly population. Clin Neuropsychol. 2004;18:385–94. doi: 10.1080/1385404049052421. [DOI] [PubMed] [Google Scholar]
- 334.Ardila A, Rosselli M, Matute E, Inozemtseva O. Gender differences in cognitive development. Dev Psychol. 2011;47:984–90. doi: 10.1037/a0023819. [DOI] [PubMed] [Google Scholar]
- 335.Bracco L, Bessi V, Alari F, Sforza A, Barilaro A, Marinoni M. Cerebral hemodynamic lateralization during memory tasks as assessed by functional transcranial Doppler (fTCD) sonography: effects of gender and healthy aging. Cortex. 2011;47:750–8. doi: 10.1016/j.cortex.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 336.McGivern RF, Adams B, Handa RJ, Pineda JA. Men and women exhibit a differential bias for processing movement versus objects. PLoS One. 2012;7:e32238. doi: 10.1371/journal.pone.0032238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.McGugin RW, Richler JJ, Herzmann G, Speegle M, Gauthier I. The Vanderbilt Expertise Test reveals domain-general and domain-specific sex effects in object recognition. Vision Res. 2012;69:10–22. doi: 10.1016/j.visres.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Trahan DE, Quintana JW. Analysis of gender effects upon verbal and visual memory performance in adults. Arch Clin Neuropsychol. 1990;5:325–34. [PubMed] [Google Scholar]
- 339.Mann VA, Sasanuma S, Sakuma N, Masaki S. Sex differences in cognitive abilities: a cross-cultural perspective. Neuropsychologia. 1990;28:1063–77. doi: 10.1016/0028-3932(90)90141-a. [DOI] [PubMed] [Google Scholar]
- 340.Youngjohn JR, Larrabee GJ, Crook TH., 3rd First-Last Names and the Grocery List Selective Reminding Test: two computerized measures of everyday verbal learning. Arch Clin Neuropsychol. 1991;6:287–300. [PubMed] [Google Scholar]
- 341.Savage RM, Gouvier WD. Rey Auditory-Verbal Learning Test: the effects of age and gender, and norms for delayed recall and story recognition trials. Arch Clin Neuropsychol. 1992;7:407–14. [PubMed] [Google Scholar]
- 342.Berenbaum SA, Baxter L, Seidenberg M, Hermann B. Role of the hippocampus in sex differences in verbal memory: memory outcome following left anterior temporal lobectomy. Neuropsychology. 1997;11:585–91. doi: 10.1037//0894-4105.11.4.585. [DOI] [PubMed] [Google Scholar]
- 343.Kimura D, Clarke PG. Women’s advantage on verbal memory is not restricted to concrete words. Psychol Rep. 2002;91:1137–42. doi: 10.2466/pr0.2002.91.3f.1137. [DOI] [PubMed] [Google Scholar]
- 344.Yonker JE, Eriksson E, Nilsson LG, Herlitz A. Sex differences in episodic memory: minimal influence of estradiol. Brain Cogn. 2003;52:231–8. doi: 10.1016/s0278-2626(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 345.Kimura D, Seal BN. Sex differences in recall of real or nonsense words. Psychol Rep. 2003;93:263–4. doi: 10.2466/pr0.2003.93.1.263. [DOI] [PubMed] [Google Scholar]
- 346.Neri AL, Ongaratto LL, Yassuda MS. Mini-Mental State Examination sentence writing among community-dwelling elderly adults in Brazil: text fluency and grammar complexity. Int Psychogeriatr. 2012;24:1732–7. doi: 10.1017/S104161021200097X. [DOI] [PubMed] [Google Scholar]
- 347.Munro CA, Winicki JM, Schretlen DJ, Gower EW, Turano KA, Munoz B, Keay L, Bandeen-Roche K, West SK. Sex differences in cognition in healthy elderly individuals. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2012;19:759–68. doi: 10.1080/13825585.2012.690366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Murre JM, Janssen SM, Rouw R, Meeter M. The rise and fall of immediate and delayed memory for verbal and visuospatial information from late childhood to late adulthood. Acta Psychol (Amst) 2013;142:96–107. doi: 10.1016/j.actpsy.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 349.Heinzel S, Metzger FG, Ehlis AC, Korell R, Alboji A, Haeussinger FB, Hagen K, Maetzler W, Eschweiler GW, Berg D, Fallgatter AJ. Aging-related cortical reorganization of verbal fluency processing: a functional near-infrared spectroscopy study. Neurobiol Aging. 2013;34:439–50. doi: 10.1016/j.neurobiolaging.2012.05.021. [DOI] [PubMed] [Google Scholar]