Abstract
Background
Previous reports have shown that prolonged duration of resuscitation efforts in out-of-hospital cardiac arrest (OHCA) is associated with poor neurologic outcome. This concept has recently been questioned with advancements in post-cardiac arrest care including the use of therapeutic hypothermia (TH). The aim of this study was to determine the rate of good neurologic outcome based on the duration of resuscitation efforts in OHCA patients treated with TH.
Methods
This prospective, observational, study was conducted between January 2008 and September 2012. Inclusion criteria consisted of adult non-traumatic OHCA patients who were comatose after return of spontaneous circulation (ROSC) and received TH. The primary endpoint was good neurologic outcome defined as a cerebral performance category score of 1 or 2. Downtime was calculated as the length of time between the patient being recognized as pulseless and ROSC.
Results
105 patients were treated with TH and 19 were excluded due to unknown downtime, leaving 86 patients for analysis. The median downtime was 18.5 (10.0–32.3) minutes and 33 patients (38.0%) had a good neurologic outcome. When downtime was divided into four groups (≤10 min, 11-20 min, 21-30 min, > 30 min), good neurologic outcomes were 62.5%, 37%, 25%, and 21.7%, respectively (p=0.02). However, even with downtime >20 minutes, 22.9% had a good neurologic outcome, and this percentage increased to 37.5% in patients with an initial shockable rhythm.
Conclusions
Although longer downtime is associated with worse outcome in OHCA patients, we found that comatose patients who have been successfully resuscitated and treated with TH have neurologically intact survival rates of 23% even with downtime > 20 minutes.
Keywords: Out-of-Hospital Cardiac Arrest, Therapeutic Hypothermia
1. Introduction
In the past decade, two randomized trials, three meta-analyses, and two registry-based analyses have shown that mild therapeutic hypothermia (TH) (32-34°C for 12-24 hr) in comatose survivors of cardiac arrest reduces mortality and improves neurologic outcome.1-7 As a result of these studies, the European Resuscitation Council and the American Heart Association recommend mild TH for comatose adult patients with return of spontaneous circulation (ROSC) after out-of-hospital shockable cardiac arrest [ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT)].8,9,
Animal models have shown that longer no-flow times are associated with worse neurologic outcome.10,11 However, early cardiopulmonary resuscitation (CPR) may provide sufficient cerebral circulation to allow for neurologic recovery.12 Prior clinical reports have shown that prolonged duration of resuscitation efforts in cardiac arrest is associated with poor neurologic outcome.13-17 However, there are few data addressing this question since the emergence of TH as a standard treatment for comatose patients after cardiac arrest. Clinicians may be reluctant to start TH when ROSC occurs after a prolonged resuscitation effort, in view of the commonly held belief that poor outcome occurs in this context. Longer downtime may even be considered a relative contraindication for TH in some protocols, though there is no clear evidence regarding stratification based on this parameter.18 This lack of clarity and variation in practice is in part a consequence of the lack of previous investigations addressing the probability of survival after longer downtimes particularly in the era of hypothermia.
The objectives of this study were 1) to determine the relationship between downtime and neurologically intact survival in comatose adult out-of-hospital cardiac arrest (OHCA) patients treated by TH, and 2) to evaluate the rates of survival at prolonged downtimes.
2. Methods
Study design and population
This prospective observational pilot study was conducted between January 2008 and September 2012 at an urban tertiary care teaching hospital, which is a cardiac arrest center, with 650 inpatient beds, and approximately 50,000 Emergency Department (ED) visits per year. The study population consisted of all adult (age > 18 years) OHCA patients with sustained ROSC who were comatose and treated with comprehensive post-arrest care including TH. The institutional indications for TH include adult patients experiencing OHCA (including both shockable and non-shockable initial rhythm), achieving ROSC in the field or in the ED, and presenting in a comatose state (defined as a lack of ability to follow commands) after resuscitation. Patients with life-threatening bleeding were excluded from the institutional hypothermia protocol and patients with profound shock typically were cooled after hemodynamic instability was stabilized on a steady fluid/vasopressor regimen. Exclusion criteria for the current study included traumatic cardiac arrest and patients with unknown duration of downtime in the pre-hospital setting. The hospital Institutional Review Board approved the study.
Data Collection and Patient Management
All patients who presented with OHCA were screened by trained research assistants via the hospital’s electronic medical record system. Patient demographic information including age, sex, race, and past medical history were recorded at the time of enrollment. Specific data collected regarding the arrest characteristics included the location of the arrest, total low-flow and no-flow times, whether or not the arrest was witnessed, and whether or not bystander CPR was performed. Vital signs and laboratory data including complete blood count, electrolytes, and lactate were recorded at the time of enrollment. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score was also calculated at 24 hours as a severity of illness marker.19
Definitions
We defined no-flow time as the time from recognition of cardiac arrest to the start of CPR. In witnessed arrest, time of arrest was defined as time of collapse and in unwitnessed arrest, it was defined as the time that EMS was called. Low-flow time was defined as the time between the start of CPR (by a bystander or EMS) and the time of sustained ROSC. We defined downtime as the time from the recognition of cardiac arrest to the time of sustained ROSC, or no-flow time plus low-flow time. Sustained ROSC was defined as the restoration of a palpable pulse for at least 20 minutes. If ROSC occurred for less than 20 minutes, that time period with a pulse was subtracted from the total downtime calculation if sustained ROSC was subsequently obtained. We defined prolonged downtime as duration of downtime greater than 20 minutes by consensus of the investigators. The primary endpoint was good neurologic outcome. We classified the neurologic status of patients who survived to discharge on the basis of cerebral performance category (CPC) measured at the time of hospital discharge.20 CPC scores are 1 (no significant impairment), 2 (moderate impairment but able to complete activities of daily living), 3 (severe impairment but conscious), 4 (vegetative state or coma) and 5 (death). Good neurologic outcome was defined as a CPC score of 1 (no major disability) or 2 (moderate disability).
Statistical analysis
Continuous variables are expressed as means with standard deviations, or medians and interquartile range (IQR) if the assumption of a normal distribution was violated. Categorical variables are expressed as numbers and percentages. Demographic and clinical characteristics across the strata of downtime duration (≤10, 11-20, 21-30, >30 min) were assessed using one-way ANOVA or Kruskal-Wallis, Chi-squared or Fisher’s exact test, as appropriate. Univariate and multivariate analyses were performed using logistic regression to evaluate the association between downtime and neurologic outcome. The results of multivariate logistic regression are reported as odds ratios (OR) and 95% confidence intervals (CI). A two-sided p value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA).
3. Results
During the study period, a total of 174 adult OHCA patients had successful ROSC. Of these, 113 patients were treated with TH. We excluded 8 patients with traumatic arrest and 19 patients who had unknown pre-hospital arrest duration or an undocumented initial rhythm, leaving a total of 86 patients for analysis. The median age was 64.5 (IQR 52.8–76.0) years and 66.3% were male. The median downtime was 18.5 (IQR 10.0–32.3) minutes. Thirty-three patients (38.0%) had a good neurologic outcome, defined as a CPC score of 1 or 2. When downtime was stratified into four groups (≤ 10 min, 11-20 min, 21-30 min, > 30 min), good neurologic outcome rates were 62.5%, 37%, 25%, and 21.7%, respectively (p=0.02). Other baseline characteristics, stratified by duration of downtime are described in Table 1.
Table 1.
Demographic and baseline characteristics of the out-of-hospital cardiac arrest patients treated with hypothermia, stratified by duration of downtime
| All patients (n=86) |
≤ 10 min (n=24) |
11 - 20 min (n=27) |
21 - 30 min (n=12) |
> 30 min (n=23) |
p- valu e |
|
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years | 64.5(52.8-76.0) | 69.5(54.5-77.5) | 66.0(54.0-75.0) | 69.0(46.0-81.8) | 57.0(44.0-72.0) | 0.40 |
| Male | 57(66.3) | 12(50.0) | 20(74.1) | 8(66.7) | 17(73.9) | 0.24 |
| Race (white) | 69(80.2) | 18(75.0) | 22(81.5) | 9(75.0) | 20(87.0) | 0.51 |
| Transfer – other hospital | 38 (45.8) | 9 (37.5) | 8 (33.3) | 8 (66.7) | 13 (56.5) | 0.15 |
| Co-morbidities | ||||||
| Hypertension | 41(47.7) | 13(54.2) | 15(55.6) | 6(50.0) | 7(30.4) | 0.28 |
| Diabetes mellitus | 21(24.4) | 7(29.2) | 9(33.3) | 3(25.0) | 2(8.7) | 0.21 |
| Coronary artery disease |
21(24.4) | 7(29.2) | 10(37.0) | 1(8.3) | 3(13.0) | 0.12 |
| Cancer | 3( 3.5) | 1(4.2) | 2(7.4) | 0(0.0) | 0(0.0) | 0.47 |
| No co-morbidities | 16(18.6) | 4(16.7) | 4(14.8) | 0(0.0) | 8(34.8) | 0.07 |
| Arrest characteristics | ||||||
| Location | 0.65 | |||||
| Private/home | 36(41.9) | 7(29.2) | 12(44.4) | 5(41.7) | 12(52.2) | |
| Public space | 29(33.7) | 8(33.3) | 12(44.4) | 3(25.0) | 6(26.1) | |
| Others or unknown | 21(24.4) | 9(37.5) | 3(11.1) | 4(33.3) | 5(21.7) | |
| Witnessed | 61(70.9) | 21(87.5) | 19(70.4) | 8(72.7) | 13(56.5) | 0.13 |
| Bystander CPR | 60(69.8) | 19(79.2) | 20(74.1) | 6(50.0) | 15(68.2) | 0.32 |
| First arrest rhythm | 0.51 | |||||
| Shockable | 44(51.2) | 11(45.8) | 17(63.0) | 6(50.0) | 10(43.5) | |
| Non-shockable | 42(48.8) | 13(54.2) | 10(37.0) | 6(50.0) | 13(56.5) | |
| Low-flow time, min | 15.0(8.0-28.8) | 6.0(5.0-8.0) | 13.5(10.0-15.0) | 23.0(17.0-25.0) | 40.0(35.0-62.0) | <0.0 1 |
| Downtime, min | 18.5(10.-32.3) | 7.0(5.0-10.0) | 15.0(13.0-20.0) | 25.0(24.3-27.8) | 45.0(40.0-65.0) | <0.0 1 |
| Initial vital signs | ||||||
| Systolic BP, mmHg | 117.5±31.6 | 128.2±35.3 | 116.2±29.4 | 105.5±35.6 | 115.5±27.0 | 0.23 |
| Diastolic BP, mmHg | 69.7±22.8 | 81.9±24.4 | 63.6±18.7 | 56.8±13.4 | 70.4±24.0 | 0.10 |
| Heart rate,/ minute | 96.2±26.2 | 90.5±26.8 | 104.7±30.3 | 92.1±22.1 | 94.3±21.7 | 0.24 |
|
Initial laboratory
values |
||||||
| Lactate, mmol/L | 6.6±3.4 | 4.8±2.2 | 7.0±3.7 | 8.2±4.1 | 7.1±3.1 | 0.06 |
| Glucose, mg/dL | 238.4±116.5 | 226.5±151.0 | 255.2±114.3 | 175.9±51.8 | 253.0±92.0 | 0.40 |
| APACHEII score (24 hr) |
25.5±6.3 | 25.0±5.9 | 26.1±7.9 | 23.0±5.0 | 26.8±5.5 | 0.43 |
Values are expressed as mean ± SD or median with interquartile range and n (%). CPR, cardiopulmonary resuscitation; BP, blood pressure; WBC, White blood cell count; APACHE II, acute physiology and chronic health evaluation II
We found that downtime [12.0 (7.0-22.0) vs. 23.0 (15.0-38.5), p<0.01], lactate (5.2 ± 2.8 vs. 7.2 ± 3.5, p=0.02), and APACHEII score (22.6 ± 6.1 vs. 27.4 ± 5.8, p=0.01) were significantly different in good neurologic outcome and bad neurologic outcome groups. However, downtime was the only one of these variables that was an independent predictor of decreased chance of good neurologic outcome [OR 1.04 (CI 1.01-1.07), p=0.01] after multivariate analysis.
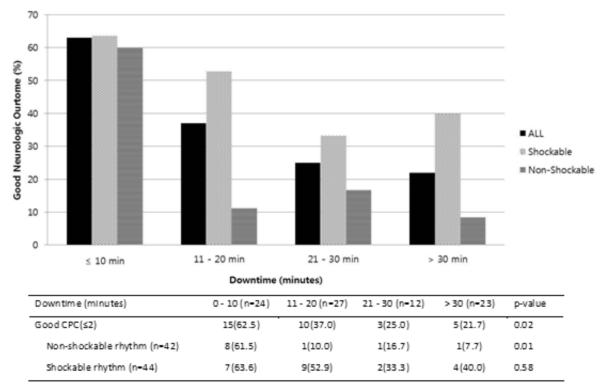
We then evaluated the rate of good neurologic outcome based on downtime, stratified by initial rhythm. Good neurologic outcome in non-shockable patients was significantly less likely with longer downtime (p=0.01), whereas good neurologic outcomes did not differ significantly in those with initial shockable rhythms (p=0.58) (Figure 1). Among non-shockable patients, only 10.3% (3/29) survived with a good neurologic outcome after a downtime greater than 10 minutes, compared to 61.5% (8/13) in those with a downtime less than 10 minutes. We then examined outcomes in only those patients with prolonged downtime. Patients with downtime > 20 minutes had a neurologically intact survival rate of 22.9% and this percentage increased to 37.5% when looking only at patients with an initial shockable rhythm. Baseline characteristics of this subgroup (downtime > 20 minutes, n=35) were then analyzed to find factors associated with favorable neurologic outcome. In this group, we found that patients with good neurologic outcome had lower lactate levels following ROSC than those with poor neurologic outcome (p=0.02) (Table 2). However, no clear distinguishing characteristics were present that allowed differentiation of patients upon initial presentation. Finally, in the population of patients with a shockable rhythm and much longer downtime (> 30 minutes), 40% (4/10) had a neurologically intact survival (Figure 1).
Figure 1.
Good neurologic outcome of the out-of-hospital cardiac arrest patients treated with hypothermia, stratified by initial rhythm and duration of downtime
CPC, cerebral performance score, reported as median with interquartile range.
Table 2.
Demographic characteristics of the prolonged downtime out-of-hospital cardiac arrest patients treated with hypothermia
| All patients (n=35) |
Good CPC (≤2) (n=8) |
Poor CPC (≥3) (n=27) |
p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 60.0(44.0-77.0) | 52.5(36.8-74.8) | 60.0(53.0-77.0) | 0.19 |
| Male | 25 (71.4) | 6 (75.0) | 19 (70.4) | 1.00 |
| Race (white) | 29 (82.9) | 8 (100.0) | 21 (77.8) | 0.34 |
| Transferred | 21 (60.0) | 6 (75.0) | 15 (55.6) | 0.43 |
| Co-morbidities | ||||
| Hypertension | 13 (37.1) | 4 (50.0) | 9 (33.3) | 0.43 |
| Diabetes mellitus | 5 (14.3) | 1 (12.5) | 4 (14.8) | 1.00 |
| Coronary artery disease | 4 (11.4) | 1 (12.5) | 3 (11.1) | 1.00 |
| No co-morbidities | 8 (22.9) | 1 (12.5) | 7 (25.9) | 0.65 |
| Arrest characteristics | ||||
| Location | 0.63 | |||
| Private/home | 17 (48.6) | 4 (50.0) | 13 (48.1) | |
| Public space | 9 (25.7) | 3 (37.5) | 6 (22.2) | |
| Others or unknown | 9 (25.7) | 1 (12.5) | 8 (29.6) | |
| Witnessed | 21 (61.8) | 4 (57.1) | 17 (63.0) | 1.00 |
| Bystander CPR | 21 (61.8) | 4 (57.1) | 17 (63.0) | 0.68 |
| Shockable rhythm | 16 (45.7) | 6 (75.0) | 10 (37.0) | 0.10 |
| Initial vital signs | ||||
| Systolic blood pressure, mmHg |
111.9 ± 30.1 | 121.6 ± 28.7 | 153 ± 33.2 | 0.53 |
| Diastolic blood pressure, mmHg |
66.1 ± 22.0 | 77.0 ± 22.8 | 65.9 ± 22.0 | 0.38 |
| Heart rate, beats per minute | 93.5 ± 21.5 | 92.5 ± 25.2 | 98.3 ± 26.7 | 0.50 |
| Initial laboratory values | ||||
| Lactate, mmol/L | 7.2 ± 3.5 | 5.2 ± 2.8 | 7.4 ± 3.4 | 0.02 |
| Sodium, mmol/L | 140.3 ± 3.9 | 138.8 ± 3.2 | 140.1 ± 3.4 | 0.89 |
| Potassium, mmol/L | 4.5 ± 1.0 | 4.1 ± 0.9 | 4.6 ± 1.1 | 0.38 |
| Glucose, mmol/L | 12.9 ± 5.0 | 12.2 ± 5.1 | 13.9 ± 7.2 | 0.76 |
| APACHEII score (24 hr) | 25.5 ± 5.5 | 22.7 ± 6.0 | 26.4 ± 5.1 | 0.13 |
Values are expressed as mean ± SD and n (%). Statistical analysis was performed by Mann-Whitney U test or Fisher’s s exact test.
CPR, cardiopulmonary resuscitation; WBC, White blood cell count; APACHE II, acute physiology and chronic health evaluation II
4. Discussion
This study presents an analysis of downtime with respect to the impact on neurologically intact survival. We found that the overall rate of neurologically intact survival in comatose adult OHCA patients treated by TH was 38.0% and good neurologic outcome was significantly associated with downtime. Among patients with downtime >20 minutes, 22.9% had a good neurologic outcome, and this percentage increased to 37.5% in patients with an initial shockable rhythm.
Previous reports have shown that prolonged duration of resuscitation effort in cardiac arrest is more likely to result in unfavorable neurologic outcome.13-15,17 Laurent et al.21 identified a longer time interval as a risk factor for hemodynamic instability after ROSC, and Jorgensen22 demonstrated that cardiac arrest patients requiring CPR for more than 20 minutes rarely survived. However, these studies were done before TH was the standard of care, and thus outcome in patients requiring prolonged resuscitation who are treated with TH remains uncertain. In the present study, when downtime was stratified into four groups (≤ 10 min, 11-20 min, 21-30 min, > 30 min), neurologically intact survival rates gradually decreased (62.5%, 37%, 25%, and 21.7%, respectively, p=0.02) and downtime was an independent predictor of decreased chance of good neurologic outcome. Such results indicate that downtime is a major determinant of recovery with good neurologic outcome even after TH. However, within this context, a substantial number of survivors with good neurological outcome existed even at downtimes > 30 minutes. The capacity to have good neurologic outcome with prolonged downtime in our sample was similar to recent findings from large studies which analyzed data from the American Heart Association’s Get With the Guidelines Resuscitation, a prospective multicenter registry of in-hospital cardiac arrest.23,24 Goldberger and colleagues 23 report that hospitals where the duration of the cardiopulmonary resuscitation effort is longer on average have significantly better survival rates. In another study analyzing data from in-hospital pediatric cardiac arrest, longer CPR duration was independently associated with lower survival. However, 16.2% of survivors received CPR for >35 minutes; of these survivors, 60% had a good neurologic outcome.24
Detailed analysis of the effect of downtime stratified by initial rhythm revealed a high frequency of good neurologic outcome in both shockable and non-shockable rhythms when the downtime was less than 10 minutes. However, the frequency of good neurologic outcome appears to drop rapidly in the non-shockable group as compared to the shockable group as the time interval increases (Figure 1). In this dataset, neurologic outcome did not differ significantly by downtime when patients initially had a shockable rhythm. This finding suggests that patients with an initially shockable rhythm have a particularly good chance of neurologically intact survival even after a prolonged cardiac arrest.
As noted, we found that even with prolonged downtime, patients had a reasonable chance of good neurologic recovery. In order to determine whether neurologically intact survivors could be differentiated upon presentation, we evaluated the subgroup of patients with prolonged downtime (> 20 minutes) for factors present on admission that were associated with good neurologic outcome. In this subgroup, patients with good neurologic outcome had slightly lower lactate levels, however no other associations or outcome predictors were found. Specifically, we did not find an association between comorbidities and outcome in this group. Thus, we were not able to find baseline characteristics to identify patients who would ultimately achieve good neurologic outcome in this group.
Our study was limited by relatively small sample size and the observational design. This was also a single-center study, and results may not be generalizable to other settings. Some selection bias may have occurred if individual clinicians opted not to use TH for a patient with a longer down time, although this is not part of the criteria for TH as used at our hospital. Further data are needed to validate these findings. Another potential concern is that our data included non-sustained ROSC. Since we used the Utstein definition of sustained ROSC as a palpable pulse for > 20 minutes, the possibility remains that our downtime calculation was influenced by patients who had return of pulse and then re-arrested prior to achieving sustained ROSC. When this occurred, we excluded the time the patient had a palpable pulse between arrest periods from our downtime calculation. In patients with un-witnessed arrests we designated the no-flow time as zero. This may have led to an underestimation of no-flow and total downtime in some patients. Finally, 11.4% patients receiving TH had early withdrawal of aggressive care. Our standard guideline in the hospital is to wait for 72 hours. However, reasons do emerge that drive clinical teams to withdraw care earlier than that point, which may have influenced our outcome.
5. Conclusion
Although longer downtime is associated with worse outcome in OHCA patients, we found that comatose patients who have been successfully resuscitated and treated with TH have neurologically intact survival rates of 23% even with prolonged downtime (> 20 minutes)
Supplementary Material
Acknowledgements
Dr. Michael W. Donnino is supported by NHLBI (1K02HL107447-01A1) and NIH (R21AT005119-01). The authors wish to thank Francesca Montillo for her editorial contribution in submitting this manuscript.
Footnotes
6. Conflict of Interest The authors have no conflicts to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- 1.Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. The New England journal of medicine. 2002 Feb 21;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 2.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. The New England journal of medicine. 2002 Feb 21;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 3.Cheung KW, Green RS, Magee KD. Systematic review of randomized controlled trials of therapeutic hypothermia as a neuroprotectant in post cardiac arrest patients. Cjem. 2006 Sep;8(5):329–337. doi: 10.1017/s1481803500013981. [DOI] [PubMed] [Google Scholar]
- 4.Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Critical care medicine. 2005 Feb;33(2):414–418. doi: 10.1097/01.ccm.0000153410.87750.53. [DOI] [PubMed] [Google Scholar]
- 5.Sanders AB. Therapeutic hypothermia after cardiac arrest. Current opinion in critical care. 2006 Jun;12(3):213–217. doi: 10.1097/01.ccx.0000224864.93829.d4. [DOI] [PubMed] [Google Scholar]
- 6.Arrich J. Clinical application of mild therapeutic hypothermia after cardiac arrest. Critical care medicine. 2007 Apr;35(4):1041–1047. doi: 10.1097/01.CCM.0000259383.48324.35. [DOI] [PubMed] [Google Scholar]
- 7.Oksanen T, Pettila V, Hynynen M, Varpula T. Therapeutic hypothermia after cardiac arrest: implementation and outcome in Finnish intensive care units. Acta anaesthesiologica Scandinavica. 2007 Aug;51(7):866–871. doi: 10.1111/j.1399-6576.2007.01365.x. [DOI] [PubMed] [Google Scholar]
- 8.Deakin CD, Nolan JP, Soar J, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation. 2010 Oct;81(10):1305–1352. doi: 10.1016/j.resuscitation.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010 Nov 2;122(18 Suppl 3):S768–786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 10.Radovsky A, Safar P, Sterz F, Leonov Y, Reich H, Kuboyama K. Regional prevalence and distribution of ischemic neurons in dog brains 96 hours after cardiac arrest of 0 to 20 minutes. Stroke; a journal of cerebral circulation. 1995 Nov;26(11):2127–2133. doi: 10.1161/01.str.26.11.2127. discussion 2133-2124. [DOI] [PubMed] [Google Scholar]
- 11.Brockman SK, Jude JR. The tolerance of the dog brain to total arrest of circulation. Bulletin of the Johns Hopkins Hospital. 1960 Feb;106:74–80. [PubMed] [Google Scholar]
- 12.Sanders AB, Kern KB, Bragg S, Ewy GA. Neurologic benefits from the use of early cardiopulmonary resuscitation. Annals of emergency medicine. 1987 Feb;16(2):142–146. doi: 10.1016/s0196-0644(87)80002-1. [DOI] [PubMed] [Google Scholar]
- 13.Cummins RO, Eisenberg MS, Hallstrom AP, Litwin PE. Survival of out-of-hospital cardiac arrest with early initiation of cardiopulmonary resuscitation. The American journal of emergency medicine. 1985 Mar;3(2):114–119. doi: 10.1016/0735-6757(85)90032-4. [DOI] [PubMed] [Google Scholar]
- 14.Pionkowski RS, Thompson BM, Gruchow HW, Aprahamian C, Darin JC. Resuscitation time in ventricular fibrillation--a prognostic indicator. Annals of emergency medicine. 1983 Dec;12(12):733–738. doi: 10.1016/s0196-0644(83)80245-5. [DOI] [PubMed] [Google Scholar]
- 15.Schultz SC, Cullinane DC, Pasquale MD, Magnant C, Evans SR. Predicting in-hospital mortality during cardiopulmonary resuscitation. Resuscitation. 1996 Nov;33(1):13–17. doi: 10.1016/s0300-9572(96)00986-0. [DOI] [PubMed] [Google Scholar]
- 16.Axelsson C, Claesson A, Engdahl J, et al. Outcome after out-of-hospital cardiac arrest witnessed by EMS: changes over time and factors of importance for outcome in Sweden. Resuscitation. 2012 Oct;83(10):1253–1258. doi: 10.1016/j.resuscitation.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 17.Herlitz J, Svensson L, Engdahl J, Angquist KA, Silfverstolpe J, Holmberg S. Association between interval between call for ambulance and return of spontaneous circulation and survival in out-of-hospital cardiac arrest. Resuscitation. 2006 Oct;71(1):40–46. doi: 10.1016/j.resuscitation.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Post-Cardiac Arrest Care/Therapeutic Hypothermia Resources 2013 http://www.med.upenn.edu/resuscitation/hypothermia/protocols.shtml. http://www.med.upenn.edu/resuscitation/hypothermia/protocols.shtml.
- 19.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical care medicine. 1985 Oct;13(10):818–829. [PubMed] [Google Scholar]
- 20.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975 Mar 1;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 21.Laurent I, Monchi M, Chiche JD, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. Journal of the American College of Cardiology. 2002 Dec 18;40(12):2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen EO. Neurological and circulatory outcomes of cardiopulmonary resuscitation in progress: influence of pre-arrest and arrest factors. Resuscitation. 1998 Jan;36(1):45–49. doi: 10.1016/s0300-9572(97)00066-x. [DOI] [PubMed] [Google Scholar]
- 23.Goldberger ZD, Chan PS, Berg RA, et al. Duration of resuscitation efforts and survival after in-hospital cardiac arrest: an observational study. Lancet. 2012 Oct 27;380(9852):1473–1481. doi: 10.1016/S0140-6736(12)60862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matos RIWS, Nadkarni VM, Huang H-H, Berg R, Meaney PA. Duration of CPR and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.112.125625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.