Abstract
Objective
A current neuroanatomical model of anxiety posits that greater structural connectivity between the amygdala and ventral prefrontal cortex (vPFC) facilitates regulatory control over the amygdala and helps reduce anxiety. However, some neuroimaging studies have reported contradictory findings, demonstrating a positive rather than negative association between trait anxiety and amygdala-vPFC white matter integrity. To help reconcile these findings, we tested the regulatory hypothesis of anxiety circuitry using aging as a model of white matter decline in the amygdala-vPFC pathway.
Methods
We used probabilistic tractography to trace connections between the amygdala and vPFC in 21 younger, 18 middle-aged, and 15 healthy older adults. The resulting tract estimates were used to extract three indices of white-matter integrity: fractional anisotropy (FA), radial diffusivity (RD) and axial diffusivity (AD). The relationship between these amygdala-vPFC structural connectivity measures and age and State-Trait Anxiety Inventory (STAI) scores were assessed.
Results
The tractography results revealed age-related decline in the FA (p = .005) and radial diffusivity (p = .002) of the amygdala-vPFC pathway. Contrary to the regulatory hypothesis, we found a positive rather than negative association between trait anxiety and right amygdala-vPFC FA (p = .01).
Conclusion
These findings argue against the notion that greater amygdala-vPFC structural integrity facilitates better anxiety outcomes in healthy adults. Instead, our results suggest that white matter degeneration in this network relates to lower anxiety in older adults.
Keywords: amygdala, prefrontal cortex, anxiety, probabilistic tractography, aging
Anxiety is an unpleasant emotional state that involves physical, cognitive, and behavioral manifestations such as impaired emotion regulation, especially in interpersonal, ambiguous, and dangerous situations (Endler et al., 1992). Studies investigating the neurobiological basis of anxiety have implicated the amygdala in various negative affective responses, including the fear and vigilance that are characteristic of anxiety (Kapp et al., 1994; Oler et al., 2009; Pessoa and Adolphs, 2010; Whalen, 1998). An increasing number of studies have linked anxiety to complex functional and structural neural circuitry that is centered on the amygdala (Kim et al., 2011a). With regard to structural networks, the amygdala is densely and reciprocally connected with many brain regions involved in emotion and stress regulation, such as the prefrontal cortex (PFC; Dolcos et al., 2011). It has been suggested that an altered balance between activity in the amygdala and PFC could be a neural mechanism underlying anxiety (Bishop, 2008).
Converging findings from animal and human research suggest that anxiety-related behaviors involve top-down regulatory influences from the vPFC to the amygdala (Banks et al., 2007; Ghashghaei et al., 2007; Stein et al., 2007a). This framework is supported by functional magnetic resonance imaging (fMRI) studies showing that successful emotion regulation is associated with lower amygdala activity and greater vPFC activity (Hariri et al., 2000; Hartley and Phelps, 2010; Wager et al., 2008). Other fMRI studies have linked amygdala-vPFC circuitry to the negative interpretation of emotionally ambiguous expressions, a threat-related perceptual bias that manifests in normal and pathological levels of anxiety (Bishop, 2007).
Since these affective processes are affected in both normal and pathological anxiety, it follows that the amygdalar circuitry implicated in these behaviors is also implicated in anxiety (Milad et al., 2006). Several fMRI studies have demonstrated a negative association between trait anxiety and amygdala-vPFC functional connectivity, both at rest and during task-based paradigms (Bishop et al., 2004; Hare et al., 2008; Simpson et al., 2001). Another study found that greater resting-state functional connectivity (rsFC) between the amygdala and vPFC predicted lower trait and state anxiety in psychiatrically healthy individuals (Kim et al., 2011a). Together, these studies suggest that higher anxiety is associated with reduced functional connectivity between the amygdala and vPFC.
Studies also suggest that anxiety-related decreases in amygdala-vPFC functional connectivity are associated with underlying structural declines. In accordance with functional findings, both human and animal studies suggest that structural connectivity between the amygdala and ventral prefrontal cortex (vPFC) also plays an important role in behaviors that are compromised in anxiety, including emotion regulation and fear conditioning (Cisler et al., 2009; Hare et al., 2008; Phelps et al., 2004). In psychiatrically healthy individuals, trait anxiety has been shown to be negatively associated with both the white matter integrity and volume of the left uncinate fasciculus, a dense white matter bundle that connects the amygdala to the vPFC (Baur et al., 2012; Bracht et al., 2009; Kim and Whalen, 2009). Patients with social anxiety disorder (SAD) also exhibit lower uncinate fasciculus integrity compared with healthy, age-matched controls (Phan et al., 2009).
To account for these findings, Kim et al. (2011a) proposed that the vPFC exerts a “top-down” influence on the amygdala, wherein better regulatory control is facilitated by both increased structural connectivity and functional connectivity between these regions (Ghashghaei and Barbas, 2002; Kim and Whalen, 2009; Kim et al., 2011b). However other neuroimaging studies have reported findings that contradict this regulatory model. Several diffusion tensor imaging (DTI) studies have demonstrated that higher trait anxiety scores are associated with increased rather than decreased structural integrity of the uncinate fasciculus (Modi et al., 2013; Montag et al., 2012). Similarly, another DTI study showed that the severity of anxiety in individuals with obsessive-compulsive disorder (OCD) was positively associated with the integrity of the internal capsule, a pathway that may encompass some amygdala-vPFC connections that are not part of the uncinate fasciculus (Lochner et al., 2012). Thus, while the amygdala-vPFC pathway is consistently implicated in anxiety, the significance of the structural integrity of this circuitry to anxiety remains unclear.
Whereas most of these findings were derived from younger adults, less is known about how changes in this pathway relate to anxiety in older adults. While anxiety is characterized by an attentional bias towards processing aversive information (Mathews and MacLeod, 2002), older adults demonstrate a positivity effect in which, relative to younger adults, they show preferences for positive over negative information in both attention and memory (Charles et al., 2003; Mather and Carstensen, 2003). Indeed, several investigations have reported age-related reductions in trait anxiety and anxiety disorders such as post-traumatic stress disorder (PTSD) and SAD (Lindesay et al., 1989; Regier et al., 1990). Given evidence that successful emotion and anxiety regulation relies on prefrontal control over the amygdala, a logical assumption would be that older adults have greater structural integrity in this circuitry than younger adults. Yet previous DTI studies have instead revealed significant age-related white matter decline between the amygdala and prefrontal cortex (Burzynska et al., 2010; Davis et al., 2009a), particularly in measures of fractional anisotropy (FA) and radial diffusivity (RD), which are sensitive to changes in myelin integrity (Schmierer et al., 2004). Age-related white matter decline is particularly robust in the frontal lobe, with pathways linking the amygdala to the vPFC declining early on (Head et al., 2004; Malykhin et al., 2011; Salat et al., 2005). However, if the regulatory model is correct, age-related decline in amygdala-vPFC structural connectivity should be associated with increased anxiety. Here, we used aging as a model of amygdala-vPFC structural decline to test the hypothesis that the structural integrity of this pathway relates to trait anxiety.
In order to test this hypothesis, we utilized probabilistic tractography, a structural imaging technique that enables white matter pathways to be traced in vivo (Behrens et al., 2007). Tractography is performed by tracing white matter “streamlines” between a pair of brain regions based on patterns of water diffusion in brain tissue. Since water diffusion is fastest along the length of axons, this technique can be used to estimate the topography and integrity of neuroanatomical connections. In turn, these tract estimates can be used to extract white matter indices, such as FA, RD, and axial diffusivity (AD), which serve as scalar measures of structural integrity. White matter tractography has been successfully implemented in previous DTI studies to examine white matter connections between the amygdala and frontal cortex (Bach et al., 2011; Saygin et al., 2011). To date, the majority of anxiety studies have focused on white matter decline in the uncinate fasciculus. However, some tractography studies have delineated additional structural connections between the amygdala and vPFC that course through the extreme capsule and more medially through peri-striatal regions, such as the substantia innominata (Croxson et al., 2005; Johansen-Berg et al., 2008; Kim and Whalen, 2009). These findings suggest that the uncinate fasciculus region-of-interest might not capture the full extent of this anxiety circuit. Thus, we used probabilistic tractography to trace participant-specific white matter pathways between the amygdala and vPFC.
In the present study, we used aging as a model of structural decline in the amygdala-vPFC pathway to test whether structural changes contribute to higher trait anxiety. Trait anxiety represents an individual’s generalized and long-lasting predisposition to respond to stress with apprehension and foreboding (Lueck, 2007), suggesting that it may be linked to more stable brain characteristics, such as the structural rather than functional integrity of circuits underlying stress and emotion processing. To quantify age-related changes in amygdala-vPFC structural connectivity, we analyzed tract FA, RD, AD and volume in three age groups ranging from younger to older adults. Only healthy, non-clinically-diagnosed participants were selected for analysis in order to compare our results to earlier findings in healthy younger adults (Kim and Whalen, 2009). We predicted that mean amygdala-vPFC FA and RD would decline with age. To test the regulatory anxiety model (Kim et al., 2011a), we also examined whether this structural change was associated with a concomitant increase in trait anxiety.
Methods
The behavioral and imaging data for this study were obtained from the Nathan Kline Institute’s (NKI) Rockland Sample, a component of the 1000 Functional Connectomes Project (http://fcon_1000.projects.nitrc.org/index.html). Participants completed semi-structured diagnostic psychiatric interviews and a variety of cognitive and behavioral assessments in order to thoroughly explore brain-behavior relationships.
Participants
A group of 54 healthy, right-handed male and female participants were selected from the NKI dataset according to several criteria. None of the participants exhibited hypertensive or prehypertensive systolic or diastolic blood pressure values, and all scored within the normal range on the Beck Depressive Index (BDI; Beck et al., 1961). The participants’ DTI and T1-weighted structural images were also closely examined to ensure they were free of image artifacts resulting from head motion, signal drop-out, blurring, or structural abnormalities. The selected participants were divided into three different age groups: 21 younger adults (age 19–29), 18 middle-aged adults (age 40–50), and 15 older adults (age 60–85). Details of the group assignments are described in Table 1.
Table 1.
Participant characteristics
| Age Group | Mage | Age Range | N | Males | Females | BDI | Trait Anxiety |
|---|---|---|---|---|---|---|---|
| YA | 23(2.66) | 19 – 29 | 21 | 13 | 8 | 3.38(3.91) | 34.1(9.82) |
| MA | 44(3.24) | 40 – 50 | 18 | 11 | 7 | 3.67(5.86) | 35.11(5.86) |
| OA | 71(8.12) | 60 – 85 | 15 | 6 | 9 | 3.93(3.71) | 28.33(6.97) |
Notes: YA = younger adults; MA = middle-aged adults; OA = older adults; BDI = Beck’s Depression Inventory (Beck et al., 1961); Trait Anxiety was assessed by State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983). Standard deviations are displayed in parentheses.
Behavioral Data
Trait anxiety was evaluated using the State-Trait Anxiety Inventory (STAI), a common measure of adult anxiety that distinguishes anxiety from depressive symptoms (Spielberger et al., 1983). The STAI is a 40-item assessment that applies a multifaceted definition of anxiety by dissociating trait anxiety from state anxiety. Trait anxiety is defined as a stable aspect of personality that describes an individual’s tendency to respond to a situation with anxiety, while state anxiety refers to a transitory state of emotional arousal (Endler and Kocovski, 2001). Higher scores on the STAI indicate higher trait anxiety levels. All participants fell within the normal range for anxiety (M = 32.83, SD = 9.75). Scores on the BDI scale were also used to assess whether participants fell within the clinical range for severe depression, i.e., scores 29–63. All participants fell within the non-severely-depressed range (M = 3.63, SD = 4.52). One male middle-aged adult (BDI = 23) and one female older adult (BDI = 15) who had depression scores that qualified as “mild depression” (BDI = 14–19) were kept for analysis.
A Shapiro-Wilk test of normality determined that both BDI and trait anxiety scores violated the assumption of normality (ps < .05). Given this result, Spearman’s rho correlations were used to examine associations between gender, BDI and trait anxiety. Gender and BDI were both significantly correlated with trait anxiety (rho = −.31, p = .024, rho = .36, p = .008, respectively). Thus, BDI and gender were modeled as nuisance covariates in subsequent analyses.
Image Acquisition
Diffusion tensor imaging
A 64-direction diffusion tensor imaging sequence was implemented using parallel imaging acceleration (GRAPPA), factor = 3. A total of 76 diffusion-weighted images were acquired (axial slices = 58; TR/TE = 10/91ms; FOV = 256mm; b-value = 1000 s/mm2; in-plane resolution = 2×2mm2; slice thickness = 2mm; slice gap = 0mm). The total acquisition time for the protocol was 13 minutes and 32 seconds.
T1-weighted structural imaging
A high-resolution T1-weighted anatomical image (MPRAGE) was also obtained to facilitate diffusion image co-registration (slabs = 1; slices = 192; TR/TE/TI= 2500/3.5/1200ms; FOV = 256m; flip angle = 8 degrees; isotropic voxel dimensions = 1mm3; multi-slice mode = single-shot). The total acquisition time for this anatomical scan was 10 minutes and 42 seconds.
Image Preprocessing
DTI and MRI preprocessing was carried out using tools from FSL Version 4.1.6 (FMRIB Software Library, www.fmrib.ox.ac.uk/fsl). The Brain Extraction Tool (BET) was used to remove non-brain tissue from the diffusion-weighted images and high-resolution T1 structural images. The effects of eddy currents and head movement were reduced and corrected by aligning the diffusion-weighted images to a non-diffusion reference image using linear registration with 12 degrees of freedom. FSL’s FNIRT tool was used to perform non-linear registration between each participant’s T1-image and a 2mm MNI standard brain. The resulting transformation matrices were concatenated and inverted to create standard-to-diffusion space and diffusion-to-standard space transformation matrices, respectively. The results of each preprocessing step were carefully inspected to verify their accuracy.
Anatomical definition of amygdala
Separate left and right amygdala masks were hand-drawn onto each participant’s T1-weighted image in the coronal plane according to tracing procedures described by Allen et al. (2005). Superior, inferior, medial and lateral boundaries were first demarcated, and the amygdala was carefully traced in the medial temporal lobe as an ovoid-shaped grey matter mass and separated from neighboring grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF). The anterior boundary of the amygdala was arbitrarily defined in a coronal slice exactly three slices posterior to the slice where the frontal lobe merges with the temporal lobe. In more anterior slices, the superior boundary of the amygdala was defined as the CSF within the temporal horn of the lateral ventricle, whereas the visible grey-white matter boundary served as the superior border in more posterior slices. The dorsomedial boundary was defined as CSF. The lateral boundary was defined as the border between amygdala grey matter and parahippocampal white matter. In more anterior coronal slices, the inferior boundary was first demarcated by parahippocampal white matter and extended dorso-medially until the line connected with CSF. As the amygdala ascended above the emerging hippocampal grey matter in more posterior slices, the inferior boundary was traced along the white matter strand of the alveus. The final amygdala masks were visually inspected by trained researchers to verify their accuracy.
Anatomical definition of vPFC
The processing pipeline used to create the vPFC masks is depicted in Figure 1. The left and right hemisphere vPFC anatomical masks were created in MNI standard space. These masks were generated by combining anatomical gyri defined by the Laboratory of NeuroImaging (LONI) Probabilistic Brain Atlas (LPBA40; http://www.loni.ucla.edu/Atlases/LPBA40; Shattuck et al., 2007) and the Harvard-Oxford Cortical Structural Atlas (http://www.cma.mgh.harvard.edu/fsl_atlas.html). First, a mask of the entire prefrontal cortex was created by combining gyri from the LONI atlas corresponding to the inferior, medial, and superior frontal gyri, the lateral and medial orbitofrontal gyri, and the gyrus rectus. In addition, the Harvard-Oxford Atlas version of the anterior cingulate cortex (ACC) was also included in this mask. The resulting frontal lobe mask was partitioned into a ventral-only portion, with the ventral mask bounded by an axial slice at MNI Z-coordinate = 37 mm. This superior boundary was defined in the mid-sagittal plane by extending a line posteriorly from the point where the medial orbital sulcus meets the frontal pole back to the genu of the corpus callosum. The standard-space vPFC mask was thresholded at 50% GM tissue-type probability to increase the likelihood that the voxels belonged to GM.
Figure 1.
The processing pipeline used to create the anatomical masks for tractography. The amygdala masks were hand-drawn on each participant’s high-resolution T1-weighted structural image by trained researchers. Each participant’s thalamus “stop” masks were segmented from their T1-weighted structural image. The ventral prefrontal cortex (vPFC) masks were created by merging each participant’s probabilistic grey-matter mask (thresholded at 35%) with the MNI-space-defined vPFC mask, which had been dilated to encapsulate white matter streamlines passing near the cortical boundary (Bach et al., 2011).
To encompass white matter fibers passing near grey matter, the vPFC masks were dilated by 4mm in every direction (Bach et al., 2011). The dilation was used to account for the low resolution of the diffusion images and partial voluming at the WM-GM border. The resulting left and right hemisphere masks were combined and the overlap was subtracted in order to reduce any inter-hemispheric overlap produced by dilation. FSL’s automated segmentation tool (FAST) was used to segment each participant’s T1-weighted image into probabilistic tissue-type masks corresponding to GM, WM, and CSF. The GM masks were thresholded to include voxels with at least a 35% probability of belonging to GM and subsequently binarized. Each standard-space-defined vPFC mask was then written into each participant’s structural space and merged with their respective GM masks in order to produce participant-specific GM vPFC masks. Each participant’s vPFC mask was then linearly transformed from structural to diffusion space using 7 degrees of freedom. These participant-specific vPFC masks served as seed and target cortical masks in the subsequent tractography and ROI analyses.
DTI Analysis
Probabilistic tractography
Probabilistic tractography between the amygdala and the vPFC was performed in each participant’s native diffusion space using FSL’s probtrackx tool. Tractography was carried out separately for the left and right brain hemispheres. Probabilistic connectivity distributions were generated for each brain voxel by repeatedly sampling ipsilateral pathways between the amygdala and vPFC (Behrens et al., 2007; Johansen-Berg and Behrens, 2009). FSL’s FIRST tool was used to parcellate the left and right thalami from every individual’s T1-weighted structural image. The thalami were used as exclusion mask to exclude indirect connections between the amygdala and vPFC. To increase the reliability of the estimated amygdala-vPFC pathway, fiber tracking was initiated in both directions (i.e., amygdala-to-vPFC and vPFC-to-amygdala). A total of 5000 streamline samples were sent out from each voxel in the amygdala or vPFC seed region. Every sample was tracked to its ipsilateral target with a step length of 0.5mm and a curvature threshold of 0.2. Fiber tracking was also constrained to voxels with an FA value > 0.2 to ensure that streamlines passed through white matter. The bidirectional fiber tracking resulted in two tracts in which each voxel’s value represented the total number of successful streamline samples (i.e., the waytotal; successful samples out of N seed voxels x 5000) that passed through it. To calculate the probability that each voxel belonged to the amygdala-vPFC pathway, the amygdalo-frontal and fronto-amygdala tracts were normalized by their respective waytotals and combined according to the following formula: (Hughes et al., 2012). The resulting amygdala-vPFC tract was thresholded at 0.01 to remove any spurious connections and binarized for subsequent analyses.
Group probability maps
To acquire reliable spatial estimates of the amygdala-vPFC pathways for each age group, group probability maps were created. For every individual, the estimated amygdala-vPFC tracts were binarized. These binary tracts were summed across all of the participants within the three age groups: young adults, middle-aged adults and older adults. The group probability maps were thresholded such that each voxel along the putative amygdala-vPFC tract was present in at least 25% of the participants within that age group.
FA, RD, AD and volume of amygdala-vPFC pathway
To estimate the structural integrity of the amygdala-vPFC pathway, each participant’s amygdala-vPFC tract was applied to his or her respective FA, RD, and AD maps and mean values were extracted. AD refers to the principal eigenvector (λ1) and is believed to reflect the integrity of axon (Glenn et al., 2003). RD refers to the average of the two eigenvectors perpendicular to the principal eigenvector [(λ2+ λ3)/2] and is sensitive to myelin integrity (Schmierer et al., 2004). Whereas high FA values indicate greater structural integrity, high RD and AD values indicate lower pathway integrity.
As a measure of the trackability, or reliability, of the white matter connections across age groups, the volume of the estimated amygdala-vPFC tracts was acquired in each participant’s native diffusion space. The SIENAX tool in FSL was used to determine each participant’s intracranial volume (ICV). These ICV values were then used to normalize the native-space tract volumes to enable cross-participant comparisons in stereotaxic space.
To investigate the effect of age on mean amygdala-vPFC FA, RD, AD, and tract volume, separate two-way repeated-measures ANOVAs were carried out with hemisphere (2: left and right) as a within-subjects factor and age category (3: younger adults, middle-aged adults, older adults) and gender (2: male and female) as between-subjects factors. Bonferroni-corrected post-hoc t-tests were performed to test for significant main effects of age group, hemisphere, and gender.
Robust linear regression analysis: associations between age, anxiety, and amygdala-vPFC FA
To examine the relationship between trait anxiety and mean amygdala-vPFC pathway FA, four separate robust linear regression analyses were conducted. Given the non-normal, skewed distribution of BDI scores across the group, we used a robust regression technique, a modified Theil-Sen estimator, to test for relationships between the mean right/left amygdala-VPFC and trait anxiety (Wilcox, 2004). This robust linear regression approach is more insensitive to outliers than simple linear regression and has been shown to yield more accurate results for skewed and heteroskedastic data. Separate linear regressions were performed for the left and right brain hemispheres due to significant covariance in the white matter measures (ps > .05). In the first two regressions (one per hemisphere), gender and BDI were modeled as nuisance covariates (Baur et al., 2012; Kim and Whalen, 2009). In the second two regressions (one per hemisphere), gender, BDI and age were modeled as covariates to determine whether amygdala-vPFC pathway FA could account for unique variance in trait anxiety scores above and beyond the influence of age. Since four robust regressions were performed, a Bonferroni correction of p < .0125 was used to assess the statistical significance of the results (p < .05/4 tests = .0125).
Results
Amygdala-vPFC Probabilistic Tractography Results
The bidirectional probabilistic tractography results were consistent with previous findings of several fronto-temporal white matter pathways between the amygdala and vPFC. Specifically, these connections included the uncinate fasciculus, extreme capsule, and more distinct medial connections adjacent to the ventral striatum (Croxson et al., 2005; Johansen-Berg et al., 2008; Kim and Whalen, 2009). The tractography results are displayed in Figures 2 and 3. To illustrate the white matter paths captured by tractography, a comparison of the spatial distribution between our group-estimated tract and an uncinate-fasciculus-only ROI derived from the JHU White Matter Tractography Atlas in FSL is displayed in Figure 2. The tractography algorithm delineated white matter connections that passed more medially than the uncinate fasciculus and into peri-striatal regions, such as the substantia innominata.
Figure 2.
Comparison of the topography of the amygdala-vPFC pathway and uncinate fasciculus. The 3D rendering depicts the mean amygdala-vPFC pathway produced by probabilistic tractography (green) and the uncincate fasiculus (orange) acquired from the JHU White-Matter Tractography Atlas. For illustrative purposes, the tractography pathway was thresholded at 20% of the 54 participants and the uncinate fasciculus was thresholded at 10%. The tractography results revealed white matter pathways consistent with the underlying anatomy.
Figure 3.
Amygdala-vPFC probability maps. Voxels within the amygdala-vPFC pathway are color-coded based on the percentage of participants in whom they appeared. The pathway in each brain slice is thresholded to reflect voxels that were present in 25–75% of participants in the entire group.
Amygdala-vPFC Group Probability Maps
To determine the inter-participant reliability of the amygdala-vPFC tractography results, we created probability maps across the entire group (see Figure 3). These group probability maps represent how consistently each brain voxel appeared in the amygdala-vPFC pathway across the participants. The probability maps revealed that the tractography performed relatively well across all of the participants in discerning various amygdala-vPFC pathways that were consistent with the underlying anatomy.
Age Differences in White Matter Measures
We performed a two-way repeated-measures ANOVA to examine the effects of age on mean FA, RD, AD and tract volume in the left and right amygdala-vPFC structural pathways. Brain hemisphere was modeled as a within-subjects factor, while both gender and age were modeled as between-subjects factors. The results of these analyses are displayed in Figure 4.
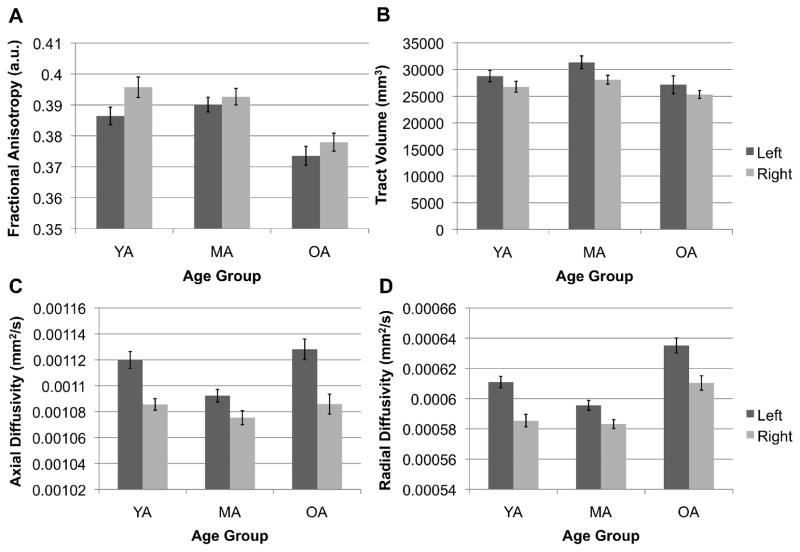
Figure 4.
Comparison of mean amygdala-vPFC (A) fractional anisotropy, (B) tract volume, (C) axial diffusivity and (D) radial diffusivity across age groups by hemisphere. Bars represent the standard errors of the means. YA = younger adults, MA = middle-aged adults, OA = older adults. Note that the mean white matter estimates on the Y-axis do not start at zero.
Fractional anisotropy results
Mean amygdala-vPFC FA significantly decreased with age, F(2,48) = 5.88, p = .005, η2 = .2. Bonferroni-corrected follow-up t-tests revealed that mean amygdala-vPFC FA was significantly higher in younger (M = .39; SD = .021) and middle-aged adults (M = .39; SD = .017) than in older adults (M = .38; SD = .022; YA > OA: p = .008, MA > OA: p = .018). However, mean FA did not significantly differ between younger and middle-aged adults. We also found a significant main effect of gender on mean amygdala-vPFC FA, F(1,48) = 5.38, p = .025, η2 = .1, such that males exhibited significantly greater mean amygdala-vPFC FA than did females. We did not observe a significant main effect of hemisphere nor any interaction effects (ps > .10).
Radial diffusivity results
Mean amygdala-vPFC radial diffusivity significantly decreased with age, F(2,48) = 6.81, p = .002, η2 = .22, but did not differ between males and females, F(1,48) = 1.94, p = .17, η2 = .039. This age-related trend followed a U-shaped trajectory, with radial diffusivity being lowest in middle-aged adults and highest in older adults (Figure 4D). This pattern is consistent with evidence of delayed myelination in this prefrontal pathway, with myelination reaching its peak around age 35 and not rapidly decreasing until the age of 55 (Bartzokis et al., 2012). A follow-up t-test revealed that mean amygdala-vPFC radial diffusivity was significantly higher in older than younger (p = .013) and middle-aged (p = .003) adults. However, mean amygdala-vPFC radial diffusivity did not significantly differ between younger and middle-aged adults (p > .10). We also found a significant main effect of hemisphere on mean amygdala-vPFC radial diffusivity, F(1,48) = 62.78, p < .001, η2 = .57, with higher radial diffusivity in the left than right hemisphere. We did not observe any significant interaction effects.
Axial diffusivity results
Mean amygdala-vPFC axial diffusivity did not significantly differ between age groups, F(2,48) = 1.09, p = .35, η2 = .043, or genders, F(1,48) = 0.3, p = .59, η2 = .006, suggesting that the axonal integrity of this pathway remains relatively intact across the lifespan (Figure 4C). However, we found a significant main effect of hemisphere, F(1,48) = 35.55, p < .001, η2 = .43. A Bonferroni-corrected follow-up t-test revealed that this hemisphere effect was driven by greater axial diffusivity in the left than right hemisphere. We did not observe any significant interaction effects.
Tract volume results
The ICV-normalized amygdala-vPFC tract volumes did not significantly differ between age groups or genders (ps > .05). We also did not observe any significant interaction effects. These results indicate that, in spite of white matter integrity decline in older adults, the trackability of this pathway was relatively stable across age groups (see Figure 2).
Association between Amygdala-vPFC FA and Trait Anxiety
To test our hypotheses that age, amygdala-vPFC FA and trait anxiety would be associated with each other, we performed robust linear correlations using a modified Theil-Sen estimator while controlling for the effects of gender and BDI scores (Baur et al., 2012). Contrary to the negative correlations seen in Kim and Whalen (2009), we found positive associations between mean amygdala-vPFC FA and trait anxiety for both the right (β = .35, p = .01) and left (β = .34, p = .033) hemispheres. Given our Bonferroni-correction cut-off of p < .0125, only the relationship in the right amygdala-vPFC pathway was deemed significant.
To examine whether age alone could account for the direct influence of mean right amygdala-vPFC FA on trait anxiety, we conducted an additional robust linear regression while also controlling for age. We found a trend towards a positive correlation between mean right amygdala-vPFC FA and trait anxiety (β = .16, p = .18). Since age, mean right amygdala-vPFC FA and trait anxiety were all inter-correlated, we performed a follow-up Sobel test to explore the strength of a potential age mediation effect. The age mediation effect was not significant (p > .2).
Discussion
Our findings argue against the notion that structural integrity of the amygdala-vPFC pathway helps facilitate better anxiety outcomes in healthy adults. Whereas this pathway is typically conceptualized as a regulatory circuit, we instead found that amygdala-vPFC FA was significantly positively correlated with trait anxiety. We argue that, even if the positive relationship between amygdala-vPFC FA and trait anxiety is driven by age influences over both variables, it is an important finding when considering the regulatory model that posits that greater structural connectivity between the amygdala and vPFC facilitates regulatory control over the amygdala and helps reduce anxiety. At the very least, it provides a boundary condition on when such a positive relationship should occur. This study expands upon previously mixed findings regarding the relationship between amygdala-vPFC structural connectivity and trait anxiety, suggesting that caution should be taken when interpreting the significance of this circuitry to anxiety.
As predicted, the DTI analysis revealed a negative association between age and the white matter integrity of amygdala-vPFC structural connectivity. This age-related decrease in amygdala-vPFC FA is consistent with previous evidence of age-related white matter decline along the amygdala-vPFC pathway (Burzynska et al., 2010). We determined that this age effect was predominantly driven by a decrease in radial rather than axial diffusivity, suggesting that age-related changes in white matter between the amygdala and vPFC was more likely associated with myelin degeneration rather than a loss of axonal integrity. Consistent with our results, previous DTI studies have indicated that age-related degeneration of white matter primarily results from axonal demyelination and is characteristic of normal aging (Davis et al., 2009a; Madden et al., 2009). Notably, higher anisotropy can also occur in the absence of myelin, suggesting that age-related increases in FA could result from other axonal structural features, such as the packing density of axons within a given voxel (Beaulieu, 2002). Thus, while it is tempting to speculate that our RD finding relates to demyelination, the underlying physiological changes remain unclear. One recent DTI study in older adults demonstrated a negative association between anxiety symptoms and white matter health in the superior longitudinal fasciculus, but not in the uncinate fasciculus, suggesting that structural decline in alternative brain pathways contributes to anxiety in later adulthood (Bijanki et al., 2013). This relationship was only observed in older participants with atherosclerosis, a vascular disease that has been associated with anxious symptoms (Stillman et al., 2012). By comparison, none of our participants had issues with vascular health, and we found a positive association between trait anxiety and FA the amygdala-vPFC pathway. Nonetheless, it is noteworthy that the relationship between white matter integrity and anxiety appears to become increasingly complex in older adults, because they are more likely to have widespread diffusion abnormalities that signify actual physiological changes or additional sources of diffusion image artifacts.
In addition to declining with age, the integrity of amygdala-vPFC connections was positively associated with trait anxiety. Importantly, this finding contradicts the prevailing regulatory model of anxiety, which posits that greater white matter integrity affords greater top-down control of the amygdala by the vPFC (Kim et al., 2011a). According to this model, the vPFC regulates the output of the amygdala to control anxiety, so less structural connectivity disables top-down control over the amygdala and, consequently, leads to increases in anxiety. Whereas several studies have demonstrated a negative correlation between trait anxiety and structural integrity of the amygdala-vPFC pathway (Baur et al., 2012; Kim and Whalen, 2009), other studies have instead reported a positive relationship between trait anxiety and amygdala-vPFC FA (Modi et al., 2013; Montag et al., 2012).
One explanation for this positive association is that many of the investigations demonstrating a negative relationship used an uncinate fasciculus ROI, which encompasses some but not all of the white matter connections between the amygdala and vPFC (Croxson et al., 2005; Johansen-Berg et al., 2008; Kim and Whalen, 2009). In previous research, one of these more posterior tracts, the external capsule, has shown increased RD in older adults, suggesting that this area is possibly impacted by normal age-related demyelination (Bennett et al., 2010). Our data supported this finding, though our single-value measure of RD spanned the entire length of the amygdala-vPFC pathway. In contrast to our tracts delineated by probabilistic tractography, the uncinate fasciculus branches toward the lenticular nucleus and the insula (Papagno, 2011). Recent evidence suggests that both structural and functional connectivity between the left amygdala and anterior insula is associated with both normal and pathological levels of anxiety (Baur et al., 2013). For instance, anxiety-prone individuals show greater insula activation during the presentation of emotional stimuli (Ball et al., 2012; Stein et al., 2007b). In line with these findings, other studies have shown increased functional connectivity between the amygdala and insula in threatening situations (Rosso et al., 2010). An important advantage of our study is that we utilized probabilistic tractography to specifically delineate amygdala-vPFC connections. As a result, we were able to examine anxiety-structure relationships unique to prefrontal cortical connections.
An alternative to the regulatory model is that anxiety arises from higher-than-normal amygdala activity as opposed to decreased regulatory activity of the vPFC. Probabilistic tractography enabled us to trace white matter connections between the amygdala and vPFC that course more medially than the uncinate fasiculus and pass through the substantia innominata and bed nucleus of the stria terminalis (BNST). A wealth of evidence from rodent (Walker and Davis, 2008), monkey (Fox et al., 2008) and human (Alvarez et al., 2010) studies has linked these regions to maintaining anxiety during sustained threat, thereby implicating their involvement in more trait - as opposed to state - aspects of anxiety responses. Specifically, prolonged anxiety has been associated with activity in an extended network consisting of amygdala sub-nuclei, the BNST and hypothalamus (Davis et al., 2009b). Notably, in healthy younger adults, Kim and Whalen (2009) localized a significant positive association between amygdala-vPFC pathway FA and amygdala reactivity to fearful faces, a putative biomarker of sensitivity to anxiety, to voxels within the substantia innominata (Kim and Whalen, 2009). While individual differences in participants’ amygdala reactivity did not correlate with trait anxiety in their study, this result aligns with our current findings of a positive association between amygdala-based white matter networks and anxiety. The age-related reduction in amygdala-vPFC pathway integrity we observed might therefore represent the uncoupling of an anxiety-sustaining circuit, which could relate to better anxiety outcomes in older adults.
In addition to peri-striatal regions, the amygdala and medial orbitofrontal gyrus (mOFC) – a portion of the vPFC - share reciprocal functional and anatomical connections (Ghashghaei et al., 2007; Milad and Quirk, 2002), suggesting that both feedback and feedforward interactions influence anxiety processes. Supporting this reciprocity, patients with social anxiety disorder exhibit enhanced effective connectivity from the amygdala to the mOFC and vice versa, indicating that an altered balance in amygdala-mOFC activity contributes to pathological anxiety (Liao et al., 2010). This finding agrees with a separate effective connectivity study in healthy adults in which the amygdala appeared to modulate subgenual vPFC activity, whereas the vPFC exerted top-down influences over the amygdala (Stein et al., 2007a). Bottom-up signals from the amygdala to the subgenual sector of the vPFC are believed to be a mechanism by which the amygdala labels the emotional significance of events (Phillips et al., 2003). Enhanced amygdalar output might therefore mislabel the motivational significance of non-threatening stimuli, leading to maladaptive attentional processing and a state of distress. Thus, while many studies point to de-regulation of the amygdala as a source of anxiety, it remains unclear how dysfunctional crosstalk between the amygdala and vPFC relates to underlying structural changes. The prevailing regulatory model of anxiety posits that lower amygdala-vPFC structural integrity disrupts inhibitory feedback and leads to greater anxiety (Kim et al., 2011a), yet because the pathway is bidirectional, it is also possible that greater amygdala-vPFC white matter integrity supports increased threat-related processing biases initiated by the amygdala.
Supporting the importance of feedforward aspects of amygdala-vPFC circuitry, both animal and human studies have implicated this pathway in the body’s adaptive stress response (Herman et al., 2005). Whereas the regulatory model predicts that the amygdala-vPFC pathway serves to inhibit stress responses, a number of findings suggest the opposite, showing that this pathway may in fact amplify stress (Myers-Schulz and Koenigs, 2012). For example, increased functional coupling between the amygdala and ventromedial PFC (vmPFC) – a sector of the PFC that fell within the larger vPFC mask used in our study - has been reported in individuals with a polymorphism of the human serotonin transporter gene, a genetic variant associated with greater amygdala reactivity to aversive stimuli and increased anxiety-related temperament (Heinz et al., 2004). In rodents, a dorsal-ventral dissociation exists such that dorsal medial PFC activity relates to the inhibition of amygdala responses, whereas more vmPFC activity is associated with enhanced hypothalamo-pituitary axis output (Cerqueira et al., 2008; Myers-Schulz and Koenigs, 2012). Similarly, human patients with vmPFC lesions show reduced skin conductance responses and lower autonomic reactivity to social stimuli (Damasio et al., 1990). Together these findings support a positive association between greater autonomic arousal and amygdala-vPFC structural connectivity in which more efficient communication between the amygdala and vPFC is associated with an enhancement rather than inhibition of anxiety and stress responses. Thus, while it is evident that the amygdala-vPFC circuitry is centrally involved in anxiety, it is conceivable that greater myelination in this pathway also permits greater amygdala-driven modulation of prefrontal function (Bishop, 2007). One limitation of our study is that probabilistic tractography cannot inform the directionality of neural communication between brain regions, so we were unable to test the regulatory versus facilitatory nature of the amygdala-vPFC circuit.
According to the current regulatory model of anxiety, age-related degeneration in the white matter connecting the amygdala and vPFC should disrupt top-down regulation of the amygdala, leading to poorer anxiety outcomes in older adults. However, our findings contradict this hypothesis by showing that, in spite of an age-related decrease in amygdala-vPFC structural integrity, trait anxiety decreased with age. Previous studies have reported mixed findings regarding age-related changes in anxiety, so it is unclear whether age is associated with either normal or pathological anxiety (Flint, 1994). Consistent with our results, many investigations have reported age-related reductions in trait anxiety (Lindesay et al., 1989; Regier et al., 1990). In the current study, it is possible that the lower trait anxiety observed in older adults resulted from an inability of the STAI to capture alternative sources of anxiety in older adults. While there are age group-specific scales that assess anxiety-related brain changes across the lifespan, using the STAI allowed for a direct comparison between older and younger adults. This decision was also supported by one study showing that 43% of psychiatrically healthy geriatric inpatients had clinically significant scores on the STAI; moreover, a factor analysis revealed that this finding was driven primarily by worries of well-being (Kvaal et al., 2001), suggesting that the symptoms assessed by the STAI are sufficient to identify high-anxiety individuals in the early and late lifespan.
In addition, we observed significant sex differences in amygdala-vPFC FA, suggesting that these connections may facilitate different anxiety outcomes in males and females. One fMRI study showed that stress-induced patterns of amygdala functional connectivity differ between males and females (Mather et al., 2010). Although sex differences in the relationship between the structure of this pathway and anxiety have not been directly studied, one investigation found that a positive correlation between trait anxiety and the integrity of the uncinate fasciculus was driven primarily by males (Montag et al., 2012). Intriguingly, higher trait anxiety has been associated with greater amygdala responses to unattended fearful faces in females than males (Dickie and Armony, 2008), which may account for females being more susceptible to anxiety and depression than males (Leach et al., 2008). Testing these sex differences using a combination of tractography and effective functional connectivity would enlighten our understanding of how males and females differentially experience and regulate their anxiety.
Several study limitations warrant further consideration. First, we acknowledge that our sample sizes in both groups were moderate. Nonetheless, the significance of our brain-behavior results, particularly showing a clear positive rather negative association between amygdala-vPFC connectivity and trait anxiety – highlights the need to revisit the widely accepted regulatory model of this pathway and consider alternative explanations. Second, our cross-sectional samples prevents us from making causal inferences, necessitating follow-up longitudinal studies to confirm that our results reflect age-related developmental changes in white matter integrity that lead to shifts in trait anxiety, rather than some other causal pathway.
We chose to examine a non-anxious population for two reasons: first, examining a non-clinical sample with the STAI enabled us to directly compare our aging results against an earlier study investigating the relationship between white matter integrity and STAI scores in younger adults; second, we were interested in determining how patterns of age-related white matter decline relate to the decreases in trait anxiety which may occur in healthy aging. Compared with younger adults, healthy older adults tend to exhibit a positivity effect in which they allocate mental resources away from aversive, anxiety-provoking stimuli in favor of positive stimuli (Isaacowitz et al., 2006; Knight et al., 2007; Mather and Carstensen, 2003). These findings raise the question of whether normal age-related decreases in attention to and memory for negative information might in part be related to underlying changes in the circuitry associated with threat-related processing biases (for a discussion of related issues, see Nashiro et al., 2012). Supporting this view, one study demonstrated that a positivity effect in healthy older adults’ attention was moderated by trait anxiety, with high-anxiety individuals initially avoiding but subsequently prolonging their attention to negative words (Lee and Knight, 2009). This prolonged threat processing among those with higher anxiety is consistent with our findings showing that individuals with relatively intact amygdala-vPFC connections – including more medial connections previously implicated in sustained anxious states (Davis et al., 2009b) - also exhibited higher trait anxiety.
Conclusion
To test the current amygdala-vPFC regulatory model of anxiety, we examined a group of individuals in which we expected reduced amygdala-vPFC structural integrity. Contrary to the regulatory model, we found that age-related structural decline in amygdala-vPFC connections was instead associated with a decrease in trait anxiety. Furthermore, we observed a significant positive association between trait anxiety and amygdala-vPFC FA. Given previously mixed findings regarding the relationship between anxiety and amygdala-vPFC structural integrity, our findings emphasize the need for caution when interpreting the structural basis of normal and pathological instances of anxiety. Whereas the regulatory model is useful for conceptualizing interactions between the amygdala and vPFC, it is also possible that more efficient communication between these regions supports amplified, rather than suppressed, anxiety and stress-related responses. In light of our aging results, this raises the possibility that structural decline in amygdala-based circuits instead relates to better anxiety outcomes in older adults. Exploring this structure-function relationship in more detail will provide a more comprehensive understanding of anxiety circuitry in both research and clinical settings.
Acknowledgments
This research was supported by grants R01AG038043, RO1AG025340, and K02 AG032309.
References
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiology of aging. 2005;26(9):1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;55(1):389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Behrens TE, Garrido L, Weiskopf N, Dolan RJ. Deep and superficial amygdala nuclei projections revealed in vivo by probabilistic tractography. Journal of Neuroscience. 2011;31(2):618–623. doi: 10.1523/JNEUROSCI.2744-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball TM, Sullivan S, Flagan T, Hitchcock CA, Simmons A, Paulus MP, Stein MB. Selective effects of social anxiety, anxiety sensitivity, and negative affectivity on the neural bases of emotional face processing. NeuroImage. 2012;59(2):1879–1887. doi: 10.1016/j.neuroimage.2011.08.074. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Heydari P, Couvrette A, Lee GJ, Kalashyan G, Altshuler LL. Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biological Psychiatry. 2012;72(12):1026–1034. doi: 10.1016/j.biopsych.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Baur V, Hänggi J, Jäncke L. Volumetric associations between uncinate fasciculus, amygdala, and trait anxiety. BMC Neuroscience. 2012;13(4):1–8. doi: 10.1186/1471-2202-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur V, Hänggi J, Langer N, Jäncke L. Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biological Psychiatry. 2013;73(1):85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson MD, Mock JMD, Erbaugh JMD. An inventory for measuring depression. Archives of General Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beekman ATF, Bremmer MA, Deeg DJH, van Bolkom AJLM, Smit JH, De Beurs E, van Tilburg W. Anxiety disorders in later life: A report from the Longitudinal Aging Study Amsterdam. International Journal of Geriatric Psychiatry. 1998;13:717–726. doi: 10.1002/(sici)1099-1166(1998100)13:10<717::aid-gps857>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Human brain mapping. 2010;31(3):378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijanki KR, Stillman AN, Arndt S, Magnotta VA, Fiedorowicz JG, Haynes WG, Moser DJ. White matter fractional anisotropy is inversely related to anxious symptoms in older adults with atherosclerosis. International journal of geriatric psychiatry. 2013 doi: 10.1002/gps.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: An integrative account. Trends in Cognitive Sciences. 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature neuroscience. 2008;12(1):92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bracht T, Tüscher O, Schnell S, Kreher B, Rüsch N, Glauche V, Saur D. Extraction of prefronto-amygdalar pathways by combining probability maps. Psychiatry Research: Neuroimaging. 2009;174(3):217–222. doi: 10.1016/j.pscychresns.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. NeuroImage. 2010;49(3):2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Almeida OF, Sousa N. The stressed prefrontal cortex. Left? Right! Brain Behavior and Immunity. 2008;22(5):630–638. doi: 10.1016/j.bbi.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47(4):1448. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: the forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132(2):310. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Olatunji BO, Feldner MT, Forsyth JP. Emotion regulation and the anxiety disorders: An integrative review. Journal of Psychopathology and Behavioral Assessment. 2009;32(1):68–82. doi: 10.1007/s10862-009-9161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TEJ, Robson MD, Pinsk MA, Gross CG, Rushworth MFS. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. Journal of Neuroscience. 2005;25(39):8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioral Brain Research. 1990;41(2):81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009a;46(2):530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2009b;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie EW, Armony JL. Amygdala responses to unattended fearful faces: Interaction between sex and trait anxiety. Psychiatry research. 2008;162(1):51. doi: 10.1016/j.pscychresns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Iordan AD, Dolcos S. Neural correlates of emotion-cognition interactions: A review of evidence from brain imaging investigations. Journal of Cognitive Psychology. 23(6):669–694. doi: 10.1080/20445911.2011.594433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler NS, Kocovski NL. State and trait anxiety revisited. Journal of Anxiety Disorders. 2001;15(3):231–245. doi: 10.1016/s0887-6185(01)00060-3. [DOI] [PubMed] [Google Scholar]
- Endler NS, Cox BJ, Parker JD, Bagby RM. Self-reports of depression and state-trait anxiety: Evidence for differential assessment. Journal of Personality and Social Psychology. 1992;63(5):832–838. doi: 10.1037//0022-3514.63.5.832. [DOI] [PubMed] [Google Scholar]
- Flint AJ. Epidemiology and comorbidity of anxiety disorders in the elderly. American Journal of Psychiatry. 1994;151(5):640–649. doi: 10.1176/ajp.151.5.640. [DOI] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PloS one. 2008;3(7):e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn OA, Henry RG, Berman JI, Chang PC, Miller SP, Vigneron DB, Barkovich AJ. DTI-based three-dimensional tractography detects differences in the pyramidal tracts of infants and children with congenital hemiparesis. Journal of Magnetic Resonance Imaging. 2003;18(6):641–648. doi: 10.1002/jmri.10420. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. NeuroReport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: The neural circuitry of emotion regulation. Neuropsychopharmacology. 2010;35(1):136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Bucker RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14(4):14410–14423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Büchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature neuroscience. 2004;8(1):20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hughes EJ, Bond J, Svrckova P, Makropoulos A, Ball G, Sharp DJ, Counsell SJ. Regional changes in thalamic shape and volume with increasing age. Neuroimage. 2012;63(3):1134–1142. doi: 10.1016/j.neuroimage.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye tracking study. Psychology and Aging. 2006;21:40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cerebral Cortex. 2008;18(6):1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TEJ. Diffusion MRI: From quantitative measurement to in-vivo neuroanatomy. Academic Press; 2009. [Google Scholar]
- Kapp BS, Supple WF, Whalen PJ. Effects of electrical stimulation of the amygdaloid central nucleus on neocortical arousal in the rabbit. Behavioral Neuroscience. 1994;108(1):81–93. doi: 10.1037//0735-7044.108.1.81. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience. 2009;29(37):11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Merchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: From normal to pathological anxiety. Behavioural Brain Research. 2011a;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex. 2011b;21(7):1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Seymour TL, Gaunt JT, Baker C, Nesmith K, Mather M. Aging and goal-directed emotional attention: Distraction reverses emotional biases. Emotion. 2007;7(4):705–714. doi: 10.1037/1528-3542.7.4.705. [DOI] [PubMed] [Google Scholar]
- Kvaal K, Laake K, Endegal K. Psychometric properties of the state part of the Spielberger State-Trait Anxiety Inventory (STAI) in geriatric patients. International Journal of Geriatric Psychiatry. 2001;16(10):980–986. doi: 10.1002/gps.458. [DOI] [PubMed] [Google Scholar]
- Leach LS, Christensen H, Mackinnon AJ, Windsor TD, Butterworth P. Gender differences in depression and anxiety across the adult lifespan: the role of psychosocial mediators. Social psychiatry and psychiatric epidemiology. 2008;43(12):983–998. doi: 10.1007/s00127-008-0388-z. [DOI] [PubMed] [Google Scholar]
- Lee LO, Knight BG. Attentional bias for threat in older adults: moderation of the positivity bias by trait anxiety and stimulus modality. Psychology and aging. 2009;24(3):741. doi: 10.1037/a0016409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Qiu C, Gentili C, Walter M, Pan Z, Ding J, Chen H. Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state FMRI study. PLoS One. 2010;5(12):e15238. doi: 10.1371/journal.pone.0015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindesay J, Briggs K, Murphy E. The Guy’s/Age Concern survey. Prevalence rates of cognitive impairment in depression and anxiety in an urban elderly community. British Journal of Psychiatry. 1989;155(3):317–329. [PubMed] [Google Scholar]
- Lochner CL, Fouche J, du Plessis S, Spottiswoode B, Soraya S, Fineberg N, Stein DJ. Evidence for fractional anisotropy and mean diffusivity white matter abnormalities in the internal capsule and cingulum in patients with obsessive compulsive disorder. Journal of Psychiatry & Neuroscience. 2012;37(3):193–199. doi: 10.1503/jpn.110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueck MD. Anxiety levels: Do they influence the perception of time? UW-L Journal of Undergraduate Research. 2007;10:1–4. [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychology Review. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin N, Vahidy S, Michielse S, Coupland N, Camicioli R, Seres P, Carter R. Structural organization of the prefrontal white matter pathways in the adult and aging brain measured by diffusion tensor imaging. Brain Structure & Function. 2011;216(4):417–431. doi: 10.1007/s00429-011-0321-1. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychological Science. 2003;14(5):409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Lighthall NR, Nga L, Gorlick MA. Sex differences in how stress affects brain activity during face viewing. NeuroReport. 2010;21:933–937. doi: 10.1097/WNR.0b013e32833ddd92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Induced processing biases have causal effects on anxiety. Cognition & Emotion. 2002;16(3):331–354. [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biological Psychiatry. 2006;73(1):61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Modi S, Trivedi R, Singh K, Kumar P, Rathore RKS, Tripathi RP, Khushu S. Individual differences in trait anxiety are associated with white matter integrity in fornix and uncinate fasciculus: Preliminary evidence from a DTI based tractography study. Behavioral Brain Research. 2013;238:188–192. doi: 10.1016/j.bbr.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Montag C, Reuter M, Weber B, Marketta S, Schoene-bake JC. Individual differences in trait anxiety are associated with white matter tract integrity in the left temporal lobe in healthy males but not females. Neuroscience. 2012;217:77–83. doi: 10.1016/j.neuroscience.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Myers-Schulz, Koenigs Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Molecular Psychiatry. 2012;17(2):132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Mather M. Age differences in brain activity during emotion processing: Reflections of age-related decline or increased emotion regulation? Gerontology. 2012;(58):156–163. doi: 10.1159/000328465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Christian BT, Murali D, Oakes TR, Kalin NH. Serotonin transporter availability in the amygdala and bed nucleus of the stria terminalis predicts anxious temperament and brain glucose metabolic activity. Journal of Neuroscience. 2009;29(32):9961–9966. doi: 10.1523/JNEUROSCI.0795-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagno C. Naming and the role of the uncinate fasciculus in language function. Current Neurology and Neuroscience Reports. 2011;11(6):553–559. doi: 10.1007/s11910-011-0219-6. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience. 2010;11:773–782. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, Arfanakis K. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biological Psychiatry. 2009;66(7):691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biological psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Regier DA, Narrow WE, Rae DS. The epidemiology of anxiety disorders: The epidemiologic catchment area (ECA) experience. Journal of Psychiatric Research. 1990;24:3–14. doi: 10.1016/0022-3956(90)90031-k. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Makris N, Britton JC, Price LM, Gold AL, Zai, Rauch SL. Anxiety sensitivity correlates with two indices of right anterior insula structure in specific animal phobia. Depression and Anxiety. 2010;27(12):1104–1110. doi: 10.1002/da.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. NeuroImage. 2009;44(4):1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin ZM, Osher DE, Augustinack J, Fischl B, Gabrieli JDE. Connectivity-based segmentation of human amygdala nuclei using probabilistic tractography. NeuroImage. 2011;56(3):1353–1361. doi: 10.1016/j.neuroimage.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck DW, Mirza M, Adisetiyo V, Hojatkashani C, Salamon G, Narr KL, Toga AW. Construction of a 3D probabilistic atlas of human cortical structures. NeuroImage. 2007;39(3):1064–1080. doi: 10.1016/j.neuroimage.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Annals of neurology. 2004;56(3):407–415. doi: 10.1002/ana.20202. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory STAI (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. NeuroImage. 2007a;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Stein M, Simmons A, Feinstein J, Paulus M. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007b;164(2):318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Stillman AN, Rowe KC, Arndt S, Moser DJ. Anxious symptoms and cognitive function in non-demented older adults: an inverse relationship. International journal of geriatric psychiatry. 2012;27(8):792–798. doi: 10.1002/gps.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Structure and Function. 2008;213(1–2):29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7(6):177–188. [Google Scholar]
- Wilcox RR. Some results on extensions and modifications of the Theil-Sen estimator. British Journal of Mathematical and Statistical Psychology. 2004;57:265–280. doi: 10.1348/0007110042307230. [DOI] [PubMed] [Google Scholar]