Abstract
Cardiopulmonary resuscitation (CPR) guidelines assume that cardiac arrest victims can be treated with a uniform chest compression (CC) depth and a standardized interval administration of vasopressor drugs. This non-personalized approach does not incorporate a patient’s individualized response into ongoing resuscitative efforts. In previously reported porcine models of hypoxic and normoxic ventricular fibrillation (VF), a hemodynamic-directed resuscitation improved short-term survival compared to current practice guidelines. Skilled in-hospital rescuers should be trained to tailor resuscitation efforts to the individual patient’s physiology. Such a strategy would be a major paradigm shift in the treatment of in-hospital cardiac arrest victims.
Keywords: Arterial blood pressure, Cardiac arrest, Cardiopulmonary resuscitation
1. Introduction
“Performing CPR without measuring the effects is like flying an airplane without an altimeter ”
- Dr. Max Harry Weil at the Fourth Wolf Creek Conference, April 1996
In the United States, approximately 200,000 patients each year receive professional cardiopulmonary resuscitation (CPR) for a cardiac arrest during their hospitalization.1 Most of these arrests now occur in intensive care units (ICUs),2,3 perhaps due to the successful implementation of early warning systems and medical emergency teams. In these highly monitored ICUs, patients will often have invasive monitoring available that could guide resuscitation quality. Yet our current training programs focus on a uniform approach to resuscitation care that does not incorporate a patient’s individualized response into ongoing resuscitative efforts.4,5
In this article, we will review the existing literature from both animal and human studies regarding hemodynamics – specifically coronary perfusion pressure (CPP) – during CPR. Like Max Harry Weil, we believe that measuring the effects of CPR performance is critically important for optimizing outcomes. For patients with invasive arterial blood pressure monitoring at the time of CPR, we believe that titrating chest compression (CC) force and advanced life support medications to arterial blood pressures can improve outcomes. Such a personalized approach to resuscitation medicine is feasible, implementable, and may lead to improved survival outcomes.
2. Coronary perfusion pressure is critically important for successful CPR
2.1. Animal models
While this article will discuss CPP as a primary determinant of resuscitation survival, myocardial blood flow is truly the primary determinant of CPR success.6 However, obtaining measurements of myocardial blood flow during actual cardiac arrest resuscitation is not practical. Conversely, CPP – the mathematical difference between the arterial diastolic pressure and the right atrial diastolic pressure – can be made available to healthcare providers via frequently used invasive clinical devices (intra-arterial and central venous catheters). As CPP is the primary driving force for myocardial blood flow,7 it may be a useful clinical surrogate to guide resuscitation quality.
The notion that CPP determines successful survival dates back to the turn of the 20th century. In 1906, Crile and Dolley described their experience using adrenaline to improve outcomes from anesthesia- or asphyxia-induced cardiac arrest.8 In this landmark article, they describe the experiments that led them to conclude that the “basic problem in resuscitation [is] … securing a coronary perfusion pressure from thirty to forty millimeters of mercury.” In their experiments, the addition of adrenaline to closed chest cardiac massage and fluid administration raised arterial diastolic blood pressure and improved survival outcomes significantly. To the best of our knowledge, this was one of the first reports supporting CPP monitoring during CPR.
In 1988, Kern and colleagues again demonstrated that CPP during CPR is a powerful predictor of 24-h survival.6 In a canine model of ventricular fibrillation (VF) cardiac arrest, they were able to demonstrate that CPPs were higher in resuscitated animals compared to non-resuscitated animals after 5, 10, 15, and 20 min of VF. They further evaluated the predictive ability of CPP at 10 min of CPR and found significant differences in CPPs between animals (1) never resuscitated, (2) that died before 24 h, and (3) that survived 24 h. Specifically, failure to obtain a CPP of at least 20 mmHg was an excellent predictor of poor survival (negative predictive value = 96%). These findings led them to conclude that CPP is a useful measure of CPR effectiveness that should be used to optimize resuscitation efforts.
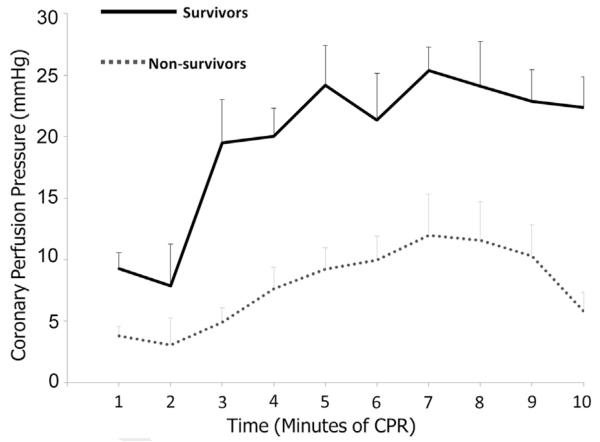
More recently in 2013, building on the work of Crile, Dolley, Kern and numerous others, Sutton,9 Friess10 and colleagues evaluated a resuscitative approach that specifically altered CPR quality and vasoactive drug administration to hemodynamic targets. To the best of our knowledge, this series of studies was the first to evaluate a resuscitative approach targeted to hemodynamic goals. Specifically, in both hypoxic and normoxic models of VF, animals were randomized to receive one of three CPR strategies with the objective to demonstrate that a resuscitative approach targeted to hemodynamics would improve short term survival compared to existing care recommendations. In the Hemodynamic-Directed Care group, CC depth was titrated to a systolic blood pressure of 100 mmHg and vasopressors to maintain CPP > 20 mmHg. There were two comparator groups utilized in these studies: (1) “realistic AHA care” – CC depth of 33 mm (based on data of CC depth actually attained while attempting to follow AHA Guidelines11) with Advanced Life Support (ACLS) epinephrine dosing every 4 min; and (2) “optimal AHA care4,5” – CC depth of 51 mm with ACLS epinephrine dosing every 4 min. In both models, 45-min ICU survival was higher when a Hemodynamic Directed resuscitative approach was utilized compared to either realistic or optimal depth-directed care with fixed epinephrine dosing. Importantly, there were no differences in the overall total amount of vasoactive medications administered across groups, suggesting that it was not the amount of drug given, but the “right amount of drug at the right time during the resuscitation” that led to improved outcomes. The authors also found higher CPPs over time in survivors compared to non-survivors, providing mechanistic validity to this new resuscitative model/approach (Fig. 1). The authors concluded that such an approach is feasible and shows promise that should be evaluated further.
Fig. 1.
Mean coronary perfusion pressure during each minute of CPR between survivors and non-survivors after hypoxic ventricular fibrillation. Similar results were also seen in normoxic VF model.10 Error bars represent SEM. Modified from Sutton et al.,9 Resuscitation 2013.
2.2. Human data
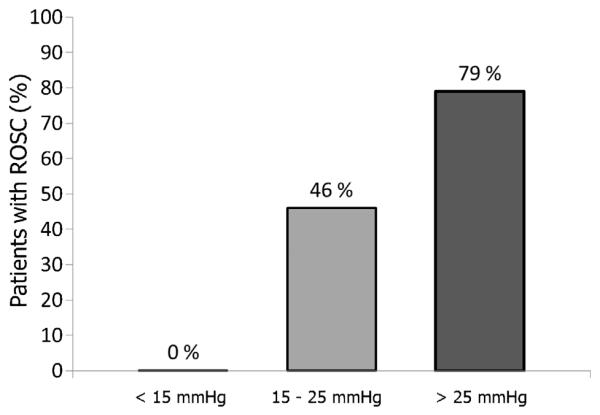
In 1990, Paradis and colleagues reported a positive association between CPP and human survival (Fig. 2).12 In this study of 24 patients, both initial CPP (13.4 ± 8.5 vs. 1.6 ± 8.5 mmHg) and maximal CPP (25.6 ± 7.7 vs. 8.4 ± 10.0 mmHg) were higher in those with return of spontaneous circulation (ROSC) compared to those without ROSC. The authors also reported c-indexes (ability to discriminate between ROSC vs. non ROSC cases) for selected hemodynamic variables in this study. Maximal CPP was calculated as having the highest c-index (i.e., best discriminatory properties, c = 0.93) as compared to initial CPP (0.837) and maximal diastolic pressure (0.708). In this small dataset, a maximal CPP of 15 mmHg was chosen as the best therapeutic cutoff because its perfect negative predictive value (i.e., no survivors with CPP < 15 mmHg) may indicate a futile resuscitation, while its positive predictive value of 57% would suggest that continued resuscitative efforts will lead to success in more than 50% of cases. As concluded by the authors, this study substantiated the large amount of animal data that existed at the time indicating the importance of CPP during CPR.
Fig. 2.
Percentage of patients achieving return of spontaneous circulation (ROSC) during adult cardiac arrest resuscitation. Modified from Paradis et al.,12 Journal of the American Medical Association 1990.
It is important to note that no prospective human study has demonstrated that targeting CPP during resuscitation will lead to improved survival. There is also no data supporting the best CPP target for infants and children. And while further investigations are warranted, these research endeavors will undoubtedly be fraught with difficulty. In a large multicenter trial determining whether a given resuscitation was driven by Guideline care vs. hemodynamic care (i.e., actual treatment assignment) when an arterial line is in place at the time of arrest would be particularly difficult and require a substantial research infrastructure.
3. Monitoring CPP during CPR is feasible
3.1. In-hospital cardiac arrest is an important public health problem
Cardiopulmonary resuscitation training has focused on treatment of out-of-hospital cardiac arrests, a well-known major public health problem.13 Each year in the USA, professional CPR is provided for approximately 175,000 people in the pre-hospital setting.14 Therefore, CPR Guidelines were developed with a simplified “one-size” fits all approach to serve laypersons and professionals in any setting. More recently, data from the AHA’s Get with the Guidelines-Resuscitation (GWTG-R) National Registry established that professional CPR is provided to approximately 200,000 in-hospital cardiac arrests in the USA annually as well.1 Therefore, in-hospital professional CPR is as common as out-of-hospital professional CPR. These epidemiologic data raise the possibility that we should re-evaluate in-hospital CPR training program paradigms. In-hospital ICU teams have the skills and information to titrate CPR performance to invasive hemodynamic parameters, including coronary perfusion pressure, and should be trained to do so effectively.
3.2. In-hospital cardiac arrests are primarily ICU events
With implementation of early warning systems and medical emergency teams, the proportion of in-hospital cardiac arrests occurring in ICU versus ward settings has increased over the last decade to ~95% among children2 and >60% among adults.3 Cardiac arrests on general inpatient wards are now considered sentinel events in safe, high-quality organizations. For example, the influential Child Healthcare Corporation of America has specifically targeted non-ICU arrests as a potentially avoidable sentinel event.15
Importantly, many patients with cardiac arrests in ICUs have invasive monitoring in place at the time of arrest available to guide resuscitation quality. Nearly half of children receiving ICU CPR in the GWTG-R registry have invasive arterial blood pressure monitoring in place at the time of the event.2 Similarly, over 30% of adults with ICU cardiac arrests in the GWTG-R registry are on vasopressor infusions, a reasonable indication of the need for continuous invasive pressure monitoring.3 Therefore, in-hospital resuscitation teams could be trained to titrate CPR efforts to invasive physiological monitoring during resuscitation.
As a further step in the transformation of CPR training to focus on hemodynamic directed care at the bedside, Wolfe and colleagues developed a CPR training manikin with biofidelic arterial blood pressure feedback.16 The feedback was termed biofidelic because the relationship between CC depth and arterial blood pressure in the manikin was derived from actual pediatric CPR data.17 Building on previous work by this group regarding Rolling Refreshers (brief CPR re-trainings with a manikin on a rolling cart18), the authors demonstrated that an arterial blood pressure targeted “Rolling Refresher” was effective to improve CPR skill acquisition and retention – without need for interval monthly retraining as in their previous studies. The authors concluded that the success of this program, maintaining retention without need for monthly retraining, was due in part to the use of a familiar “clinical” endpoint as the training target. Rather than training toward absolute depth targets, ICU providers were more successful targeting and titrating to blood pressure goals as they would in numerous other critical situations, such as heart failure, sepsis, or hemorrhagic shock.
4. Expert consensus now recommends physiological monitoring
In a recent consensus statement,19 monitoring a patient’s response to resuscitation was recommended. The authors provided a hierarchal and situational contextualization of physiological monitoring based upon the available data most closely related to myocardial blood flow. In this consensus statement, CPP was recommended as the primary physiological target (goal > 20 mmHg) when both arterial and central venous catheters are in place at the time of the arrest, followed by arterial diastolic pressure (goal > 25 mmHg) when an arterial catheter is available without a central venous catheter. Recognizing that invasive mechanical ventilation is much more common than arterial pressure monitoring, the consensus statement also recommended capnography monitoring third tier (goal end tidal carbon dioxide > 20 mmHg) when an arterial line is not in place at the time of arrest. While end tidal monitoring has limitations, it is a good surrogate marker of pulmonary blood flow and cardiac output,20 and clearly better than no quality monitoring.
5. Conclusions
During the last ten years, the inpatient culture in the USA has successfully focused on identifying and transferring at risk adult and pediatric patients to intensive care units for monitoring and treatment of respiratory failure and shock. As a result, patients frequently have invasive monitoring available at the time of arrest. Surrogates for myocardial blood flow, the primary determinant of successfully CPR, such as coronary perfusion pressure or diastolic blood pressure, should be used to measure the effectiveness of CPR. Nearly 20 years after Dr. Weil’s controversial “altimeter” statement, the time has arrived for in-hospital healthcare providers to routinely use available hemodynamic monitoring during CPR. This simple transformational change to hemodynamic data driven “personalized” resuscitation could be life-saving.
Acknowledgements
The studies conducted were supported by the National Institute of Child Health and Human Development (RMS K23), the National Institute of Neurological Disorders and Stroke (SHF K08), and the Russell Raphaely Endowed Chair of Pediatric Critical Care Medicine at CHOP.
Abbreviations
- AHA
American Heart Association
- CPR
cardiopulmonary resuscitation
- CC
chest compression
Footnotes
A Spanish translated version of the abstract of this article appears as Appendix in the final online version at http://dx.doi.org/10.1016/j.resuscitation.2014.04.015.
Conflicts of interest
Vinay Nadkarni and Matt Maltese receive unrestricted research grant support from the Laerdal Foundation for Acute Care Medicine.
References
- 1.Merchant RM, Yang L, Becker LB, et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39:2401–6. doi: 10.1097/CCM.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg RA, Sutton RM, Holubkov R, et al. Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med. 2013;41:2292–7. doi: 10.1097/CCM.0b013e31828cf0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girotra S, Nallamothu BK, Spertus JA, et al. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367:1912–20. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(Suppl. 3):S729–67. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 5.Kleinman ME, Chameides L, Schexnayder SM, et al. Part 14: Pediatric advanced life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(Suppl. 3):S876–908. doi: 10.1161/CIRCULATIONAHA.110.971101. [DOI] [PubMed] [Google Scholar]
- 6.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: a predictor of 24-h survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16:241–50. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 7.Sanders AB, Ewy GA, Taft TV. Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med. 1984;12:871–3. doi: 10.1097/00003246-198410000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Crile G, Dolley DH. An experimental research into the resuscitation of dogs killed by anesthetics and asphyxia. J Exp Med. 1906;8:713–25. doi: 10.1084/jem.8.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton RM, Friess SH, Bhalala U, et al. Hemodynamic directed CPR improves short-term survival from asphyxia-associated cardiac arrest. Resuscitation. 2013;84:696–701. doi: 10.1016/j.resuscitation.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friess SH, Sutton RM, Bhalala U, et al. Hemodynamic directed cardiopulmonary resuscitation improves short-term survival from ventricular fibrillation cardiac arrest. Crit Care Med. 2013;41:2698–704. doi: 10.1097/CCM.0b013e318298ad6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293:305–10. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 12.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–13. [PubMed] [Google Scholar]
- 13.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the united states, 1989 to 1998. Circulation. 2001;104:2158–63. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 14.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute for healthcare improvement. 2012. [Google Scholar]
- 16.Wolfe H, Fischman E, Legkobitova V, et al. Titration of CPR quality to patient physiology: biofidelic hemodynamic-directed booster trainings improve CPR performance. Crit Care Med. 2012;40 abstract 607. [Google Scholar]
- 17.Sutton RM, French B, Nishisaki A, et al. American Heart Association cardiopulmonary resuscitation quality targets are associated with improved arterial blood pressure during pediatric cardiac arrest. Resuscitation. 2013;84:168–72. doi: 10.1016/j.resuscitation.2012.08.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton RM, Niles D, Meaney PA, et al. Low-dose, high-frequency CPR training improves skill retention of in-hospital pediatric providers. Pediatrics. 2011;128:e145–51. doi: 10.1542/peds.2010-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meaney PA, Bobrow BJ, Mancini ME, et al. Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128:417–35. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 20.Weil MH, Bisera J, Trevino RP, Rackow EC. Cardiac output and end-tidal carbon dioxide. Crit Care Med. 1985;13:907–9. doi: 10.1097/00003246-198511000-00011. [DOI] [PubMed] [Google Scholar]