Abstract
Objective
Hemolysis, occurring during cardiopulmonary bypass (CPB), is associated with lipid peroxidation and postoperative acute kidney injury (AKI). Acetaminophen (ApAP) inhibits lipid peroxidation catalyzed by hemeproteins and in an animal model attenuated rhabdomyolysis-induced AKI. This pilot study tests the hypothesis that ApAP attenuates lipid peroxidation in children undergoing CPB.
Design
Single center prospective randomized double blinded study.
Setting
University-affiliated pediatric hospital.
Patients
Thirty children undergoing elective surgical correction of a congenital heart defect.
Interventions
Patients were randomized to ApAP (OFIRMEV® (acetaminophen) injection, Cadence Pharmaceuticals, San Diego, CA) or placebo every 6 hours for 4 doses starting before the onset of CPB.
Measurement and Main Results
Markers of hemolysis, lipid peroxidation (isofurans and F2-isoprostanes) and AKI were measured throughout the perioperative period. CPB was associated with a significant increase in free hemoglobin (from a pre-bypass level of 9.8±6.2 mg/dl to a peak of 201.5±42.6 mg/dl post-bypass). Plasma and urine isofuran and F2-isoprostane concentrations increased significantly during surgery. The magnitude of increase in plasma isofurans was greater than the magnitude in increase in plasma F2-isoprostanes. ApAP attenuated the increase in plasma isofurans compared to placebo (P=0.02 for effect of study drug). There was no significant effect of ApAP on plasma F2-isoprostanes or urinary makers of lipid peroxidation. ApAP did not affect postoperative creatinine, urinary neutrophil gelatinase-associated lipocalin or prevalence of AKI.
Conclusion
CPB in children is associated with hemolysis and lipid peroxidation. ApAP attenuated the increase in plasma isofuran concentrations. Future studies are needed to establish whether other therapies that attenuate or prevent the effects of free hemoglobin result in more effective inhibition of lipid peroxidation in patients undergoing CPB.
Keywords: acetaminophen, lipid peroxidation, cardiac surgery, pediatrics, cardiopulmonary bypass, hemoglobin, acute kidney injury
Introduction
Hemolysis frequently occurs during CPB and leads to an increase in free hemoglobin.(1, 2) The adverse effects of free hemoglobin is countered by haptoglobin that binds free hemoglobin to form a complex that is degraded by hemo-oxygenase-1.(3) Evidence that free hemoglobin contributes to postoperative acute kidney injury (AKI) is suggested by the association between hemolysis and postoperative AKI,(1, 4, 5) the nephrotoxic effects of hemoglobin-based oxygen carriers,(6) and the protective effect of haptoglobin on renal function.(7–9) Moreover, corticosteroids can induce haptoglobin synthesis and prevent complications associated with cell free hemoglobin.(8) Although the mechanism/s whereby free hemoglobin causes AKI is still debated, we have previously demonstrated that hemoglobinemia is associated with lipid peroxidation and that patients that develop postoperative AKI have greater hemoglobinemia and lipid peroxidation compared to controls.(1) These findings suggest a potential role of hemeprotein-mediated lipid peroxidation in the pathogenesis of postoperative AKI.
OFIRMEV® (acetaminophen, ApAP) injection (Cadence Pharmaceuticals, San Diego, CA) was approved by the US FDA in November 2010 for the treatment of acute pain and fever in children and adults and is used in patients undergoing cardiac surgery as part of a multimodal pain management regimen.(10, 11) ApAP acts as a ferryl protein reductant, thus inhibiting hemeprotein-catalyzed lipid peroxidation.(12, 13) ApAP inhibits oxidation of arachidonic acid catalyzed by hemoglobin with an IC50 of 17.7±2.5μM (13) which is well within the therapeutic range for humans (5–30μg/mL; 33–200μM). In an animal model of rhabdomyolysis-induced kidney injury, ApAP significantly decreased markers of lipid peroxidation and protected kidney function.(13) Moreover, an observational study in critically ill patients suggests that receiving ApAP in the setting of increased free hemoglobin was independently associated with a protective effect against death and lower plasma concentrations of F2-isoprostanes.(14) This pilot study tests the hypothesis that ApAP attenuates lipid peroxidation in pediatric patients undergoing CPB.
Methods
Thirty (15 male and 15 female with an age range of 2–144 months) children participated in the study (ClinicalTrials.gov Identifier: NCT01228305). This study was approved by the Vanderbilt University Institutional Review Board for Research on Human Patients and conducted according to the Declaration of Helsinki. A legal guardian of all patients provided written informed consent, and patients greater than 7 years of age additionally signed an assent form. Patients were eligible for the study if they were: 1) less than 18 years of age, and 2) undergoing elective cardiac surgery requiring CPB for surgical correction of their congenital heart lesion. Patients were excluded for the following reasons: 1) patients with single ventricle physiology because these patients undergo procedures that frequently require deep hypothermic circulatory arrest (DHCA) that results in global reperfusion oxidant injury, 2) inability of the patient’s legal guardian to understand the nature, scope, and possible consequences of the study, 3) severe neurological abnormalities at baseline, 4) weight less than 3kg, 5) inability of the patient to comply with the protocol i.e. children in whom it was deemed unsafe to have the extra blood draws, 6) patients with major non-cardiac congenital malformations, developmental disorders or serious chronic disorders, 7) previous adverse reaction to ApAP, 8) history of chronic liver disease, and 9) chronic renal insufficiency.
Protocol
Patients were randomly assigned by the investigational pharmacy to treatment (IV ApAP or matching IV placebo). IV ApAP (OFIRMEV® (acetaminophen) injection, Cadence Pharmaceuticals, San Diego, CA) was given at a standard dose of 15 mg/kg IV for children >=2 years of age and 12.5mg/kg IV for children 29 days to <2 years of age (OFIRMEV prescriber information). Study drug was given every 6 hours, with the first dose in the operating room and before the onset of CPB, for a total of 4 doses over a 24 hour study period. Drug administration was double-blind. The study drug was prepared and labeled by the investigational pharmacy in an identical fashion. Unblinding of study drug only occurred after completion of the statistical analyses. ApAP plasma concentrations were measured after CPB to assess plasma concentrations achieved during peak hemolysis only after completion of the study and unblinding occurred. While the patient was receiving study drug (24 hour period), no open label ApAP was administered.
Anesthesia and Cardiopulmonary Bypass
Anesthesia and CPB protocols have been previously described.(15) Aminocaproic acid (antifibrinolytic), methylprednisolone and dexmedetomidine (alpha-2 agonist) were given intraoperatively at the discretion of the attending anesthesiologist. Briefly, the circuit was primed with a mixture of albumin and plasmalyte-A. Packed red blood cells (PRBC) were added to the prime taking into account the patients age, weight, preoperative hematocrit and surgical procedure. Plasma was added to the prime for patients less than 10kg. After pre-bypass ultrafiltration (pre-BUF) of this mixture, mannitol, sodium bicarbonate, calcium chloride, and heparin were added to the washed circuit prime in doses adjusted for body surface area. Aortic cross-clamping, with administration of cold high potassium blood cardioplegia solution was delivered at 20 min intervals for myocardial protection in all patients. Deep hypothermic circulatory arrest was not performed. Modified ultrafiltration (MUF), with a pediatric hemoconcentrator (Terumo Cardiovascular Systems Corporation, Ann Arbor, MI) was performed after CPB in all patients.
Blood Sampling and Biochemical Assays
Blood samples were obtained for measurement of free hemoglobin, haptoglobin, F2-isoprostanes, and isofurans. F2-isoprostanes and isofurans are sensitive and specific markers of lipid peroxidation in vivo,(13, 16, 17) and are increased after CPB.(1, 18) Measurement of both isofuran and F2-isoprostane concentrations provide the most reliable approach to assess oxidative stress status under conditions of varying concentrations of oxygen because the formation of F2-isoprostanes is suppressed by elevated concentrations of oxygen.(17) All blood samples were collected on ice and centrifuged immediately at 0°C for 20 minutes. Plasma was then separated and stored at –80°C until the time of assay. Urine samples were obtained for measurement of F2-isoprostanes, isofurans and neutrophil gelatinase-associated lipocalin (NGAL), a marker of acute kidney injury. Samples were collected at four time points: 1) after induction of anesthesia, prior to CPB and administration of study drug (pre-bypass), 2) following 30 min of CPB, 3) after protamine administration (post-bypass) and 4) 24 hours after administration of first ApAP dose on postoperative day 1 (POD1). Free hemoglobin was determined using the 2-wavelength method s previously described.(19) Haptoglobin concentrations were measured using a commercially available ELISA kit (Abcam, Cambridge, MA) according to the manufacturer’s instructions. Free F2-isoprostane (non-esterified) and isofuran concentrations were determined by gas chromatography-mass spectrometry (GCMS) as previously described.(17, 20) To account for differences in renal function among subjects, urine F2-isoprostane and isofuran concentrations were normalized to creatinine clearance ([pg/mL] x [plasma creatinine (mg/dL) / urine creatinine (mg/dL)]) and are expressed as pg/mL Cr.Cl as previously described.(13, 21) Urine NGAL was measured using a commercially available ELISA assay (Bioporta Diagnostics, Gentofte, Denmark). ApAP concentrations were quantified with the Roche COBAS INTEGRA 800 System analyzer (Roche Diagnostics Corporation, Indianapolis, IN).
Clinical Data
Clinical outcome data collected included urine output, blood loss (chest tube drainage), blood products transfused, need for surgical re-exploration, time to tracheal extubation, and incidence of acute kidney injury (AKI). The definition of AKI was based on the Acute Kidney Injury Network (AKIN) consensus guidelines for the staging of AKI: stage 1 AKI, 0.3 mg/dl increase in serum creatinine concentration or increase to more than or equal to 1.5- to 2-fold from baseline; stage 2 AKI, increase in serum creatinine > 2- to 3-fold from baseline; and stage 3 AKI, increase in serum creatinine > 3-fold from baseline. Any patient that developed AKI stage 1 or more within 72 hours were defined as having postoperative AKI. (22, 23)
Statistical Analysis
Data are presented as means ± standard error of the mean (SEM) unless otherwise indicated. Sample size calculations were based on preliminary data of markers of lipid peroxidation in adults and assuming ApAP will reduce these markers by 40%. With these assumptions, a sample size of 14 in each group had 90% power to detect a difference in means of 23 using a two group Satterthwaite t-test with a 0.05 two-sided significance level. Categorical data were compared between groups using Chi-squared or Fischer’s exact tests, as appropriate. Continuous baseline data were compared using Student’s t-test or Mann-Whitney U test, as appropriate. Comparison of free hemoglobin, haptoglobin and markers of lipid peroxidation between groups (Placebo vs ApAP group) was made using a general linear model-repeated measures analysis of variance (ANOVA) in which the within patient variable was time and the between patient variable was study drug. Biomarkers that were not normally distributed were log-transformed prior to analysis. A 2-tailed P value less than 0.05 was considered statistically significant. Statistical analyses were performed with the statistical package SPSS for Windows (Version 21.0, IBM, New York, NY).
Results
Pre-Randomization Patient Characteristics
All pre-randomization patient characteristics (Table 1) were comparable between the two study groups except preoperative hematocrit was lower in the placebo group.
Table 1.
Pre-Randomization Patient Characteristics
| Placebo (N=15) | ApAP (N=15) | P-value | |
|---|---|---|---|
| Age (months) | 34.1±8.9 | 33.1±9.2 | 0.97a |
| Gender, Male, N (%) | 7 (46.7) | 8 (53.3) | 0.72d |
| Race, White, N (%) | 11 (73.3) | 13 (86.7) | 0.51d |
| Weight (kg) | 15.9±3.8 | 14.5±2.6 | 0.84a |
| Weight (percentile for age) | 41.4±10.2 | 35.5±6.7 | 0.71a |
| Mean arterial pressure (mmHg) | 75.4±3.6 | 72.7±2.4 | 0.55b |
| Heart Rate (BPM) | 104.7±6.1 | 110.2±6.1 | 0.52b |
| Pulse oximetry saturation (%) | 98±1.0 | 98.0±0.7 | 0.52a |
| Hematocrit (%) | 35.7±0.9 | 38.5±0.7 | 0.046a |
| Platelet count (k/μL) | 345.4±25.7 | 301.3±13.7 | 0.23a |
| Creatinine (mg/dL) | 0.32±0.02 | 0.33±0.03 | 0.69b |
| Potassium (meq/L) | 4.5±0.19 | 4.9±0.15 | 0.20b |
| Prior cardiac surgery, N (%) | 1 (6.7) | 1 (6.7) | 1.0c |
| Preoperative Medications, N (%) | |||
| Digoxin | 3 (20) | 1 (6.7) | 0.60c |
| Diuretic | 4 (26.7) | 1 (6.7) | 0.33c |
| Congenital heart defect, N (%) | 0.67d | ||
| Atrial septal defect | 6 (40) | 7 (46.7) | |
| Ventricular septal defect | 4 (26.7) | 2 (13.3) | |
| Tetralogy of Fallot | 3 (20) | 5 (33.3) | |
| Other | 2 (13.3) | 1 (6.7) | |
| RACHS Score, N (%) | 0.52d | ||
| Category 1 | 6 (40) | 7 (46.7) | |
| Category 2 | 9 (60) | 7 (46.7) | |
| Category 3 | 0 | 1 (6.7) | |
RACHS, Risk adjusted congenital heart surgery score.
Mann Whitney U test;
t-test;
Fischer Exact test,
Pearson Chi-Square test
Intra- and Postoperative Patient Characteristics
The use of PRBC and plasma in the pump prime, age of PRBC used in pump prime, CPB time, cross-clamp time, use of aminocaproic acid, steroids, dexmedetomidine, cells saver volume, modified ultrafiltration volume, the amount of blood products given, blood loss as measured by chest tube output in 24 hours, urine output, need for surgical re-exploration, time to extubation and hospital length of stay were not significantly different between the study groups (Table 2). ApAP concentrations measured post-bypass were 3.8±0.4 μg/mL (25.0±2.9 μM).
Table 2.
Intraoperative and Postoperative Patient Characteristics
| Placebo (N=15) | ApAP (N=15) | P-value | |
|---|---|---|---|
| PRBC in pump prime, N (%) | 13 (86.7) | 13 (86.7) | 1.0c |
| Age of PRBC used in pump prime (days) | 7.4±1.2 | 8.9±1.4 | 0.48a |
| Fresh frozen plasma in pump prime, N (%) | 7 (46.7) | 6 (40.0) | 0.71d |
| CPB time (min) | 77.1±7.9 | 80.2±13.0 | 0.53a |
| Cross-clamp time, (min) | 44.7±7.6 | 33.8±6.5 | 0.25a |
| Aminocaproic Acid, N (%) | 2 (13.3) | 1 (6.7) | 1.0c |
| Pre-pump steroids, N (%) | 3 (20) | 5 (33.3) | 0.68c |
| Dexmedetomidine, N (%) | 12 (80) | 11 (73.3) | 1.0c |
| Cell Saver volume (mL/kg) | 11.1±2.1 | 10.0±1.9 | 0.93a |
| MUF volume (mL/kg) | 27.7±5.4 | 30.5±4.7 | 0.60a |
| Total Transfusions (mL/kg) | |||
| PRBC | 48.9±11.1 | 44.9±11.3 | 0.95a |
| Platelets | 5.6±2.5 | 4.0±1.6 | 0.96a |
| Fresh frozen plasma | 11.0±3.3 | 7.3±2.7 | 0.39a |
| Urine output first 24 hours (mL/kg) | 41.5±4.1 | 42.8±3.9 | 0.95a |
| Chest tube output in 24 hours (mL/kg) | 23.4±5.0 | 22.8±4.0 | 0.98a |
| Surgical re-exploration, N (%) | 1 (6.7) | 0 | 1.0c |
| Time to extubation (hours) | 16.2±7.6 | 7.4±1.6 | 0.32a |
| Hospital length of stay (days) | 5.6±0.8 | 4.6±0.3 | 0.48a |
| Highest postoperative creatinine (mg/dL) | 0.48±0.03 | 0.51±0.03 | 0.52b |
| Acute kidney injury within 72hrs, N (%) | 8 (53.3) | 8 (53.3) | 1.0d |
| Highest AKI Stage within 72hrs, N (%) | 0.64d | ||
| No injury | 7 (46.7) | 7 (46.7) | |
| Stage 1 | 7 (46.7) | 5 (33.3) | |
| Stage 2 | 1 (6.7) | 2 (13.3) | |
| Stage 3 | 0 | 1 (6.7) | |
PRBC, packed red blood cells; CPB, cardiopulmonary bypass; MUF, modified ultrafiltration. The definition of acute kidney injury (AKI) was based on the Acute Kidney Injury Network (AKIN) consensus guidelines for the staging of AKI: stage 1 AKI, 0.3 mg/dl increase in serum creatinine concentration or increase to more than or equal to 1.5- to 2-fold from baseline; stage 2 AKI, increase in serum creatinine > 2- to 3-fold from baseline; and stage 3 AKI, increase in serum creatinine > 3-fold from baseline.
Mann Whitney U test;
t test;
Fischer Exact test;
Pearson Chi-Square test
Markers of Hemolysis
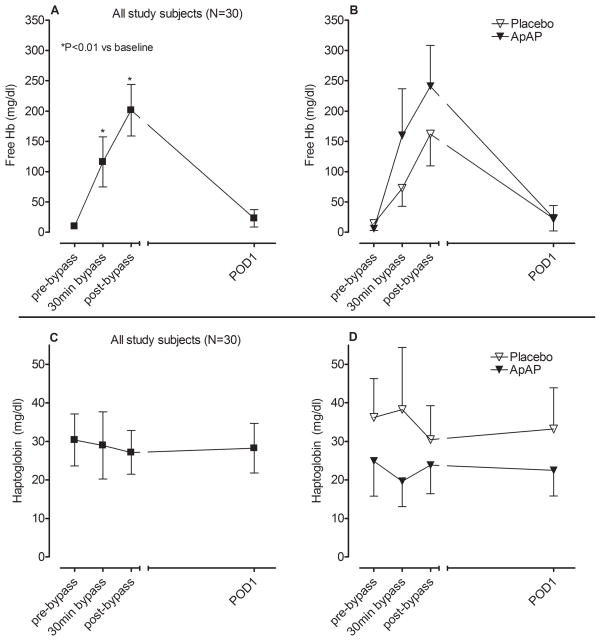
CPB was associated with an increase in free hemoglobin (P<0.001 for effect of time, Figure 1A) indicating hemolysis. The degree of hemolysis trended to be higher in patients that received ApAP compared to the placebo group (P=0.07 for effect of study drug, Figure 1B). Haptoglobin concentrations did not change significantly over the course of the study (P=0.94 for effect of time, Figure 1C) and there was no significant difference between the two study groups (P=0.29 for effect of study drug, Figure 1D). Because haptoglobin concentrations did not decrease as expected during CPB we explored potential causes. We considered both the administration of corticosteroids (induce haptoglobin synthesis (8)) and plasma (contains haptoglobin) priming of the pump as potential contributing factors. Patients that received pre-bypass steroids had higher post-bypass haptoglobin concentrations compared to those that did not (51.1±13.9 vs 18.4±4.9 mg/dL respectively, P=0.04). Haptoglobin concentrations remained elevated in patients that received a plasma pump prime (from a baseline of 36.4±13.0 to 48.6±17.1 mg/dl at 30min of bypass, P=0.75) compared to a significant decrease in patients that did not receive a plasma pump prime (from a baseline of 25.4±6.3 to 11.9±3.2 mg/dl at 30min of bypass, P=0.02).
Figure 1.
Hemolysis, as indicated by free hemoglobin concentrations, A) in all study patients and B) by study drug group. Haptoglobin concentrations in C) all study patients and D) by study drug group. POD 1 indicates postoperative days 1 and ApAP indicates acetaminophen.
Markers of Lipid Peroxidation
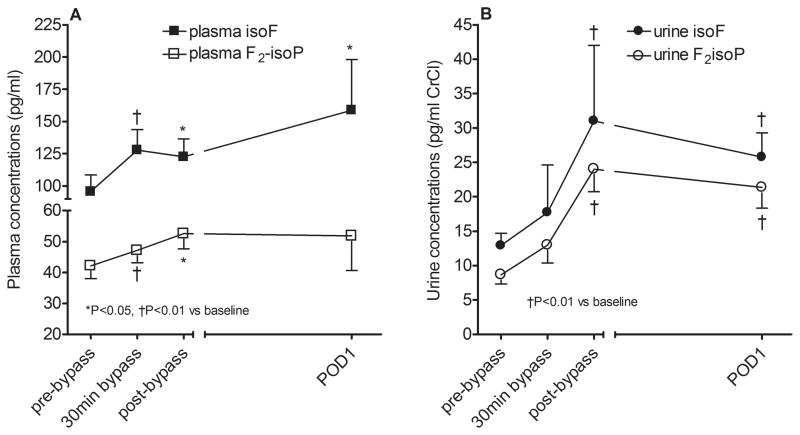
Both plasma isofuran and F2-isoprostane concentrations increased significantly during surgery (P=0.03 and P=0.05 respectively for effect of time; Figure 2A). Plasma isofuran concentrations remained elevated on POD1 whereas F2-isoprostane concentrations returned to baseline levels. The magnitude of increase for POD1 plasma isofurans was significantly greater compared to the magnitude of increase for plasma F2-isoprotanes (2.6±0.7 fold versus 1.6±0.4 fold increase from baseline, P=0.02). Similar to plasma markers of lipid peroxidation, urine isofurans and F2-isoprostanes increased significantly during surgery (P=0.001 and P=0.003 respectively for effect of time; Figure 2B) and remained elevated on POD1.
Figure 2.
Lipid peroxidation as quantified by isofuran (isoF) and F2-isoprostane (F2isoP) concentrations in A) plasma and B) urine for all study patients. 30min bypass indicates 30min of cardiopulmonary bypass and POD1 indicates postoperative day 1.
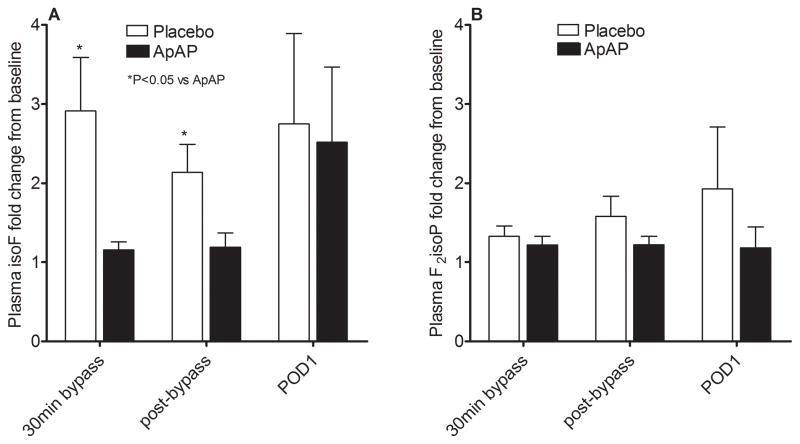
Because baseline concentrations of plasma F2-isoprostanes (37.7±5.7 vs 46.6±5.0 pg/mL, P=0.21) and isofurans (85.2±21.0 vs 106.4±14.6 pg/mL, P=0.12) tended to be lower in the placebo group compared to the ApAP group, we calculated the fold change from baseline for these biomarkers. The administration of ApAP attenuated the increase in plasma isofurans (P=0.02 for effect of study drug; Figure 3A). The effect of ApAP on isofuran concentrations was predominantly seen during the intraoperative period and disappeared by POD1. The increase in plasma F2-isoprostanes was attenuated by ApAP but was not statistically significant (P=0.16 for effect of study drug; Figure 3B). In addition, ApAP did not affect the increase in urine isofuran or F2-isoprostane concentrations (P=0.99 and P=0.47 respectively for effect of study drug). There was a significant correlation in post-bypass free Hb and post-bypass fold change in plasma F2-isoprostane concentrations (r2=0.32, P=0.03) in the placebo group but not in the ApAP group.
Figure 3.
Fold change in A) plasma isofuran (isoF) and B) plasma F2-isoprostane (F2-isoP) concentrations from baseline. Acetaminophen (ApAP) attenuated the increase in isoF (P=0.02 for effect of study drug) but not F2isoP (P=0.16 for effect of study drug). 30min bypass indicates 30min of cardiopulmonary bypass.
Clinical Outcomes
Sixteen patients (53%) developed AKI (Table 2). There were no significant differences in the highest postoperative creatinine, the prevalence of AKI, or the AKI stage between the placebo and ApAP groups. No patient required postoperative dialysis. The change in urine NGAL concentrations was not significantly different between the placebo and ApAP group (29.0±14.2 vs 44.6±37.0 ng/mL respectively, P=0.66). Patients that developed AKI tended to have higher post-bypass urine NGAL concentrations compared to patients that did not developed AKI (77.9±32.1 vs 20.7±6.6 ng/mL respectively, P=0.15). Peak free hemoglobin concentrations were significantly higher in patients that subsequently developed AKI compared to patients that did not (308.5±67.8 vs 79.1±20.8 mg/dl, P=0.02).
Discussion
This study examined the effect of ApAP, a ferryl protein reductant, on markers of lipid peroxidation in children undergoing cardiac surgery requiring CPB. CPB was associated with significant hemolysis and lipid peroxidation. ApAP attenuated the increase in plasma isofuran concentrations but did not affect postoperative creatinine or urine NGAL concentrations.
Our finding of hemolysis, with a subsequent increase in free hemoglobin during CPB, is consistent with prior studies.(1, 4, 24) The cause for the greater degree of hemolysis observed in ApAP group is not known. The duration of CPB, transfusion of PRBC and age of PRBC used for pump prime were similar between the two groups. Although ApAP can cause hemolysis in the presence of glucose-6-phosphate dehydrogenase deficiency, it is in the setting of an overdose; our patients received standard ApAP doses and blood levels at the end of bypass were below toxic levels. The haptoglobin response during CPB is variable with most studies indicating a decrease in haptoglobin concentrations,(7, 25, 26) while in another study haptoglobin concentrations remained within normal limits at the end of CPB.(27) The haptoglobin response observed in our study may in part be explained by the administration of pre-bypass steroids that can induce haptoglobin synthesis as well as plasma priming of the pump that resulted in higher intraoperative haptoglobin concentrations.
Consistent with prior studies, markers of lipid peroxidation increased significantly during CPB, and isofuran concentrations increased to a greater extent compared to F2-isoprostane concentrations.(1, 18, 24) Oxygen administration, which is common after surgery, most likely accounted for the relative increased formation of isofurans compared to isoprostanes because the formation of F2-isoprostanes is suppressed by elevated concentrations of oxygen.(17) ApAP attenuated the intraoperative increase in plasma isofuran concentrations even though patients in the ApAP group tended to have greater hemolysis. The lack of effect of ApAP on plasma F2-isoprostane concentrations could be the result of the small sample size and being underpowered to detect the small reduction in plasma F2-isoprostane concentrations observed in the ApAP group. The modest effect of ApAP on plasma markers of lipid peroxidation and the lack of effect on urinary markers of lipid peroxidation could be explained by several reasons. Firstly, despite administering the intravenous formulation of ApAP at doses usually recommended in the literature, concentrations achieved post-bypass were sub-therapeutic but still above the IC50 for ApAP inhibition of hemoglobin catalyzed oxidation of arachidonic acid. The low concentrations of ApAP post-bypass may be due to the increased volume of distribution of bypass which was not accounted for in dosing ApAP. Secondly, isofurans and F2-isoprostanes represent global free radical–induced lipid peroxidation and not only lipid peroxidation mediated by hemeprotein redox cycling. Because of this, ApAP would not be expected to completely or near completely diminish lipid peroxidation in these patients. Thirdly, haptoglobin concentrations remained elevated during CPB and could have attenuated hemeprotein-mediated redox cycling by scavenging free hemoglobin.
Although the study was not powered to assess the effect of ApAP on postoperative AKI, we did an exploratory analysis of AKI because ApAP inhibits hemeprotein-mediated lipid peroxidation and protects kidney function in an animal model.(13) While ApAP attenuated intraoperative plasma isofuran concentrations there were no significant differences in creatinine or NGAL concentrations or the prevalence of AKI between the ApAP and placebo groups. The lack of effect of ApAP on postoperative kidney function is in keeping with the failure of other anti-oxidants such as N-acetylcysteine, vitamin C and vitamin E to protect the kidney after cardiac surgery.(28)
Limitations
Several factors reduce enthusiasm for future studies investigating the effect of ApAP on AKI following cardiac surgery. Firstly, we observed only a modest effect of ApAP on intraoperative lipid peroxidation and no effect on urinary markers of lipid peroxidation. Secondly, we did not observe an effect of ApAP on markers of AKI although we are underpowered. Thirdly, higher sustained concentrations of ApAP throughout the period of maximum hemolysis may be more effective in inhibiting hemeprotein-mediated lipid peroxidation but we are limited by the approved ApAP dosing regimen. Although enthusiasm for ApAP as ferryl protein reductant during cardiac surgery may be reduced, we cannot exclude the possibility that more effective inhibition of hemeprotein-mediated lipid peroxidation may reduce markers of AKI and reduce the prevalence of AKI in children undergoing CPB. Deferoxamine, which is not only an iron chelator but also a ferryl protein reductant, may be more effective in reducing lipid peroxidation compared to ApAP and deserves further study.(29) The extrapolation of our findings are limited by the small sample size and larger studies in different populations are necessary to validate or refute our results. Lastly, we did not measure ascorbate concentrations (indicator of the endogenous anti-oxidant status) although prior studies indicate decrease concentrations in children undergoing CPB.(24)
In summary, CPB in children is associated with hemolysis and lipid peroxidation. Although ApAP attenuated the increase in plasma isofuran concentrations it did not affect postoperative creatinine or urine NGAL concentrations. Future studies are needed to establish whether other therapies that attenuate or prevent the effects of free hemoglobin result in more effective inhibition of lipid peroxidation in patients undergoing CPB.
Acknowledgments
Funding Sources: This research was funded by the NIH [P50 GM015431-43 and T32HL105334-02], by the Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06 and by an Investigator Initiated Trial grant from Cadence Pharmaceuticals.
We would like to thank Jeff Petro and Anthony DeMatteo for their technical assistance and Patricia Wright RN for her nursing assistance.
Footnotes
Clinical Trial Registration Information: NCT01228305
References
- 1.Billings FTt, Ball SK, Roberts LJ, 2nd, et al. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. 2011;50:1480–1487. doi: 10.1016/j.freeradbiomed.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vercaemst L. Hemolysis in cardiac surgery patients undergoing cardiopulmonary bypass: A review in search of a treatment algorithm. J Extra Corpor Technol. 2008;40:257–267. [PMC free article] [PubMed] [Google Scholar]
- 3.Kato GJ. Haptoglobin halts hemoglobins havoc. J Clin Invest. 2009;119:2140–2142. doi: 10.1172/JCI40258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, et al. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 2010;77:913–920. doi: 10.1038/ki.2010.24. [DOI] [PubMed] [Google Scholar]
- 5.Gbadegesin R, Zhao S, Charpie J, et al. Significance of hemolysis on extracorporeal life support after cardiac surgery in children. Pediatr Nephrol. 2009;24:589–595. doi: 10.1007/s00467-008-1047-z. [DOI] [PubMed] [Google Scholar]
- 6.Hill SE, Gottschalk LI, Grichnik K. Safety and preliminary efficacy of hemoglobin raffimer for patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2002;16:695–702. doi: 10.1053/jcan.2002.128416. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka K, Kanamori Y, Sato T, et al. Administration of haptoglobin during cardiopulmonary bypass surgery. ASAIO Trans. 1991;37:M482–483. [PubMed] [Google Scholar]
- 8.Boretti FS, Buehler PW, D’Agnillo F, et al. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest. 2009;119:2271–2280. doi: 10.1172/JCI39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baek JH, D’Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattabriga I, Pacini D, Lamazza G, et al. Intravenous paracetamol as adjunctive treatment for postoperative pain after cardiac surgery: A double blind randomized controlled trial. Eur J Cardiothorac Surg. 2007;32:527–531. doi: 10.1016/j.ejcts.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Iodice FG, Thomas M, Walker I, et al. Analgesia in fast-track paediatric cardiac patients. Eur J Cardiothorac Surg. 2011;40:610–613. doi: 10.1016/j.ejcts.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Aronoff DM, Oates JA, Boutaud O. New insights into the mechanism of action of acetaminophen: Its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin h2 synthases. Clin Pharmacol Ther. 2006;79:9–19. doi: 10.1016/j.clpt.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci U S A. 2010;107:2699–2704. doi: 10.1073/pnas.0910174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janz DR, Bastarache JA, Peterson JF, et al. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: An observational study*. Crit Care Med. 2013;41:784–790. doi: 10.1097/CCM.0b013e3182741a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming GA, Billings FTt, Klein TM, et al. Angiotensin-converting enzyme inhibition alters the inflammatory and fibrinolytic response to cardiopulmonary bypass in children. Pediatr Crit Care Med. 2011;12:532–538. doi: 10.1097/PCC.0b013e3181fe3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 17.Fessel JP, Porter NA, Moore KP, et al. Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc Natl Acad Sci U S A. 2002;99:16713–16718. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albers E, Donahue BS, Milne G, et al. Perioperative plasma f(2)-isoprostane levels correlate with markers of impaired ventilation in infants with single-ventricle physiology undergoing stage 2 surgical palliation on the cardiopulmonary bypass. Pediatr Cardiol. 2012;33:562–568. doi: 10.1007/s00246-012-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unger J, Filippi G, Patsch W. Measurements of free hemoglobin and hemolysis index: Edta- or lithium-heparinate plasma? Clin Chem. 2007;53:1717–1718. doi: 10.1373/clinchem.2007.091421. [DOI] [PubMed] [Google Scholar]
- 20.Milne GL, Sanchez SC, Musiek ES, et al. Quantification of f2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 21.Moore KP, Holt SG, Patel RP, et al. A causative role for redox cycling of myoglobin and its inhibition by alkalinization in the pathogenesis and treatment of rhabdomyolysis-induced renal failure. J Biol Chem. 1998;273:31731–31737. doi: 10.1074/jbc.273.48.31731. [DOI] [PubMed] [Google Scholar]
- 22.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan CJ, Zappitelli M, Robertson CM, et al. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr. 2013;162:120–127. e121. doi: 10.1016/j.jpeds.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 24.Christen S, Finckh B, Lykkesfeldt J, et al. Oxidative stress precedes peak systemic inflammatory response in pediatric patients undergoing cardiopulmonary bypass operation. Free Radic Biol Med. 2005;38:1323–1332. doi: 10.1016/j.freeradbiomed.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Leijala M, Peltola K, Aronen M, et al. Comparison of hollow fibre membrane oxygenators during cardiopulmonary bypass in children: Dideco masterflo versus terumo capiox ii. Perfusion. 1990;5:33–43. doi: 10.1177/026765919000500105. [DOI] [PubMed] [Google Scholar]
- 26.Morgan IS, Codispoti M, Sanger K, et al. Superiority of centrifugal pump over roller pump in paediatric cardiac surgery: Prospective randomised trial. European Journal of Cardio-Thoracic Surgery. 1998;13:526–532. doi: 10.1016/s1010-7940(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 27.Chukwuemeka AO, Turtle MR, Trivedi UH, et al. A clinical evaluation of platelet function, haemolysis and oxygen transfer during cardiopulmonary bypass comparing the quantum hf-6700 to the hf-5700 hollow fibre membrane oxygenator. Perfusion. 2000;15:479–484. doi: 10.1177/026765910001500602. [DOI] [PubMed] [Google Scholar]
- 28.Alsabbagh MM, Asmar A, Ejaz NI, et al. Update on clinical trials for the prevention of acute kidney injury in patients undergoing cardiac surgery. American journal of surgery. 2013;206:86–95. doi: 10.1016/j.amjsurg.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Reeder BJ, Wilson MT. Desferrioxamine inhibits production of cytotoxic heme to protein cross-linked myoglobin: A mechanism to protect against oxidative stress without iron chelation. Chem Res Toxicol. 2005;18:1004–1011. doi: 10.1021/tx049660y. [DOI] [PubMed] [Google Scholar]