Abstract
Outcomes for patients with glioblastoma remain poor despite aggressive multimodal therapy. Immunotherapy with genetically modified T cells expressing chimeric antigen receptors (CARs) targeting IL13R[.alpha]2, HER2, EGFRvIII, or EphA2 has shown promise for the treatment of glioma in preclinical models. Based on IL13Rα2-targeted immunotoxins that contain IL13 molecules with one or two amino acid substitutions (IL13 muteins) to confer specificity to IL13Rα2, investigators have constructed CARs with IL13 muteins as antigen binding domains. While the specificity of IL13 muteins in the context of immunotoxins is well characterized, limited information is available for CAR T cells. We constructed four 2nd generation CARs with IL13 muteins with one or two amino acid substitutions. T cells expressing all four CARs recognized IL13Rα1 or IL13Rα2 recombinant protein in contrast to control protein (IL4R) as judged by IFNα production. IL13Rα2 protein induced significantly more IL2, indicating that IL13 mutein-CAR T cells have a higher affinity to IL13Rα2 than IL13Rα1. In cytotoxcity assays, CAR T cells killed IL13Rα1- and/or IL13Rα2-positive cells in contrast to IL13Rα1- and IL13Rα2-negative controls. While we observed no significant differences between IL13 mutein CAR T cells in vitro, only T cells expressing IL13 mutein CARs with an E13K amino acid substitution had antitumor activity in vivo that resulted in a survival advantage of treated animals. Our study highlights that the specificity/avidity of ligands is context-dependent and that evaluating CAR T cells in preclinical animal model is critical to assess their potential benefit.
Keywords: Cancer, CAR, GBM, IL13Rα1, IL13Rα2, Immunotherapy, T cells
INTRODUCTION
The outcome for glioblastoma (GBM) remains poor and immunotherapy with vaccines or GBM-specific T cells is one attractive strategy to improve survival, since it does not rely on the cytotoxic mechanisms employed by conventional therapies such as chemotherapy and radiation [1-4]. Genetic modification with chimeric antigen receptors (CARs) allows for the rapid generation of tumor-specific T cells [5]. For GBM-targeted T-cell therapy several antigens are actively being pursued including interleukin 13 receptor alpha 2 (IL13Rα2), human epidermal growth factor receptor 2 (HER2), epidermal growth factor variant III (EGFRvIII), and erythropoietin-producing hepatocellular carcinoma A2 (EphA2) [6-11]. The majority of clinical studies with CAR T cells so far have focused on targeting CD19-positive hematological malignancies, and while dramatic clinical responses were observed, several patients also developed life-threatening cytokine storms [12-17]. Other reported side effects of CAR T-cell therapies include a death of one patient, who received 1010 HER2-CAR T cells after a lymphodepleting conditioning regimen, and local inflammatory responses at non-malignant tissue sites, which expressed the targeted antigen [18, 19].
Hence, one important aspect of preclinical testing of CAR T cells is to evaluate their specificity. CARs consist of an ectodomain, which is most commonly derived from a single chain variable fragment (scFv), a transmembrane domain, and an endomain. The endodomain contains either one (1st generation), two (2nd generation), or three (3rd generation) signaling domains derived from CD3ζ and/or co-stimulatory molecules [5]. Current IL13Rα2-specific CARs do not contain scFvs, but an IL13 mutein with one or two amino acid substitutions to preferentially redirect T cells to IL13Rα2 [8, 11]. While one study showed that T cells expressing a 1st generation CAR with an IL13 mutein, which contained an E13Y amino acid substitution, did not recognize IL13Rα1-positive cells [8], another study indicated cross-reactivity to IL13Rα1 of T cells expressing a 2nd generation CAR containing an IL13 mutein with E13K and R109K amino acid substitutions [11].
Here we report the construction of four 2nd generation CARs containing IL13 muteins with one (E13K or E13Y) or two amino acid substitution (E13K.K105R; E13Y.K105R). We show that all four CARs recognize IL13Rα1 and IL13Rα2 ex vivo, and that only CARs with an E13K or E13K.K105R amino acid substitutions have antitumor activity in vivo that is associated with a survival advantage of treated animals.
MATERIAL AND METHODS
Blood donors and cell lines
Blood samples were obtained from healthy subjects on a protocol approved by the Institutional Review Board of Baylor College of Medicine. The cell lines U373, U87, T98G, A431, 293T and Raji were purchased from the American Type Culture Collection (ATCC; Manassas, VA). SNT16 cells were kindly provided by Dr. Norio Shimizu (Tokyo Medical and Dental University, Tokyo, Japan). The generation of U373 cells expressing an enhanced green fluorescent protein firefly luciferase fusion gene (U373.eGFP.ffLuc) was previously reported [7]. To generate Raji cells expressing IL13Rα1 or IL13Rα2 we cloned cDNAs encoding IL13Rα1 or IL13Rα2 (Origene, Rockville, MD) into pCDH-CMVMCS-EF1-GFP+puro (System Bioscience, Mountainview, CA). Cloning was verified by sequencing (Seqwright, Houston, TX). Raji cells were transduced with VSVG-pseudotyped lentiviral vectors to generate Raji-GFP, Raji-IL13Rα1, and Raji-IL13Rα2. Cell lines were grown in RPMI or DMEM (Thermo Scientific HyClone, Waltham, MA; Lonza, Basel, Switzerland) with 10% fetal calf serum (FCS; HyClone, Logan, UT) and 2 mM GlutaMAX-I (Invitrogen, Carlsbad, CA).
Generation of IL13-mutein CARs
Codon-optimized mini genes flanked by 5’ NcoI and 3’ BamHI sites were synthesized by GeneArt (Invitrogen, Carlsbad, CA) containing the immunoglobulin heavy-chain leader peptide [20] and IL13 muteins with one (E13K; E13Y) or two amino acid substitutions (E13K.K105R; E13Y.K105R). IL13 muteins were subcloned into an SFG retroviral vector containing the human IgG1-CH2CH3 domain, a CD28 transmembrane domain, and costimulatory domains derived from CD28 and the CD3ζ-chain [21, 22]. Cloning was verified by sequencing (Seqwright, Houston, TX). The construction of the control CAR specific for murine and human fibroblast activation protein (mhFAP) has been described elsewhere [23].
Retrovirus production and transduction of T cells
RD114-pseudotyped retroviral particles were generated as previously described [6]. The protocol to transduce T cells with retroviral particles has been described in detail [7]. To activate T cells, non-tissue culture treated 24 well plates were coated with 0.5 mL OKT3 (1μg/mL) and CD28 (1μg/ml) monoclonal antibodies (BD Biosciences, Mountain View, CA) for 24 hours. On day 1, the antibody solution was removed, and wells were washed with complete media before plating 1x106 peripheral blood mononuclear cells (PBMCs) per well. On day 2, recombinant human interleukin-2 (IL2; Proleukin; Chiron, Emeryville, CA) was added at a final concentration of 100 units/mL, and a separate non-tissue culture treated 24 well plate was coated with 1 mL of RetroNectin® (7μg/mL; Clontech, Mountainview, CA). On day 3, the RetroNectin® solution was removed and wells were washed with complete media. Each well was coated twice with 0.5 mL of retroviral supernatant for 30 minutes before adding 1.5 mL retroviral supernatant, 0.5 mL of activated PBMCs (2.5x105 cells) and IL2 (final concentration of 50 units/mL). Forty eight to 72 h post transduction cells were transferred to a new 24-well pate and expanded in the presence of 50 to 100 units/mL of IL2 for 10 to 15 days before use. Non-transduced (NT) T cells, used as controls, were activated with OKT3/CD28 and expanded in parallel with 50-100 U/mL IL2.
Flow cytometry
A FACSCalibur instrument (Becton Dickinson, San Jose, CA) and CellQuest software (Becton Dickinson, San Jose, CA) were used for all flow cytometric analyses, analyzing >10,000 events; in all cases, negative controls included isotype antibodies. Cells were washed once with PBS containing 1% fetal bovine serum (FACS buffer) before addition of antibodies. After 30 min of incubation at 4°C in the dark, the cells were washed once and fixed in 0.5% paraformaldehyde/FACS buffer before analysis. T cells were analyzed with fluorescein-conjugated monoclonal antibodies (Becton Dickinson, San Jose, CA) directed against CD3,CD4, CD8, CD45RA, CD45RO, CD56, CD62L and CCR7 surface proteins. To determine surface expression of CARs on T cells a CH2CH3 Cy5 antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) was used. To determine IL13Rα1 and IL13Rα2 expression of tumor cells polyclonal goat antibodies specific for IL13Rα1 and IL13Rα2 (R&D Systems, Minneapolis, MN) were used as primary antibodies and rabbit anti-goat Alexa (Jackson ImmunoResearch Laboratories, West Grove, PA) was used as a secondary antibody.
Co-culture assays
Recombinant protein co-culture assay
Non-tissue culture 24-well plates were precoated with recombinant human IL13Rα1-, IL13Rα2-, or IL4R-Fc chimera, (R&D Systems, Minneapolis, MN) at a final concentration of 15.6 to 500 ng/well. Plates were washed once using RPMI, and IL13 mutein-CAR and NT T cells were plated. After 24 hours (h) of culture supernatant was harvested and IFNγ and IL2 release was measured by ELISA per the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Cell culture co-culture assay
IL13 mutein-CAR T cells were cocultured with genetically modified Raji cells expressing human IL13Rα1, IL13Rα2 or GFP at a 1:4 effector to target (E:T) ratio in a 48-well plate. NT and mhFAP-CAR T cells served as controls. After 24h of incubation, culture supernatants were harvested, and the presence of IFNγ and IL2 was determined by ELISA as per the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Cytotoxicity assays
Standard chromium (51Cr) release assays were performed as previously described [24]. Briefly, 51Cr-labeled target cells were mixed with decreasing numbers of effector cells at E:T ratios of 40:1, 20:1, 10:1, and 5:1. Target cells incubated in complete medium alone or in 1% Triton X-100 were used to determine spontaneous and maximum 51Cr release. After 4 h, released radioactivity was measured in a gamma counter (Cobra Quantum; Perkin-Elmer). The mean percentage of specific lysis of triplicate wells was calculated according to the following formula: (test release − spontaneous release)/ (maximal release − spontaneous release) × 100.
Quantitative real time PCR
RNA was isolated from different tumor cell lines using RNeasy Mini Kit (Qiagen, Valencia, CA). Relative quantification of IL-13Rα1, IL-13Rα2, and GAPDH mRNA expression was done using TaqMan® One-Step RT-PCR Master Mix Reagents (Applied Biosystems, Branchburg, NJ) using the primer/probes for human or murine IL13Rα1, IL13Rα2, and GAPDH (all from Applied Biosystems).
Orthotopic xenograft SCID mouse model
All animal experiments followed a protocol approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. For animal experiments ICR-SCID mice (IcrTac:ICR-Prkdcscid; Taconic, Hudson, NY) were used and we used our previously described the surgical procedure [7]. Briefly, U373.eGFP.ffLuc cells (1 × 105 in 2.0 μL) were injected 3 mm deep to the bregma, corresponding to the center of the right caudate nucleus over 5 minutes. Seven days after tumor cell injection, animals were treated with 2 × 106 NT or IL13 mutein-CAR T cells from the same donor in 2 μL to the same tumor coordinates.
Bioluminescence imaging
Isofluorane anesthetized animals were imaged using the IVIS® system (IVIS, Xenogen Corp., Alameda, CA) as previously described [7]. The photons emitted from the luciferase-expressing tumor cells were quantified using Living Image software (Caliper Life Sciences, Hopkinton, MA). A pseudo-color image representing light intensity (blue least intense and red most intense) was generated and superimposed over the grayscale reference image. Mice were euthanized when the tumor radiance was greater than 1×109 on two occasions or when they met euthanasia criteria (neurological deficits, weight loss, signs of distress) in accordance with the Center for Comparative Medicine at Baylor College of Medicine.
Statistical analysis
All in vitro experiments were performed in triplicate, GraphPad Prism 5 software (GraphPad software, Inc., La Jolla, CA) was used for statistical analysis. Measurement data were presented as mean ± standard deviation (SD). The differences between means were tested by appropriate tests including t-test or non-parametric Mann Whitney U test. The significance level used was P < 0.05. For the mouse experiments, fold changes in tumor radiance from baseline at each time point were calculated. Survival determined from the time of tumor cell injection was analyzed by the Kaplan-Meier method.
RESULTS
Generation of IL13 mutein-CAR T cells
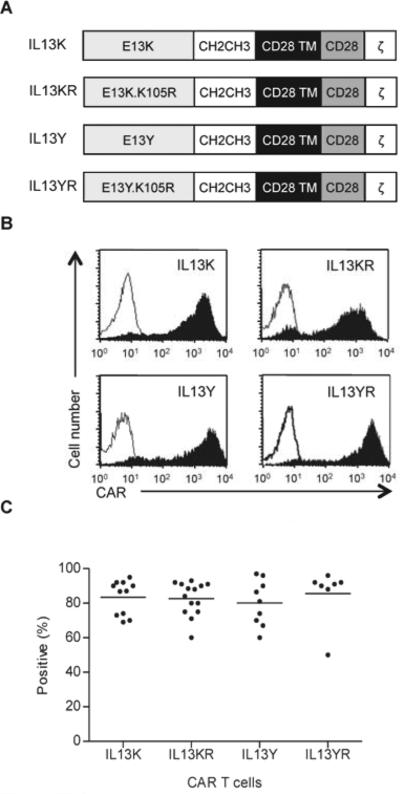
We constructed four SFG retroviral vectors encoding IL13 mutein-CARs. While the IL13 mutein-CARs had identical IgG1-CH2CH3 spacers, CD28 transmembrane domains, and signaling domains derived from CD28 and the TCR-ζ chain, their extracellular binding domain contained IL13-muteins with increased affinity to IL13Rα2. Two had single amino acid substitutions at position 13 (E13K or E13Y; IL13Y-CAR, IL13K-CAR), and two had an additional amino acid substitution at position 105 (K105R; IL13YR-CAR, IL13KR-CAR) (Fig.1A). CD3/CD28-activated T cells from healthy donors were transduced with RD114-pseudotyped retroviral particles encoding the four different IL13 mutein-CARs. Five to 6 days post transduction FACS analysis was performed to determine CAR expression; 80.2 to 85.6% (n=7-14, Fig. 1B,C) of the T cells expressed IL13 mutein-CARs with no significant differences between all four constructs. There was no difference in T-cell phenotype, and transduced T cells included CD4-positive and CD8-positive subpopulations (mean 34.8±3.73%; mean 65.9±2.4%), and central (CD3+/CD62L+: 47.1±8.6%) as well and effector memory T cells (CD3+/CD62L-/CCR7-: 33.0±8.5%) (Supplementary Fig. 1).
Figure 1. Generation of IL13 mutein-CAR T cells.
(A) Scheme of IL13 mutein-CARs. All four constructs consisted of an IgG1-CH2CH3 domain, a CD28 transmembrane (TM) domain, and costimulatory domains derived from CD28 and the CD3 ζ-chain. The binding domain contained different IL13 muteins with one (E13K or E13Y) or two amino acid substitution (E13K.K105R; E13Y.K105R). (C,B) CAR expression was confirmed using FACS analysis. Representative plots (B) and summary data (C) is shown (mean 80.2% – 85.6%, n=7-14 per CAR construct).
IL13 mutein-CAR T cells recognize IL13Rα1 and IL13Rα2
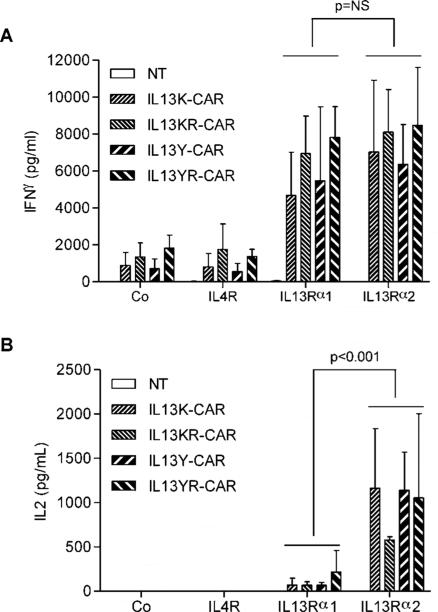
To evaluate the specificity of T cells expressing the different IL13 mutein-CARs we initially took advantage of recombinant proteins encoding IL13Rα1, IL13Rα2, and IL4R. IL13 mutein-CAR or non-transduced (NT) T cells were incubated on IL13Rα1- or IL13Rα2-coated plates with IL4R- or non-coated plates serving as controls. T cells expressing all four CAR constructs produced significant levels of IFNγ (p<0.001) when stimulated with recombinant IL13Rα1 or IL13Rα2 proteins in comparison to IL4R stimulated T cells (Fig. 2A). However, only stimulation with IL13Rα2 resulted in significant IL2 production (p<0.001) (Fig. 2B). No significant IFNγ or IL2 production was observed in co-cultures with NT-T cells. These studies were performed with 24-well plates, which were coated with 500μg of recombinant proteins per well. To evaluate if there was a difference in IFN[.gamma] secretion with lower protein concentrations, IL13Rα1 or IL13Rα2 recombinant proteins were serially diluted. Starting at 125[.proportional]g IL13Rα2/well IL13Y- and IL13K-CAR T cells secreted significant amounts of IFN[.gamma], while 250[.proportional]g IL13Rα1/well was needed to induce IFN[.gamma] secretion. At ≤ 62.5μg/well neither protein induced significant IFN[.gamma] secretion (Supplementary Fig. 2).
Figure 2. IL13 mutein-CAR T cells release cytokines after stimulation with recombinant IL13Rα1 and IL13Rα2 proteins.
IL13 mutein-CAR or non-transduced (NT) T cells were stimulated with recombinant IL13Rα1, IL13Rα2, or IL4R proteins. After 24h IFNγ (A) or IL2 (B) was measured by ELISA (n=3). T cells expressing all four CAR constructs expressed significant levels of IFNγ (p<0.001) when stimulated with recombinant IL13Rα2 or IL13Rα1 proteins in comparison to IL4R stimulated T cells. Only stimulation with recombinant IL13Rα2 protein induced significant levels of IL2 (p<0.001).
IL13 mutein-CAR T cells recognize and kill IL13Rα2-positive cells
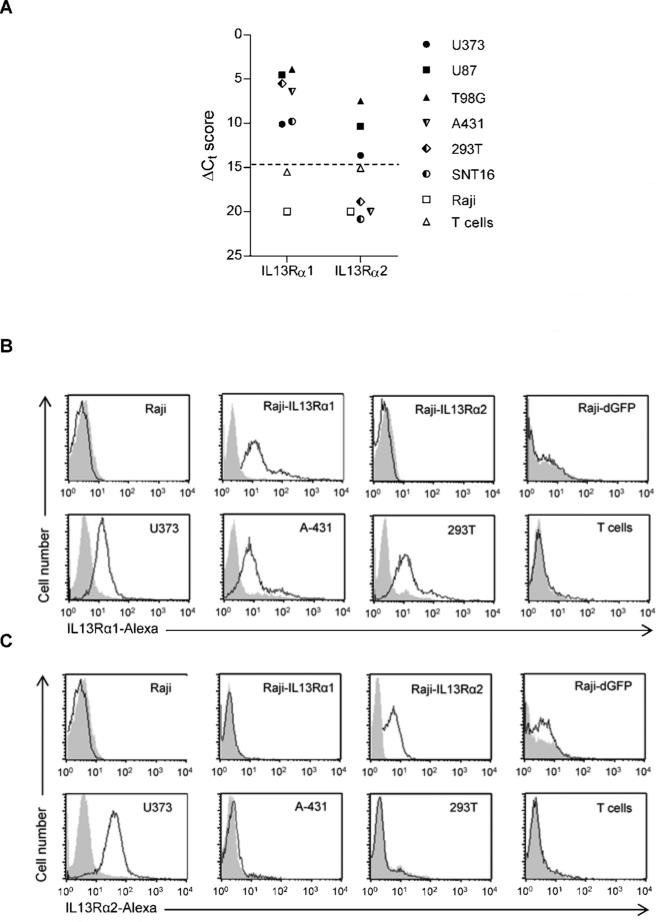
We screened a panel of cell lines for the expression of IL13Rα1 and IL13Rα2 using qRT-PCR and FACS analysis (3 glioma cell lines: U87, U373, T98G; 2 lymphoma cell lines: SNT16, Raji; the epidermoid carcinoma cell line A431; 293T cells and NT T cells). U87, U373, T98G expressed IL13Rα1 and IL13Rα2; SNT16, A431, and 293T expressed only IL13Rα1, and Raji and NT T cells were negative for both (Fig. 3A-C).
Figure 3. Expression of IL13Rα1 and IL13Rα2 in cell lines.
(A) qRT-PCR for IL13Rα1 or IL13Rα2. FACS analysis for IL13Rα1 (B) or IL13Rα2 (C).
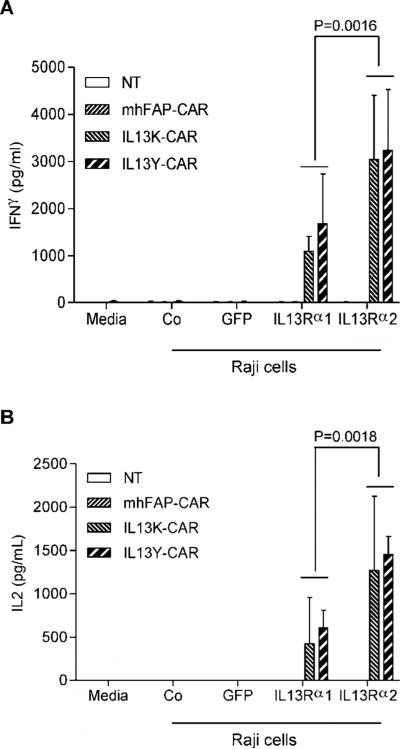
Since none of the cell lines only expressed IL13Rα2, we genetically modified Raji cells with a lentiviral vector encoding IL13Rα2 and GFP (Raji-IL13Rα2; Fig. 3C). To assess IL13Rα1 and IL13Rα2 recognition by IL13 mutein-CAR T cells in the same cell background, we generated Raji cells expressing IL13Rα1 and GFP (Raji-IL13Rα1) and as controls Raji cells expressing GFP (Raji-GFP) (Fig. 3B). Stimulation of T cells, expressing IL13 mutein-CARs with E13Y or E13K amino acid substitutions (IL13Y- or IL13K-CARs), with Raji-IL13Rα1 or Raji-IL13Rα2 cells resulted in significant production of IFNγ (p<0.001) and IL2 (p<0.001) in comparison to stimulations with Raji or Raji-GFP cells. However, Raji-IL13Rα2 cells induced significant higher levels of IFN[.gamma] (p=0.0016) and IL2 (p=0.0018) in comparison to Raji-IL13Rα1 cells (Fig. 4A,B). No significant IFNγ or IL2 production was observed from NT T cells or T cells expressing a CAR specific for an unrelated antigen (mhFAP).
Figure 4. IL13 mutein-CAR T cells recognize IL13Rα1- and IL13Rα2-positive cells.
(A,B) IL13 mutein-CAR T cells (IL13Y-CAR, IL13K-CAR) were cocultured with genetically modified Raji cells expressing IL13Rα1, IL13Rα2 or GFP, or unmodified Raji cells (Co) at a 1:4 E:T ratio. NT and mhFAP-CAR T cells served as controls. After 24h IFNγ (A) or IL2 (B) was measured by ELISA (n=3). Only stimulation of IL13 mutein-CAR T cells with Raji-IL13Rα1 or Raji-IL13Rα2 cells resulted in significant production of IFNγ (p<0.001) and of IL2 (p<0.001). Raji-IL13Rα2 cells induced significant higher levels of IFNγ (p=0.0016) and IL2 (p=0.0018) in comparison to Raji-IL13Rα1 cells.
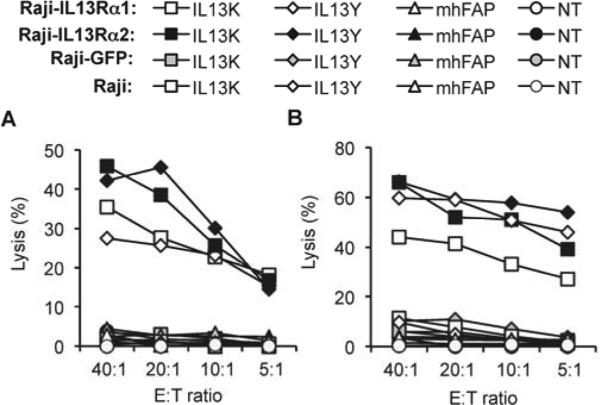
IL13Y- and IL13K-CAR T cells also killed Raji-IL13Rα1 and Raji-IL13Rα2 cells in contrast to Raji and Raji-GFP cells (Fig. 5). NT T cells and mhFAP-CAR T cells had no cytolytic activity, demonstrating that cell killing depends on the cell surface expression of IL13Rα1 or IL13Rα2 on target cells and IL13 mutein-CARs on T cells. We confirmed for all four IL13 mutein-CAR constructs that T cells expressing these CARs kill cells that express IL13Rα1 and IL13Rα2 (U373) and cell lines (A431 or 293T) that only express IL13Rα1 (Supplementary Fig. 3). These results indicate that T cells expressing IL13 mutein-CARs recognize IL13Rα1- and Il13Rα2-positive target cells as judged by cytokine production and cytolytic activity.
Figure 5. IL13 mutein-CAR T cells kill IL13Rα1- and IL13Rα2-positive cells.
(A,B) IL13 mutein-CAR T cells (IL13Y-CAR, IL13K-CAR) killed Raji-IL13Rα1and Raji-IL13Rα2 cells in contrast to Raji and Raji-GFP cells in a standard 4h cytotoxicity assay. Controls (NT T cells or mhFAP-CAR T cells) exhibited no cytolytic activity (results for 2 donors are shown).
Treatment of glioma xenografts with T cells expressing IL13 mutein-CARs with E13K amino acid substitutions results in improved overall survival
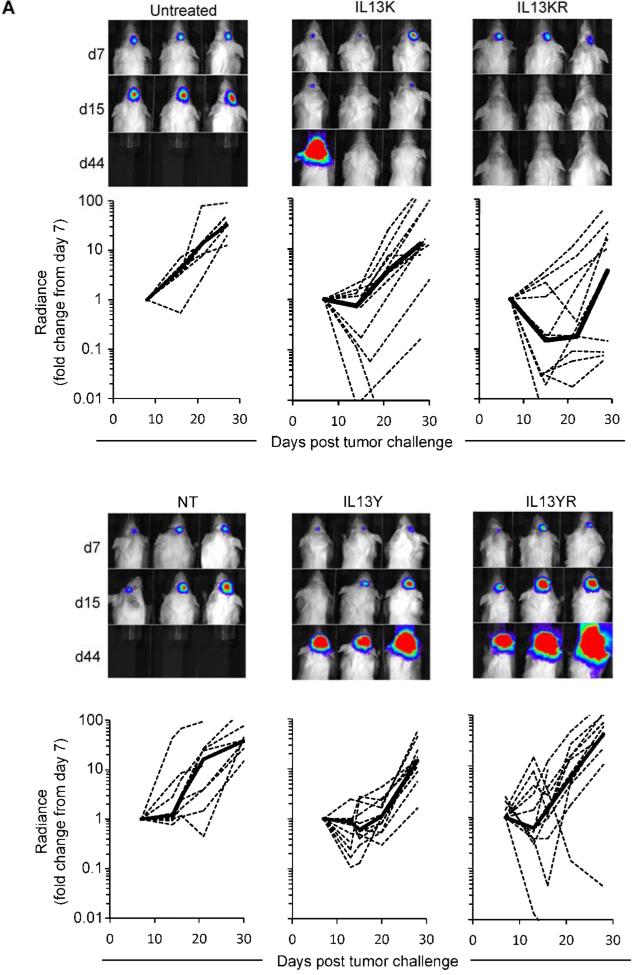
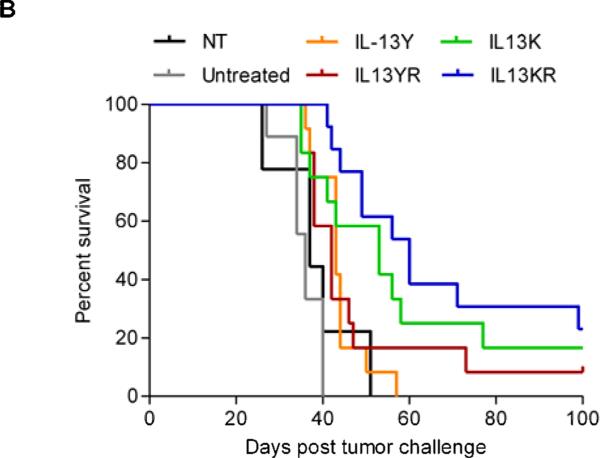
Since the recognition of IL13Rα1 will most likely limit the application of IL13 mutein-CAR T cells to local administration, we used the orthotopic U373 glioma xenograft model in which T cells are directly injected into tumors (20). 1 × 105 U373.eGFP.ffLuc cells were injected sterotactically into brains of SCID mice on day 0. On day 7, 2 × 106 IL13 mutein-CAR T cells were injected intracranially into the previous stereotactic tumor coordinates (n=11 per CAR construct). Mice treated with NT T cells (n=9) and untreated mice (n=10) served as controls. Response to therapy was defined as a greater than 75% reduction in bioluminescence signal on day 7 post T-cell injection. Three of 11 mice treated with IL13Y-CAR T cells, 3 of 11 mice treated with YR-CAR T cells, 4 of 11 mice treated with IL13K-CAR, and 7 of 11 mice treated with IL13KR-CAR T cells had a response. NT-T cells had no antitumor activity (Fig. 6A). While IL13K- or IL13KR-specific T-cell therapy resulted in a significant increase in overall survival in comparison to injection of NT-T cells (NT-T cells vs IL13K-CAR T cells, p=0.009; NT-T cells vs IL13KR-CAR T cells, p<0.001), IL13Y- or IL13YR-CAR T-cell therapy did not (NT-T cells vs IL13Y-CAR T cells, p=0.322; NT-T cells vs IL13KR-CAR T cells, p=0.253; Fig. 6B).
Figure 6. Treatment of glioma xenograft with T cells expressing IL13 mutein-CARs with E13K amino acid substitutions results in improved overall survival.
U373 glioma bearing mice were treated on day 7 with IL13K- (n=11), IL13Y- (n=11), IL13KR- (n=11), or IL13YR-CAR (n=11) T cells. Untreated mice (n=10) or mice treated with NT T cells (n=9) served as controls. (A) Representative images for each group and quantitative bioluminescence (radiance = photons/sec/cm2/sr) imaging data for all mice are shown (dotted lines: individual mice; solid lines: median). (B) Kaplan-Meier survival analysis (NT-T cells vs IL13K-CAR T cells, p=0.009; NT-T cells vs IL13KR-CAR T cells, p<0.001; NT-T cells vs IL13Y-CAR T cells, p=0.322; NT-T cells vs IL13KR-CAR T cells, p=0.253).
DISCUSSION
Here we describe the construction of CARs with IL13-muteins as ectodomains and demonstrate that these CARs not only recognize IL13Rα2 but also IL13Rα1. While we observed no functional difference between IL13 mutein-CARs in vitro, only CARs with an E13K amino acid substitution had antitumor activity in an orthotopic xenograft SCID mouse model of GBM resulting in a survival advantage of treated animals.
IL13Rα2 is a promising immunotherapy target for GBM [25]. The receptor has been targeted with immunotoxins, vaccines, and antigen-specific T cells with encouraging results [8, 11, 26-28]. To redirect immunotoxin to IL13Rα2, investigators have taken advantage of IL13 muteins that have increased binding affinity to IL13Rα2 [29, 30]. IL13 contains 4 α−helices (A, B, C, D), and amino acid substitutions at position 13 (E13Y or E13K) within α-helix A greatly decreases the ability of IL13 to bind to IL13Rα1. In addition, amino acid substitutions in α-helix D (K105R, R109K) increase the affinity of IL13 to IL13Rα2 [30, 31]. Pseudomonas-based IL13 mutein immunotoxins have shown preferential killing of IL13Rα2-positive cells, and based on these findings 2 groups of investigators have generated IL13-mutein-based CARs [8, 11]. CD8-positive T-cell clones expressing a 1st generation CAR with an E13Y IL13 mutein only recognized IL13Rα2-positive target cells, but these T-cell clones did not produce IL2 upon stimulation. In vivo these cells had anti-glioma activity, however the targeted glioma cell line was genetically modified to express IL2 [8]. Kong et al. reported the construction of a 2nd generation CAR with an E13K.R109K IL13 mutein. T cells expressing this CAR recognized IL13Rα1-positive cells in co-culture assays and had antitumor activity in the U251 (U373) xenograft model with a long-term, disease-free survival rate of ~30% [11].
Our study now extends these findings and clarifies the specificity of CARs containing IL13 muteins. We constructed 4 CARs that contained 2 or 4 amino acid substitutions. Two CARs contained E13Y or E13K amino acid substitutions in α-helix A and 2 CARs had an additional amino acid substitution in α-helix D (K105R). We used K105R instead of R109K since the resulting IL13 mutein has a higher affinity towards IL13Rα2 [30]. We demonstrate that T cells expressing IL13 mutein-CARs recognize IL13Rα1 and IL13Rα2 recombinant proteins and kill Raji cells that express either IL13Rα1 or IL13Rα2. The killing of IL13Rα1-positive target cells was unexpected since the recognition of IL13Rα1 by IL13 mutein-based immunotoxins is greatly reduced [29, 30, 32]. Our results suggest that the overall avidity of IL13 mutein CARs arrayed on the cell surface of T cells is greater towards IL13Rα1 than IL13 mutein-based immunotoxins, which only contain a single binding site. Nevertheless, IL13Rα2 recombinant protein and IL13Rα2-expressing Raji cells induced significantly higher levels of IL2 by IL13 mutein-CAR T cells than their IL13Rα1 counterparts. Since effector function of CAR T cells depends in part on the affinity of the CAR [33], our results suggest that IL13 mutein-CAR T cells have a higher affinity towards IL13Rα2 than IL13Rα1.
Our finding that IL13 mutein-CAR T cells recognize IL13Rα1 should not preclude their local administration in humans, due to the clinical experience with the local, convection- enhanced delivery of a pseudomonas-immunotoxin into GBMs, which recognize IL13Rα1 and IL13Rα2 (Cintredekin besudotox) [28]. No dose-limiting toxicity was observed, and patients with optimally positioned catheters had a median survival of 55.6 weeks, which is encouraging in the setting of progressive high grade glioma [28]. In addition, we observed no side effects post injection of IL13 mutein-CAR T cells, which is especially encouraging since our CARs recognize murine IL13Rα1 and IL13Rα2 (Supplementary Fig. 4). However, infused IL13 mutein-CAR T cells persisted for less than one week, and additional safety studies are needed with IL13 mutein-CAR T cells, that expand and persist for several weeks in a xenograft or immune competent mouse model. Another strategy to minimize potential side effects is the use of T cells that express a CAR containing an IL13Rα2-specific scFv ectodomain. Two groups have reported the isolation of IL13Rα2-specific scFvs [34, 35] and in the future these scFvs should be evaluated in the context of CAR T-cell therapy.
In vivo, only the injection of T cells, expressing IL13 mutein-CARs with an E13K amino acid substitution, was associated with improved survival. This was surprising since we did not observe any significant difference between E13Y- and E13K-mutein CAR T cells in vitro. A recent study suggest that structural components of CARs, which are not involved in signaling, influence the in vivo function of CAR T cells [33]. Accordingly, amino acids with different side chains (K: positive side chain; Y: hydrophobic side chain) in the antigen-binding domain of a CAR could potentially impact CAR T-cell function in vivo. The long-term survival rate of ~ 20% was similar to the reported rate by Kong et al., who used a 2nd generation E13K.R109K IL13 mutein-CAR targeting the same glioma cell line in vivo [11]. To evaluate how long T cells persist in vivo, T cells were ‘double transduced’ with retroviral vectors encoding the IL13KR CAR or eGFP.ffLuc. CAR T cells persisted for less than one week at the tumor site, as judged by bioluminescence imaging (Supplementary Fig. 5), indicating that limited T-cell persistence at the tumor site is most likely responsible for tumor progression. Since we could not detect bioluminescence signal outside the glioma site, T cells did not migrate away from gliomas, but most likely underwent apoptosis. Inclusion of additional costimulatory domains besides CD28 might improve persistence of infused T cells [22, 36]. In addition, targeting multiple tumor antigens might improve antitumor effects. For example, we have shown that targeting glioma with IL13 mutein- and HER2-CAR T cells results in enhanced antitumor effects in the U373 xenograft model [37].
In conclusion, we have generated IL13 mutein-based CARs and show that these CARs recognize not only IL13Rα2 but also IL13Rα1. T cells expressing IL13 mutein-CARs with E13K amino acid substitutions had antitumor activity in vivo resulting in a survival advantage of treated animals. Our study highlights that the specificity/avidity of ligands is context-dependent and provides the rationale to develop IL13Rα2-specific CARs with an IL13Rα2-specific scFv as an antigen recognition domain to prevent cross reactivity to IL13Rα1. In addition, our results indicate that evaluating CAR T cells in preclinical animal model is critical to assess their potential benefit.
Supplementary Material
Acknowledgements
This work was supported by The Dana Foundation (SG and NA), The Clayton Foundation for Research (SG and NA), and the James S McDonnell Foundation (SG). KKHC is a Melnick scholar and was supported by NIH grants 5T32HL092332 and 5T32GM007330.
ABBREVIATIONS
- CAR
chimeric antigen receptor
- EGFRvIII
epidermal growth factor variant III
- EphA2
erythropoietin-producing hepatocellular carcinoma A2
- FACS
fluorescence activated cell sorting
- GBM
glioblastoma
- GFP
green fluorescent protein
- HER2
human epidermal growth factor receptor 2
- IFN
interferon
- IL
interleukin
- IL13Rα1
interleukin 13 receptor alpha 1
- IL13Rα2
interleukin 13 receptor alpha 2
- scFv
single chain variable fragment
- NT
non-transduced
- PBMC
peripheral blood mononuclear cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The Center for Cell and Gene Therapy has a Research Collaboration with Celgene and bluebird bio. SK, KKHC, ZY, NA, and SG have patent applications in the field of T-cell and gene modified T-cell therapy for cancer.
References
- 1.Okada H, Kohanbash G, Zhu X, Kastenhuber ER, Hoji A, Ueda R, et al. Immunotherapeutic approaches for glioma. Crit Rev Immunol. 2009;29:1–42. doi: 10.1615/critrevimmunol.v29.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell DA, Sampson JH. Toward effective immunotherapy for the treatment of malignant brain tumors. Neurotherapeutics. 2009;6:527–38. doi: 10.1016/j.nurt.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow KK, Gottschalk S. Cellular immunotherapy for high-grade glioma. Immunotherapy. 2011;3:423–34. doi: 10.2217/imt.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finocchiaro G, Pellegatta S. Immunotherapy for glioma: getting closer to the clinical arena? Curr Opin Neurol. 2011;24:641–7. doi: 10.1097/WCO.0b013e32834cbb17. [DOI] [PubMed] [Google Scholar]
- 5.Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med. 2012;14:405–15. doi: 10.1002/jgm.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res. 2010;16:474–85. doi: 10.1158/1078-0432.CCR-09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, et al. T Cells Redirected to EphA2 for the Immunotherapy of Glioblastoma. Mol Ther. 2013;21:629–37. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–6. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 9.Bullain SS, Sahin A, Szentirmai O, Sanchez C, Lin N, Baratta E, et al. Genetically engineered T cells to target EGFRvIII expressing glioblastoma. J Neurooncol. 2009 doi: 10.1007/s11060-009-9889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther. 2012;23:1043–53. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong S, Sengupta S, Tyler B, Bais AJ, Ma Q, Doucette S, et al. Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clin Cancer Res. 2012;18:5949–60. doi: 10.1158/1078-0432.CCR-12-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–8. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 20.Wels W, Harwerth IM, Zwickl M, Hardman N, Groner B, Hynes NE. Construction, bacterial expression and characterization of a bifunctional single-chain antibody-phosphatase fusion protein targeted to the human erbB-2 receptor. Biotechnology (N Y ) 1992;10:1128–32. doi: 10.1038/nbt1092-1128. [DOI] [PubMed] [Google Scholar]
- 21.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–7. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–41. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Kakarla S, Chow KK, Mata M, Shaffer DR, Song XT, Wu MF, et al. Antitumor Effects of Chimeric Receptor Engineered Human T Cells Directed to Tumor Stroma. Mol Ther. 2013 doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottschalk S, Edwards OL, Sili U, Huls MH, Goltsova T, Davis AR, et al. Generating CTL against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive Immunotherapy of EBV-associated malignancies. Blood. 2003;101:1905–12. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen V, Conyers JM, Zhu D, Gibo DM, Dorsey JF, Debinski W, et al. IL-13Ralpha2-Targeted Therapy Escapees: Biologic and Therapeutic Implications. Transl Oncol. 2011;4:390–400. doi: 10.1593/tlo.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwami K, Shimato S, Ohno M, Okada H, Nakahara N, Sato Y, et al. Peptide-pulsed dendritic cell vaccination targeting interleukin-13 receptor alpha2 chain in recurrent malignant glioma patients with HLA-A*24/A*02 allele. Cytotherapy. 2012 doi: 10.3109/14653249.2012.666633. [DOI] [PubMed] [Google Scholar]
- 27.Brown CE, Starr R, Naranjo A, Wright C, Bading J, Ressler J, et al. Adoptive Transfer of Autologous IL13-zetakine+ Engineered T Cell Clones for the Treatment of Recurrent Glioblastoma: Lessons from the Clinic. Molecular Therapy. 2011;19:S136–S137. [Google Scholar]
- 28.Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–44. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 29.Nash KT, Thompson JP, Debinski W. Molecular targeting of malignant gliomas with novel multiply-mutated interleukin 13-based cytotoxins. Crit Rev Oncol Hematol. 2001;39:87–98. doi: 10.1016/s1040-8428(01)00124-x. [DOI] [PubMed] [Google Scholar]
- 30.Madhankumar AB, Mintz A, Debinski W. Interleukin 13 mutants of enhanced avidity toward the glioma-associated receptor, IL13Ralpha2. Neoplasia. 2004;6:15–22. doi: 10.1016/s1476-5586(04)80049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debinski W, Thompson JP. Retargeting interleukin 13 for radioimmunodetection and radioimmunotherapy of human high-grade gliomas. Clin Cancer Res. 1999;5:3143s–7s. [PubMed] [Google Scholar]
- 32.Thompson JP, Debinski W. Mutants of interleukin 13 with altered reactivity toward interleukin 13 receptors. J Biol Chem. 1999;274:29944–50. doi: 10.1074/jbc.274.42.29944. [DOI] [PubMed] [Google Scholar]
- 33.Hudecek M, Lupo Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen M, Rader C, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T-cells. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kioi M, Seetharam S, Puri RK. Targeting IL-13Ralpha2-positive cancer with a novel recombinant immunotoxin composed of a single-chain antibody and mutated Pseudomonas exotoxin. Mol Cancer Ther. 2008;7:1579–87. doi: 10.1158/1535-7163.MCT-07-2131. [DOI] [PubMed] [Google Scholar]
- 35.Balyasnikova IV, Wainwright DA, Solomaha E, Lee G, Han Y, Thaci B, et al. Characterization and immunotherapeutic implications for a novel antibody targeting interleukin (IL)-13 receptor alpha2. J Biol Chem. 2012;287:30215–27. doi: 10.1074/jbc.M112.370015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21:2087–101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.