Abstract
Background
Saliva contains a number of protective factors such as mucins, immunoglobulins (e.g., IgA, IgG, and IgM), and enzymes (e.g., lysozyme and lactoperoxidases) that play an important role in the maintenance of oral health. The aim of this study was to compare levels of sIgA, histatin-5, and lactoperoxidase in saliva of adolescents with dental caries.
Material/Methods
Thirty-five adolescents (age 18 years) from high school were examined. Eight subjects with DMF=3 (Group I) and 27 adolescents with DMF>11 (Group II) were enrolled for this study. Clinical evaluation procedures comprised oral examination (including tooth, periodontal, and oral mucosal status) and collection of saliva samples. Saliva was collected for enzyme-linked immunosorbent assay (ELISA) and was used for determination of sIgA, histatin-5, and lactoperoxidase levels.
Results
Our results showed that adolescents with very high intensity of dental caries (DMF>11) had increased levels of sIgA, histatin-5, and lactoperoxidase compared to adolescents with lower intensity of caries. The increase was statistically significant (p<0.05).
Conclusions
We suggest that high intensity of caries is associated with increased levels of some salivary components – sIgA, histatin-5 and lactoperoxidase – that possess strong bactericidal or bacteriostatic effects, resulting in aggregation of oral bacteria and their clearance from the oral cavity.
MeSH Keywords: Dental Caries Susceptibility, Histatins, Lactoperoxidase, Saliva
Background
Saliva contains a number of protective factors such as mucins, immunoglobulins (e.g., IgA, IgG, and IgM), and enzymes (e.g., lysozyme and lactoperoxidases) that play an important role in the maintenance of oral health and are the first line of defense of the host against pathogens such as Streptococcus mutans, Streptococcus sobrinus, and Streptococcus sanguis [1]. Other protective factors include saliva flow, buffering capacity of saliva, protective dietary components, and non-cariogenic sweeteners [2]. The capacity is based on the phosphate system, the carbonic acid system, and the bicarbonate system [3]. Disorders in the dynamic balance between pathological and protective factors lead to dental caries.
The various immunoglobulins can control the growth of cariogenic oral microflora [3]. One of them is secretory immunoglobulin A (sIgA), which plays the main role in prevention of dental caries by inhibition of bacterial adherence, inactivation of bacterial enzymes and toxins, or by acting in synergy with other factors such as lysozyme and lactoferrin [4–6]. Salivary sIgA differs from serum IgA by having a dimeric structure, compared to the monomeric structure of the latter [7].
Another protective factor in saliva is histatins. The predominant human histatins are histatin-1, histatin-3, and histatin-5 [8]. They exhibit cidal activities against a broad range of pathogenic fungi, including Candida albicans, Cryptococcus neoformans, and Aspergillus fumigates. These proteins may represent major components of the non-immune host defense system involved in the maintenance of oral health. Histatin-5 is a salivary peptide, which contains 24 amino acids and it has antifungal properties against the opportunistic yeast Candida albicans. The mechanism of action is still unclear but mitochondria seem to be the specific intracellular target leading to membrane damage and cell death [9–11]. Besides fungicidal and fungistatic properties, antibacterial properties have been attributed to histatins based on their killing and growth-inhibitory activity against several species of oral bacteria [12].
Salivary lactoperoxidase (LPO) is a protein with unique enzymatic activity. The main role of the enzyme is protection of salivary proteins from bacterial degradation. LPO works in conjunction with thiocyanate and hydrogen peroxide. The products of that interaction inhibit glucose metabolism and oxidize bacterial sulfhydryl groups [13].
The aim of the study was to compare sIgA, histatin-5, and lactoperoxidase levels between adolescents with low and high caries activity. There is no data about the level of these 3 specific components in saliva of adolescents with dental caries disease in a single study.
Material and Methods
Study population
Thirty-five adolescents (age 18 years) from a high school were examined. Their caries intensity index DMF (decayed/missing/filled; D+M+F/number of the examined) was determined and the study population was then divided into 2 groups. Group I was composed of 8 adolescents with DMF=3 (low intensity of dental caries) and Group II was 27 adolescents with DMF>11 (high intensity of dental caries). All subjects had DMF higher than value 0. Clinical evaluation procedures were performed and included periodontal and mucosal status, evaluation of malocclusion, and collection of unstimulated whole saliva samples.
The research protocol was approved by the Committee for Ethics and Supervision on Human Research, Medical University of Bialystok, with informed consent from the patients.
Saliva collection
Unstimulated whole saliva was collected by a standard method into sterilized tubes, which were then placed on ice. Samples from the subjects were taken between 9:00 A.M and 11:00 A.M. Before collecting the saliva, all subjects abstained from eating and drinking for 2 h. Saliva samples were homogenized and clarified by centrifugation at 10 000 × g for 15 min at 4°C. The aliquots of clarified supernatants were kept at –70°C until needed for measurements.
Analysis of salivary IgA
The high-sensitivity assay kit (USCNK) was used to determine the level of sIgA in the saliva samples. The microtiter plates had been pre-coated with a monoclonal antibody specific to sIgA. Standards and samples were added to the appropriate microtiter plate wells and incubated for 1 h at room temperature. After washing away any unbound substances, peroxidase-labeled antibody was added to each microplate well and incubated for 1 h at room temperature. After another aspiration and washing step, a TMB substrate solution was added to each well. The enzyme-substrate reaction was terminated by the addition of a sulfuric acid solution and the color change was measured at a wavelength of 450 nm. The assay was performed in duplicate and the concentration of sIgA in the samples was then determined by comparing the O.D. of the samples to the standard curve.
Determination of histatin-5
Saliva samples were determined by using an enzyme-linked immunosorbent assay kit (Cusabio Biotech Co., LTD.). The microtiter plate had been pre-coated with an antibody specific to histatin-5. Standards and samples (100 μL each) were pipetted into the wells in duplicate and incubated for 2 h at 37°C. After the incubation step, biotin-conjugated antibody working solution was added to each well. After washing away any unbound substances, avidin conjugated to horseradish peroxidase (HRP) working solution (100 μL) was added to each well for 1 h at 37°C. Then a substrate solution (90 μL) was added to the wells for 30 min; the color developed in proportion to the amount of histatin-5 bound in the initial step. Color development was stopped by sulfuric acid solution, and the intensity of the color was measured spectrophotometrically at a wavelength of 450 nm. The concentrations of histatin-5 in the samples were calculated from the standard curve.
Determination of human lactoperoxidase (LPO)
Saliva samples were determined by using an enzyme-linked immunosorbent assay kit (Uscn Life Science, Inc.). The microtiter plate had been pre-coated with an antibody specific to LPO. Standards and samples (100 μL each) were then added into the wells in duplicate and incubated for 2 h at 37°C. After incubation, biotin-conjugated antibody working solution was added to each well. After washing away any unbound substances, avidin conjugated to horseradish peroxidase (HRP) working solution (100 μL) was added to each well for 1 h at 37°C. Then a TMB substrate solution (90 μL) was added to the wells for 30 min; the color developed in proportion to the amount of LPO bound in the initial step. Color development was stopped by addition of sulfuric acid solution, and the intensity of the color was measured spectrophotometrically at a wavelength of 450 nm. The minimum detectable dose (MDD) of human LPO was typically less than 8.1 nmol/L. The concentrations of histatin-5 in the samples were calculated from the standard curve.
Statistical analysis
The Mann-Whitney U nonparametric test for unrelated results of sIgA, histatin-5, and LPO was used to compare differences between the groups. All differences were considered significant when p<0.05. Statistical analysis was carried out using the Statistica 8.0 (StatSoft, USA).
Results
The salivary IgA, histatin-5, and LPO median and range of Group I and II are presented in Table 1. The minimum concentration of sIgA in Group I was 2.1 mg/dL and it was lower compared to minimal concentration in Group II (7.2 mg/dL). The maximum value of sIgA in Group I was 22.2 mg/dL and 23.8 mg/dL in Group II. Figure 1 shows the sIgA levels in both groups. The median of sIgA was 8.10 mg/dL in Group I and it was lower in comparison with adolescents from Group II, in which the median was 13 mg/dL. The difference was statistically significant (p=0.003, Table 1).
Table 1.
Median with range of sIgA, histatin-5, and LPO in whole unstimulated saliva.
| Salivary components | Group I with DMF=3 (N=8) | Group II with DMF>11 (N=27) | P |
|---|---|---|---|
| sIgA (mg/dL) | 8.10 (2.1–22.2) | 13 (7.2–23.8) | 0.003* |
| Histatin-5 (ng/mL) | 16.89 (14.11–29.95) | 66.84 (14.11–649) | 0.015* |
| LPO (nmol/L) | 2148 (1305–3248) | 3047 (1515–3313) | 0.02* |
Significant difference:
p<0.05.
Figure 1.
Salivary concentration of sIgA in Group I (with DMF=3) and Group II (with DMF>11) using ELISA technique. ** P<0.05 – differences statistically significant between Group I and Group II.
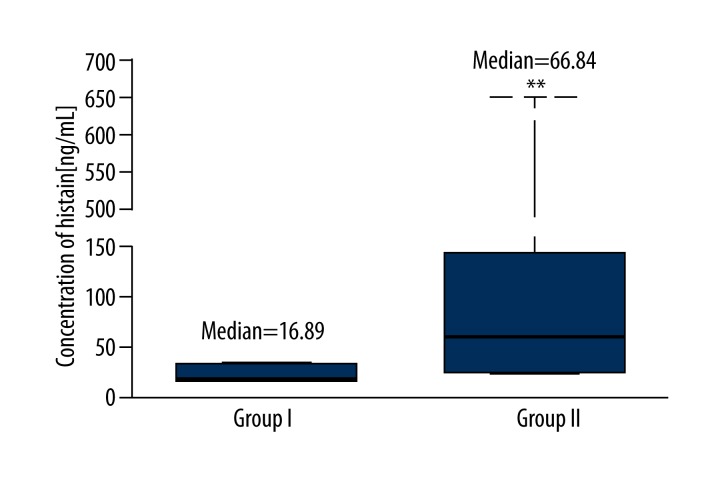
Figure 2 shows the histatin-5 levels in both groups. The median of histatin-5 was 16.89 ng/mL in Group I and 66.84 ng/mL in Group II, which had higher intensity of caries. The level of histatin-5 was significantly higher in Group II (p=0.015, Table 1). The minimum concentration of histatin-5 was 14.11 ng/mL in both groups, but the maximum concentration was higher in Group II, in which the value was 649 ng/mL (Table 1).
Figure 2.
Salivary concentration of histatin-5 in Group I (with DMF=3) and Group II (with DMF>11) using ELISA technique. ** P<0.05 – differences statistically significant between Group I and Group II.
Results of LPO determination in groups are presented in Figure 3. The median of LPO was 2148 nmol/L in Group I and it was lower compared to Group II, in which the median of LPO was 3047 nmol/L. The difference was statistically significant (p=0.02, Table 1). The minimum and maximum was 1305 and 3248 nmol/L in Group I (with DMF=3). The values were lower compared to minimum (1515 nmol/L) and maximum (3313 nmol/L) in Group II (with DMF>11) (Table 1).
Figure 3.
Salivary concentration of LPO in Group I (with DMF=3) and Group II (with DMF>11) using technique ELISA. ** P<0.05 – differences statistically significant between Group I and Group II.
There was no significant Spearman’s correlation between analyzed salivary components (Tables 2 and 3).
Table 2.
Spearman’s correlations in Group I with DMF=3.
| Parameters | sIgA | Histatin-5 | LPO | |
|---|---|---|---|---|
| sIgA | r | – | −0.02 | −0.21 |
| p | – | 0.96 | 0.567 | |
| Histatin-5 | r | −0.02 | – | 0.44 |
| p | 0.96 | – | 0.208 | |
| LPO | r | −0.21 | 0.44 | – |
| p | 0.567 | 0.208 | – | |
Table 3.
Spearman’s correlations in Group II with DMF>11.
| Parameters | sIgA | Histatin-5 | LPO | |
|---|---|---|---|---|
| sIgA | r | – | 0.32 | −0.37 |
| p | – | 0.146 | 0.089 | |
| Histatin-5 | r | 0.32 | – | −0.06 |
| p | 0.146 | – | 0.789 | |
| LPO | r | −0.37 | −0.06 | − |
| p | 0.089 | 0.789 | – | |
Discussion
Dental caries is an infectious and is the most common disease affecting children and adolescents worldwide [14]. It is caused by many factors, but the main microbial cause of the disease is Streptococcus mutans, which possesses a very strong cariogenic potential. The literature reports a positive correlation between S. mutans and dental caries disease.
Saliva and its composition play a vital role in the immune defense against pathogenic bacteria and takes part in demineralization and remineralization of the tooth structure.
The major salivary components are immunoglobulins (sIgA), proteins (histatin-5), and enzymes (LPO). They have very strong antimicrobial properties. The aim of the study was to measure the levels of selected salivary components with bactericidal potential. Our results showed significantly higher levels of sIgA in unstimulated whole saliva of adolescents with high activity of caries compared to subjects with low caries activity.
Several studies reported different results regarding the concentration of sIgA in dental caries patients. Our results are similar to those reported by Thaweboon et al., Bruno et al., and de Farias et al. [15–17]. They demonstrated that increase in caries activity is associated with an increase of sIgA levels. Some studies have reported a correlation between levels of sIgA and the age of subjects. Jafarzadeh et al. observed age-dependent changes in salivary IgA, showing that mean sIgA levels were significantly higher in older subjects compared to subjects aged 1–10 years, although they proved that the mean level of sIgA slightly decreased in subjects aged 61–70 years [1]. Challacombe et al. showed higher levels of sIgA in older people [18]. Some authors have emphasized links between age-dependent changes in salivary IgA and changes in cytokine production. Recently, we have shown higher levels of pro-inflammatory cytokines (IL-6, IL-8, and TNF-α) and selected mucins in saliva of adolescents with high intensity of caries [19,20].
Bagharian et al. found higher levels of sIgA in the saliva of children who were colonized for less than 6 months with S. mutans and their DMF was lower compared to those who had harbored S. mutans for longer than 24 months and had high DMF score [21].
Histatins are a group of small, cationic, histidine-rich peptides secreted by human salivary glands. Histatins have antifungal and bactericidal activity, and have been shown to reduce the clinical signs of gingival inflammation and plaque formation. Researchers have investigated its possible role in periodontal disease. One study showed that the levels of histatin increase from health to periodontitis, but the levels of histatin in the gingival crevicular fluid and saliva had no correlation with severity of periodontal disease [22]. We observed higher levels of histatin-5 and LPO in adolescents with high intensity of caries. All differences were statistically significant.
Lactoperoxidase, a member of the mammalian heme peroxidase family, uses hydrogen peroxide (H2O2) to catalyze the oxidation of thiocyanate (SCN–) and produce hypothiocyanite (OSCN−), a biocidal compound [23]. The lactoperoxidase system is known to inhibit many Gram-negative bacteria, including H. pylori, in vitro [24]. Haukioja et al. proved that H. pylori strain ATCC 43504T and 5 clinical isolates were efficiently inhibited by the peroxidase system, with high concentrations of H2O2 in buffer. The inhibition was stronger at lower pH. However, in human saliva these high concentrations of H2O2 generated less hypothiocyanite, the antibacterial product of the peroxidase system, and the effects of the peroxidase system were weaker [25].
The effect of lactoperoxidase on the antimicrobial effectiveness was checked by Welk et al., who discovered that tested thiocyanate and H2O2 mixtures showed no relevant antimicrobial effect, but by adding LPO the mixtures they became effective bactericidal and fungicidal agents [26].
Our research proves that sIgA acts in conjunction with other salivary components. SIgA together with LPO and histatin-5 may enhance antibacterial properties. The network of interactions between salivary components needs further investigation.
Conclusions
We suggested that high-intensity caries is associated with increased levels of some salivary components – sIgA, histatin-5, and lactoperoxidase – that possess strong bactericidal or bacteriostatic effects resulting in aggregation of oral bacteria and their clearance from the oral cavity. More studies should be carried out to discover and explain molecular pathways involved in dental caries.
Footnotes
Source of support: This investigation was supported by research grant 134-15607P from Medical University of Białystok, Poland
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Jafarzadeh A, Sadeghi M, Karam GA, Vazirinejad R. Salivary IgA and IgE levels in healthy subjects: relation to age and gender. Braz Oral Res. 2010;24:21–27. doi: 10.1590/s1806-83242010000100004. [DOI] [PubMed] [Google Scholar]
- 2.Featherstone JD. The continuum of dental caries-evidence for a dynamic disease process. J Dent Res. 2004;83:39–42. doi: 10.1177/154405910408301s08. [DOI] [PubMed] [Google Scholar]
- 3.Kuriakose S, Sundaresan C, Mathai V, et al. A comparative study of salivary buffering capacity, flow rate, resting pH, and salivary immunoglobulin A in children with rampant caries and caries-resistant children. J Indian Soc Pedod Prev Dent. 2013;31:69–73. doi: 10.4103/0970-4388.115697. [DOI] [PubMed] [Google Scholar]
- 4.Ranadheer E, Nayak UA, Reddy NV, Rao VA. The relationship between salivary IgA levels and dental caries in children. J Indian Soc Pedod Prev Dent. 2011;29:106–12. doi: 10.4103/0970-4388.84681. [DOI] [PubMed] [Google Scholar]
- 5.Chawda JG, Chaduvula N, Patel HR, et al. Salivary sIgA and dental caries activity. Indian Pediatr. 2011;48:719–21. doi: 10.1007/s13312-011-0113-y. [DOI] [PubMed] [Google Scholar]
- 6.Newbrun E. Current concepts of caries etiology. In: Newbrun E, editor. Cariology. 3rd ed. Baltimore: Williams and Wilkins; 1978. p. 43. [Google Scholar]
- 7.Shifa S, Muthu MS, Amarlal D, Rathna Prabhu V. Quantitative assessment of IgA levels in unstimulated whole saliva of caries-free and caries-active children. J Indian Soc Pedod Prevent Dent. 2008;26:158–61. doi: 10.4103/0970-4388.44031. [DOI] [PubMed] [Google Scholar]
- 8.Xu T, Levitz SM, Diamond RD, Oppenheim FG. Anticandidal activity of major human salivary histatins. Infect Immun. 1991;59:2549–54. doi: 10.1128/iai.59.8.2549-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helmerhorst EJ, Troxler RF, Oppenheim FG. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc Natl Acad Sci USA. 2001;98:14637–42. doi: 10.1073/pnas.141366998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan SA, Fidel PL, Jr, Thunayyan AA, et al. Impaired histatin-5 levels and salivary antimicrobial activity against C. albicans in HIV infected individuals. J AIDS Clin Res. 2013;4:1000193. doi: 10.4172/2155-6113.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edgerton M, Koshlukova SE. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv Dent Res. 2000;14:16–21. doi: 10.1177/08959374000140010201. [DOI] [PubMed] [Google Scholar]
- 12.Gusman H, Travis J, Helmerhorst EJ, et al. Salivary histatin 5 is an inhibitor of both host and bacterial enzymes implicated in periodontal disease. Infect Immun. 2001;69:1402–8. doi: 10.1128/IAI.69.3.1402-1408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part1) J Clin Pediatr Dent. 2003;28:47–52. doi: 10.17796/jcpd.28.1.yg6m443046k50u20. [DOI] [PubMed] [Google Scholar]
- 14.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 15.Thaweboon S, Thaweboon B, Nakornchai S, Jitmaitree S. Salivary secretory IgA, pH, flow rates, mutans streptococci and Candida in children with rampant caries. Southeast Asian J Trop Med Public Health. 2008;39:893–99. [PubMed] [Google Scholar]
- 16.Bruno B, Pezzini A, Menegazzi M. Salivary levels of immunoglobulin and dental caries in children. Boll Soc Ital Biol Sper. 1985;61:381–86. [PubMed] [Google Scholar]
- 17.De Farias DG, Bezerra AC. Salivary antibodies, amylase and protein from children with early childhood caries. Clin Oral Investig. 2003;7:154–57. doi: 10.1007/s00784-003-0222-7. [DOI] [PubMed] [Google Scholar]
- 18.Challacombe SJ, Percival RS, Marsh PD. Age-related changes in immunoglobulin isotypes in whole and parotid saliva and serum in healthy individuals. Oral Microbiol Immunol. 1995;10:202–7. doi: 10.1111/j.1399-302x.1995.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 19.Gornowicz A, Bielawska A, Bielawski K, et al. Pro-inflammatory cytokines in saliva of adolescents with dental caries disease. Ann Agric Environ Med. 2012;19:711–16. [PubMed] [Google Scholar]
- 20.Gabryel-Porowska H, Gornowicz A, Bielawska A, et al. Mucin levels in saliva of adolescents with dental caries. Med Sci Monit. 2014;20:72–77. doi: 10.12659/MSM.889718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagherian A, Jafarzadeh A, Rezaeian M, et al. Comparison of the salivary immunoglobulin concentration levels between children with early childhood caries and caries-free children. Iran J Immunol. 2008;5:217–21. doi: 10.22034/iji.2008.17171. [DOI] [PubMed] [Google Scholar]
- 22.Bhadbhade SJ, Acharya AB, Thakur SL. Salivary and gingival crevicular fluid histatin in periodontal health and disease. J Clin Exp Dent. 2013;5:174–78. doi: 10.4317/jced.51106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fragoso MA, Torbati A, Fregien N, Conner GE. Molecular heterogeneity and alternative splicing of human lactoperoxidase. Arch Biochem Biophys. 2009;482:52–57. doi: 10.1016/j.abb.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin K, Yamauchi K, Teraguchi S, et al. Susceptibility of Helicobacter pylori and its urease activity to the peroxidase-hydrogen peroxide-thiocyanate antimicrobial system. J Med Microbiol. 2002;51:231–37. doi: 10.1099/0022-1317-51-3-231. [DOI] [PubMed] [Google Scholar]
- 25.Haukioja A, Ihalin R, Loimaranta V, et al. Sensitivity of Helicobacter pylori to an innate defence mechanism, the lactoperoxidase system, in buffer and in human whole saliva. J Med Microbiol. 2004;53:855–60. doi: 10.1099/jmm.0.45548-0. [DOI] [PubMed] [Google Scholar]
- 26.Welk A, Meller Ch, Schubert R, et al. Effect of lactoperoxidase on the antimicrobial effectiveness of the thiocyanate hydrogen peroxide combination in a quantitative suspension test. BMC Microbiol. 2009;9:134. doi: 10.1186/1471-2180-9-134. [DOI] [PMC free article] [PubMed] [Google Scholar]