Abstract
Background
The use of bone marrow-derived mesenchymal stromal cells (MSCs) as a cellular therapy for various diseases, such as graft-versus-host-disease, diabetes, ischemic cardiomyopathy, and Crohn's disease has produced promising results in early-phase clinical trials. However, for widespread application and use in later phase studies, manufacture of these cells needs to be cost effective, safe, and reproducible. Current methods of manufacturing in flasks or cell factories are labor-intensive, involve a large number of open procedures, and require prolonged culture times.
Methods
We evaluated the Quantum Cell Expansion system for the expansion of large numbers of MSCs from unprocessed bone marrow in a functionally closed system and compared the results to a flask-based method currently in clinical trials.
Results
After only two passages, we were able to expand a mean of 6.6×108 MSCs from 25 mL of bone marrow reproducibly. The mean expansion time was 21 days, and cells obtained were able to differentiate into all three lineages: chondrocytes, osteoblasts, and adipocytes. The Quantum was able to generate the target cell number of 2.0×108 cells in an average of 9-fewer days and in half the number of passages required during flask-based expansion. We estimated the Quantum would involve 133 open procedures versus 54,400 in flasks when manufacturing for a clinical trial. Quantum-expanded MSCs infused into an ischemic stroke rat model were therapeutically active.
Discussion
The Quantum is a novel method of generating high numbers of MSCs in less time and at lower passages when compared to flasks. In the Quantum, the risk of contamination is substantially reduced due to the substantial decrease in open procedures.
Keywords: Cell Culture Expansion, Good Manufacturing Practices (GMP), Mesenchymal Stromal Cells (MSC), Quantum, Stroke
Introduction
Mesenchymal stromal cells (MSCs) show promise in therapeutic applications, including inflammatory and immune-based diseases such as Crohn's disease or graft-versus-host disease, as well as in regenerative medicine treatments such as osteogenica imperfecta, burns, myocardial infarction, and stroke.(1-7)
MSCs can be enriched and expanded from numerous sources, including bone marrow, cord blood, and adipose tissue, and have the potential to differentiate into chondrocytes, osteoblasts, and adipocytes.(8-11) When grown under appropriate conditions the tri-lineage potential of these cells is maintained. However, during expansion, the telomeres shorten and unbiased differentiation into the three lineages could become polarized.(12) Therefore, for therapeutic applications, obtaining clinically-relevant numbers of cells with a minimum number of cell passages and doublings is essential.
Current methods for generating large numbers of MSCs have usually involved traditional flask-based methods and cell factories. Use of hundreds of cell culture flasks to generate the required numbers of cells is extremely laborious, and involves thousands of open events, which increase the possibility of contamination. While cell factories overcome some of these issues,(13, 14) they can be technically challenging, even for experienced users.(15) For example, visualizing cells is difficult due to the multiple layers, and in our experience, a good cell recovery is challenging when using these devices with MSCs. For these reasons, manufacture of MSCs is generally restricted to established cell therapy centers with considerable experience, resources, and Good Manufacturing Practices (GMP) facilities.(16, 17)
Despite these limitations, there remains considerable interest in using MSCs for a diverse range of therapeutic applications. This interest is likely to continue since allogeneic MSCs may provide an “off the shelf” source of cells due to their lack of expression of Human Leukocyte Antigen (HLA)-class II and co-stimulatory molecules, which limits the immune response of the recipient to these cells.(18, 19) Therefore, large banks of MSCs can be prepared, making the cells rapidly available for use in early stage clinical trials, or eventually as a licensed drug. Generation of such cell banks using the current flask-based technologies would be extremely labor-intensive and expensive.
One alternative could be the Quantum Cell Expansion System (henceforth referred to as Bioreactor) by Terumo BCT, a self-contained system including a hollow fiber bioreactor. Although this system has been reported previously, (20),(21) large-scale production of MSCs (>2.0×108) using the Bioreactor and a head-to-head comparison of flasks versus the Bioreactor have not been done. Furthermore, MSCs expanded in the Bioreactor have not been tested for efficacy in an animal model. Here we report the use of the Bioreactor to generate large numbers of allogeneic MSCs that could be banked for multi-patient use. We demonstrate that these MSCs are functional in a rat model of ischemic stroke. In this study, we aim to compare the use of the Bioreactor with the traditional flask-based method for MSC production. The primary endpoint for this study is a comparison of MSCs expanded in the Bioreactor compared to flasks and the secondary endpoint is the function of these cells in vitro and in vivo.
Methods
Preparation of D-5 medium
Expired apheresis platelets from eligible donors (Gulf Coast Regional Blood Service, Houston, TX, USA) were pooled and frozen in 30 mL aliquots at -80°C. They were then thawed, centrifuged, and the supernatant was added (5% platelet lysate) to Dulbecco's Modified Eagle Medium (DMEM, Lonza, Walkersville, MD, USA) containing 2.1 units/mL heparin (APP Pharmaceutical, Schaumburg, IL), 2mM GlutaMax (Invitrogen, Carlsbad, CA, USA), and 10mM N-acetylcysteine (Sigma, St. Louis, MO, USA). The complete medium was then filtered through a 0.2 μm filter.(22)
Bone marrow processing
Twenty five to one hundred mL of bone marrow (BM) aspirate from normal donors was purchased from Lonza and was shipped on cold packs overnight. Twenty-five mL tubes of BM were pooled in cell processing bags (Baxter, Deerfield, IL, USA). If the aspirate was intended for flask-based culture, the bone marrow mononuclear cell (BMMC) fraction was enriched using a Ficoll density gradient (GE Healthcare, Pittsburgh, PA, USA) on a Sepax cell separation device (Biosafe, Geneva, CH). In total five BM aspirates were used; three were used to generate MSCs in both the Bioreactor and in flasks and of the remaining two, one was used for the Bioreactor only and one was used for flasks only.
Plating of BMMC in flasks and expansion of MSCs
BMMC were resuspended in D-5 medium and plated at 5×105 cells/cm2 in T-175 cm2 flasks (defined as Passage 1).(15) Upon reaching 70-80% confluence, the monolayer was washed, harvested using TrypLE Select (Gibco, Carlsbad, CA, USA) and the cells were split 1:4 into new T-175 flasks. Although variable, the typical seeding density was 2×103-2×104 cells/cm2. Cells were maintained in incubators at 37°C and 5% CO2 in air. After 3-5 passages, cells were harvested and frozen in cryopreservation medium containing a final concentration of 5% human serum albumin (HSA) (Baxter), 10% dimethyl sulfoxide (DMSO – Cryoserv, Bioniche Pharma, Lakewood, IL, USA), and 85% Plasmalyte (Baxter).
Bioreactor cell expansion device
The Bioreactor (Figure 1a, Terumo BCT, Lakewood, CO, USA) is a functionally closed system consisting of a disposable hollow fiber bioreactor enclosed in a stand-alone incubator. It is fed via two circulation loops: the intracapillary loop and the extracapillary loop, both of which have inlets for media and reagents, or cells (intracapillary loop only). Waste is removed into a waste bag. The entire process is computerized and controlled by a touch screen interface, allowing the user to control medium perfusion rate, harvest time, media washouts and other tasks associated with the growth of MSC. With the exception of filling inlet bags with TrypLE Select and Fibronectin and transferring harvested cells from the harvest bag to the cell inlet bag (all of which are done prior to loading onto the Bioreactor), all procedures associated with the Bioreactor are closed or protected by a 0.2 μm sterile barrier filter.
Figure 1.
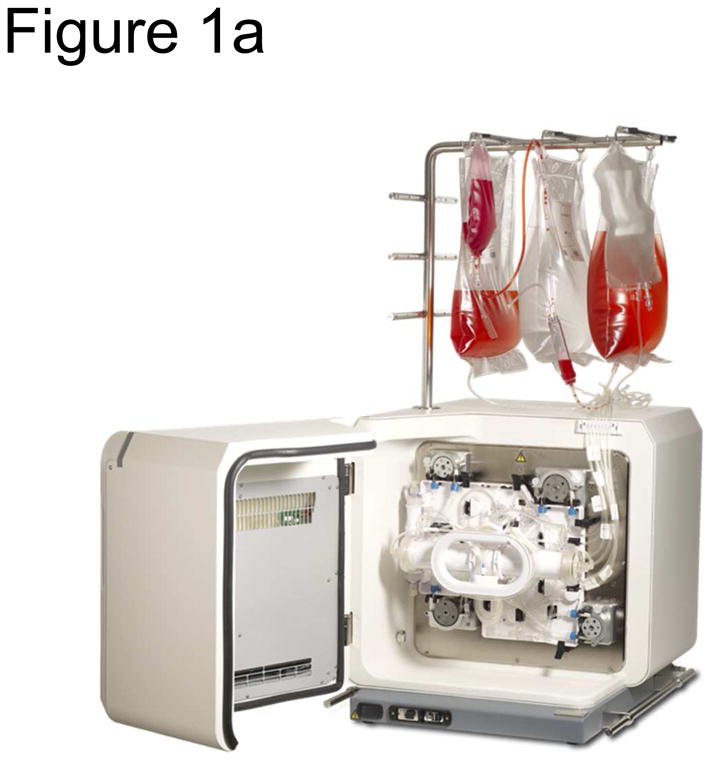
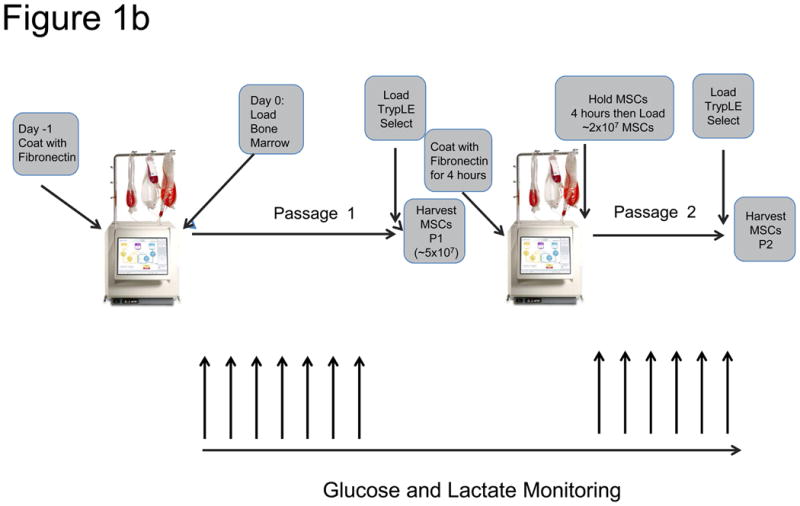
The Bioreactor Cell Expansion System a) The Bioreactor instrument is shown, illustrating a hollow fiber-contained bioreactor, touch-screen interface, peristaltic pumps, valves, as well as cell inlet, media, and waste bags. b) The procedure for expanding MSCs in the Bioreactor is depicted schematically. One day before loading bone marrow into the system, the disposable expansion set is loaded onto the Bioreactor and the system is primed and the bioreactor coated with fibronectin. After approximately 18 hours, the fibronectin is washed out with media and bone marrow (filtered through a 200 μm filter) is loaded into the system. Cells are allowed to adhere for ∼48 hours, after which the cells are fed continuously with media, starting at a rate of 0.1 ml/min and ending at 0.4 ml/min. The media is adjusted according to glucose and lactate concentration in the media sample taken from the system. When the lactate levels reach 4.0 mM while at a feed rate of 0.4 ml/min, the cells are harvested within 24-48 hours. Cells are harvested by washing out the system with PBS, loading TrypLE Select, incubating for 15 minutes, and washing out the cells with media. At this point were counted and loaded into a new expansion set that had been primed and coated with Fibronectin either overnight or for 4 hours after the initial harvest. Twenty to thirty-five million MSCs were then loaded into the new expansion set and the process was repeated. However, the cells were allowed to reach a lactate level of 8 mM at a feed rate of 1.6 ml/min before harvest.
Priming and Coating the cell expansion set (bioreactor)
Either 4 or ∼18 hours before addition of cells to the device, a cell expansion set was loaded onto the Bioreactor device (Figure 1a,b) and the system was primed by passage of phosphate-buffered saline (PBS – Hyclone, Thermo Fisher, Rockford, IL, USA) through the system. After priming, 5 mg of Fibronectin (BD Biosciences, San Jose, CA) was loaded into the bioreactor and allowed to incubate until the cells were loaded after 4 or ∼18 hours. Before loading the cells, the PBS was washed out of the system and replaced with D-5 medium. The entire MSC manufacture process in the Bioreactor is depicted in Figure 1b. Gas was provided to the system as a pre-mixed supply of 5% O2, 5% CO2 and 90% N2.
Loading bone marrow into the Bioreactor Cell Expansion System (Passage 1)
Twenty-five mL of BM was transferred to a Bioreactor cell inlet bag, passed through a 200 μm BM filter (Terumo BCT), and loaded into the bioreactor. In experiments comparing expansion in flasks to Bioreactor-expanded MSC, 50 ml of BM was pooled and split between the Bioreactor and the Sepax device (for use in the flask culture system). After loading BM into the Bioreactor, BMMC were allowed to attach for 48 hours, after which cells were continuously fed with fresh medium.
Feeding MSCs
Cells were fed via continuous introduction of media into the Bioreactor, resulting in a passive removal of waste into the waste bag. Cells were fed according to manufacturer's recommendations. Media was first fed at a rate of 0.1 mL/minute (min). Glucose and Lactate measurements were typically measured twice daily (Aviva Accu-Chek meter, Roche Diagnostics, Indianapolis, IN, USA and LactatePlus Lactate Meter, Nova Biomedical, Waltham, MA, USA, respectively). Once the lactate concentration reached 4 mM, the inlet rate was doubled. During the first passage, the inlet rate was doubled each time the lactate concentration rose to 4 mM until the inlet rate reached 0.4 mL/min. At this inlet rate, cells were harvested when the lactate concentration remained above 4 mM for 24-48 hours. For the second and subsequent passages, the inlet rate was doubled as before, but cells were harvested when the rate reached 1.6 mL/min and the lactate concentration reached 8 mM.
Cell harvest
Approximately 24 hours after reaching the final lactate threshold, 180ml TrypLE Select was loaded into a cell inlet bag and connected to the “reagent” line of the expansion set. The system was washed with 2.5 volumes of PBS and the TrypLE Select was then loaded into the system. After incubating with the cells for 15 minutes, the TrypLE Select and the harvested cells were washed into the cell harvest bag using fresh medium that was also added to the system.
Loading MSCs into the Bioreactor Cell Expansion System (Passage 2+)
After first passage, 2.0-3.5×107 (ideally 2.0×107 or ∼1×104/cm2) MSCs were loaded into a new expansion set. Cells were either loaded into a second Bioreactor device which had an expansion set that had been primed and coated with fibronectin 24 hours previously, or they were loaded into the same Bioreactor equipped with a new expansion set that was coated with fibronectin for only 4 hours, during which the harvested cells were held at ambient temperature in a gas-permeable AFC cell processing bag (Houston, TX, USA) on a rocking platform. Once fibronectin coating was finished, the MSCs were loaded using the “Load Cells with Distribution” task as per Terumo's recommendation.
Measuring growth kinetics
MSCs were plated in 96-well culture plates at 1×103 cells/well. Population doubling time was measured during the cell growth log phase using CyQUANT, a fluorescence-based proliferation assay (Invitrogen). The cells were labeled at initiation with the CyQUANT reagent and tested daily for 7 days. Fluorescence was measured using a microplate reader (Safire2™, Tecan, San Jose, CA). A standard curve for each sample was generated by plotting known numbers of MSC on 96-well tissue culture plates against fluorescence intensity values obtained after labeling with the CyQUANT reagent.
Enumeration of colony forming units - fibroblasts (CFU-F)
Colony-forming cells were enumerated at each cell passage by plating duplicate 75 cm2 flasks at low cell density (20 cells/cm2) in Alpha-modified Minimum Essential Medium containing ribo- and deoxyribonucleotides (Invitrogen) supplemented with 10% Fetal Bovine Serum.(23) Colony-forming cells were allowed to grow for two weeks and were then washed twice with PBS. Cultures were fixed in ethanol for 30 minutes at room temperature and stained with Giemsa stain. Colonies containing at least 40 cells were counted under a stereomicroscope. The results are presented as the absolute number of colonies.
Differentiation potential assay
MSCs were tested for their ability to differentiate into chondrocytes, osteoblasts, and adipocytes as described by Pittenger et al.(11)
Phenotyping
MSCs were directly stained for the positive markers CD73 (clone 5E10), CD90 (clone AD2), and CD105 (clone 266) as well as lineage markers CD45 (2D1), CD34 (8G12), CD14 (clone MΦP9), CD19 (cloneSJ25C1), and HLA-DRII (clone G46-6) per the International Society for Cellular Therapy (ISCT) position paper on MSCs.(24) To further characterize the MSCs, they were also stained for the MSC markers MSCA-1 (clone W8B2) and CD271 (clone C40-1457). With the exception of MSCA-1 (Miltenyi Biotec, Auburn, CA, USA), all antibodies were purchased from BD Biosciences and were preconjugated. At least fifty thousand total events were acquired on a FACSCanto-II using FACSDiva Software v6.3.1.
MSC-mediated T Cells suppression
MSC lines were irradiated and plated in titrated numbers. Peripheral blood mononuclear cells (PBMCs) from healthy donors were labeled with carboxyfluorescein succinimidyl ester (CFSE, Sigma). CFSE-labeled PBMCs were then cultured alone (1:0 PBMC:MSC ratio) for use as a positive control or co-cultured with titrated numbers of MSCs ranging from 1:1 down to a 1:0.05 PBMC:MSC ratio. Soluble anti-human CD28 monoclonal antibodies (RnD Systems, Minneapolis, MN, USA) were used to stimulate T cell populations. After four days in culture, cells were harvested and stained with anti-huCD4 APC (RnD Systems) to gauge the proliferation of CD4+ T cells by flow cytometry. Data acquisition was performed with an Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA). CD4+ T cell proliferation (%CD4+/CFSE-low cells) was measured using a negative control gate set with non-stimulated PBMCs co-cultured at a 1:0.05 PBMC:MSC ratio (%CD4+/CFSE-high cells).
Rat model of ischemic stroke and MSC administration
Adult male Long-Evans rats were subjected to transient middle cerebral artery occlusion (MCAo) for 90 minutes. On day 7 after MCAo, animals showing neurological deficits were randomized to one of three groups: (1) Saline (n=5); (2) Bioreactor-expanded MSCs (n=8); or (3) flask-expanded MSCs (n=7). The animals were weighed and then anesthetized followed by infusion of freshly thawed Bioreactor (P2)- or Flask-expanded MSCs (P4) in 1 mL of PBS or PBS alone. The cells or PBS were infused IV via the femoral vein at a rate of 200 μl/min. The dose of cells was 1×106 cells/kg. The animal surgeon was blinded to the treatment by wrapping the syringe in translucent Parafilm.
Animals underwent suture occlusion of the MCA for 90 minutes and were evaluated on the cylinder test (see below) at days 7 (before randomization), 14, 28, and 35 after stroke. At day 7, animals that showed impairments on the cylinder test were randomized to one of three groups: (1) Saline (n=5); (2) Bioreactor-generated MSCs (n=8); or (3) flask-generated MSCs (n=7).
Cylinder Test
To test for functional deficits and recovery of the animals, we conducted the Cylinder test.(25) This test was performed on the day before MCAo surgery, and days 7 (before treatment), 14, 28, and 35 after surgery. Briefly, the animals were placed in a Plexiglas cylinder. The number of times the animal touches the cylinder wall with its impaired or non-impaired front paws were recorded. The final score was calculated as (non-impaired forelimb movement – impaired forelimb movement)/(non-impaired forelimb movement + impaired forelimb movement + both movement). A total of 20 consecutive touches were recorded in the 5-minute test.
Statistical analysis
The student's t-test was used to test for the difference between groups, assuming equal variance. Mean values +/- standard error of the mean (SEM) were used unless otherwise noted. For behavior test, data was shown Mean±SE and the Normality Test (Shapiro-Wilk) was used to test among three groups at the same timepoint first, followed by the comparison of Mann-Whitney Rank Sum Test. P <0.05 was considered statistically significant.
Results
MSC expansion
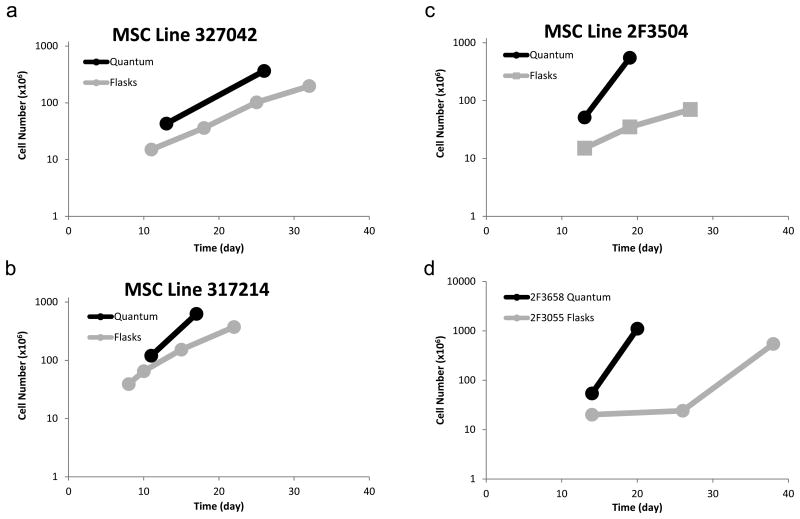
To compare the expansion of MSCs in the Bioreactor versus flasks, BM-derived MSCs were expanded in flasks as per published methods that are currently in use in a Phase 1 clinical trial (15), as well as in the Bioreactor. In flasks, BMMC were seeded at 5×105 cells per cm2 in T-175 cm2 flasks and split 1:4 when 70-80% confluent for 3-5 passages. After 3-5 passages, the average yield in flasks was 2.9×108 MSCs (Figure 2a,b,c,d) requiring 128-192 T-175 cm2 flasks. The average time-to-final harvest was 29.8 days in flasks (range: 22-38 days), varying according to passage number when harvested and the growth kinetics of the cells.
Figure 2.
Cell expansion in the Bioreactor and in flasks. MSCs were expanded for 2 passages (Bioreactor) or 4 passages (flasks). Cells were harvested and counted using a hemocytometer. a,b,c) Comparison of MSCs generated from the same bone marrow in either the Bioreactor (black lines) or flasks (grey lines). d) Two additional bone marrow MSC donors that were expanded only in either flasks (grey) or the Bioreactor (black).
To expand MSCs in the Bioreactor, 25 mL of unfractionated bone marrow was loaded into the Bioreactor and the perfusion rate of medium was based on glucose and lactate measurements. After only two passages in the Bioreactor, the average yield was 6.6×108 MSCs (Figure 2a,b,c,d). The average time-to-final harvest in the Bioreactor was 20.5 days (P <0.05) (range: 17 – 26 days, Figure 2a,b,c,d), with the second passage taking approximately 6 days (range: 6-7 days).
Kinetics of MSC expansion
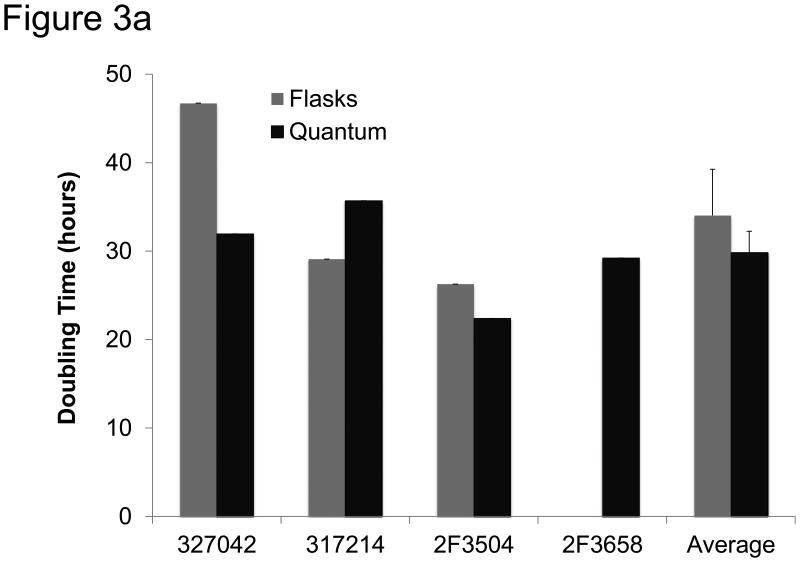
The kinetics of expansion were evaluated in cells cultured using each method. In this assay cells were plated in 96 well plates at 300 cells/well and their proliferation rate was measured using CyQUANT, a fluorescence-based population doubling time assay (Invitrogen). The average doubling time was faster, but not significantly, in cells expanded in the Bioreactor. Cells in the Bioreactor doubled every 30 hours (±2.4), compared to cells expanded in flasks which doubled every 34 hours (±5.2) (Figure 3a).
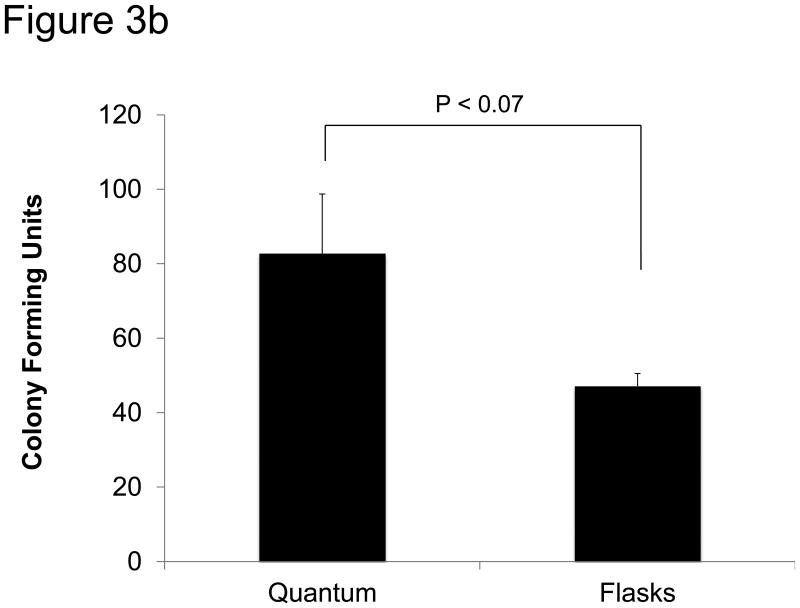
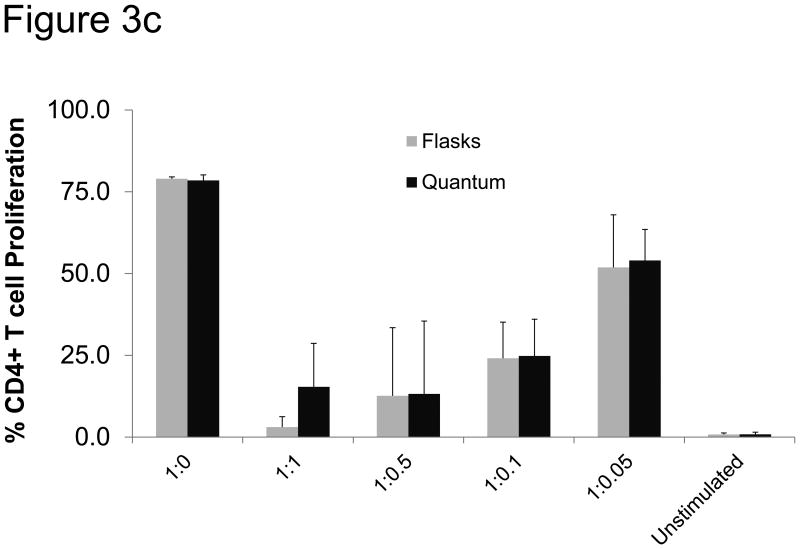
Figure 3.
Functional analysis of MSCs. a) MSCs were expanded for 2-4 passages and then tested for growth kinetics using CyQUANT. Shown are three MSC donors grown in both flasks and the Bioreactor as well as MSC donor 2F3658, which was only grown in the Bioreactor. Shown to the right is the mean doubling time in flasks and in the Bioreactor. The error bars represent standard error from the mean. b) Colony-forming units (CFU) were measured by plating 20 MSCs/cm2 in T75 cm2 flasks. After two weeks clusters containing more than 40 cells were considered a colony and scored. Shown are the absolute numbers of colonies from MSCs expanded in the Bioreactor and in flasks. c) MSCs expanded in the Bioreactor (black) and in flasks (grey) co-cultured with CFSE-labeled, anti-CD28-activated PBMC. After 4 days cells were harvested and the number of proliferating CD4+ T cells was measured via flow cytometry. Shown is the percentage of proliferating CD4+ T cells at different ratios of T cells to MSCs. “Unstimulated” depicts PBMCs that were not stimulated with anti-CD28.
Colony formation
An additional test of MSCs is their propensity to form colonies as measured by colony-forming units (CFU). MSCs were harvested from flasks or the Bioreactor and immediately plated at 20 cells/cm2 in T-75 cm2 flasks. After 2 weeks, the cells were stained with Giemsa stain and the number of colonies was counted. As shown in Figure 3b, the average CFU in the Bioreactor was greater than the number of CFU in cells expanded in flasks, but this difference was not statistically significant.
Suppression of T cell proliferation
One of the proposed mechanisms by which MSCs exert their immunomodulatory effects is by suppressing T cell proliferation. To test whether cells generated in the Bioreactor and flasks were able to inhibit T cell proliferation, MSCs were combined with human PBMCs at different ratios, after which the T cells were stimulated with anti-CD28 monoclonal antibodies. As shown in Figure 3c, MSCs expanded from three independent lots of bone marrow expanded in Bioreactor or flasks were able to suppress the proliferation of CD4+ T cells at ratios as low as 1 MSC : 20 T cells in a similar manner.
Tri-lineage potential
While a definitive assay has yet to be established to test MSC potency, there are certain functional assays that have been used to further define the cells as MSC. According to the ISCT position paper(24), the cells must show the potential to differentiate into chondrocytes, osteoblasts, and adipocytes. MSCs expanded either in the Bioreactor or in flasks were tested for their ability to differentiate into each of these lineages. All MSC lines expanded in the Bioreactor and in flasks were able to differentiate into each of the three lineages with no qualitative difference between the Bioreactor and flasks for any of the lineages (Supplementary Figure 3).
Phenotype
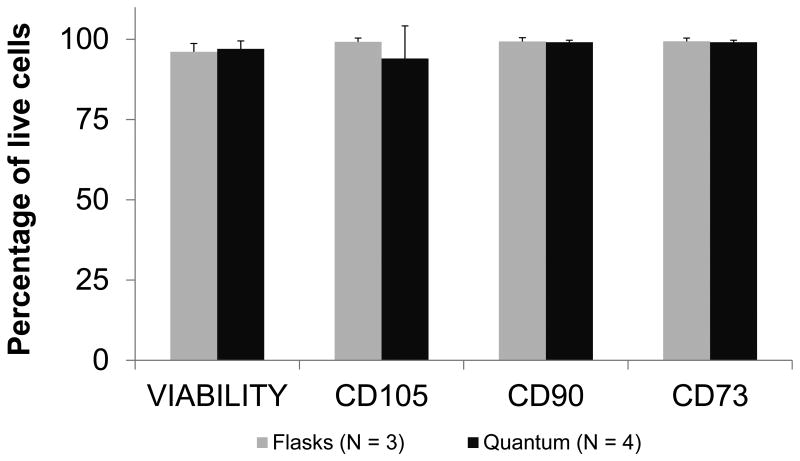
Cells expanded in the Bioreactor as well as in flasks met ISCT standards(24) for expression of CD73, CD90, CD105, and lack of expression of hematopoietic lineage markers and HLA-DR (Figure 4, Table 1, and Supplementary Figure 4a,b,c). Cells were compared at passage 2 in the Bioreactor and passage 4 in flasks, corresponding to the point at which the cells met release criteria for both cell number and phenotype and hence could have been used clinically. For a comparison of passage 2 cells see Supplementary Figure 4c. Cells harvested from the Bioreactor at P2 had a viability of 97.1% while those harvested from the flasks at P4 were 91.0% viable as determined by staining for the viability marker 7AAD. MSCs were also stained for CD271, a low affinity nerve growth factor receptor belonging to the tumor necrosis factor receptor superfamily and marker of proliferative potential, as well as mesenchymal stromal cell antigen-1 (MSCA-1). MSCA-1 and CD271 were found to be expressed at similar levels in MSCs cultured in the Bioreactor and in flasks (Table 1).
Figure 4.
Phenotype of expanded MSCs. Shown is the percentage of live cells stained for the surface antigens CD105, CD90, and CD73, as well as the viability. Data shown are the mean percentage of cells expanded in the Bioreactor (n = 4) shown in black and in flasks (n = 3) in grey. Error bars represent the standard error of the mean.
Table 1. Phenotype of MSCs expanded in flasks and in the Bioreactor after 4 passages (Flasks) or 2 passages (Bioreactor).
| Flasks (%) | Bioreactor (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 327042 | 317214 | 2F3055 | 2F3504 | Mean (SEM) | 327042 | 317214 | 2F3658 | 2F3504 | Mean (SEM) | |
| CD73 | 98.82 | 99.96 | 99.93 | 99.93 | 99.66 ± 0.22 | 98.57 | 99.40 | 98.60 | 99.75 | 99.08 ±0.26 |
| CD90 | 98.85 | 99.95 | 99.85 | 99.87 | 99.63 ± 0.20 | 98.52 | 99.29 | 98.90 | 99.80 | 99.13 ± 0.24 |
| CD105 | 98.85 | 99.95 | 99.86 | 93.47 | 98.03 ± 1.19 | 98.52 | 99.29 | 78.80 | 99.47 | 94.02 ± 4.40 |
| CD271 | 99.96 | 0.99 | 0.98 | 3.48 | 26.35 ± 19.01 | 99.90 | 0.97 | N/A | 94.62 | 65.16 ± 22.72 |
| MSCA-1 | 66.00 | 70.03 | 99.98 | 99.84 | 83.96 ± 7.16 | 76.29 | 54.40 | N/A | 99.47 | 76.72 ± 9.20 |
| Viability | 81.46 | 98.70 | 97.95 | 85.91 | 91.01 ± 3.35 | 93.45 | 98.20 | 99.18 | 97.71 | 97.13 ± 1.10 |
SEM: Standard error of the mean
Functional activity of MSCs in a rat model of ischemic stroke
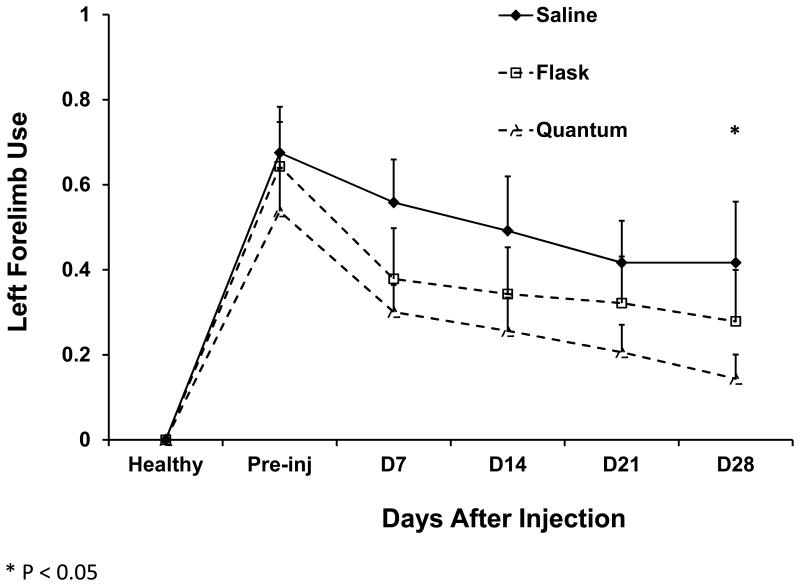
Unlike other therapeutic cells such as T cells or NK cells, a definitive potency assay or marker of efficacy has yet to be established for MSCs. Despite their similar phenotype and tri-lineage potential, MSCs exhibit great variability from lot-to-lot even when cultured using the same methods. Cells expanded by different culture techniques may be expected to exhibit even greater variability. Therefore, to test whether MSCs expanded in the Bioreactor were as potent as those grown in flasks, we used a well-established rat model of ischemic stroke in which flask-generated or Bioreactor-generated MSCs were administered seven days after the onset of induced stroke. The therapeutic benefit of the MSCs was then measured using the Cylinder test.(25) As shown in Figure 5, Bioreactor-generated MSCs had a significant therapeutic benefit when compared to saline placebo controls. Flask-generated MSCs trended towards significance but, given the small number of animals, were not significantly different than placebo treated animals.
Figure 5.
Therapeutic effect of MSCs infused into a rat model of ischemic stroke. Seven days after MCAo, rats were infused saline (n = 5, solid triangles) or MSCs expanded in flasks (n = 7, open squares) or in the Bioreactor (n=8, open triangles). Every 7 days the rats were tested using the Cylinder test for their left forelimb use. Shown is the mean left forelimb use at each time point. * represents a statistical difference (P<0.05) between Bioreactor-expanded MSC- and saline-treated rats. Error bars represent standard error.
Cost of manufacturing cells for a Phase I clinical trial
When preparing a cellular therapy product, an important concern is the cost of manufacturing. We compared the cost of preparing a dose of 2.0×108 cells in the Bioreactor versus flask-based culture. As seen in Table 2, the cost of production of cells in the Bioreactor ($18,418) and in flasks ($19,313) was similar. However, the cost of labor—a scarce resource in most cell production facilities—in flasks was 82% higher than with the Bioreactor.
Table 2.
Reagents, supplies, labor, and costs associated with the expansion of 200 million MSCs for clinical use.
| T-175 cm2 Flask | Bioreactor | |
|---|---|---|
| Culture Time | 30 days | 21 days |
| Passages | 4 | 2 |
| Media | 20 L | 12 L |
| Flasks/Bioreactors | 340 | 2 |
| Pipettes | 2040 | 2 |
| TrypLE Select | 3.4 L | 0.4 L |
| PBS | 8.5 L | 4 L |
| Number of Staff | 5 | 2 |
| Total Labor Hours | 101 | 21 |
| Harvest time | 6 hours | 0.75 hours |
| Release Testing | $2,577.13 | $1,687.13 |
| Cost | $19,313 | $18,418 |
The greatest difference in labor costs occurred during the harvest procedures. Harvesting flasks at the fourth passage involves washing, dissociation, neutralization, and finally harvesting over 100 flasks. To do this efficiently, our group has traditionally employed at least 5 staff members working concurrently. Even with 5 staff members, the harvest, in addition to the necessary documentation and sampling for release testing, takes over 6 hours. In the Bioreactor, TrypLE Select was added to the reagent line. Preexisting tasks were selected using the touch screen and the system then automatically washed the bioreactor with PBS, added the TrypLE Select, and then harvested the dislodged cells into MSC medium. Including the 15 minute incubation with TrypLE Select, the entire harvesting time was approximately 45 minutes, more than 5 hours less than the time required to harvest over 100 flasks.
Open Events in Flasks versus the Bioreactor
One of the greatest limitations of small-scale cell culture and of early-stage cell therapies in general is that cells are typically grown in small vessels that require multiple manipulations and are repeatedly exposed to the environment containing potentially harmful pathogens and contaminants. Although FDA regulations require that cells intended for patients are manufactured in a well-controlled and monitored environment, cells exposed to the environment – regardless of the precautions taken—are at a greater risk of pathogenic contamination. To compare the traditional, flask-based method of cell culture to cells grown in the Bioreactor, we calculated the number of open events that would be required to manufacture sufficient cells for a phase I clinical trial with 60 patients at a dose level of 3×106 cells/kg in average-sized patients (75 kg). Table 3 shows the number of open events required using T-175 cm2 flasks and the Bioreactor for each expansion and to treat the entire cohort of patients for a total of approximately 13.5 billion MSCs. Overall the Bioreactor requires an estimated 0.02% of the open procedures occurring in an equivalent flask-based process.
Table 3.
Number of open events associated with the production of MSCs for a clinical trial treating sixty, 75 kg patients at 3×106/kg.
| T-175 cm2 Flask | Quantum | ||
|---|---|---|---|
| Per Flask/Expansion Set | Seeding Cells | 2 | 3 |
| Exchanging Media | 2 | 1 | |
| Cell Dissociation | 6 | 3 | |
| Total | 10 | 7 | |
| Per Donor (340 Flasks) | 340 Flasks | 3,400 | 7 |
| Number of Donors / Expansion Sets | 16 Donors* | 19 Expansion Sets (1 donor) | |
| Total | 54,400 | 133 |
To keep passage numbers below 5, at least 16 donors would be necessary to reach the required cell number.
Discussion
Here we compare for the first time the feasibility of expanding high numbers of MSC for use in the clinical and allogeneic setting in the Bioreactor versus using traditional culture flasks and a method currently in Phase I clinical trials. Cells expanded in the Bioreactor retained tri-lineage differentiation and expressed standard phenotypic markers of MSCs. When compared directly to MSCs grown in flasks, those expanded in the Bioreactor exceeded the number grown in flasks after only two passages. Flask-expanded MSCs required at least 4 passages to produce clinically-relevant numbers (2.0 ×108). When the cost of producing 2.0×108 MSC was compared in the two systems, they were found to be similar. The cost of the Bioreactor expansion sets is offset by the increased labor cost associate with using flasks. Importantly, the number of open events is reduced by over 99% when the Bioreactor is used. For investigators without access to clean rooms, or for facilities with limited staff and space, the self-contained design of the Bioreactor conserves resources by eliminating the need for multiple incubators.
MSCs are under intensive investigation for the treatment of diseases ranging from diabetes to stroke.(26, 27) Some of their popularity is a result of their safety profile in multiple clinical trials and the relative ease with which they can be manufactured. However, the production of MSCs, as with any cellular product, requires extensive resources, space, labor and GMP-compliance. These requirements may restrict investigators from translating promising research into clinical trials, even for MSC that require relatively limited manipulation. The Bioreactor system could address many of these concerns because 1) it is functionally closed so it minimizes the need for qualified airspace, 2) it is semi-automated so the amount of labor is markedly less than when generating MSC in flasks, and 3) the harvest time is 88% less than that needed for flasks, and requires 4 fewer processing technologists. The decreased harvest time may account for the improved viability of Bioreactor cells versus flask-generated cells for which the final harvest at P4 requires processing of over 100 flasks. Cells from flasks harvested at P2 had similar viability to those from the Bioreactor, indicating that the time required to harvest the large number of flasks at P4 may adversely impact viability. The inferior yield from flasks may also be attributed to the favorable hypoxic environment in the Bioreactor. However, in our experience, flasks and Quantum cells grown under hypoxic conditions were not markedly different from those grown under normal conditions (Supplementary figure 1). Instead, it is likely that the better monitoring of lactate and glucose conditions and the continuous feed of fresh media promoted better overall survival and proliferation.
Other bioreactor systems are available that address some of the aforementioned issues. We have not tested other bioreactors, so we cannot attest to their advantages or disadvantages first hand. However, based upon published descriptions, The Xpansion system from ATMI is an example of a single-use bioreactor that functions similarly to a 10-stack cell factory, but is designed with 200 layers packed closely with a central line for gas diffusion(28). Although this system shares many advantages of the Bioreactor, the Xpansion lacks the flexibility of the Quantum, which allows the user to program feed times and rates that can either be held constant or change depending upon the conditions. The Quantum can theoretically be programmed to be entirely automated, starting when the cells are loaded and ending once the cells are harvested. Another benefit of the design is that ultrafiltration (a force being applied across the semi-permeable membrane resulting from an increased flow rate on the extracapillary side of the membrane in relation to the intracapillary side) can be used in addition to enzymatic dissociation to ensure complete removal of cells during harvest. The most widely-used alternative to conventional flask-based expansion of MSCs is the use of cell factories. Other groups have reported the successful generation of large numbers of MSCs (>1×109 cells after 3-4 passages) by utilizing up to 8, 10-stack cell factories concurrently.(13, 14) Although our experience with this approach is limited, it uses fewer consumables than the flask-based approach and has fewer open events, though the labor required is still considerable.
One limitation of the Quantum is that the disposable bioreactor must be coated with Fibronectin before addition of the cells. Between the harvest of passage 1 and the loading of cells for passage 2, the cells would either need to be frozen or a second Bioreactor would have to be available to perform the Fibronectin coating of the second bioreactor. To avoid this limitation and show that two Bioreactors are not required, we successfully validated a procedure in which we held MSCs in a gas-permeable AFC bag on a rocking platform at room temperature for 4-6 hours while the old expansion set was unloaded, the new expansion set loaded, the system primed, and the bioreactor coated with Fibronectin. In our hands, cells held under these conditions were >90% viable and when placed back in culture were able to grow normally, with no difference when compared to the control (data not shown). Another limitation of the device is that although we are able to manufacture an average of >600×106 MSCs per run, this number would not be sufficient to support large, late phase clinical trials. This could be addressed by the development of a larger disposable or the use of multiple devices. An additional concern is that residual ethylene oxide in the expansion set, used by the manufacturer to sterilize the system, could lead to chromosomal instability, though safety data for this has recently been published.(29)
In our experiments, we loaded whole bone marrow (filtered through a 200 μm filter) into the Bioreactor system and washed away non-adherent cells after 3-4 days. However, companies such as Kaneka have manufactured MSC filters that greatly enrich the starting population. MSCs grown after filtering with the Kaneka filter showed more rapid growth in flasks.(30) Our preliminary data (Supplementary Figure 2) show that even when the same starting material is used such as Kaneka-filtered MSCs, the expansion in the Bioreactor is still more efficient than in flasks. Whether first enriching MSC – or even BMMC via Sepax – would improve the final yield in the Bioreactor still needs to be further tested. Although we observed a mean 66-fold expansion of expanded cells in the Bioreactor at passage-2, the 2.1 m2 (about 120 T-175 cm2 flasks) of the surface area within the bioreactor is not uniformly utilized, thus decreasing the potential yield. Further optimization of the seeding density and loading protocol will likely further improve the efficiency of expansion.
Overall, the self-contained Quantum is appealing for institutions looking to manufacture MSCs for clinical trials. The ability to produce large numbers of cells from each expansion set would allow the production of an allogeneic master cell bank with minimal chance of contamination compared to flasks. Moreover, because the system is almost entirely closed, there are fewer supplies to track and fewer manipulations to perform.
In conclusion, MSCs expanded in the Bioreactor were phenotypically and functionally similar to those expanded in flasks; however, MSCs expanded in the Quantum bioreactor achieved higher cell numbers more rapidly than those expanded in flasks and had more cell doublings at the time of harvest. When used in a rat model of ischemic stroke, animals receiving Bioreactor-grown cells showed significantly better outcomes than those infused with saline. Therefore, the Quantum could be a viable alternative to flask-based expansion of MSCs in vivo.
Supplementary Material
S1. The same lot of cells were thawed and 2×107 cells were placed in one Quantum supplied with a gas mixture of 20% oxygen or 5% oxygen. When the lactate exceeded 3 mM the Quantums were harvested and the cells were counted.
S2. 50 mL of bone marrow was filtered per Kaneka's recommendation and split into either flasks or the Quantum. Cells were cultured as described in the materials and methods and then harvested after 2 passages (Quantum) or 4 passages (Flasks).
S3. Trilineage potential of MSCs. Shown are MSCs after treatment with lineage-specific stimuli (see materials and methods). Shown is staining for adipogenesis (top), osteogenesis(middle), and chondrogenesis(bottom).
S4. Phenotyping of a representative example of MSCs. (A) Positive markers for MSCs (CD73, CD90, CD105) as well as the negative marker HLA-DR. (B) Lineage markers(FITC) and individual positive markers for CD73, CD90, and CD105. (C) Phenotypic comparison of cells expanded at P2 in the Quantum (black bars) and flasks (grey bars).
Acknowledgments
The authors would like to thank Terumo BCT as well as Dr. Helen Heslop and Dr. Malcolm Brenner for their guidance and support of this project. This work was funded by a Production Assistance for Cellular Therapy (PACT) contract from the NIH-NHLBI to Baylor College of Medicine Center for Cell and Gene Therapy, Contract No. HHSN268201000007C as well as a Cancer Prevention Research Institute of Texas award RP110553 to Malcolm Brenner.
Abbreviations
- MSC
Mesenchymal Stromal Cells
- BM
Bone Marrow
- GMP
Good Manufacturing Practices
- HLA
Human Leukocyte Antigen
- DMEM
Dulbecco's Modified Eagle Medium
- BMMC
Bone Marrow Mononuclear Cells
- DMSO
Dimethyl Sulfoxide
- PBS
Phosphate Buffered Saline
- Bioreactor
Quantum Cell Expansion System
- Quantum
Quantum Cell Expansion System
- Min
Minute
- P
Passage
- ISCT
International Society for Cellular Therapy
- CFSE
Carboxyfluorescein Succinimidyl Ester
- PBMC
Peripheral Blood Mononuclear Cells
- MCAo
Middle Cerebral Artery Occlusion
- CFU
Colony-Forming Units
- FDA
Food and Drug Administration
Footnotes
Conflict of Interest: Brent Rice is an employee of Terumo BCT.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature reviews Immunology. 2008;8(9):726–36. doi: 10.1038/nri2395. Epub 2009/01/28. [DOI] [PubMed] [Google Scholar]
- 2.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–506. doi: 10.1182/blood-2007-02-069716. Epub 2007/08/01. [DOI] [PubMed] [Google Scholar]
- 3.Philippe B, Luc S, Valerie PB, Jerome R, Alessandra BR, Louis C. Culture and Use of Mesenchymal Stromal Cells in Phase I and II Clinical Trials. Stem cells international. 2010;2010:503593. doi: 10.4061/2010/503593. Epub 2010/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflammation & allergy drug targets. 2009;8(2):110–23. doi: 10.2174/187152809788462635. Epub 2009/06/18. [DOI] [PubMed] [Google Scholar]
- 5.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):8932–7. doi: 10.1073/pnas.132252399. Epub 2002/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiser J, Zhang XY, Hemenway CS, Mondal D, Pradhan L, La Russa VF. Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert opinion on biological therapy. 2005;5(12):1571–84. doi: 10.1517/14712598.5.12.1571. Epub 2005/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11(5):503–15. doi: 10.1080/14653240903193806. Epub 2009/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–402. doi: 10.1182/blood.v98.8.2396. Epub 2001/10/06. [DOI] [PubMed] [Google Scholar]
- 9.Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. International journal of clinical and experimental medicine. 2010;3(4):248–69. Epub 2010/11/13. [PMC free article] [PubMed] [Google Scholar]
- 10.Gotherstrom C, Ringden O, Westgren M, Tammik C, Le Blanc K. Immunomodulatory effects of human foetal liver-derived mesenchymal stem cells. Bone marrow transplantation. 2003;32(3):265–72. doi: 10.1038/sj.bmt.1704111. Epub 2003/07/15. [DOI] [PubMed] [Google Scholar]
- 11.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. Epub 1999/04/02. [DOI] [PubMed] [Google Scholar]
- 12.Vidal MA, Walker NJ, Napoli E, Borjesson DL. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue. Stem cells and development. 2012;21(2):273–83. doi: 10.1089/scd.2010.0589. Epub 2011/03/18. [DOI] [PubMed] [Google Scholar]
- 13.Connick P, Kolappan M, Patani R, Scott MA, Crawley C, He XL, et al. The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pre-test: post-test study with blinded outcome assessments. Trials. 2011;12:62. doi: 10.1186/1745-6215-12-62. Epub 2011/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabatino M, Ren J, David-Ocampo V, England L, McGann M, Tran M, et al. The establishment of a bank of stored clinical bone marrow stromal cell products. Journal of translational medicine. 2012;10:23. doi: 10.1186/1479-5876-10-23. Epub 2012/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanley PJ, Mei Z, da Graca Cabreira-Hansen M, Klis M, Li W, Zhao Y, et al. Manufacturing mesenchymal stromal cells for phase I clinical trials. Cytotherapy. 2013;15(4):416–22. doi: 10.1016/j.jcyt.2012.09.007. Epub 2013/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bieback K, Kinzebach S, Karagianni M. Translating research into clinical scale manufacturing of mesenchymal stromal cells. Stem cells international. 2011;2010:193519. doi: 10.4061/2010/193519. Epub 2011/02/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sensebe L, Bourin P, Tarte K. Good manufacturing practices production of mesenchymal stem/stromal cells. Human gene therapy. 2011;22(1):19–26. doi: 10.1089/hum.2010.197. Epub 2010/10/30. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nature medicine. 1999;5(3):309–13. doi: 10.1038/6529. Epub 1999/03/23. [DOI] [PubMed] [Google Scholar]
- 19.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. Journal of biomedical science. 2005;12(1):47–57. doi: 10.1007/s11373-004-8183-7. Epub 2005/05/03. [DOI] [PubMed] [Google Scholar]
- 20.Nold P, Brendel C, Neubauer A, Bein G, Hackstein H. Good manufacturing practice-compliant animal-free expansion of human bone marrow derived mesenchymal stroma cells in a closed hollow-fiber-based bioreactor. Biochemical and biophysical research communications. 2013;430(1):325–30. doi: 10.1016/j.bbrc.2012.11.001. Epub 2012/11/14. [DOI] [PubMed] [Google Scholar]
- 21.Rojewski MT, Fekete N, Baila S, Nguyen K, Furst D, Antwiler D, et al. GMP-compliant isolation and expansion of bone marrow-derived MSCs in the closed, automated device Quantum Cell Expansion system. Cell transplantation. 2012 doi: 10.3727/096368912X657990. Epub 2012/10/31. [DOI] [PubMed] [Google Scholar]
- 22.Schallmoser K, Strunk D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. Journal of visualized experiments : JoVE. 2009;(32) doi: 10.3791/1523. Epub 2009/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Experimental hematology. 1974;2(2):83–92. Epub 1974/01/01. [PubMed] [Google Scholar]
- 24.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. Epub 2006/08/23. [DOI] [PubMed] [Google Scholar]
- 25.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39(5):777–87. doi: 10.1016/s0028-3908(00)00005-8. Epub 2000/03/04. [DOI] [PubMed] [Google Scholar]
- 26.Pileggi A. Mesenchymal stem cells for the treatment of diabetes. Diabetes. 2012;61(6):1355–6. doi: 10.2337/db12-0355. Epub 2012/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–106. doi: 10.1002/stem.430. Epub 2010/05/28. [DOI] [PubMed] [Google Scholar]
- 28.Jean-Francois Michiels ME. Scaling up Stem Cells. Utilizing a Multiplate Bioreactor to Preserve the Integrity of the Culture. Genetic Engineering and Biotechnology News. 2013;33(2) Internet. [Google Scholar]
- 29.Jones M, Varella-Garcia M, Skokan M, Bryce S, Schowinsky J, Peters R, et al. Genetic stability of bone marrow-derived human mesenchymal stromal cells in the Quantum System. Cytotherapy. 2013;15(11):1323–39. doi: 10.1016/j.jcyt.2013.05.024. Epub 2013/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otsuru S, Hofmann TJ, Olson TS, Dominici M, Horwitz EM. Improved isolation and expansion of bone marrow mesenchymal stromal cells using a novel marrow filter device. Cytotherapy. 2013;15(2):146–53. doi: 10.1016/j.jcyt.2012.10.012. Epub 2013/01/17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. The same lot of cells were thawed and 2×107 cells were placed in one Quantum supplied with a gas mixture of 20% oxygen or 5% oxygen. When the lactate exceeded 3 mM the Quantums were harvested and the cells were counted.
S2. 50 mL of bone marrow was filtered per Kaneka's recommendation and split into either flasks or the Quantum. Cells were cultured as described in the materials and methods and then harvested after 2 passages (Quantum) or 4 passages (Flasks).
S3. Trilineage potential of MSCs. Shown are MSCs after treatment with lineage-specific stimuli (see materials and methods). Shown is staining for adipogenesis (top), osteogenesis(middle), and chondrogenesis(bottom).
S4. Phenotyping of a representative example of MSCs. (A) Positive markers for MSCs (CD73, CD90, CD105) as well as the negative marker HLA-DR. (B) Lineage markers(FITC) and individual positive markers for CD73, CD90, and CD105. (C) Phenotypic comparison of cells expanded at P2 in the Quantum (black bars) and flasks (grey bars).