Abstract
Promoting mesenchymal stem cell (MSC) proliferation has numerous applications in stem cell therapies, particularly in the area of regenerative medicine. In order for cell-based regenerative approaches to be realized, MSC proliferation must be achieved in a controlled manner without compromising stem cell differentiation capacities. Here we demonstrate that 6-bromoindirubin-3’-oxime (BIO) increases MSC β-catenin activity 106-fold and stem cell-associated gene expression ~33-fold respectively over untreated controls. Subsequently, BIO treatment increases MSC populations 1.8-fold in typical 2D culture conditions, as well as 1.3-fold when encapsulated within hydrogels compared to untreated cells. Furthermore, we demonstrate that BIO treatment does not reduce MSC multipotency, where MSCs maintain their ability to differentiate into osteoblasts, chondrocytes, and adipocytes using standard conditions. Taken together, our results demonstrate BIOs potential utility as a proliferative agent for cell transplantation and tissue regeneration.
Keywords: mesenchymal stem cells, hydrogel biomaterials, 6-bromoindirubin-3’-oxime, proliferation, pluripotency, regenerative medicine
1. Introduction
The self-renewal capacity of mesenchymal stem cells (MSCs), also referred to as marrow stromal cells or multipotent mesenchymal stem cells was first identified in the early 1960s (Becker et al., 1963). It is well established that MSCs maintain self-renewal capacities in vitro during early passage number (Bonab et al., 2006; Pittenger et al., 1999; Shahdadfar et al., 2005) but undergo senescence upon extensive passages (>8) (Bork et al., 2010; Roobrouck et al., 2008; Wagner et al., 2008). Many reports have supported multilineage differentiation capacity, highlighting their ability to undergo osteogenic, chondrogenic, and adipogenic differentiation (Elisseeff et al., 2005; Jaiswal et al., 1997; Mackay et al., 1998; McBeath et al., 2004). Advancements in purification and amplification techniques have enabled MSCs to be isolated from fat, muscle, and bone marrow (Elisseeff et al., 2005; Pittenger et al., 1999). Most recently, preclinical models have demonstrated MSC therapeutic efficacy in the regeneration of a variety of musculoskeletal tissues (Banfi et al., 2000; Elisseeff et al., 2005; Murphy et al., 2003; Xie et al., 2007). Unfortunately, MSCs are sparse, accounting for only 0.1-1% of the total bone marrow cell population (Tsutsumi et al., 2001). Cell-based therapies that exploit MSCs require long culture periods to obtain adequate numbers of cells. From one bone marrow aspirate (~10 mL), it takes ~3 weeks to culture ~13×106 MSCs, the typical cell population used in articular cartilage repair strategies (Wakitani et al., 2002). Furthermore, this approximation does not take into account MSC proliferative quiescence in vivo (Baxter et al., 2004; Bruder et al., 1997), or the diminished MSC prevalence in the elderly, the target population for a number of MSC therapeutic strategies (Kasten et al., 2008; Wakitani et al., 2002). Reducing this waiting period to expedite patient treatment requires methods to efficiently and reproducibly expand MSCs ex vivo (Tsutsumi et al., 2001).
Numerous methods have been investigated to enhance MSC proliferation (Ball et al., 2007; Fierro et al., 2007; Ogawa et al., 2010; Rodrigues et al., 2010; Stewart et al., 2010; Tsutsumi et al., 2001). Often growth factors are utilized, including transforming growth factor beta 1 and 3 (TGFβ-1, -3) (Ogawa et al., 2010; Rodrigues et al., 2010), bone morphogenic protein 3 (BMP-3) (Rodrigues et al., 2010; Stewart et al., 2010), basic fibroblast growth factor (bFGF) (Rodrigues et al., 2010; Tsutsumi et al., 2001), vascular endothelial growth factor (VEGF) (Ball et al., 2007), and platelet-derived growth factor (PDGF) (Fierro et al., 2007). However these approaches are expensive (Awad et al., 2007) and limited by transient cellular responses (Bonewald and Dallas, 1994), compromised differentiation potential (Luu et al., 2007), and increased population heterogeneity (Shahdadfar et al., 2005). Furthermore, growth factor-treated MSCs have been shown to exhibit senescent phenotypes, reduced proliferation and homing capacities, and telomere shortening that may further hamper cell therapeutic applications (Baxter et al., 2004; Bruder et al., 1997).
As an alternative to growth factors, small molecule drugs have also been used to promote stem cell proliferation (Chen et al., 2006; Meijer et al., 2003; Pevsner-Fischer et al., 2007; Polychronopoulos et al., 2004; Ying et al., 2008; Yu et al., 2011). Small molecules are not readily susceptible to protease degradation or unfolding and can be synthesized using synthetic chemistry techniques, greatly reducing their production costs as compared to recombinant growth factors (Benoit et al., 2006b). For example, Pevsner-Fischer et al. observed 1.4-fold increases in MSC proliferation 48 hr after treatment with the small molecule Pam3Cys, a synthetic Toll-like receptor ligand (Pevsner-Fischer et al., 2007). Similarly, embryonic stem cell (ESC) proliferation has been increased using the rat sarcoma GTPase-activating protein/extracellular signal-regulated kinase 1 (RasGAP/ERK1) inhibitor Pluripotin (Chen et al., 2006), the TGF-β/Activin/Nodal receptor inhibitor A83-01 (Yu et al., 2011), and both CHIR99021 (Ying et al., 2008) and BIO (Meijer et al., 2003; Polychronopoulos et al., 2004), agonists of Wnt/β-catenin signaling. Furthermore, small molecule drugs can be incorporated into controlled release strategies without compromising their bioactivity, making them more readily applicable for in situ tissue engineering strategies. For example, hydrolytic degradation of lactic acid ester tethers have been used to control the release of both fluvastatin (Benoit et al., 2007b; Benoit et al., 2006b) and dexamethasone (Nuttelman et al., 2006) from poly(ethylene glycol) (PEG) hydrogels to initiate MSC osteogenic differentiation.
Amongst the cohort of small molecule drugs explored to increase proliferation, BIO acts as a glycogen synthase kinase 3 beta (GSK3β) specific inhibitor and prevents proteosomal degradation of β-catenin (Meijer et al., 2003; Polychronopoulos et al., 2004) (Supplemental Figure S1). Increased cytosolic concentrations of active β-catenin translocate into the nucleus and bind to the Transcription Factor/Lymphoid Enhancing-Binding Factor (TCF/LEF) transcription factors, resulting in Wnt target gene expression (Sato et al., 2004; Sineva and Pospelov, 2010; Tseng et al., 2006). Previously, BIO has been shown to agonize Wnt/β-catenin signaling, and enhance self-renewal and pluripotency of ESCs (Sato et al., 2004; Sineva and Pospelov, 2010) and mammalian cardiomyocytes (Tseng et al., 2006). However, the effect of BIO on MSCs has not been reported.
In this work, we sought to investigate BIO's effect on MSC expansion in both tissue culture-plated (2D) MSCs as well as MSCs encapsulated in PEG hydrogels (3D). Following BIO-treatment MSC proliferation and Wnt target gene expression was monitored over 6 weeks. In addition, BIO-treated cells assessed for maintenance of multilineage potential in 2D and 3D culture to further support the use of BIO for MSC-based stem cell therapies.
2. Materials and Methods
All materials were purchased from Sigma-Aldrich unless otherwise specified.
2.1 Synthesis of 6-Bromoindirubin-3’-oxime (BIO)
The small molecule BIO was synthesized as previously described (Meijer et al., 2003; Polychronopoulos et al., 2004) (Supplemental Figure S2A). The resulting product was verified via 1H-NMR and matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectroscopy (m/z 356 g/mol).
2.2 Synthesis of Poly(ethylene glycol) (PEG) Macromers
Hydrolytically-Degradable PEG Macromers
To synthesize degradable PEGPLADM tri-block copolymers [poly(lactide)-b-PEG-b-poly(lactide) dimethacrylate], linear PEG (Alfa Aesar, MW 10 kDa, n=227) was functionalized with d,l-lactide as previously described (Sawhney et al., 1993) (Supplemental Figure S2B). PEG was reacted with d,l-lactide at a molar ratio of 1:2 (d,l-lactide:PEG) in the presence of stannous octoate catalyst for 4 hrs in a 140 °C oil bath. The reaction was cooled and the product (PEGPLA) was precipitated three times in cold ethyl ether, collected by filtration, and dried under vacuum. 1H-NMR analysis was used to determine the number of lactide (δ=5.19, 2H) units per PEG macromer (δ=3.64, 908H). PLA NMR analysis revealed m~2.
Methacrylation was performed as described previously (Lin-Gibson et al., 2004). Methacrylic anhydride was combined with PEGPLA at a molar ratio of 5:1 (methacrylic anhydride:PEGPLA) in a glass scintillation vial and microwaved (1100 W, Sharp) for 5 min. The reaction was cooled and the product (PEGPLADM) was dissolved in dichloromethane, precipitated three times in cold ethyl ether, collected by filtration, and dried under vacuum. 1H-NMR analysis was used to determine the number of methacrylate functional groups (δ=5.6, 1H/end and 6.1, 1H/end) per PEG macromer (δ=3.64, 908H) and the percent methacrylation was determined to be >95%.
Synthesis of Acrylate-PEG-RGDS
The amino acid sequence Arg-Gly-Asp-Ser (RGDS; 433 Da, EMD Chemicals, San Diego CA) was coupled to acrylated-PEG through the amino terminus by dissolving 20 mg of peptide in 2 mL of dimethyl sulfoxide (DMSO) and adding a single drop of N,N-Diisopropylethylamine. To this, acrylate-PEG-N-Hydroxysuccinimide (acrylate-PEG-NHS, MW 3500 Da, Jenkem Technology, Beijing China, m/z 3707 Da, p=79) was added at a molar ratio of 1:1.1 (peptide:acrylate-PEG-NHS). The reaction was vortexed for 1 min and allowed to incubate overnight with gentle agitation. The product (acrylate-PEG-RGDS, Supplemental Figure S2C) was dialyzed against deionized water (molecular weight cutoff = 1000 Da, Spectrum Labs, Rancho Dominguez CA), lyophilized, analyzed via MALDI-TOF (m/z Na+, 4070 Da), and stored at 4 °C.
2.3 The effect of BIO on two-dimensionally treated mesenchymal stem cell (MSC) functions
Cell Culture
MSCs were isolated from human donor bone marrow obtained from Lonza (Walkersville, MD) as described previously (Pittenger et al., 1999). MSCs were grown at 37 °C and 5% CO2 in growth media (GM) consisting of low-glucose Dulbecco's Modified Eagle Medium (DMEM, Thermo) supplemented with 10% Fetal Bovine Serum (FBS) (Atlanta Biologicals, Lawrenceville, GA, USA), 100 units/ml penicillin (Lonza), 100 μg/ml streptomycin (Lonza), 0.25 μg/ml amphotericin B (Lonza) (low-glucose GM). In this study MSCs from a single donor at passage 3 were used. It should be noted however that similar results as presented herein were obtained in preliminary studies using MSCs from at least one other donor cell population at passages ranging from 2 to 5 (data not shown).
Mouse embryonic fibroblasts (C3H10T1/2, Clone 8) were obtained from American Type Culture Collection (ATCC) (Manassas, VA). C3H10T1/2s were grown at 37 °C and 5% CO2 in Basal Medium Eagle (cellgro) supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B (BME media). In this study C3H10T1/2s at passage 10 were used instead of MSCs for transfection experiments. It has been demonstrated that C3H10T1/2s exhibit enhanced transfection efficiencies and comparable differentiation capacities as MSCs, making them an ideal and often used surrogate cell type for these experiments (Bilkovski et al., 2010; Bowers and Lane, 2008; Reznikoff et al., 1973; Shea et al., 2003; Tang and Lane, 2012; Zhao et al., 2009).
Cell Treatment with BIO
MSCs or C3H10T1/2s were seeded (3,000 cell/cm2) in 24-well tissue culture plates (Greiner Bio-One, Monroe NC) and allowed to become adherent over 24 hr. 10 μL of BIO in DMSO was added to 1 mL of culture media to achieve final BIO concentrations of 0, 2, and 5 μM. Cells were cultured with BIO-containing media for 24 hr after which media was replaced by media without BIO.
Analyzing β-catenin activity in C3H10T1/2s
C3H10T1/2s seeded in 48-wells plates were transfected with 0.4 μg/well of TOPFlash or FOPFlash reporter plasmid (Addgene Plasmid #12456 and #12457 (Biechele and Moon, 2008)) using Lipofectamine LTX and PLUS reagents (Invitrogen) for 3 hrs at 37 °C and 5% CO2. Transfection media was then removed and replaced with medium containing 0, 2, or 5 μM BIO. Cells were incubated at 37 °C and 5% CO2 for 24 hr, after which BIO containing media was removed and replaced with normal BME media. Total luminescence was measured (BioTek Synergy Mx plate-reader) prior to BIO treatment (day 0), as well as on days 1, 2, 3, 4, and 5 after treatment using the Luciferase Assay Kit (lysis reagent and luciferase reagent, Promega, Madison WI). Total luminescence was normalized to cellular DNA concentration measured using a Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen) to account for well-to-well variability in cell density.
Analyzing the effects of BIO on 2D MSC proliferation
To assess 2D MSC proliferation, cells were trypsinized and counted using trypan blue exclusion and a hemocytometer. Average cell counts were normalized to data collected on day 0 to illustrate the effect of BIO on MSC proliferation over time.
Assessment of MSC cell layer thickness
MSC cell layer thickness was measured using the z-directional controls of a FV1000 Olympus Laser Scanning Confocal Microscope. On days 1, 14, and 40 cells, seeded on glass bottom 24-well plates (MatTek, Ashland MA) were stained using a fluorescent LIVE/DEAD Viability/Cytotoxicity kit (Invitrogen). Cell layer thickness was recorded as the distance from the cell-plate interface to the focal plane where live fluorescently stained cells were no longer visible.
Analyzing the effects of BIO on MSC differentiation
MSCs seeded (3,000 cell/cm2) in 24-well plates and treated with BIO were allowed to proliferate for 3 weeks before being cultured for an additional 3 weeks in standard osteogenic, chondrogenic, and adipogenic induction media (Jaiswal et al., 1997; Mackay et al., 1998; McBeath et al., 2004). For osteogenic differentiation MSCs were grown in GM supplemented with 100 nM dexamethasone, 10 mM β-glycerophosphate, and 50 μM ascorbic acid-2-phosphate (2-phospho-L-ascorbic acid) (Jaiswal et al., 1997). Similarly, for adipogenic differentiation, MSCs were cultured with adipogenic supplements, switching between 3 days in adipogenic differentiation medium (high-glucose GM, 1 μM dexamethasone, 0.2 mM indomethacin (Alfa Aesar), 10 μg/mL insulin, and 0.5 mM methylisobutylxanthine (Calbiochem, La Jolla CA) and 1 day in adipogenic maintenance medium (high-glucose GM, 10 μg/mL insulin) (McBeath et al., 2004). Differentiated cell cultures were fixed in 4% paraformaldehyde and stained for mineralization (von Kossa, osteogenic), or the presence of lipid droplets (oil red o, adipogenic). For chondrogenic differentiation, BIO-proliferated MSCs were placed into pellet cultures (250,000 cells/pellet) and cultured for 3 weeks in GM supplemented with 10 ng/mL TGF-β3 (PeproTech, Rocky Hill NJ), 100 nM dexamethasone, 50 μg/mL ascorbic acid-2-phosphate, 40 μg/mL proline, and 0.1% v/v ITS+premix (BD Biosciences, Bedford MA) (Mackay et al., 1998). Pellet cultures were fixed in 4% paraformaldehyde, cryosectioned, stained for glycosaminoglycan production (toluidine blue, chondrogenic). Images were taken on a Motic AE20 inverted light microscope using a modified Canon EOS Rebel T2i.
Evaluation of BIO-mediated alterations in MSC gene expression
Reverse transcription polymerase chain reaction (RT-PCR) was used to assess gene expression of MSCs over time. Sample RNA was isolated using the ENZA Total RNA Kit (Omega) and RNA concentration was normalized across groups to account for experimental variations in cell number between treated and untreated populations. RT was performed using the iScript cDNA Synthesis Kit (Bio-Rad) incubated at 25 °C for 5 min, 42 °C for 30 min, and terminated at 85 °C for 5 min. PCR was performed using a CFX96 Real-Time PCR System (Bio-Rad), monitoring sybrgreen products (SsoFastEvaGreen Master Mix, Bio-Rad). Primers for β-actin, sex determining region Y-box 2 (Sox2), homeobox transcription factor NANOG (NANOG), octamer-binding transcription factor 4 (Oct4), G1/S-specific cyclin D1 (Cyclin D1), core binding factor alpha-1 (Cbfa1), sex determining region Y-box 9 (Sox9), and peroxisome proliferator-activated receptor gamma (PPARγ) were used (Supplemental Figure S3). The PCR parameters used were as follows: hold at 95 °C for 5 min, followed by 40 cycles of: 95 °C for 15 sec denaturation, 60 °C (β-actin, Sox2, Cyclin, D1, Sox9), or 57 °C (NANOG, Oct4, Cbfa1), or 55 °C (PPARγ) for 60 sec annealing, and 72°C for 20 sec extension. Threshold cycle (CT) analysis was used to quantify PCR products normalized to the cellular housekeeping gene β-actin. Relative gene quantification was performed using the Pfaffl Method, taking into account the variation between calculated primer efficiencies (Pfaffl, 2001).
2.4 The effect of BIO on PEG-encapsulated MSC Function
Photoencapsulation of MSCs in PEG hydrogels
A 10 wt% solution of PEGPLADM was prepared in phosphate buffered saline with 2.0 mM acrylate-PEG-RGDS to maintain MSC viability through integrin interactions (Benoit et al., 2007a; Benoit et al., 2007b; Benoit et al., 2007c; Benoit et al., 2008). The photoinitiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate was synthesized as previously described (Fairbanks et al., 2009) and added at a final concentration of 0.05 wt%. Trypsinized MSCs added to the PEG macromer solution to achieve a final concentration of 25 million cells/mL. Using a sterile 1 mL syringe with the tip removed as a mold, 40 μL of PEG/cell solution was added and exposed to long-wavelength 365 nm light (5 mW/cm2 intensity) for 10 min at room temperature. Encapsulated MSC viability, BIO treatment, and assessment of gene expression were performed as described in Section 2.3 with exception of cell lysate collection. As 2D proliferation results revealed no statistical significance between 2 and 5 μM treatment, encapsulated MSCs were treated with only 5 μM BIO in an effort to reduce sample size. For assessment of gene expression PEG/cell constructs were homogenized in lysis buffer and centrifuged to pellet polymerized PEG. The supernatant was then collected for analysis.
Encapsulated MSC proliferation analysis
Encapsulated MSC proliferation was assessed using total cellular DNA concentration, assuming constant DNA per cell, using a Quant-iT PicoGreen dsDNA Assay Kit. Average encapsulated DNA concentrations were normalized to data collected on day 0 to illustrate BIOs effect on MSC proliferation over time.
Encapsulated MSC differentiation analysis
MSCs were treated with BIO, and allowed to proliferate for 21 days in 2D before being encapsulated in PEGPLADM hydrogels and differentiated osteogenically, chondrogenically, and adipogenically using standard induction media (Jaiswal et al., 1997; Mackay et al., 1998; McBeath et al., 2004). Samples were collected for gene analysis, or fixed in 4% paraformaldehyde, cryosectioned, and stained for mineralization, glycosaminoglycan production, or the presence of lipid droplets.
2.5 Statistical Analysis
All data is presented as mean +/- standard deviation with at least three replicate samples averaged for each data point. Statistics were assessed with GraphPad Prism Software using a two-way ANOVA with Bonferroni post-hoc analysis.
3. Results
3.1 BIO-mediated agonism of Wnt/ β-catenin signaling
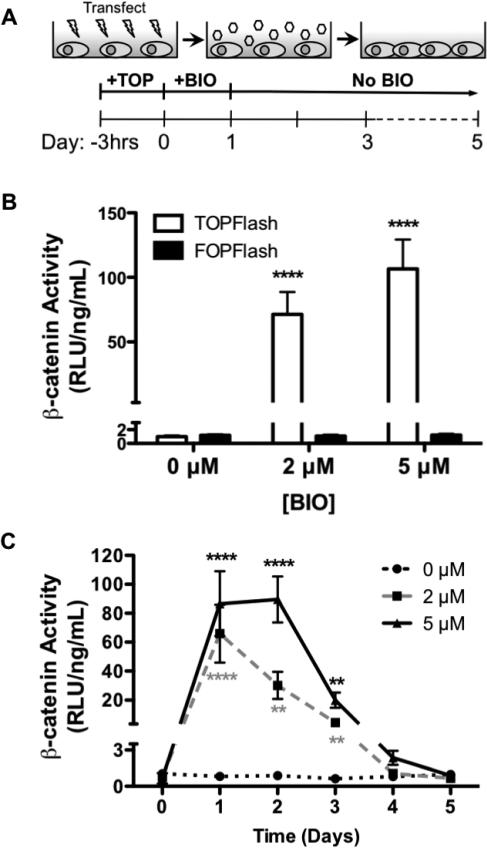
BIO-mediated enhancement of β-catenin activity is well established (Meijer et al., 2003; Sato et al., 2004; Sineva and Pospelov, 2010). Herein, the efficacy of BIO as an agonist of Wnt/β-catenin signaling was quantified using a luminescent reporter plasmid specific for active β-catenin (Figure 1A) (Biechele and Moon, 2008). Mouse embryonic fibroblasts (C3H10T1/2) were used as a surrogate for MSCs due to poor MSC transfection rates observed in our studies and in literature (Bilkovski et al., 2010; Bowers and Lane, 2008; Reznikoff et al., 1973; Tang and Lane, 2012). Previously, it has been demonstrated that C3H10T1/2s have similar multi-lineage potential as MSCs (e.g., can be differentiated into chondrogenic, osteogenic, and adipogenic lineages) (Shea et al., 2003; Zhao et al., 2009). β-catenin activity (represented as TOPFlash relative luminescence units/ng DNA) supported dose-dependent BIO-mediated increases after 24 hr BIO treatments. As illustrated in Figure 1B, cells exhibited a 71- and 106-fold increase in β-catenin activity compared to untreated controls immediately after treatment with 2 and 5 μM BIO, respectively. Furthermore, β-catenin activation persisted for 3 days after BIO treatment before returning to basal levels (Figure 1C).
Figure 1. BIO Treatment Enhanced Active Nuclear β-catenin Concentrations.
C3H10T1/2 transfection with DNA reporter plasmids (A). Active β-catenin (TOPFlash) shows a BIO-mediated increase compared to controls (FOPFlash) (****P<0.0001 vs. FOPFlash) (mean +/− SD) (B). Increased active β-catenin persists for 3 days after BIO-treatment before returning to basal levels (**p<0.01, ****p<0.0001 vs. 0 μM) (mean +/− SD) (C).
3.2 Proliferative effect of BIO on tissue culture plated (2D) MSCs
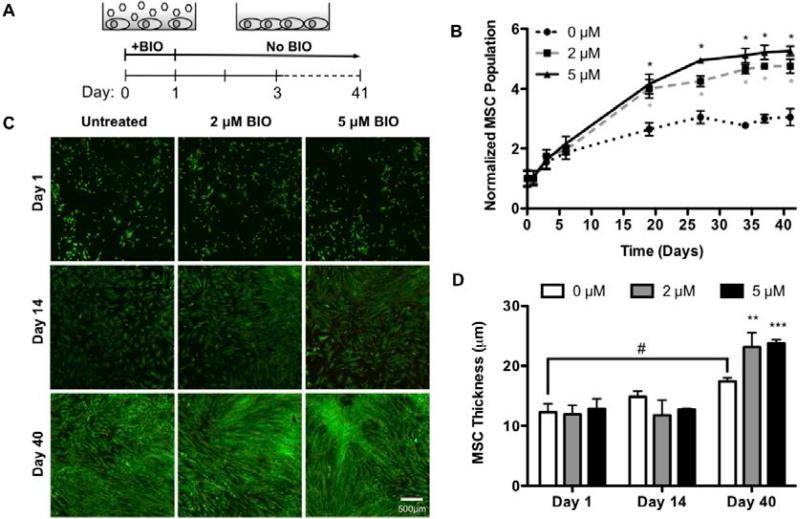
Wnt/β-catenin signaling has been shown to regulate proliferation and survival in numerous cell types, including ESCs and endothelial cells (Masckauchan et al., 2005; Sineva and Pospelov, 2010). As an agonist of Wnt/β-catenin signaling, BIO has been shown to increase ESC (Sato et al., 2004; Sineva and Pospelov, 2010) and cardiomyocyte (Tseng et al., 2006) proliferation. To evaluate the effect of BIO on MSC proliferation, cells were counted manually just before and at several time points (1, 3, 6, 19, 27, 34, 37, and 41 days) after treatment with 0, 2, or 5 μM BIO (Figure 2A). The results, shown in Figure 2B, were normalized to day 0 cell counts. Normalized cell populations increased in BIO dose-dependent fashions over 41 day culture periods. Compared to day 0, at day 41, MSCs treated with 2 or 5 μM BIO exhibited a 4.5- and a 5.5-fold increase in cell number, respectively. Comparatively, untreated MSC exhibited a 3.0-fold increase in cell number.
Figure 2. BIO Enhances Proliferation of Tissue Culture Plated (2D) MSCs.
2D MSC culture after 24 hr BIO-treatment (A). Cells were shown to undergo a BIO-mediated increase in proliferation (*p<0.0001 vs. 0 μM) (mean +/− SD) (B). LIVE/DEAD (live = green, dead = red) confocal imaging of 2D BIO-treated MSCs revealed increased cell density (Bar = 500 μM) (C), and cell layer thickness compared to untreated controls (#p<0.05 vs. day 1 0 μM, **p<0.01, ***p<0.001 vs. 0 μM) (mean +/− SD) (D).
By day 18 of the proliferation study, all experimental groups (0, 2, and 5 μM) had reached confluence based on visual inspection. To further investigate how BIO was increasing MSC proliferation beyond the limits of contact inhibition, cell morphology was examined using LIVE/DEAD staining and confocal microscopy. Qualitative assessment of images taken on day 14 showed greater cell density for BIO-treated cells than for untreated controls (Figure 2C). This trend continued at day 40 where there were noticeably denser cell clusters in the BIO-treated cultures, while the untreated controls looked uniformly confluent. Closer analysis of the 2 and 5 μM BIO treated cultures at day 40 revealed these denser cell regions had thicknesses of 23.5 μm and 24.0 μm respectively, as compared to the untreated controls (17.5 μm, Figure 2D). Measurements made at day 1 and 14 respectively showed no statistically significant differences between groups. Comparison between days 1 and 40 for the untreated MSC controls showed a slight and statistically significant increase in cell layer thickness. This is attributed to cell crowding as the cultures reach confluence.
3.3 Proliferation of BIO-treated MSCs encapsulated in PEG hydrogels (3D)
Having demonstrated that BIO enhanced MSC proliferation in 2D culture conditions, we sought to assess if this proliferative effect could be translated to cells within hydrogel microenvironments. PEG hydrogels are commonly used in tissue engineering applications (Benoit et al., 2007b; Benoit et al., 2006b; Elisseeff et al., 2005; Lin and Anseth, 2009; Nuttelman et al., 2006). They are highly hydrophilic, biologically inert, are easily modified to incorporate biomolecules and cell-adhesive ligands, and resemble soft tissues with respect to mechanical properties, water content, and elasticity (Benoit et al., 2007b; Benoit et al., 2006b; Elisseeff et al., 2005; Lin and Anseth, 2009; Nuttelman et al., 2006). Furthermore, hydrogels can be easily modified to incorporate controlled drug release mechanisms (Benoit et al., 2007b; Benoit et al., 2006b; Nuttelman et al., 2006).
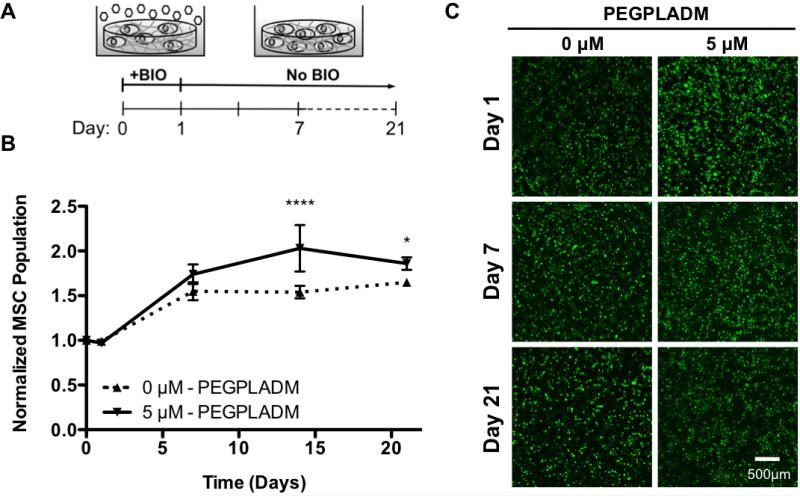
To accommodate MSC proliferation and expansion, hydrolytically degradable poly(lactide)-b-PEG-b-poly(lactide) dimethacrylate (PEGPLADM) tri-block copolymers were utilized (Benoit et al., 2006a). PEGPLADM-encapsulated MSCs were treated with 0 or 5 μM BIO for 24 hr (Figure 3A). As 2D proliferation results revealed no statistical significance between 2 and 5 μM BIO treatment (Figure 2B) 2 μM treatment was not investigated in 3D. Just before, and 1, 7, 14, and 21 days after BIO treatment, MSC proliferation was assessed by quantifying DNA content within hydrogels. The results shown in Figure 3B were normalized to initial DNA concentrations (day 0), and are a reflection of relative cell number assuming constant DNA per cell. BIO-treated MSCs exhibited statistically significant 1.3-fold increases in cell numbers compared to untreated controls. Additionally, BIO-treated MSC viability (>90%) was verified by LIVE/DEAD confocal imaging throughout the culture period, as illustrated in Figure 3C (Benoit et al., 2007c).
Figure 3. BIO Enhances Proliferation of PEGPLADM Encapsulated (3D) MSCs.
3D PEGPLADM-encapsulated MSC culture after 24 hr BIO-treatment (A). Encapsulated MSCs were shown to undergo a BIO-mediated increase in proliferation (*P<0.05, ****p<0.0001 vs. 0 μM) (mean +/− SD) (B). Representative LIVE/DEAD (live = green, dead = red) confocal images of encapsulated BIO-treated MSC cultures (Bar = 500 μm) (C).
3.4 BIO-treated MSC gene expression (2D)
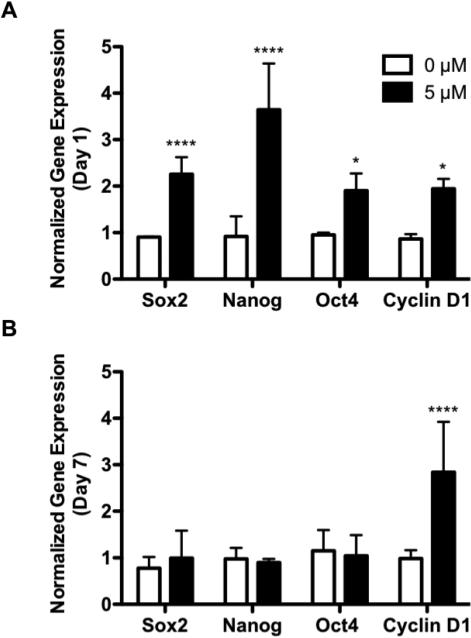
To more closely examine how BIO-mediated increases of active β-catenin concentrations affect MSC phenotype and proliferation, expression of Wnt target genes were analyzed. Specifically, stem cell-associated transcription factors Sox2, NANOG, Oct4, as well as the cell cycle regulator Cyclin D1. These genes were selected, as their elevated expression and subsequent enhancements in pluripotency and proliferation (Kashyap et al., 2009; Kelly et al., 2011; Yi et al., 2011; Yu et al., 2007) have previously been shown in BIO-treated ESCs (Sato et al., 2004). RNA was collected just before, and several days (1, 3, 6, 19, 27, 34, 37, and 41) after BIO-treatment. Gene profiles (Supplemental Figure S4) were normalized to both the housekeeping gene β-actin as well as untreated controls, and are expressed as a fold-increase over initial gene expression prior to BIO treatment (day 0).
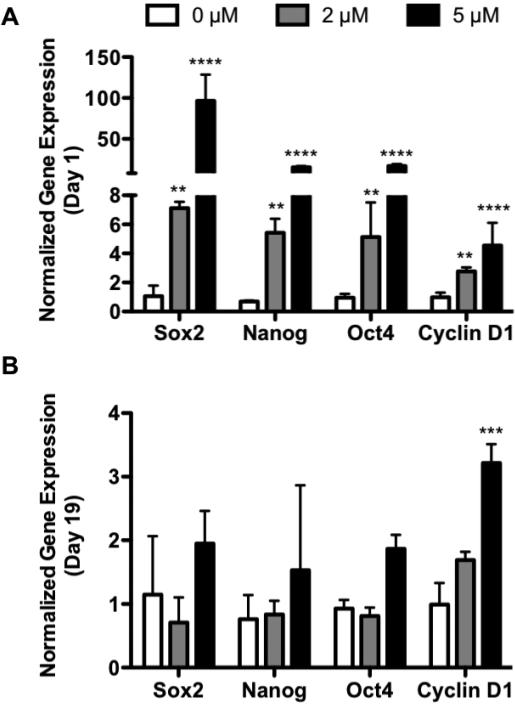
Immediately after BIO-treatment (day 1), an increase in transcription factor expression was observed (Figure 4A). Sox2 expression was elevated 7.1- and 96.4-fold with 2 and 5 μM BIO treatment, compared to untreated controls (Figure 4A). Similarly, NANOG expression was increased to 5.4- and 15.3-fold (Figure 4A), and Oct4 expression was increased to 5.1- and 16.9-fold untreated controls (Figure 4A). The expression of these transcription factors remained up-regulated in a BIO dose-dependent fashion through day 3 before returning to basal levels (Supplemental Figure S4A-C). By day 19, BIO-treated MSC Sox2, NANOG, and Oct4 expression levels were not significantly increased over untreated controls (Figure 4B). This spike in stem cell-associated transcription factor expression directly coincided with enhanced β-catenin activity (Figure 1C). This is indicative of BIO-mediated activation of canonical Wnt/β-catenin signaling, and subsequent transcription of Wnt target genes.
Figure 4. BIO Enhances 2D MSC Expression Sox2, NANOG, Oct4, and Cyclin D1.
Profiles of Wnt target genes Sox2, NANOG, Oct4, and Cyclin D1 following 24 hr BIO treatment (Day 1) (A), and 18 days post-BIO treatment (Day 19) (B) of 2D cultured MSCs. Profiles are normalized to the housekeeping gene β-actin and 0 μM controls, and are expressed as a ratio of initial gene expression (day 0) prior to BIO treatment (**p<0.01, ***p<0.001, ****p<0.0001 vs. 0 μM) (mean +/− SD).
Cyclin D1 was increased immediately after 2 and 5 μM BIO treatments (day 1) to 2.8- and 4.5-fold untreated controls (Figure 4A). Unlike Sox2, NANOG, and Oct4, for 5 μM BIO treatment, Cyclin D1 remained up-regulated through day 19 (3.2-fold vs. untreated controls) (Figure 4B; Supplemental Figure S4D). Day 19 was significant as it corresponded to the time of greatest BIO-mediated MSC proliferation (Figure 2B). Therefore, it is possible that elevated Cyclin D1 is the primary executor for the observed BIO-mediated increases in MSC proliferation.
3.5 BIO-treated MSC gene expression in PEG hydrogels (3D)
Stem cell-associated transcription factor expression for 5 μM BIO-treated, encapsulated MSCs were also analyzed. Immediately following BIO-treatment (day 1), relative Sox2, NANOG, and Oct4 gene expression increased to 2.3-, 3.6-, and 1.9-fold untreated controls, respectively (Figure 5A). However, as observed in 2D, Sox2, NANOG, and Oct4 expression returned to basal levels by day 7 (Figure 5B; Supplemental Figure S5A-C), which coincided with the time of greatest BIO-mediated, encapsulated MSC proliferation (Figure 3B).
Figure 5. BIO Enhances 3D MSC Expression of Sox2, NANOG, Oct4, and Cyclin D1.
Profiles of Wnt target genes Sox2, NANOG, Oct4, and Cyclin D1 following 24 hr BIO treatment (Day 1) (A), and 6 days post-BIO treatment (Day 7) (B) of encapsulated MSCs. Profiles are normalized to the housekeeping gene β-actin and 0 μM controls, and are expressed as a ratio of initial gene expression (day 0) prior to BIO treatment (*p<0.05, ****p<0.0001 vs. 0 μM) (mean +/− SD).
Cyclin D1 expression increased 1.9-fold compared to untreated controls for 5 μM BIO-treated encapsulated MSCs (Figure 5A). As in 2D, Cyclin D1 remained significantly up regulated through the period of encapsulated MSC proliferation (Supplemental Figure S5D). Cyclin D1 expression at day 7 was 2.8-fold that of untreated controls for 5 μM BIO-treated PEGPLADM networks (Figure 5B). In combination with expression profiles obtained in 2D, this data further supported the hypothesis that BIO increased MSC proliferation via Wnt/β-catenin-mediated transcription and sustained up-regulation of Cyclin D1.
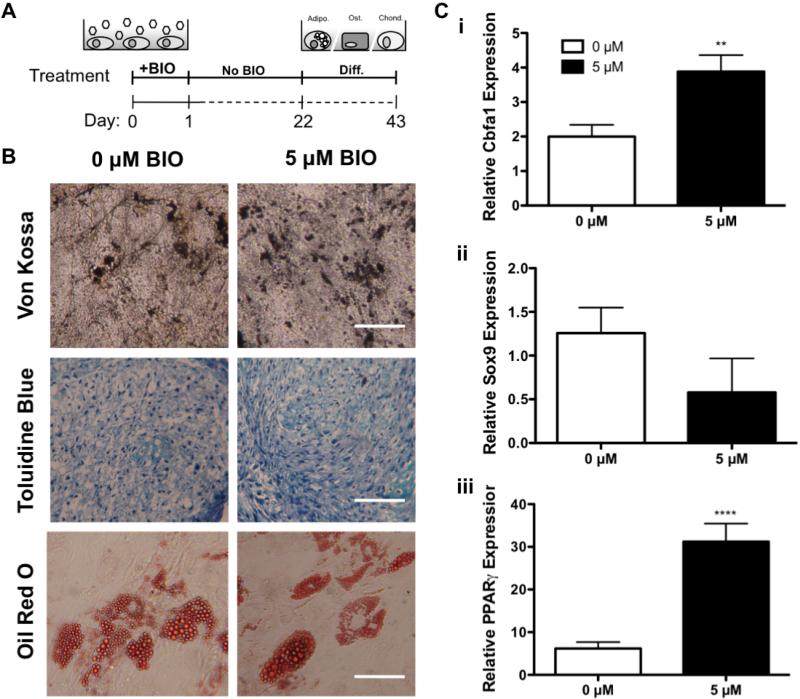
3.6 Controlled differentiation of BIO-treated MSCs
The ability to increase MSC populations has countless applications in stem cell therapies, including regenerative medicine. Therefore, it is imperative that expanded MSCs maintain their ability to differentiate into tissue-specific cell types. To evaluate the multilineage potential of BIO-treated MSCs, cells were cultured for 3 weeks to exploit BIOs proliferative effects (Figure 2B). Cells were then differentiated into osteogenic, chondrogenic, and adipogenic lineages using standard culture conditions (Jaiswal et al., 1997; Mackay et al., 1998; McBeath et al., 2004) (Figure 6A). Qualitative histological analysis showed no effect of BIO-mediated proliferation on histological stains used to evaluate differentiation. Identical mineralization, glycosaminoglycan production, and lipid droplet accumulation were observed between BIO-treated and untreated groups (Figure 6B). In parallel, Cbfa1 (Figure 6Ci, osteogenic marker), Sox9 (Figure 6Cii, chondrogenic marker), and PPARγ (Figure 6Ciii, adipogenic marker) gene expression were analyzed. Gene expression was normalized to both the housekeeping gene β-actin as well as undifferentiated controls. Results are shown as fold-increases over initial expression obtained after BIO treatment but prior to differentiation.
Figure 6. BIO-treated MSCs Retain Multipotent Potential in 2D.
Differentiation of 2D BIO-treated MSCs (A). BIO-treated MSCs were allowed to proliferate for 3 weeks before induction of osteogenesis (von Kossa, hydroxyapatite), chondrogenesis (toluidine blue, glycosaminoglycans), and adipogenesis (oil red o, lipid droplets) (Bar = 100 μM) (B). Subsequent gene analysis for markers of osteogenesis (Cbfa1) (Ci), chondrogenesis (Sox9) (Cii), and adipogeneis (PPARγ) (Ciii) normalized to both the housekeeping gene β-actin and undifferentiated 0 μM controls (**p<0.01, ****p<0.0001 vs. 0 μM) (mean +/−SD).
BIO-treated MSCs exhibited no reduction in differentiation capacity as compared to untreated controls, demonstrating that BIO did not negatively affect MSC multipotency. BIO-treated, osteogenic-differentiated, MSCs exhibited 2.1-fold increases in relative Cbfa1 expression compared to untreated, differentiated MSCs (Figure 6Ci). Similarly, BIO-treated, adipogenic-differentiated MSCs exhibited 5.0-fold increases in relative PPARγ expression compared to untreated controls (Figure 6Ciii). Although not statistically significant, Sox9 expression for 5 μM BIO-treated, chondrogenic-differentiated MSCs was reduced slightly to 0.5-fold that of chondrogenic-differentiated controls that were not treated with BIO (Figure 6Cii). In addition to demonstrating that BIO-treatment does not alter MSC multipotency, this data suggests that BIO may enhance MSC differentiation capacity for osteogenic and adipogenic lineages.
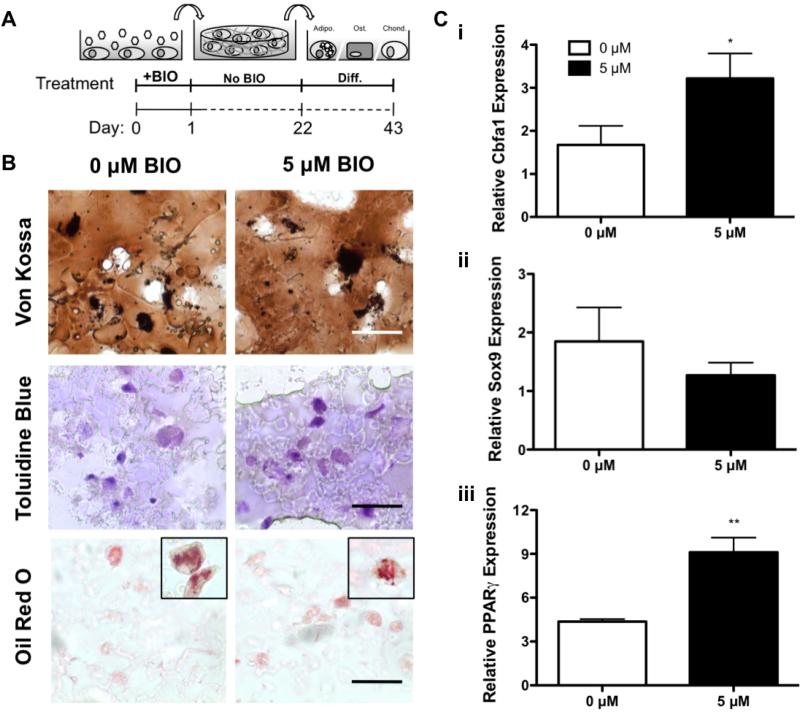
3.7 Differentiation of PEG hydrogel encapsulated BIO-treated MSCs
To evaluate the multipotency of BIO-treated MSCs within PEG-based hydrogels, treated cells were expanded in 2D for 3 weeks and encapsulated in PEGPLADM hydrogels (Figure 7A). Subsequently, encapsulated MSCs were treated with common media supplements to induce osteogenic, chondrogenic, and adipogenic differentiation (Jaiswal et al., 1997; Mackay et al., 1998; McBeath et al., 2004). Qualitative histological analysis showed no effect of BIO on encapsulated MSC differentiation capacity. Identical mineralization, glycosaminoglycan production, and lipid droplet accumulation were observed between BIO-treated and untreated groups (Figure 7B). Furthermore, gene expression for osteogenesis (Figure 7Ci), chondrogenesis (Figure 7Cii), and adipogenesis (Figure 7Ciii) was normalized to housekeeping gene β-actin as well as undifferentiated, untreated controls.
Figure 7. BIO-treated MSCs Retain Multipotent Potential in 3D.
Differentiation of BIO-treated encapsulated MSCs (A). BIO-treated MSCs underwent proliferation in 2D for 3 weeks before encapsulation and subsequent induction of osteogenesis (von Kossa, hydroxyapatite), chondrogenesis (toluidine blue, glycosaminoglycans), and adipogenesis (oil red o, lipid droplets) (Bar = 50 μM) (B). Subsequent gene analysis for markers of osteogenesis (Cbfa1) (Ci), chondrogenesis (Sox9) (Cii), and adipogeneis (PPARγ) (Ciii) normalized to both the housekeeping gene β-actin and undifferentiated 0 μM controls (*p<0.05, **p<0.01 vs. 0 μM) (mean +/− SD).
Encapsulated MSCs treated with BIO exhibited no reduction in differentiation capacity as compared to untreated controls, further supporting that BIO did not negatively affect MSC multipotency. BIO-treated MSCs treated with osteogenic supplements exhibited 1.9-fold increases in relative Cbfa1 expression compared to differentiated MSCs that were not BIO-treated (Figure 7Ci). Similarly, BIO-treated MSCs differentiated using adipogenic supplements exhibited 2.1-fold increases in relative PPARγ expression compared to untreated controls (Figure 7Ciii). Although not statistically significant, Sox9 expression for BIO-treated chondrogenic differentiated MSCs was reduced to 0.7-fold that of untreated, chondrogenic differentiated controls (Figure 7Cii).
4. Discussion
The focus of this study was to investigate the effect of BIO, a small molecule GSK3β inhibitor, on MSC expansion. Based on previous studies with other cell types, it was hypothesized that BIO would enhance MSC proliferation. Through experiments described herein, this hypothesis was confirmed. Our results demonstrate that BIO enhances stem cell-associated gene expression and subsequent proliferation of 2D cultured MSCs as well as MSCs encapsulated in PEG hydrogels, a common material employed in regenerative medicine applications (Benoit et al., 2007b; Benoit et al., 2006b; Elisseeff et al., 2005; Lin and Anseth, 2009; Nuttelman et al., 2006). Furthermore, treatment with BIO does not reduce MSC multipotent differentiation capacity. These results provide evidence that BIO has potential utility to expand MSCs for cell transplantation applications.
Commonly, growth factors are used to achieve ex vivo MSC expansion (Ball et al., 2007; Fierro et al., 2007; Ogawa et al., 2010; Rodrigues et al., 2010; Stewart et al., 2010; Tsutsumi et al., 2001). MSCs treated with bFGF (1ng/mL) (Tsutsumi et al., 2001), VEGF (10 ng/mL) (Ball et al., 2007), and PDGF (10 ng/mL) (Fierro et al., 2007) have been shown to exhibit 2.0-, 1.6-, and 2.4-fold increases in MSC doubling rates. Herein we demonstrated that BIO-treated MSCs exhibited up to 1.5-fold increases in doubling rates. Unlike typical growth factors, which require continuous administration (Ball et al., 2007; Fierro et al., 2007; Tsutsumi et al., 2001), BIO achieves comparable MSC expansion using only a single 24 hr exposure. Furthermore, growth factors are highly pleiotropic, and often result in multiple, unintended biological effects, such as senescence and impaired homing capacity (Baxter et al., 2004; Bonewald and Dallas, 1994; Bruder et al., 1997; Luu et al., 2007; Rodrigues et al., 2010; Shahdadfar et al., 2005). BIO, on the other hand, has been shown to act in a homogenous, reproducible fashion via GSK3β-specific inhibition (Meijer et al., 2003; Polychronopoulos et al., 2004).
Several previous studies have investigated the role of Wnt/β-catenin signaling and subsequent cellular responses (Brack et al., 2007; Kashyap et al., 2009; Krause et al., 2010; Liu et al., 2009; Wu et al., 2008; Yi et al., 2011; Yu et al., 2007; Zhang et al., 2011). Classically, Wnt target gene transcription via increased β-catenin has been associated with the induction of osteogenesis in mesenchymal cells (Krause et al., 2010; Liu et al., 2009; Wu et al., 2008). For example, Wu et al. demonstrated that over-expression of Wnt/β-catenin signaling in periosteum cells and chondrocytes results in increased osteogenesis and enhanced endochondral ossification (Wu et al., 2008). Furthermore, Wnt/β-catenin signaling has also been implicated in cellular aging of MSCs (Zhang et al., 2011) and muscle stem cells (Brack et al., 2007). However, recent evidence suggests that agonism of Wnt/β-catenin signaling also plays vital roles in maintenance of stem cell differentiation capacity (Kashyap et al., 2009; Kelly et al., 2011; Yi et al., 2011; Yu et al., 2007). While conflicting viewpoints remain, contributing effects of TCF-dependent and independent mechanisms (Kelly et al., 2011), and the post-transcriptional roles of Sox2, NANOG, and Oct4 interactions (Kashyap et al., 2009; Yi et al., 2011; Yu et al., 2007), have been cited as possible mediators of multi- and/or pluripotency. Literature has shown that nuclear transfer of post-transcriptional Sox2, NANOG, Oct4, and Lin28-homolog A (Lin28) reprograms human somatic cells to pluripotent stem cells (Yu et al., 2007). These induced cells exhibit enhanced proliferation and stem cell-associated transcription factor expression (Yu et al., 2007). Furthermore, Sox2, NANOG, and Oct4 have been shown to play an integral role in Wnt-dependent ESC self-renewal (Yi et al., 2011), acting synergistically to post-transcriptionally maintain stem cell phenotypes (Kashyap et al., 2009; Yi et al., 2011). As an agonist of Wnt/β-catenin signaling, BIO has been shown to enhance ESC pluripotency through expression of NANOG, Oct4, as well as RNA exonuclease 1 homolog-like 1 (Rex1), another stem cell-associated gene (Sato et al., 2004; Sineva and Pospelov, 2010). In addition, the effect of BIO was shown to be reversible, where removal of the drug caused transcription factor expression to return to basal levels (Sato et al., 2004). Similarly, in this work, BIO treatment transiently increased β-catenin activity and Sox2, NANOG, and Oct4 expression.
BIO-treated MSCs in 2D cultures (Figure 2B) and 3D PEG hydrogels (Figure 3B) exhibited 1.8- and 1.3-fold increases in cell population, respectively, compared to untreated controls. BIO-mediated population increases have been demonstrated in ESCs (~5.8-fold) (Sineva and Pospelov, 2010) and mammalian cardiomyocytes (~8.1-fold) (Tseng et al., 2006) in 2D culture conditions, while no work has established the effect of BIO in 3D culture. The discrepancy in proliferation between these reports and our own work is likely due to confounding or synergistic effects with other stimulatory factors employed. Sineva et al. pre-cultured ESCs using Leukemia Inhibitory Factor (LIF), an inhibitor of differentiation and known agonist of Wnt/β-catenin signaling (Sineva and Pospelov, 2010). Similarly, Tseng et al. repeatedly exposed cardiomyocytes to FGF-1. It has previously been demonstrated that embryonic germ cell proliferation is synergistically enhanced 1.3- and 3.8-fold over BIO-treated cells when supplemented with BIO+LIF and BIO+bFGF respectively (Wen et al., 2010). The observed 1.8-fold (2D) increases in MSC populations described here (Figure 2B) would result in halved culture periods (Kasten et al., 2008; Tsutsumi et al., 2001) to obtain the therapeutically-relevant cell populations needed for articular cartilage, as previously discussed (Kasten et al., 2008; Tsutsumi et al., 2001; Wakitani et al., 2002). As a result, BIO may be useful to reduce the period between injury and therapeutic intervention.
As previously noted, BIO-treated MSCs in 2D exhibited a 1.8-fold increase in cell number, while encapsulated MSCs exhibited a 1.3-fold proliferative enhancement. This discrepancy is likely due to cooperative Notch-Wnt signaling that is precluded in 3D PEG hydrogel culture. Intestinal epithelial cells of Wnt knockout mice, which effectively eliminates Notch-Wnt cooperation, exhibit a 0.55-fold reduction of proliferation (Fre et al., 2009). Reduced cell-cell contacts prevent cleavage of the Notch intracellular domain, which, in its membrane bound form actively degrades β-catenin and reduces Wnt target gene transcription (Kwon et al., 2011). Cell-cell contacts are significantly reduced when cells are encapsulated in hydrogels. This is best exemplified in LIVE/DEAD confocal images where cells appear as singular cells in 3D (Figure 3C). This is in stark contrast to traditional 2D cultured cells where cell-cell interactions are plentiful due to over-confluence (Figure 2C), Notch-Wnt cooperative signaling occurs, and Wnt gene transcription is robust. In fact, closer analysis of the 2D BIO-treated MSC cultures (Figure 2D) reveals some regions of cell stacking (not shown) as compared to the uniform monolayer of MSCs that occurs in the untreated controls. This may be a result of increases in Notch-Wnt cooperative signaling acting in a self-regulatory feed-forward manner that further enhances MSC proliferation (Fre et al., 2009; Kwon et al., 2011).
Another mechanism by which BIO may be increasing proliferation is through Cyclin D1. We demonstrate that peak rates of BIO-mediated MSC proliferation correspond directly with the greatest expression of Cyclin D1. This holds true for both 2D cultured MSCs (Figure 4B) as well as encapsulated MSCs (Figure 5B). Similar to our results, cardiomyocytes treated with BIO also increased proliferation in parallel with elevated Cyclin D1 expression (Tseng et al., 2006). Furthermore, BIO has been shown to shorten circadian periods within fibroblasts, suggesting enhanced proliferation by more rapid cell cycle progression, which assumes Cyclin D1 involvement (Vougogiannopoulou et al., 2008). Prolonged expression of Cyclin D1 (Tseng et al., 2006; Vougogiannopoulou et al., 2008) (Figures 4B and 5B), in combination with post-transcriptional activity of Sox2, Oct4, and NANOG (Kashyap et al., 2009; Kelly et al., 2011; Yi et al., 2011; Yu et al., 2007) may explain sustained, BIO-mediated, MSC proliferation (Figures 2B and 3B) beyond the time frame of enhanced Wnt/β-catenin signaling (Figure 1C). Transient BIO treatment may therefore be sufficient to jump-start the self-regulatory cycle of stem cell-associated gene expression (Kashyap et al., 2009; Kelly et al., 2011; Tseng et al., 2006; Vougogiannopoulou et al., 2008; Yi et al., 2011; Yu et al., 2007), thereby sustaining MSC proliferation and multipotency.
While increasing MSC populations using BIO is a worthy achievement, the therapeutic efficacy of this approach is lost if treated cells undergo phenotypic changes that preclude tissue-specific differentiation. Herein, we demonstrate that BIO-treated MSCs exhibit increased apparent osteogenic and adipogenic differentiation potential based on Cbfa1, and PPARγ gene expression data (Figure 6 and 7). It should be noted that increased cell-cell contacts has been shown to increase osteogenic and adipogenic differentiation of MSCs (Tang et al., 2010). In 2D MSC cultures herein, BIO-mediated MSC proliferation and resulting increases in cell-cell contacts may contribute to increased differentiation capacity or potency. However, similar increases in differentiation capacity are observed within 3D cultures in which MSCs are relatively isolated from cell-cell contacts due to the physical constraints of hydrogel encapsulation (see Figure 3C). Thus BIO-mediated Wnt/B-catenin signaling, and not increased cell number or cell-cell contacts is likely to be the dominant factor in observed enhancements in MSC differentiation capacity following BIO treatment. Statistically similar, albeit reduced chondrogenic gene expression with BIO-treatment was also observed in both 2D and 3D, and may be explained by the Wnt-inducible transcription factor Twist-related protein 1 (Twist1). Reinhold et al. previously showed that increased expression of Twist1 inhibited chondrogenesis in murine limb bud mesenchyme (Reinhold et al., 2006). Furthermore, Twist1 expression, induced by transient BIO treatment may delay the temporal expression of the chondrogenic transcription factor Sox9 and thereby delay chondrogenic differentiation within BIO-treated MSCs. As this data represents a single time point examined during differentiation we may be failing to capture alterations in temporal gene expression due to an overall increase in rates or efficiencies of differentiation within BIO-treated MSC populations. Regardless, by demonstrating that BIO-treated MSCs maintain their differentiation capacity in 2D and in 3D hydrogels, we have provided evidence to support the therapeutic utility of BIO. BIO-mediated expansion of MSCs followed by encapsulation and subsequent differentiation matches the clinical progression of MSC-based regenerative medicine approaches. Thus, to achieve clinical translation, patient-specific MSCs can be isolated, expanded by treatment with BIO, transplanted, and, with a variety of cues (Benoit et al., 2007a; Benoit and Anseth, 2005; Benoit et al., 2007b; Benoit et al., 2006a; Benoit et al., 2006b; Nuttelman et al., 2006), differentiated into tissue-specific cell types (Kasten et al., 2008).
In summary, we have demonstrated that BIO has therapeutic utility for expansion of stem cells ex vivo for regenerative medicine applications. Through increased Wnt/β-catenin signaling, and subsequent increases in Cyclin D1 expression, BIO enhances MSC proliferation and differentiation capacity in both 2D culture conditions as well as when encapsulated in PEG hydrogels.
5. Conclusion
In this work, we provide the first evidence that the small molecule GSK3β inhibitor, BIO, increases MSC proliferation. We demonstrate that BIO increases β-catenin activity in a dose-dependent manner. Agonism of Wnt/β-catenin increases gene expression of several Wnt target genes, including Sox2, NANOG, Oct4, and Cyclin D1. MSC proliferation was increased in typical 2D culture conditions and when MSCs were encapsulated in PEG hydrogels. Finally, BIO-treated MSCs maintain their multipotency, as indicated through osteogenic, chondrogenic, and adipogenic differentiation assays, examining both histological staining and gene expression. In summary, BIO-mediated MSC proliferation can be achieved in 2D and 3D, making BIO, in combination with tissue engineering scaffolds, a viable therapeutic strategy for musculoskeletal tissue regeneration.
Supplementary Material
Acknowledgments
Funding was received from the Orthopaedic Research and Education Foundation /Musculoskeletal Transplant Foundation (OREF/MTF), the Rochester/Finger Lakes Eye & Tissue Bank (RETB/FLETB), and the NIH (T32 Training in Orthopaedic Research (NIH-T32AR053459) to MDH). The authors also wish to thank Dr. Tori Matthews and Teresa Sherman for their assistance in plasmid isolation, as well as Dr. Yufeng Dong for human primer development, Dr. Jennifer Jonason and Dr. Tzong-jen Sheu for transfection troubleshooting, and Dr. James McGrath for use of laboratory equipment.
Contributor Information
Michael D. Hoffman, University of Rochester, Department of Biomedical Engineering University of Rochester Medical Center, Center for Musculoskeletal Research 207 Robert B. Goergen Hall, Box 270168 Rochester, NY 14627-0168 hoffman@bme.rochester.edu
Danielle S.W. Benoit, University of Rochester, Departments of Biomedical Engineering, Chemical Engineering University of Rochester Medical Center, Department of Orthopaedics, and Center for Musculoskeletal Research 207 Robert B. Goergen Hall, Box 270168 Rochester, NY 14627-0168.
References
- Awad HA, Zhang X, Reynolds DG, Guldberg RE, O'Keefe RJ, Schwarz EM. Recent advances in gene delivery for structural bone allografts. Tissue Eng. 2007;13:1973–1985. doi: 10.1089/ten.2006.0107. [DOI] [PubMed] [Google Scholar]
- Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol. 2007;177:489–500. doi: 10.1083/jcb.200608093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- Benoit DS, Tripodi MC, Blanchette JO, Langer SJ, Leinwand LA, Anseth KS. Integrin-linked kinase production prevents anoikis in human mesenchymal stem cells. J Biomed Mater Res A. 2007a;81:259–268. doi: 10.1002/jbm.a.31292. [DOI] [PubMed] [Google Scholar]
- Benoit DSW, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomater. 2005;1:461–470. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Benoit DSW, Collins SD, Anseth KS. Multifunctional hydrogels that promote osteogenic human mesenchymal stem cell differentiation through stimulation and sequestering of bone morphogenic protein 2. Adv Funct Mater. 2007b;17:2085–2093. doi: 10.1002/adfm.200700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit DSW, Durney AR, Anseth KS. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006a;12:1663–1673. doi: 10.1089/ten.2006.12.1663. [DOI] [PubMed] [Google Scholar]
- Benoit DSW, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007c;28:66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Benoit DSW, Nuttelman CR, Collins SD, Anseth KS. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006b;27:6102–6110. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biechele TL, Moon RT. Assaying beta-catenin/TCF transcription with beta-catenin/TCF transcription-based reporter constructs. Methods Mol Biol. 2008;468:99–110. doi: 10.1007/978-1-59745-249-6_8. [DOI] [PubMed] [Google Scholar]
- Bilkovski R, Schulte DM, Oberhauser F, Gomolka M, Udelhoven M, Hettich MM, Roth B, Heidenreich A, Gutschow C, Krone W, Laudes M. Role of WNT-5a in the determination of human mesenchymal stem cells into preadipocytes. J Biol Chem. 2010;285:6170–6178. doi: 10.1074/jbc.M109.054338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF, Dallas SL. Role of active and latent transforming growth factor beta in bone formation. J Cell Biochem. 1994;55:350–357. doi: 10.1002/jcb.240550312. [DOI] [PubMed] [Google Scholar]
- Bork S, Pfister S, Witt H, Horn P, Korn B, Ho AD, Wagner W. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell. 2010;9:54–63. doi: 10.1111/j.1474-9726.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers RR, Lane MD. Wnt signaling and adipocyte lineage commitment. Cell Cycle. 2008;7:1191–1196. doi: 10.4161/cc.7.9.5815. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Chen S, Do JT, Zhang Q, Yao S, Yan F, Peters EC, Scholer HR, Schultz PG, Ding S. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci U S A. 2006;103:17266–17271. doi: 10.1073/pnas.0608156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisseeff J, Puleo C, Yang F, Sharma B. Advances in skeletal tissue engineering with hydrogels. Orthod Craniofac Res. 2005;8:150–161. doi: 10.1111/j.1601-6343.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials. 2009;30:6702–6707. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro F, Illmer T, Jing D, Schleyer E, Ehninger G, Boxberger S, Bornhauser M. Inhibition of platelet-derived growth factor receptorbeta by imatinib mesylate suppresses proliferation and alters differentiation of human mesenchymal stem cells in vitro. Cell Prolif. 2007;40:355–366. doi: 10.1111/j.1365-2184.2007.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Pallavi SK, Huyghe M, Lae M, Janssen KP, Robine S, Artavanis-Tsakonas S, Louvard D. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci U S A. 2009;106:6309–6314. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- Kashyap V, Rezende NC, Scotland KB, Shaffer SM, Persson JL, Gudas LJ, Mongan NP. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009;18:1093–1108. doi: 10.1089/scd.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten P, Beyen I, Egermann M, Suda AJ, Moghaddam AA, Zimmermann G, Luginbuhl R. Instant stem cell therapy: characterization and concentration of human mesenchymal stem cells in vitro. Eur Cell Mater. 2008;16:47–55. doi: 10.22203/ecm.v016a06. [DOI] [PubMed] [Google Scholar]
- Kelly KF, Ng DY, Jayakumaran G, Wood GA, Koide H, Doble BW. beta-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell. 2011;8:214–227. doi: 10.1016/j.stem.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause U, Harris S, Green A, Ylostalo J, Zeitouni S, Lee N, Gregory CA. Pharmaceutical modulation of canonical Wnt signaling in multipotent stromal cells for improved osteoinductive therapy. P Natl Acad Sci USA. 2010;107:4147–4152. doi: 10.1073/pnas.0914360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V, Srivastava D. Notch post-translationally regulates beta-catenin protein in stem and progenitor cells. Nat Cell Biol. 2011;13:1244–1251. doi: 10.1038/ncb2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Gibson S, Bencherif S, Cooper JA, Wetzel SJ, Antonucci JM, Vogel BM, Horkay F, Washburn NR. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules. 2004;5:1280–1287. doi: 10.1021/bm0498777. [DOI] [PubMed] [Google Scholar]
- Liu GZ, Vijayakumar S, Grumolato L, Arroyave R, Qiao HF, Akiri G, Aaronson SA. Canonical Wnts function as potent regulators of osteogenesis by human mesenchymal stem cells. J Cell Biol. 2009;185:67–75. doi: 10.1083/jcb.200810137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, Sharff KA, Montag AG, Haydon RC, He TC. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, Crovace C, Tarricone C, Musacchio A, Roe SM, Pearl L, Greengard P. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- Nuttelman CR, Tripodi MC, Anseth KS. Dexamethasone-functionalized gels induce osteogenic differentiation of encapsulated hMSCs. Journal of Biomedical Materials Research Part A. 2006;76:183–195. doi: 10.1002/jbm.a.30537. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Akazawa T, Tabata Y. In vitro proliferation and chondrogenic differentiation of rat bone marrow stem cells cultured with gelatin hydrogel microspheres for TGF-beta1 release. J Biomater Sci Polym Ed. 2010;21:609–621. doi: 10.1163/156856209X434638. [DOI] [PubMed] [Google Scholar]
- Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Polychronopoulos P, Magiatis P, Skaltsounis AL, Myrianthopoulos V, Mikros E, Tarricone A, Musacchio A, Roe SM, Pearl L, Leost M, Greengard P, Meijer L. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem. 2004;47:935–946. doi: 10.1021/jm031016d. [DOI] [PubMed] [Google Scholar]
- Reinhold MI, Kapadia RM, Liao Z, Naski MC. The Wnt-inducible transcription factor Twist1 inhibits chondrogenesis. J Biol Chem. 2006;281:1381–1388. doi: 10.1074/jbc.M504875200. [DOI] [PubMed] [Google Scholar]
- Reznikoff CA, Brankow DW, Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973;33:3231–3238. [PubMed] [Google Scholar]
- Rodrigues M, Griffith LG, Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1:32. doi: 10.1186/scrt32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937–1944. doi: 10.1016/j.yexcr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Sawhney AS, Pathak CP, Hubbell JA. Bioerodible Hydrogels Based on Photopolymerized Poly(Ethylene Glycol)-Co-Poly(Alpha-Hydroxy Acid) Diacrylate Macromers. Macromolecules. 1993;26:581–587. [Google Scholar]
- Shahdadfar A, Fronsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357–1366. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- Shea CM, Edgar CM, Einhorn TA, Gerstenfeld LC. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J Cell Biochem. 2003;90:1112–1127. doi: 10.1002/jcb.10734. [DOI] [PubMed] [Google Scholar]
- Sineva GS, Pospelov VA. Inhibition of GSK3beta enhances both adhesive and signalling activities of beta-catenin in mouse embryonic stem cells. Biol Cell. 2010;102:549–560. doi: 10.1042/BC20100016. [DOI] [PubMed] [Google Scholar]
- Stewart A, Guan H, Yang K. BMP-3 promotes mesenchymal stem cell proliferation through the TGF-beta/activin signaling pathway. J Cell Physiol. 2010;223:658–666. doi: 10.1002/jcp.22064. [DOI] [PubMed] [Google Scholar]
- Tang J, Peng R, Ding J. The regulation of stem cell differentiation by cell-cell contact on micropatterned material surfaces. Biomaterials. 2010;31:2470–2476. doi: 10.1016/j.biomaterials.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012;81:715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- Tseng AS, Engel FB, Keating MT. The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem Biol. 2006;13:957–963. doi: 10.1016/j.chembiol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, Takagishi K, Kato Y. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- Vougogiannopoulou K, Ferandin Y, Bettayeb K, Myrianthopoulos V, Lozach O, Fan Y, Johnson CH, Magiatis P, Skaltsounis AL, Mikros E, Meijer L. Soluble 3′,6-substituted indirubins with enhanced selectivity toward glycogen synthase kinase -3 alter circadian period. J Med Chem. 2008;51:6421–6431. doi: 10.1021/jm800648y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative Senescence of Mesenchymal Stem Cells: A Continuous and Organized Process. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- Wen J, Liu J, Song GQ, Liu LM, Tang B, Li ZY. Effects of 6-bromoindirubin-3′-oxime on the maintenance of pluripotency of porcine embryonic germ cells in combination with stem cell factor, leukemia inhibitory factor and fibroblast growth factor. Reproduction. 2010;139:1039–1046. doi: 10.1530/REP-09-0539. [DOI] [PubMed] [Google Scholar]
- Wu QQ, Chen D, Zuscik MJ, O'Keefe RJ, Rosier RN. Overexpression of Smurf2 stimulates endochondral ossification through upregulation of beta-catenin. Journal of Bone and Mineral Research. 2008;23:552–563. doi: 10.1359/JBMR.071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Reynolds D, Awad H, Rubery PT, Pelled G, Gazit D, Guldberg RE, Schwarz EM, O'Keefe RJ, Zhang X. Structural bone allograft combined with genetically engineered mesenchymal stem cells as a novel platform for bone tissue engineering. Tissue Eng. 2007;13:435–445. doi: 10.1089/ten.2006.0182. [DOI] [PubMed] [Google Scholar]
- Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol. 2011;13:762–770. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Chau KF, Vodyanik MA, Jiang J, Jiang Y. Efficient feeder-free episomal reprogramming with small molecules. PLoS One. 2011;6:e17557. doi: 10.1371/journal.pone.0017557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang DY, Wang HJ, Tan YZ. Wnt/beta-Catenin Signaling Induces the Aging of Mesenchymal Stem Cells through the DNA Damage Response and the p53/p21 Pathway. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Li G, Chan KM, Wang Y, Tang PF. Comparison of multipotent differentiation potentials of murine primary bone marrow stromal cells and mesenchymal stem cell line C3H10T1/2. Calcif Tissue Int. 2009;84:56–64. doi: 10.1007/s00223-008-9189-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.