Abstract
Acellular extracellular matrix scaffold derived from porcine urinary bladder (UBM) is decellularized material that has shown success for constructive remodeling of various tissues and organs. The regenerative effects of UBM were reported for the tympanic membrane, esophagus, trachea, larynx, pleura, and pericardium in animal studies with promising results. The aim of this study was to investigate regenerative effects of UBM to regenerate hemilarynx using a canine model. A left partial hemilaryngectomy was performed, and the surgical defects were reconstructed by insertion of UBM scaffold. Although local infection was observed in one dog in a week after implantation of the scaffold, all dogs showed good re-epithelialization with minimum complication in one month. The effect of regeneration of the larynx was evaluated 6 months after the operation. The excised larynx experiments were performed to measure phonation threshold pressure (PTP), normalized mucosal wave amplitude (NMWA), and normalized glottal gap (NGG). The results of the measurements showed that PTP was normal or near normal in 2 cases, NMWA was within normal range in 3 cases, although there were individual variations. Histologic examination was completed to evaluate structural changes of the scaffold with appearance of new cartilaginous structure. However the regenerated vocal fold mucosa is mostly scarred. The UBM scaffold has shown to be biocompatible, biodegradable, and useful for tissue regeneration of the hemilarynx with possible restoration of the vocal fold function. The vocal fold mucosa was scarred, which is the next challenge to improve.
Keywords: acellular extracellular matrix, scaffold, glottic regeneration, tissue engineering, porcine urinary bladder, laryngeal function
1. Introduction
The larynx is a composite organ consisting of different types of tissues including the cartilage, muscle and mucosa. It has critical roles in respiration, phonation and swallowing, which are required for human life. Once the larynx is damaged, these functions are deteriorated, which leads to severe handicap. For example, hemi- or total laryngectomy is still an important surgery in the treatment of laryngeal cancer, but caused postoperative disturbance or loss of voice and/or swallowing dysfunction. Although several surgical procedures have been developed to reconstruct the defect of larynx after hemilaryngectomy using skin flap, myocutaneous flap, fascial flap, thyroid gland flap, the procedure is complicated with comorbidity to the donor sites, and the postoperative laryngeal function is poor. One of the reasons for the unfavorable results is the use of grafts derived from different organs which do not have similar function to the larynx. It is regarded to be essential to regenerate naïve laryngeal tissues, although it is not an easy task to regenerate the histologically complex organ simultaneously. An appropriate scaffold is necessary to induce regeneration of complex tissues. Ideal material should be biocompatible, biodegradable, and facilitate cell influx from the surrounding tissues and circulating progenitor cells. Our group has examined the feasibility of polypropylene mesh-based scaffold for laryngeal reconstruction after hemilaryngectomy using canine model, and have reported the usefulness to recreate the framework of the larynx (Kitani et al., 2011; Yamashita et al., 2010). However, foreign body reaction and inflammation were frequently observed, probably because the material is artificial and a non-biodegradable foreign body. The reconstructed tissues were comprised mostly of scar tissue, and vibratory function of the reconstructed vocal mucosa was still poor. Recently decellularization has been developed as a technique (Gilbert et al., 2006; Ott et al., 2008), and several decellularized materials have been examined as feasible implantable materials for tissue regeneration. Acellular scaffolds prompt a modulated immune reaction as both allogeneic and xenogeneic implantable materials. Lately successful regeneration of trachea and bronchus was reported using decellularized broncho-trachea in a human case (Macchiarini et al., 2008).
Acellular extracellular matrix scaffold derived from porcine urinary bladder (UBM) is another decellularized material that has shown success for constructive remodeling of various tissues and organs (Badylak et al., 2009). The regenerative effects of UBM were reported for the tympanic membrane, esophagus, trachea, larynx, pleura, and pericardium in animal studies with promising results (Gilbert et al., 2008a, 2008b; Huber et al., 2003; Nieponice et al., 2009; Parekh et al., 2009; Robinson et al., 2005). The preliminary report of laryngeal regeneration using UBM in a canine model demonstrated a potential of UBM to reconstruct laryngeal tissues after hemilaryngectomy (Huber et al., 2003). The aim of this study was to investigate regenerative effects of the UBM for the hemilarynx in terms of histological aspect as well as functional aspect of the vocal fold.
2. Materials and methods
2.1. Preparation of UBM scaffold
Porcine urinary bladders were harvested from market weight pigs (approximately 110–130 kg) immediately after sacrifice. Residual external connective tissues, including adipose tissue, were trimmed and all residual urine was removed by repeated washes with tap water. The urothelial layer was removed by soaking of the materialin 1 N saline. The tunica serosa, tunica muscularis externa, tunica submucosa, and most of the muscularis mucosa were mechanically delaminated from the bladder tissue. The remaining basement membrane of the tunica epithelialis mucosa and the subjacent tunica propria, collectively termed UBM, were then decellularized and disinfected by immersion in 0.1% (v/v) peracetic acid (s), 4% (v/v) ethanol, and 96% (v/v) deionized water for 2 h. The UBM-ECM material was then washed twice for 15 min with phosphate-buffered saline (pH 7.4) and twice for 15 min with deionized water (Gilbert et al., 2008c).
For the multilaminate device, four layers of UBM-ECM (approximately 4 × 4 cm) were stacked, and placed upon a stainless steel mesh (Figure 1A, 1D). Two additional layers were laid on top of the original stack, starting from one edge so that they covered half of the original stack, and the rest of the sheets were held vertically above the midline. Similarly, two more sheets were placed from the opposite edge of the original stack, with the vertical portion in apposition with the other vertical sheets. Additional stainless steel mesh was laid on top of the horizontal stack of sheets with the vertical stack extending from between the two mesh pieces (Figure 1B, 1D). The four vertical sheets were trimmed to a size of approximately 1.5 × 1.5 cm without cutting the edge that connected to the horizontal stack (Figure 1C). The vertical stack was then folded into a horizontal orientation as one unit so that it was laid on top of one of the stainless steel mesh sheets. One more stainless steel mesh was placed on top of the entire construct (Figure 1D). The construct was then wrapped in cheese cloth and cotton sheets, and placed within vacuum bagging on a bench top. The vacuum bagging was sealed with vacuum tape and connected to a vacuum pump (Model D4B; Leybold, Export, PA) with a condensate trap inline. The constructs were subjected to a vacuum of 710 to 740 mm Hg for 10 to 12 h to dehydrate the UBM-ECM and form a tight mechanical bond between the layers of UBM-ECM (Figure 2) (Gilbert et al., 2008c). The cheese cloth and cotton facilitate removal of liquid from the ECM, while the stainless steel mesh serves the dual purpose of preventing the cheese cloth from mechanically binding with the ECM and creating pressure points that enhance the bonding between the ECM layers. The multilaminate device was terminally sterilized with 18.3–25 kGy electron beam irradiation.
Figure 1.
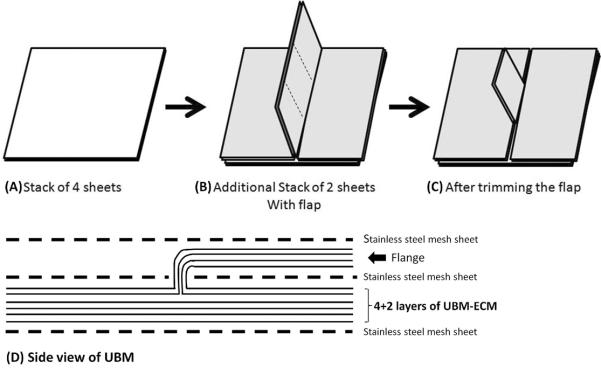
Schema of preparation of acellular extracellular matrix scaffold sheet derived from urinary bladder matrix (UBM).
Figure 2.
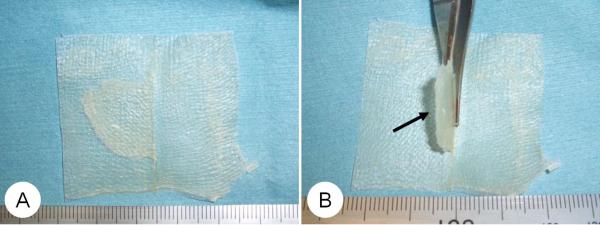
Photograph of UBM sheet. (A) The size of UBM sheet: 5cm × 5cm × 1mm. (B) A flap (1.5×1.5cm) was added for adjustment to the vocal fold ridge. Arrow: flange.
2.2. Animals and surgical procedure
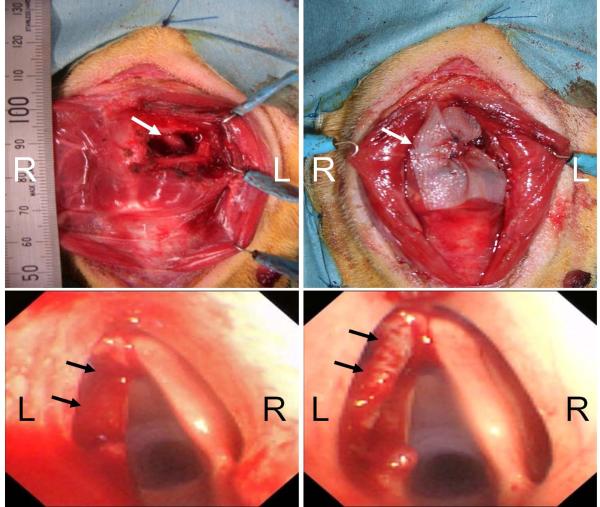
Five beagle dogs weighing 9–11 kg were used in this study. Animal care, housing and experimental procedures were conducted according to the Guideline of Animal Research Committee in Kyoto University. The animals were anesthetized with subcutaneous injections of ketamine hydrochloride (15 mg/kg) and xylazine hydrochloride (6 mg/kg). After the neck of the animal was shaved, a midline cervical skin incision was performed. The strap muscles were extracted laterally and the left side of the thyroid cartilage was exposed. A left partial hemilaryngectomy was performed by resecting the thyroid cartilage together with the vocal fold. The size of the cartilage defect was about 1.8 × 1.0 cm (Figure 3A). The vocal fold was removed from the anterior commissure to the vocal process including the mucosa in the laryngeal ventricle. The surgical defects were reconstructed by implantation of UBM scaffold (Figure 3B). The flap of UBM was rolled so as to fit the vocal fold defect, and then affixed to the defect with 3-0 absorbable sutures (PDS-II®, Ethicon; Figure 3C–D)
Figure 3.
Surgical procedure. (A) Left partial hemilaryngectomy was performed by resecting the thyroid cartilage together with the vocal fold mucosa from the anterior commissure to the vocal process. Arrow: defect of thyroid cartilage. (B) The surgical defects were reconstructed by implantation of UBM scaffold. Arrow: the UBM. (C) Endoscopic finding after the resection of vocal fold. Arrow: defect of left vocal fold. (D) Endoscopic finding after implantation of UBM scaffold. The flap of UBM fit the vocal fold ridge. Arrow: the UBM.
2.3. Endoscopic evaluation
Endoscopic examinations were performed at 1 week, 2 weeks, and then every 1month after the operation with a video-endoscopy system consisting of a video bronchoscope (BF type 1T240, Olympus Co Ltd, Tokyo) and a video processor (CV-240, Olympus Co Ltd) with a light source (CLV-U40D, Olympus Co Ltd). All examinations were performed under general anesthesia by ketamine hydrochloride and xylazine hydrochloride at the above-mentioned dosage.
2.4. Tissue harvest and outcome measurements
All beagles were sacrificed 6 months after the operation with an overdose injection of sodium pentobarbital. Larynges were harvested immediately to examine the vibratory function of the repaired glottis with excised larynx setup. The supraglottic structures, such as the epiglottis, aryepiglottic folds, and false vocal folds were removed in order to get the better image of vocal fold vibration. Arytenoid adduction was performed to medialize the vocal folds with 3-0 nylon strings. An endotracheal tube was inserted into the trachea, and the cuff was blown up to prevent air leakage. The vocal fold vibrations were generated via airflow through the tube and were recorded with a high speed digital imaging system at the rate of 2,000 frames per second (Memrecam®, NAC Image Technology, Osaka, Japan). Subglottic pressure during vibration was monitored by manometer (PG-100-101GP, Tech Jam, Japan). Phonation threshold pressure (PTP) was recorded as the minimum subglottic pressure that initiates vocal fold vibration. Our previous data indicates that normal PTP in dog is less than 5.5cmH2O (Hirano et al., 2004).
Image analyses were completed to measure the amplitude of vibration and area of glottal gap using image analysis software (Scicon Image®, USA). Figure 4 shows a schematic of the procedure, in which `d1' is the distance from the midline of the glottis to the free edge of the vocal fold at the closed phase, and `d2' is the same distance at the maximum open phase. `L' is the length from the anterior commissure to the vocal process, `a' is the area of glottal gap at closed phase. Normalized mucosal wave amplitude (NMWA) and normalized glottal gap (NGG) were determined by the following formula: NMWA = (d2−d1)/L×100, NGG = a/L2×100 Our previous data indicates that the normal range of NMWA in dogs is 8.6–16.8 (Ohno et al., 2011).
Figure 4.
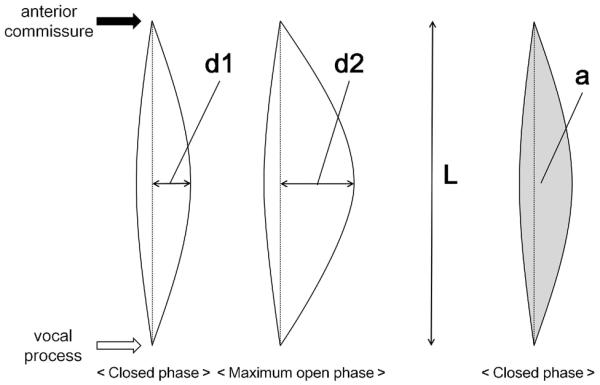
Measurement of normalized mucosal wave amplitude (NMWA) and normalized glottal gap (NGG). NMWA = (d2–d1)/L × 100, NGG = a / L2 × 100.
2.5. Histological examination
The excised larynges were fixed in 10% formaldehyde and embedded in paraffin. The 5μm serial coronal sections were made at the center portion of the newly generated vocal fold, and were stained with hematoxylin eosin (H&E) and Elastica-van-Gieson (EVG) stain. The slides were examined under light microscopy (BZ-9000, Keyence, Osaka) to evaluate the reconstructed site. Since the whole length of the vocal fold was resected and reconstructed with implant of the scaffold, the place of slides examined was thought to be the exact place of regeneration.
3. Results
3.1. Endoscopic findings
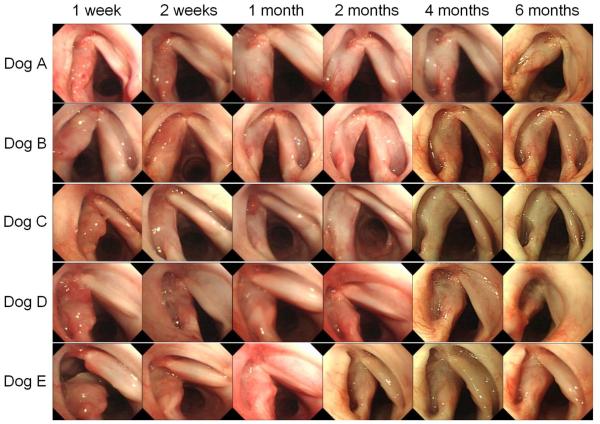
Figure 5 indicates endoscopic laryngeal findings at 1 week through 6 months after the procedure. Granulation tissue was observed at the surgical site at 1–2 weeks after the operation. Local infection was observed in one case (Dog E) at 1 week, and then it was improved at 2 weeks. All dogs showed good re-epithelialization with minimum complication in one month.
Figure 5.
Endoscopic laryngeal findings in 5 dogs at 1 week through 6 months after surgery. Granulation tissues are observed at 1 week, but they are mostly disappeared at 2 weeks. Re-epithelialization was completed within 1 month.
3.2. Vibratory data
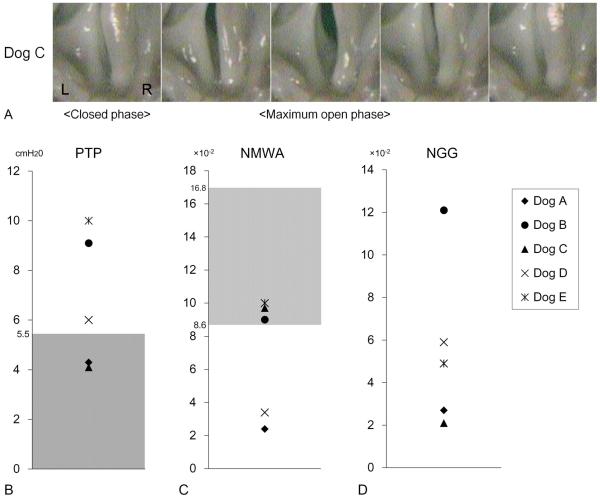
Vibratory function varied in terms of PTP, NMWA, and NGG. Figure 6A shows a representative case (Dog C) with favorite recovery of the function indicating complete glottic closure and near-normal vibration pattern with normal PTP.
Figure 6.
The results of vibratory function. (A) High speed digital imaging of vocal fold vibration in a representative case (Dog C). Left side is the regenerated site. The mucosal movement was near normal with complete glottis closure. (B) Phonation threshold pressure (PTP). (C) Normalized mucosal wave amplitude (NMWA). (D) Normalized glottal gap (NGG). Although individual variation was noted, PTP was almost normal in 3 cases, and NMWA was within normal range in 3 cases. Gray zone: normal range.
Figure 6B–D indicates overall data of each parameter with normal range derived from 3 normal canine larynges. Although data of each parameter varied, PTP was normal or near normal in 2 cases, NMWA was within normal range in 3 cases.
3.3. Histologic data
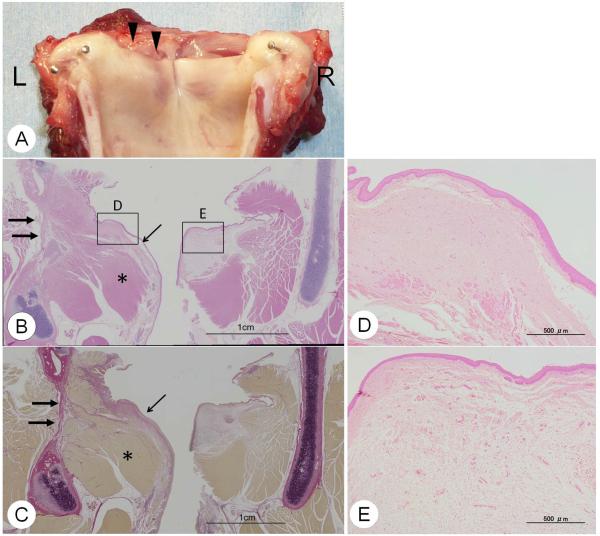
Figure 7A shows macroscopic appearance of the larynx at the time of harvest. The reconstructed vocal fold is re-epithelialized with sufficient mucosal ridge. Figure 8 indicates the high magnification of the epithelium and the original construct (UBM) before implantation. UBM was totally absorbed, and the regenerated epithelium demonstrated almost normal squamous cell epithelium.
Figure 7.
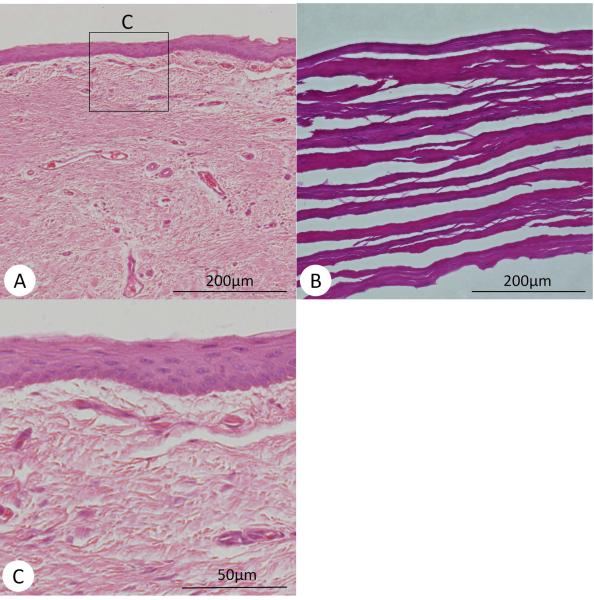
Macroscopic appearance and histological findings at 6 months after surgery. (A) Macroscopic appearance of excised larynx. Regeneration of the vocal fold is noted. Arrow: the regenerated vocal fold. (B) H&E stain, and (C) Elastica-van-Gieson stain in coronal section. The cartilage, muscle, and mucosa were separately regenerated on the reconstructed side (Left side). Thick arrow: regenerated cartilage, Thin arrow: regenerated vocal fold mucosa, Star: regenerated muscle. (D) Regenerated vocal fold (x40) and (E) Normal vocal fold(x40). Normal vocal fold shows two layers in the lamina propria, while the regenerated vocal fold shows no layer structure.
Figure 8.
Histological findings of regenerated vocal folds. (A) Regenerated epithelium and lamina propria (x100), (B) Histology of UBM before implant, (C) Higher magnification of the regenerated epithelium (x400).
The histological findings also indicated partial appearance of newly generated cartilage and muscle in the original portion (Figure 7B–C). The vocal fold was also regenerated; however, the contraction with fibrosis and no layer structure was noted in the lamina propria (Figure 7D). The lamina propria of the canine is divided into two layers (Kurita et al., 1981). The superficial layer is dense with elastic and collagenous fibers. The deep portion of the lamina propria is less dense in fiberous components and appeared to be amorphous (Figure 7E). The regenerated vocal fold showed good re-epithelialization, but the lamina propria of regenerated vocal fold was occupied with homogenous thick fibrous tissues without layer structure.
4. Discussion
Tissue engineering has three important elements for tissue regeneration: cell, scaffold and regulatory factors. Cell may be the most powerful tool to induce regeneration, but is not easy to use for laryngeal regeneration because the larynx consists of multiple different tissues and does not allow simultaneous regeneration by a single type of cell. An appropriate scaffold has a potential to regenerate composite organ by recruiting several types of cells required for recreation of each tissue. In situ tissue engineering is the concept that the scaffolding and regulatory factors recruit endogenous cells to repopulate the tissue defect and differentiate in response to local environmental cues.
Porcine-derived xenogeneic extracellular matrix (ECM) has been successfully used as a scaffold for tissue repair and reconstruction in several animal studies and human applications. This scaffold has been reported to be completely and rapidly degraded and replaced by host-derived tissues (Badylak et al., 2009). A previous preliminary study was performed to examine the effects of this scaffold for laryngeal reconstruction using canine model (Huber et al., 2003). Partial hemilaryngectomy was done by removing the thyroid cartilage and vocal fold, and then the defects were replaced with the ECM scaffold. Histologic examination showed the presence of squamous epithelial lining, reconstructed thyroid cartilage, and bundles of skeletal muscle at 3 months through 12 months after surgery. The results were encouraging, but the histologic findings indicate mixed regeneration of the tissue; for example, the vocal fold included tissues of cartilage, muscle and collagen. Functional aspects were not assessed.
The UBM used in the current study was basically developed similarly to that used in the previous study just mentioned above. The current results showed little inflammatory response at the site of implant of the UBM as well as rapid re-epithelialization, which indicated appropriate biocompatibility of this material. An advantage of the current UBM was the addition of flap that could induce better regeneration of the vocal fold, because the shape of the flap could fit the contour of the vocal fold ridge. Consequently, in contrast that the previous study indicated mixture of multiple tissues in the reconstructed vocal fold (Huber et al., 2003), which might reduce the vibratory function, our results demonstrated appropriate regeneration of each tissue including the cartilage, muscle and mucosa in the original position separately.
The mechanism of the regeneration of the glottis in the current study can be explained by the hypothesis that the scaffold recruited necessary cells and growth factors from the surrounding tissues, which caused regeneration of each tissue such as the epithelium, muscle and cartilage. This mechanism seems like ingrowth of the surrounding tissues, and it may be difficult to distinguish both mechanisms. It might be needed to set up sham control without reconstruction, but this set up is impossible because the wound after hemilaryngectomy is hardly healed without reconstruction.
Another weak point is that we did not confirm how long the scaffold existed and absorbed, because we could examine the histology only at 6 months. Extrusion of the scaffold is thought to be unlikely because the scaffold was firmly sutured to the cartilage, and also because little regeneration was expected without scaffold after hemilaryngectomy as mentioned before. From the concept of in situ tissue engineering, it was suggested that the regeneration was caused by the presence and function of the UBM scaffold.
The current results on functional aspect were encouraging with normal to near normal PTP and NMWA in approximately half of the cases. The problem is scarring of the regenerated vocal fold mucosa that causes varied recovery of vibratory function (Hirano. 2005). Further improvement is required in this aspect. Possible strategy may be the combined use of growth factors or cells. Our group has reported several studies on regeneration of scarred vocal fold using hepatocyte growth factor (Hirano et al., 2004), basic fibroblast growth factor (Suehiro et al., 2010), and mesenchymal stem cell (Kanemaru et al., 2003). Future study is needed to clarify the regenerative effects of combined use of the UBM scaffold and growth factors/cells for regeneration of the whole architecture of the larynx.
Recently decellularized laryngeal scaffold has been researched as a new material to regenerate laryngeal tissues (Hou et al., 2011, Baiguera et al., 2011). Hou et al. (2011) prepared a whole rabbit larynx scaffold including cartilage, muscle, mucosa, and vessels by decellularization with chemicals. They found that the scaffold had few intact cells, and when implanted into the omentum after recellularization with mesenchymal stem cell, vascularization and integrated cartilage frameworks were well remained. This kind of works are interesting in that they attempt to regenerate the larynx with laryngeal derived scaffold, which may make it possible to regenerate the whole larynx all at once using such scaffolds in the future. This technique has just begun and should be carefully researched. At least acellular scaffolds have a great potential for regeneration of tissue defect after hemi- or total laryngectomy.
Acknowledgement
This study was supported by the National Institute of Biomedical Innovation and the National Institutes of Health (R03 DC009655 (T.W.G.)).
Footnotes
This paper won the first place of poster award in the 91st Annual Meeting of American Bronchoesophagological Association, Chicago, 2011.
References
- Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biologic scaffold material: structure and function. Acta Biomaterialia. 2009;5(1):1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Baiguera S, Gonfiotti A, Jaus M, et al. Development of bioengineered human larynx. Biomaterials. 2011;32:4433–4442. doi: 10.1016/j.biomaterials.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Gilbert TW, Sellaro Tl, Badylak SF. Decellularization of organs and tissues. Biomaterial. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Gilbert TW, Nieponice A, Spievack AR, et al. Repair of the thoracic wall with an extracellular matrix scaffold in a canine model. J Surg Res. 2008a;147:61–67. doi: 10.1016/j.jss.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Gilbert TW, Gilbert S, Madden M, et al. Morphologic assessment of extracellular matrix scaffolds for patch tracheoplasty in a canine model. Ann Thorac Surg. 2008b;86:967–974. doi: 10.1016/j.athoracsur.2008.04.071. [DOI] [PubMed] [Google Scholar]
- Gilbert TW, Wognum S, Joyce EM, et al. Collagen fiber alignment and biaxial mechanical behavior of extracellular matrix scaffolds derived from the porcine urinary bladder. Biomaterials. 2008c;29:4775–4782. doi: 10.1016/j.biomaterials.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Bless DM, Nagai H, et al. Growth Factor Therapy for Vocal Fold Scarring in Canine Model. Ann Otol Rhinol Laryngol. 2004;113:777–785. doi: 10.1177/000348940411301002. [DOI] [PubMed] [Google Scholar]
- Hirano S. Current treatment of vocal fold scarring. Cur Opinion Otolaryngol Head Neck Surg. 2005;131:143–147. doi: 10.1097/01.moo.0000162261.49739.b7. [DOI] [PubMed] [Google Scholar]
- Hou N, Cui P, Luo J, et al. Tissue-engineered larynx using perfusion-decellularized technique and mesenchymal stem cells in a rabbit model. Acta Otolaryngol. 2011;131:645–652. doi: 10.3109/00016489.2010.547517. [DOI] [PubMed] [Google Scholar]
- Huber JE, Spievack A, Simmons-Byrd A, et al. Extracellular matrix as a scaffold for laryngeal reconstruction. Ann Otol Rhinol Laryngol. 2003;112:428–433. doi: 10.1177/000348940311200508. [DOI] [PubMed] [Google Scholar]
- Kanemaru S, Nakamura T, Omori K, et al. Regeneration of the vocal fold using autologous mesenchymal stem cells. Ann Otol Rhinol Laryngol. 2003;112:915–920. doi: 10.1177/000348940311201101. [DOI] [PubMed] [Google Scholar]
- Kitani Y, Kanemaru S, Umeda H, et al. Laryngeal Regeneration Using Tissue Engineering Techniques in a Canine Model. Ann Otol Rhino Laryngol. 2011;120:49–56. doi: 10.1177/000348941112000107. [DOI] [PubMed] [Google Scholar]
- Kurita S, Nagata K, Hirano M. A comparative study of the layer structure of the vocal fold. In: Bless DM, Abbs JH, editors. Vocal fold physiology. College-Hill Press; San Diego, Calif: 1981. pp. 3–21. [Google Scholar]
- Macchiarini P, Ungebluth P, Go T, et al. Clinical transplantation of a tissue engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- Nieponice A, McGrath K, Qureshi I, et al. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2009;69:289–296. doi: 10.1016/j.gie.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Ohno S, Hirano S, Kanemaru S, et al. Implantation of an atelocollagen sponge with autologous bone marrow derived mesenchymal stromal cells for treatment of vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2011;120:401–408. doi: 10.1177/000348941112000610. [DOI] [PubMed] [Google Scholar]
- Ott HC, Taylor DA, Thomas SM, et al. Perfusion decellularized matrix: using nature's platform to engineer a bioartificial heart. Nature Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- Parekh A, Mantle B, Banks J, et al. Repair of the Tympanic Membrane with Urinary Bladder Matrix. Laryngoscope. 2009;119:1206–1213. doi: 10.1002/lary.20233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KA, Li J, Mathison M, et al. Extracellular matrix scaffold for cardiac repair. Circulation. 2005;112:1135–1143. doi: 10.1161/CIRCULATIONAHA.104.525436. [DOI] [PubMed] [Google Scholar]
- Suehiro A, Hirano S, Kishimoto Y, et al. Treatment of acute vocal fold scar with local injection of basic fibroblast growth factor: A canine study. Acta Otolaryngol. 2010;130:844–850. doi: 10.3109/00016480903426618. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Kanemaru S, Hirano S, et al. Glottal Reconstruction With a Tissue Engineering Technique Using Polypropylene Mesh: A Canine Experiment. Ann Otol Rhino Laryngol. 2010;119:110–117. doi: 10.1177/000348941011900208. [DOI] [PubMed] [Google Scholar]