Abstract
Aims
The role of vascular smooth muscle endothelin A receptors (ETA) in development and normal physiology remains incompletely understood. To address this, mice were generated with smooth muscle-specific knockout (KO) of ETA.
Materials and Methods
Mice were homozygous for loxP-flanked exons 6–8 of the EDNRA gene (floxed) or were also hemizygous for a transgene expressing Cre recombinase under control of the smooth muscle-specific SM22 promoter (KO mice).
Key Findings
Genotyping at 17 days postnatal yielded a 10:1 ratio of floxed:KO mice. Smooth muscle actin staining of embryos at day E10.5 revealed increased tortuosity in dorsal aortae while E12.5 embryos had mandibular, vascular and thymic abnormalities. Mice surviving to weaning developed and bred normally. ETA KO mice aged 2–3 months manifested EDNRA gene recombination in all organs tested. Aortas from KO mice had a >90% reduction in ETA mRNA content, but no differences in ET-1 or ETB mRNA levels. Addition of 0.01 – 100 nM ET-1 to isolated femoral arteries from floxed, but not KO, mice dose-dependently decreased vessel diameter (up to 80% reduction in the presence of ETB blockade). Intravenous infusion of ET-1 into floxed, but not KO, mice increased mean arterial pressure (MAP) (by ~10 mmHg). Telemetric analysis revealed decreased MAP in KO mice (reduced by ~ 7–10 mmHg) when fed a high salt diet.
Significance
Smooth muscle ETA is important for normal vascular, mandibular and thymic development and is involved in the maintenance of arterial pressure under physiological conditions.
Keywords: Endothelin-1, Endothelin A receptor, Vascular Smooth Muscle
Introduction
Endothelin-1 (ET-1) was originally identified as a potent endothelium derived vasoconstrictor peptide (Yanagisawa, Kurihara et al. 1988) though now it is appreciated that a variety of tissues are able to produce ET-1 (Suzuki, Matsumoto et al. 1989; Hama, Kasuya et al. 1997). ET-1 has been shown to manifest most of its actions directly through two G protein coupled receptors, endothelin A and B receptors, which are differentially distributed throughout many tissues (Nakamichi, Ihara et al. 1992; Godfraind 1993; Karet, Kuc et al. 1993). Because circulating plasma levels of ET-1 are too low to activate its receptors (Battistini, D'Orleans-Juste et al. 1993), it is thought that local ET-1 production and signaling drive its effects. ET-1 signaling via the ETA receptors in the vascular smooth muscle and kidney has been shown to have an important regulatory role for both blood pressure and blood flow control in health and disease.
With the advent of whole body knock out (KO) mice, the developmental importance of both ET- 1 and ETA receptors has become appreciated. Whole body KO of ET-1 alters cardiovascular development in both the cardiac neural crest and larger arteries (Kurihara, Kurihara et al. 1995) and several other less obvious areas such as the thyroid, thymus and cranial/facial structures (KURIHARA, KURIHARA et al. 1995). Furthermore, whole body ETA KO mice appear to phenocopy these developmental defects (Clouthier, Hosoda et al. 1998; Clouthier, Williams et al. 2000) indicating ET-1 signaling thru the ETA receptor is critical in development of these tissues and organs. Still, mice with a conditional KO of the ETA receptor in cardiac myocytes have not recapitulated these development defects (Kedzierski, Grayburn et al. 2003) begging the question in which tissues is the ET-1 signaling via ETA receptor necessary for appropriate development.
The goal of this study was, by using a Cre LoxP system to develop a vascular smooth muscle specific conditional KO for the ETA receptor, to: (1) characterize the vascular and hemodynamic effect of vascular smooth muscle deletion of the ETA receptor and (2) determine if local vascular ET-1 via ETA receptor signaling can phenocopy developmental abnormalities seen in whole body KO models.
Materials and Methods
This investigation conforms with the Guide to the Care and Use of Laboratory Animals (version 8, revised 2011) and was approved by the University of Utah Animal Care and Use Committee.
Animal Model
Mouse breeding
Mice containing the loxP-flanked (floxed) ETA receptor gene (obtained from Dr. M. Yanagisawa at the Howard Hughes Institute at University of Texas Southwestern Medical Center) were mated with SM22-Cre mice. The floxed mice contain exons 6–8 of the ETA receptor gene flanked by loxP sites [these exons are critical to ETA receptor gene functional expression (Clouthier, Hosoda et al. 1998). The SM22-Cre mouse has been well characterized (Holtwick, Gotthardt et al. 2002) and were obtained from Jackson Labs (Bar Harbor, ME). Mice were bred to obtain floxed controls (homozygous for the floxed ETA allele) or ETA KO (homozygous for floxed ETA alleles and hemizygous for SM-22 Cre).
Genotyping
Tail DNA was prepared by standard methods and PCR was amplified using RAF102F2 5’-CCCATGCTTAGACACAACCATG-3’ and ETA genoR2 5’- GATGACAACCAAGCAGAAGACAG-3’. These primers span the loxP site upstream of exon 6. The wild-type allele product is 314 bp and floxed allele is 354 bp. For SM22-Cre, tail DNA was amplified using SM F: 5’-CAGACACCGAAGCTACTCTCCTTC C-3’ and SM R: 5’- CGCATAACCAGTGAAACAGCATTG-3’ which yields a 600 bp product. PCR products were visualized after electrophoresis through 1.5% agarose.
Screening for recombination
DNA from various organs was PCR amplified to assess target organ recombination using primers spanning exons 6–8 in the EDNRA gene: F 5’- CCCATGCTTAGACACAACCATG-3’ and R 5’-CGCTGTTGTATATCCAGTATCAGG-3’. Recombination of the EDNRA gene yields a 610 bp product; the predicted size of the unrecombined EDNRA gene is 1287 bp and, under the PCR conditions utilized, was not detectable.
Developmental Methods
Immunohistochemistry on tissue sections
Embryos were harvested at 12.5 DPC and fixed in 20 volumes zinc-buffered formalin for 2h at room temperature, followed by overnight storage in 70% ethanol at 4°C, and processing through graded alcohols to paraffin. 5µm transverse sections were collected to positively charged glass slides and cured overnight at 42°C. Following de-waxing and rehydration, slides were re-fixed in Methyl Carnoy’s fixative, washed and incubated in 3% H2O2 to block endogenous peroxidases. Sections were washed in PBS, blocked for 1h in 1% BSA+ 10% normal goat serum at room temperature, followed by incubation in monoclonal anti-smooth muscle alpha actin antibody (DAKO), in the presence of 1% BSA + 2% normal goat serum, overnight at 4°C. Slides were next washed in PBS and incubated in a 1:200 dilution of HRP-conjugated goat ant-Mouse IgG (Jacksonimmuno) for 1h at room temperature, followed by signal color development via DAB with hydrogen peroxide (Vector Labs). Sections were counter-stained in Mayer hematoxylin and blued in Scott’s solution before dehydration and mounting with Cytoseal 60. Images were taken at 200x magnification on a Zeiss Axiophot2 microscope, equipped with a Zeiss Axiocam digital camera.
Whole Mount Immunohistochemistry
Whole mount immunohistochemistry was carried out according to the methods of Brigid Hogan et al. with minor modifications . Embryos were dissected at 10.5 DPC and yolk sac fragments retained for PCR genotyping. Tissues were fixed overnight in Dent’s fixative (4:1 MEOH: DMSO) at 4°C, followed by bleaching in same by addition of 1 part 37% H2O2 for 6–10h at room temperature, and storage at −20°C in 100% MEOH. Embryos were rehydrated to PBS, blocked/permeabilized for 3h in PBSMT (PBS+ 0.5% Triton X-100 +2% nonfat milk ), and incubated overnight at 4°C in same solution with the addition of 1:200 monoclonal anti-mouse smooth muscle alpha-actin (DAKO). Tissues were washed six times in PBSMT according to protocol, and incubated overnight in PBSMT+1:200 anti-Mouse IgG HRP-conjugate (JacksonImmuno.com). Embryos were washed six times again in PBSMT in the same manner, and incubated for 30 minutes in 1 ml DAB+NiCl2 (Vector Labs) made up in PBT (PBS+0.5%Triton X-100 + 0.2% BSA). 37% H2O2 (1/1000 vol.) was added to develop signal color. Peroxidase reaction was quenched by moving tissues to fresh PBT. Tissues were post-fixed in 4% paraformaldehyde overnight at 4°C, cleared with 1:2 benzyl alcohol: benzyl benzoate, and photographed on a Leica MZ12 stereoscope equipped with a Zeiss Axiocam digital camera.
Vascular physiology phenotyping
In Vitro Isolated Artery Preparation
Normal chow fed mice were anesthetized (2–5% isoflurane) and euthanized by exsanguination via cardiac puncture. The femoral artery was excised from each leg and placed in cold (4°C) physiological saline solution (PSS) that contained 145.0 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.17 mM MgSO4, 1.2 mM NaH2PO4, 5.0 mM glucose, 2.0 mM pyruvate, 0.02 mM EDTA, 3.0 mM MOPS buffer and 1 g/100 ml BSA, pH 7.4 and surrounding tissues were cleared from artery as previously described (McCurdy, Colleran et al. 2000; Muller-Delp, Spier et al. 2002; Muller-Delp, Spier et al. 2002). Arteries were placed in the bath of the pressure myograph (DMT) containing MOPS-buffered PSS equilibrated to room air. Arteries were then cannulated on both ends with micropipettes and secured with 11-0 surgical nylon suture. After cannulation, the chambers were transferred to the stage of an inverted microscope for recording of luminal diameter and pressurized to 50 mmHg by two independent hydrostatic pressure reservoirs (Durrant, Seals et al. 2009; Lesniewski, Durrant et al. 2011; Donato, Walker et al. 2013). Leaks were detected by pressurizing the vessel and then closing the reservoirs to verify that diameter remained constant. Arterioles that did not maintain diameter were discarded. Arterioles were then warmed to 37°C and allowed to equilibrate ≥ 1hr prior to dose responses. Lumen diameter was measured prior to and after the cumulative addition of ET-1 (10−11 to 10−7 M) in the presence of the ETB receptor antagonist BQ-788 (1 µM, 1 hr) to the surrounding bath. Vessels were allowed to reach a steady state luminal diameter (~5 min) prior to the addition of the next dose of ET-1. A second set of isolated arteries were frozen and stored for ETA and ETB receptor mRNA analysis.
RNA Analysis
RNA was isolated from aortas, reverse transcribed and the resulting DNA assayed for relative expression of ETA mRNA using the Taqman Gene Expression Assay (Applied Biosystems, Carlsbad, CA, ETA probe cat# Mm01243722_m1, GAPDH probe cat# Mm03302249_g1) as previously described (Donato, Lesniewski et al. 2005).
Systemic ET-1 Vasopressor Responsiveness
Mice (n=8 per group) were surgically implanted with a carotid telemetry device (TA11-PAC10, Data Sciences International, St. Paul, MN) to monitor conscious blood pressures and allowed to recover at least 3 days prior to ET-1 study. On the day of the study, mice were anesthetized with 2% inhaled isoflurane and an indwelling jugular vein catheter (Micro-renathane 0.025″ O.D. × 0.012″ I.D., Braintree Scientific Inc., Braintree, MA, USA) was implanted. While maintained under anesthesia, an initial blood pressure recording was made for 1 min via telemetry. A 150 µl bolus of sterile saline was then infused via the jugular catheter and blood pressure recorded continuously for 3 min and averaged over each minute. Mean arterial pressure during saline infusion was not different from pre-saline infusion and was therefore used as baseline blood pressure in the analyses. Following blood pressure monitoring after saline infusion, a 150 µl bolus injection containing 0.1 nmoles/kg ET-1 was administered via the jugular catheter and blood pressure monitored continuously for 15 min and minute averages were calculated. Following 15 min of blood pressure monitoring, the mouse was euthanized by bilateral thoracotomy while under isoflurane anesthesia. The peak blood pressure response to ET-1 was used in the analysis.
Pressor Effects of High Sodium Diet
Blood pressure was recorded via telemetry. Mice aged ~3 months were anesthetized with 2% isoflurane, implanted with a radio transmitter and allowed to recover in individual cages for 5 days. Automated blood pressure and heart rate were recorded continuously thereafter with measurements taken every 10 minutes. Animals were maintained on a normal Na+ diet (0.15% Na+) or high Na+ diet (3.2% Na+). Mice were not handled during blood pressure recording periods since even small stimuli markedly affect blood pressure.
Data presentation and statistical analysis
For in vitro vascular reactivity to ET-1, vasoconstrictor responses were recorded as actual diameters and expressed as a percentage of possible constriction according to the formula:
where DS is the steady-state inner diameter recorded after each dose and DB is the initial baseline inner diameter before the first addition of ET-1. Sensitivity (EC50) was calculated by fitting a sigmoidal curve ot the dose response data using BioDataFit 1.02.
Repeated measures (RM) ANOVAs were used to determine the significance of differences between genotypes for dose responses in isolated arteries and for MAP, SBP, DBP and HR measured by telemetry in normal chow and high salt fed mice. Student’s t-tests were performed to detect group differences for all other analyses including post hoc analyses. All data are presented as mean ± S.E.M. Significance was set at P ≤ 0.05.
Results
Confirmation of smooth muscle ETA KO
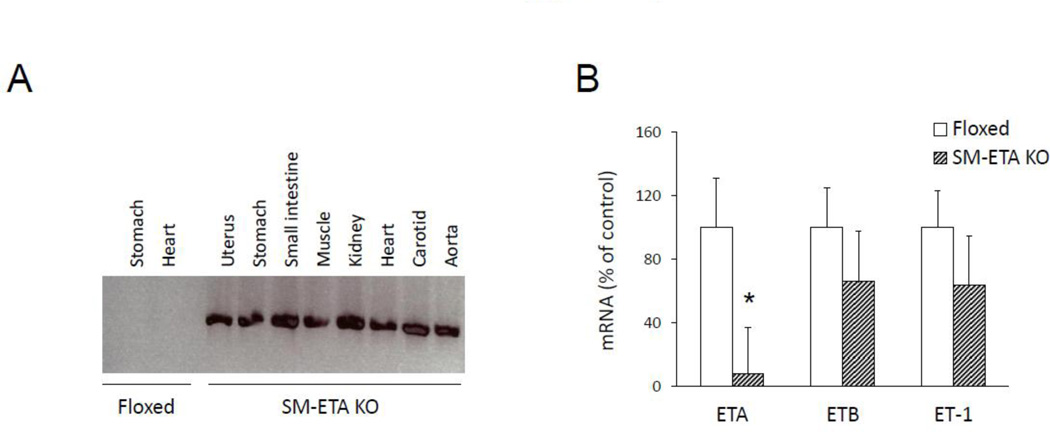
DNA recombination occurred in all tissues assessed from the SM22-Cre/floxed ETA mice (Fig 1A) and was associated with an almost total loss of ETA in aortas of the ETA KO mice compared to floxed controls (Fig 1B). This deletion of ETA was specific to the ETA isoform, as there was no concomitant reduction in either ETB or ET-1 mRNA in the aortas of the SM-ETA KO mice (Fig 1B).
Figure 1. Confirmation of successful deletion of smooth muscle endothelin A receptors (SM-ETA) in knockout mice.
DNA recombination (A) in SM-ETA knockout (KO) mice and floxed control mice. Representative of data from 5 mice of each genotype. mRNA expression (B) of ETA, endothelin receptor B (ETB) and endothelin-1 (ET-1) in aortas from floxed control and SM-ETA KO mice (N=5/genotype). * P≤0.05 vs. floxed.
Developmental effects of smooth muscle ETA deletion
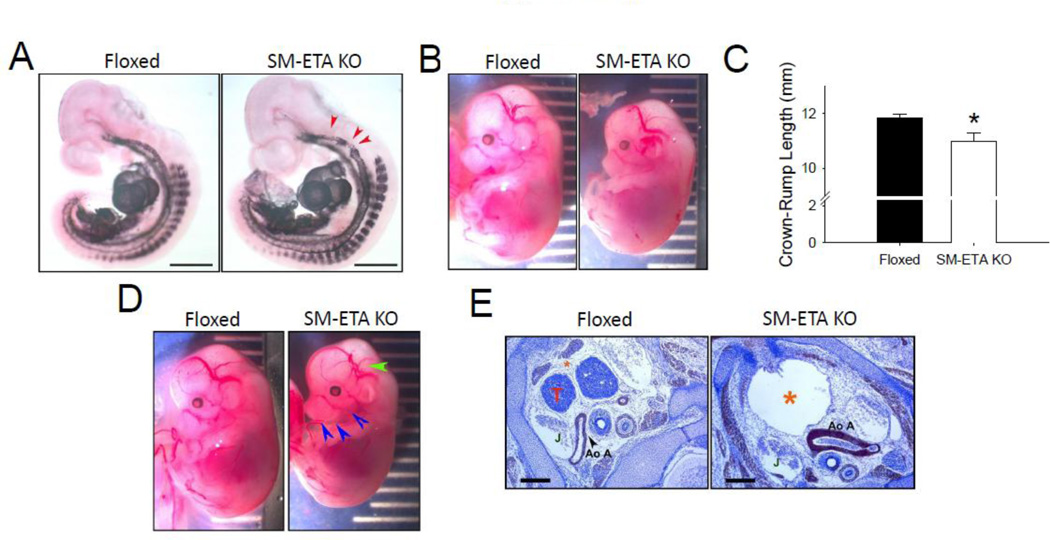
Compared to the floxed control mice, SM-ETA KO mice demonstrated an irregular smooth muscle cell investment in the pharyngeal arch region with tortuosity evident at E10.5 (Fig 2A). By day E12.5, SM-ETA KO embryos were growth retarded (Fig 2B) with reduced crown to rump length (Fig 2C). Mandibular mal-development and cranial vascular malformations (Fig 2D) in the SM-ETA KO mice were also evident by E12.5, and were concomitant with defective thymic processes, pericardial cystic space and thickened aortic arch vessels (Fig 2D). In addition to these developmental defects, the SM-ETA KO mice demonstrated decreased viability such that there were only 29 SM-ETA KO offspring compared to 202 homozygous floxed ETA mice surviving to at least postnatal day 17 from a homozygous floxed ETA/heterozygous SM22-Cre transgenic (smooth muscle ETA KO). In adult mice, there were no differences in body, heart, liver, gastrocnemius muscle, epididymal white adipose tissue mass or blood glucose concentrations (Table).
Figure 2. Developmental defects in smooth muscle endoethelin A receptor knockout (SMETA KO) mice.
At E10.5, SM-ETA KO mice demonstrate an (A) irregular smooth muscle cell investment of dorsal aortic anlagen in the pharyngeal arch region with tortuosity, demonstrated by alpha AM actin whole mount immunohistochemistry (N=3/genotype). Bar =1mm. SM-ETA KO have retarded (B) growth quantified as reduced (C) crown-rump length (C) compared to floxed control mice as well as (D) mandibular maldevelopment (blue arrows) and cranial vascular malformations (green arrow). At alpha smooth muscle actin stained transverse sections of embryos E12.5 demonstrate defective thymic processes (T, absent in SM-STA KO), and enlarged pericardial cavity (orange *), jugular vein (J) and thickened aortic arch (Ao A) vessels (N=3/genotype). * P≤0.05 vs. floxed.
Table 1.
Body and tissue masses for floxed control and smooth muscle endothelin receptor A knockout (SM-ETA KO) mice.
| Floxed | SM-ETA KO | |
|---|---|---|
| Body mass (g) | 32 ± 2 | 31 ± 3 |
| Blood glucose (mg/dl) | 135 ± 10 | 132 ± 9 |
| Heart mass (g) | 0.20 ± 0.03 | 0.16 ± 0.03 |
| Liver mass (g) | 1.75 ± 0.20 | 1.67 ± 0.14 |
| Gastrocnemius muscle mass (g) | 0.27 ± 0.04 | 0.29 ± 0.06 |
| Epididymis Adipose tissue (g) | 0.68 ± 0.17 | 0.70 ± 0.09 |
Data are means±SEM
ET-1-mediated vasoconstriction/vasopressive responses
In the presence of the ETB receptor antagonist BQ-788, the dose response to ET-1 and maximal ET-1 induced vasoconstriction was blunted ~60% (P=0.01) in the SM-ETA KO mice compared with floxed controls (Fig 3A). Sensitivity (EC50) to ET-1 did not differ between groups (Fig 3A). Maximal diameter of the isolated, pressurized femoral arteries did not differ between SMETA KO and floxed control mice (Figure 3B). Although baseline anesthetized mean arterial pressure did not differ between SM-ETA KO and floxed mice, in response to infusion of ET-1 in the intact animal, ET-1 elicited an increase in mean arterial pressure (P<0.01) that was absent in the SM-ETA KO mice (Fig 3C).
Figure 3. Endothelin-1 mediated vasoconstriction is absent in smooth muscle endothelin receptor A knockout (SM-ETA KO) mice.
Dose response to endothelin-1 (A) in the presence of the endothelin receptor B antagonist, BQ-788, in isolated femoral arteries from SM–ETA KO and floxed control mice. Sensitivity (EC50) to ET-1 is presented in the insert. Maximal femoral artery luminal diameter (B) measured in vitro (N=5/genotype). * P≤0.05 vs. floxed for dose response by RM-ANOVA, † P≤0.05 vs. floxed for maximal vasodilation by student’s t-test. Peak mean arterial pressure (C) in response to bolus infusion of saline (baseline, monitored 3 min post infusion) or endothelin-1 (ET1, monitored 15 min post infusion) in SM –ETA KO and floxed control mice (N=3–6/genotype). * P≤0.05 vs. floxed.
Responses to hemodynamic stressor of high salt diet
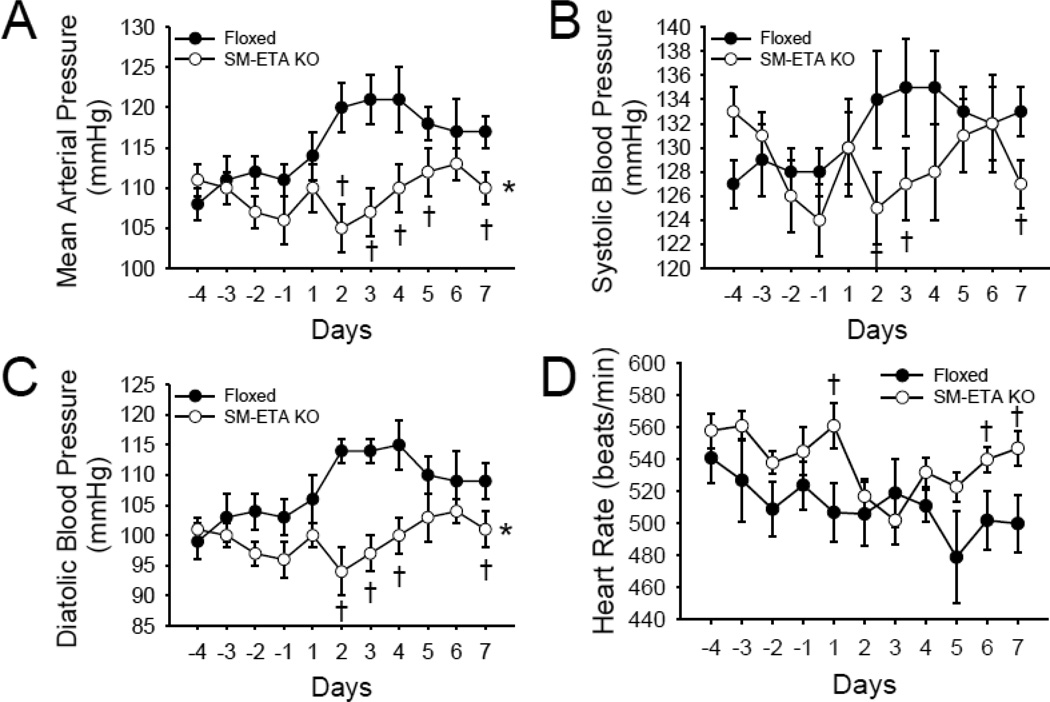
Measured in home cages by telemetry, there were no differences in mean, systolic and diastolic blood pressure or pulse rate between floxed control and SM ETA KO mice in the four days prior to dietary salt loading. After increased dietary salt intake, mean (Fig 4A), systolic (Fig 4B) and diastolic (Fig 4C) blood pressure were higher in the floxed control mice compared with SM-ETA KO mice (all P<0.05) and this effect largely persisted over 7 days of high dietary salt. Although not different when fed normal chow, when compared with floxed control mice, pulse rate was higher in SM-ETA KO mice after 1, 6 and 7 days of high dietary salt (Fig 4D).
Figure 4. Hypertensive response to high salt diet is diminished in mice with smooth muscle endothelin receptor A knockout (SM-ETA KO).
Mean (A), systolic (B), diastolic (C) blood pressure and heart rate (D) in SM-ETA KO and floxed control mice for days prior and 7 days after initiation of high salt diet. * P≤0.05 vs. floxed for dose response during high salt diet (days 1–7) assessed by RM-ANOVA, † P≤0.05 vs. floxed for individual data points by student’s t-test.
Discussion
Our results demonstrate that smooth muscle targeting of the Ednra gene utilizing a SM22-Cre and loxP flanking of the ETA receptor resulted in successful smooth muscle specific deletion of the ETA receptor in mice. Furthermore, this smooth muscle specific ETA receptor deletion results in blunted hemodynamic ET-1 vasoconstriction measured both in vitro (isolated arteries) and in vivo (via blood pressure). Salt-sensitive increases in blood pressure are dependent on an intact smooth muscle ETA receptor, as it was prevented in the smooth muscle ETA KO mice but not floxed control mice. Finally, our findings also provide broad insight into the importance of smooth muscle ET-1 signaling through the ETA receptor in development as our smooth muscle specific ETA receptor KO phenocopied whole body ETA receptor KO and ET-1 KO mice with decreased survival and developmental abnormalities in the cardiovascular system, thymus and mandible. Our findings suggest that these developmental abnormalities are the result of the abolished ET-1 signaling through the ETA receptor in the vascular smooth muscle and not other tissues.
Hemodyamic effects of smooth muscle deletion of ETA receptors
Dysregulation of ET-1 and its signaling through the ETA receptor has been implicated in disrupted hemodynamic and vascular homeostasis in a multitude of cardiovascular diseases, kidney disease and advanced age (Koyama, Tabata et al. 1989; Zoccali, Leonardis et al. 1995; Rossi, Colonna et al. 1999; Schiffrin 1999; Ihling, Szombathy et al. 2001; Donato, Gano et al. 2009; Seals, Jablonski et al. 2011). Whilst, numerous studies have been conducted on arterial vasoreactivity and hemodynamic responses of ET-1 / ETA signaling via pharmacological blockade of the ETA receptors (Maguire and Davenport 1995; Spratt, Goddard et al. 2001; Donato, Lesniewski et al. 2005), no smooth muscle specific genetic models had been created to date. Our data suggests that, utilizing a Cre recombinase on the SM22 promoter, we have successfully created a conditional smooth muscle ETA receptor knock out mouse. Our novel model supports the role of the vascular smooth muscle ETA receptor in inducing a vast majority of ET-1-induced vasoconstriction measured in vitro in isolated arteries as well as in the induction of the vasopressor response to in vivo ET-1 infusion. These results are consistent with numerous prior studies that utilized pharmacological ETA antagonists in vitro and in vivo in health and disease (Iglarz, Matrougui et al. 1998; Spratt, Goddard et al. 2001; Cowburn, Cleland et al. 2005). Finally, we aimed to determine if salt-dependent increases in blood pressure, albeit modest in the C57BL6 mouse strain, involved the ETA receptor. Here, we demonstrate that smooth muscle deletion of the ETA receptor abrogated the blood pressure response to a high salt diet compared to the floxed controls. These results are consistent with pharmacological blockade of the ETA receptor (Okada, Fukuroda et al. 1994; d’Uscio, Barton et al. 1997). In summary, we have demonstrated that our smooth muscle ETA knock out faithfully displays a hemodynamic profile that is consistent with the established role of ETA signaling in response to ET-1 exposure and dietary stressors. Thus, this mouse model provides an alternate approach that may be employed to better understand ET-1 / ETA signaling when pharmacological blockade is not a viable scientific option or when isolating smooth muscle specific effects from other tissue is of utmost importance.
Developmental effect of smooth muscle deletion of ETA receptors
Until recently, the importance of ET-1 signaling through the ETA receptor was not appreciated. With the advent of whole body genetically altered mice it has been subsequently shown that this pathway is of critical importance in the normal development of several organs and the arterial network (Kurihara, Kurihara et al. 1995; Clouthier, Hosoda et al. 1998; Yanagisawa, Hammer et al. 1998). Furthermore, it became evident that whole body deletion of either of these components of the endothelin system resulted in increased embryonic mortality compared to wild type mice (Kurihara, Kurihara et al. 1994; Clouthier, Hosoda et al. 1998). Notably, endothelial cell-specific ET-1 deletion does not cause developmental abnormalities, suggesting that ET-1 from non-endothelial cell sources can maintain normal vascular development (Kisanuki, Emoto et al. 2010). These studies established a critical role for ET-1 signaling through the ETA receptor in viable embryonic development as well as the role of ET-1/ETA receptor signaling in development of critical tissues such as the thymus and arterial system. Still, due to the ubiquitous production of ET-1 and its ETA receptor in tissues, it was unclear what tissue was critical for these developmental effects (Nakamichi, Ihara et al. 1992; Godfraind 1993; Karet, Kuc et al. 1993). Our model of smooth muscle specific ETA receptor deletion phenocopies the developmental defects previously demonstrated in whole body knock out models, i.e. that there is abnormal arterial network development, mandibular development and defective development of the thymic processes and the pericardium. Lastly, pups from each litter were vastly skewed to the wild type versus knock out, suggesting that smooth muscle deletion of the ETA receptor yields a lower than the expected Mendelian ratio of viable pups. This is a likely consequence of the abnormal embryonic aortic arch developmental previously described (Kurihara, Kurihara et al. 1995; Yanagisawa, Hammer et al. 1998). Still, smooth muscle ETA knockout mice that survived and matured had a grossly normal appearance and normal growth. While not the intent of the study, we speculate that mice that survived to adulthood likely had some difference in ETA expression during development, yet had complete vascular ETA knockout in adulthood – it is possible that the timing of ETA knockout was slightly later in these surviving mice. Taken together, our results suggest that vascular smooth muscle specific ET-1/ETA signaling is a critical pathway in vascular, mandibular, neural crest and thymus development. Therefore, the deletion of pathway components reduces embryonic viability. Importantly, the emergence of these phenotypes suggest that this mouse model may be useful for research related to human diseases such as velocardiofacial syndrome and / or DiGeorge syndrome (Kurihara, Kurihara et al. 1995; Clouthier, Hosoda et al. 1998; Kobrynski and Sullivan 2007).
Limitations
In the present study we sought to validate and characterize the developmental and hemodyanmic consequences of vascular smooth muscle ETA receptor deletion. We focused on establishing evidence of smooth muscle ETA receptors via whole aorta RT-PCR for mRNA and femoral vascular responsiveness to ET-1 in the presence of an ETB receptor antagonist. Whilst there were drastic reductions in both ETA receptor mRNA and vasoconstriction, some residual expression and vasoconstriction were observed. We attribute the minimal amounts of ETA mRNA to non-vascular smooth muscle cells within the arterial wall (i.e mast cells, fibroblasts and resident immune cells) that exhibit ETA receptors. To minimize the effects of ETB-mediated vasoconstriction masking differences in the ET-1 mediated vasoconstriction between control and ETA KO mice, we chose to perform the isolated artery dose responses in the presence of ETB inhibition. Nevertheless, it is likely that the small amount of residual vasoconstriction observed in the femoral arteries at very high doses of ET-1 is due to ET-1 out-competing the ETB antagonist for the ETB receptor. It is well known that at supraphysiological levels of ET-1 it is difficult to completely block all the vascular smooth muscle ETB receptors. An alternative explanation would be that we have only knocked down the vascular smooth muscle ETA receptor, but with the genetic technology utilized it is far more likely that our results are due to the aforementioned technical limitations. Next, we did not extensively explore alterations of ETB receptors or examine the sympathetic nervous system in this model. It may be that vascular smooth muscle ETB receptors and sympathetic nervous system outflow are altered; future studies should better phenotype the compensatory physiological mechanisms which may compensate for the deletion of vascular smooth muscle ETA receptors during development.
Conclusions
In conclusion, this novel animal model provides direct evidence for a specific role of the smooth muscle ETA receptor in embryonic development and vascular reactivity. Specifically, we demonstrate that smooth muscle ETA receptors are essential for normal embryonic development and that, in mature animals, the ETA receptor in the smooth muscle is essential for normal ET-1 mediated vasoconstriction, as well as for the induction of elevated blood pressure in response to high dietary salt intake. This novel mouse model will provide yet another tool in the endothelin researchers’ armamentarium yielding greater understanding of this pathway in neonatal development, healthy physiological function and the pathology of disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Battistini B, D'Orleans-Juste P, et al. Endothelins: circulating plasma levels and presence in other biologic fluids. Lab Invest. 1993;68(6):600–628. [PubMed] [Google Scholar]
- Clouthier DE, Hosoda K, et al. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125(5):813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Williams SC, et al. Signaling pathways crucial for craniofacial development revealed by endothelin-A receptor-deficient mice. Developmental biology. 2000;217(1):10–24. doi: 10.1006/dbio.1999.9527. [DOI] [PubMed] [Google Scholar]
- Cowburn PJ, Cleland JGF, et al. Comparison of selective ETA and ETB receptor antagonists in patients with chronic heart failure. European Journal of Heart Failure. 2005;7(1):37–42. doi: 10.1016/j.ejheart.2004.08.001. [DOI] [PubMed] [Google Scholar]
- d’Uscio LV, Barton M, et al. Structure and Function of Small Arteries in Salt-Induced Hypertension: Effects of Chronic Endothelin-Subtype-A–Receptor Blockade. Hypertension. 1997;30(4):905–911. doi: 10.1161/01.hyp.30.4.905. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Gano LB, et al. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. American Journal of Physiology - Heart and Circulatory Physiology. 2009;297(1):H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA, et al. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res. 2005;66(2):393–401. doi: 10.1016/j.cardiores.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Walker AE, et al. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. 2013;12(5):772–783. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. The Journal of Physiology. 2009;587(13):3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T. Evidence for heterogeneity of endothelin receptor distribution in human coronary artery. British journal of pharmacology. 1993;110(3):1201–1205. doi: 10.1111/j.1476-5381.1993.tb13942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Kasuya Y, et al. Role of endothelin-1 in astrocyte responses after acute brain damage. Journal of Neuroscience Research. 1997;47(6):590–602. doi: 10.1002/(sici)1097-4547(19970315)47:6<590::aid-jnr4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Holtwick R, Gotthardt M, et al. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A. 2002;99(10):7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglarz M, Matrougui K, et al. Chronic blockade of endothelin ETA receptors improves flow dependent dilation in resistance arteries of hypertensive rats. Cardiovasc Res. 1998;39(3):657–664. doi: 10.1016/s0008-6363(98)00151-5. [DOI] [PubMed] [Google Scholar]
- Ihling C, Szombathy T, et al. Coexpression of endothelin-converting enzyme-1 and endothelin- 1 in different stages of human atherosclerosis. Circulation. 2001;104(8):864–869. doi: 10.1161/hc3301.094742. [DOI] [PubMed] [Google Scholar]
- Karet F, Kuc R, et al. Novel ligands BQ123 and BQ3020 characterize endothelin receptor subtypes ETA and ETB in human kidney. Kidney international. 1993;44(1):36–42. doi: 10.1038/ki.1993.210. [DOI] [PubMed] [Google Scholar]
- Kedzierski RM, Grayburn PA, et al. Cardiomyocyte-specific endothelin A receptor knockout mice have normal cardiac function and an unaltered hypertrophic response to angiotensin II and isoproterenol. Mol Cell Biol. 2003;23(22):8226–8232. doi: 10.1128/MCB.23.22.8226-8232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki YY, Emoto N, et al. Low blood pressure in endothelial cell-specific endothelina knockout mice. Hypertension. 2010;56(1):121–128. doi: 10.1161/HYPERTENSIONAHA.109.138701. [DOI] [PubMed] [Google Scholar]
- Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromes. Lancet. 2007;370(9596):1443–1452. doi: 10.1016/S0140-6736(07)61601-8. [DOI] [PubMed] [Google Scholar]
- Koyama H, Tabata T, et al. Plasma endothelin levels in patients with uraemia. Lancet. 1989;1(8645):991–992. doi: 10.1016/s0140-6736(89)92631-7. [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Kurihara H, et al. Aortic arch malformations and ventricular septal defect in mice deficient in endothelin-1. J Clin Invest. 1995;96(1):293–300. doi: 10.1172/JCI118033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y, Kurihara H, et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Natur. 1994;368(6473):703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, et al. Aerobic exercise reverses arterial inflammation with aging in mice. American Journal of Physiology - Heart and Circulatory Physiology. 2011;301(3):H1025–H1032. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JJ, Davenport AP. ETA receptor-mediated constrictor responses to endothelin peptides in human blood vessels in vitro. Br J Pharmacol. 1995;115(1):191–197. doi: 10.1111/j.1476-5381.1995.tb16338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy MR, Colleran PN, et al. Effects of fiber composition and hindlimb unloading on the vasodilator properties of skeletal muscle arterioles. J Appl Physiol. 2000;89(1):398–405. doi: 10.1152/jappl.2000.89.1.398. (1985) [DOI] [PubMed] [Google Scholar]
- Muller-Delp J, Spier SA, et al. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;282(5):H1843–H1854. doi: 10.1152/ajpheart.00666.2001. [DOI] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, et al. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283(4):H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Nakamichi K, Ihara M, et al. Different distribution of endothelin receptor subtypes in pulmonary tissues revealed by the novel selective ligands BQ-123 and [Ala< sup>1, 3, 11, 15</sup>] ET-1. Biochemical and biophysical research communications. 1992;182(1):144–150. doi: 10.1016/s0006-291x(05)80123-8. [DOI] [PubMed] [Google Scholar]
- Okada M, Fukuroda T, et al. Antihypertensive effects of BQ-123, a selective endothelin ETA receptor antagonist, in spontaneously hypertensive rats treated with DOCA-salt. Eur J Pharmacol. 1994;259(3):339–342. doi: 10.1016/0014-2999(94)90665-3. [DOI] [PubMed] [Google Scholar]
- Rossi GP, Colonna S, et al. Endothelin-1 and its mRNA in the wall layers of human arteries ex vivo. Circulation. 1999;99(9):1147–1155. doi: 10.1161/01.cir.99.9.1147. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. State-of-the-Art lecture. Role of endothelin-1 in hypertension. Hypertension. 1999;34(4 Pt 2):876–881. doi: 10.1161/01.hyp.34.4.876. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, et al. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120(9):357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt JC, Goddard J, et al. Systemic ETA receptor antagonism with BQ-123 blocks ET-1 induced forearm vasoconstriction and decreases peripheral vascular resistance in healthy men. Br J Pharmacol. 2001;134(3):648–654. doi: 10.1038/sj.bjp.0704304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Matsumoto H, et al. Production of endothelin-1 and big-endothelin-1 by tumor cells with epithelial-like morphology. J Biochem. 1989;106(5):736–741. doi: 10.1093/oxfordjournals.jbchem.a122925. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Hammer RE, et al. Role of Endothelin-1/Endothelin-A receptor-mediated signaling pathway in the aortic arch patterning in mice. J Clin Invest. 1998;102(1):22–33. doi: 10.1172/JCI2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Zoccali C, Leonardis D, et al. Urinary and plasma endothelin 1 in essential hypertension and in hypertension secondary to renoparenchymal disease. Nephrol Dial Transplant. 1995;10(8):1320–1323. [PubMed] [Google Scholar]