Abstract
Recent evidence suggests considerable overlap between the default mode network (DMN) and regions involved in social, affective and introspective processes. We considered these overlapping regions as the social-affective part of the default mode network. In this study we established a robust mapping of the underlying brain network formed by these regions and those strongly connected to them (the extended social-affective default network: eSAD). We first seeded meta-analytic connectivity modeling and resting state analyses in the meta-analytically defined DMN regions that showed statistical overlap with regions associated with social and affective processing. Consensus connectivity of each seed was subsequently delineated by a conjunction across both connectivity analyses. We then functionally characterized the ensuing regions and performed several cluster analyses. Among the identified regions the amygdala/hippocampus formed a cluster associated with emotional processes and memory functions. The ventral striatum, anterior cingulum, subgenual cingulum and ventromedial prefrontal cortex formed a heterogeneous subgroup associated with motivation, reward and cognitive modulation of affect. Posterior cingulum/precuneus and dorsomedial prefrontal cortex were associated with mentalizing, self-reference and autobiographic information. The cluster formed by the temporo-parietal junction and anterior middle temporal sulcus/gyrus was associated with language and social cognition. Taken together, the current work highlights a robustly interconnected network that may be central to interospective, socio-affective, that is, self- and other-related mental processes.
Keywords: default mode network, meta-analytic connectivity modeling, resting state functional connectivity, social cognition, emotion
Introduction
Following the initial demonstration of brain areas, which decrease their metabolism upon the commencement of most cognitive tasks, the so-called “default mode network“ (DMN) of the human brain has become an important focus of neuroscience research. Marking the beginning of the DMN, Gusnard and Raichle (2001) observed that brain activity decreases during many tasks when compared to passive mental states (e.g. passive viewing or eyes closed). They argued that there might exist a physiological baseline of brain function in the absence of an external focus of attention. These brain regions were proposed to show decreases from baseline during goal-directed behavior and therefore might be conceptualized as default mode areas (Raichle et al., 2001). Importantly, the default mode network was conceptualized as and is still often referred to as “task-negative” network. This notion was based on increased activation during task-free states and decreased activation during the performance of cognitive tasks. In particular, this network was often distinguished from a set of “task-positive” network, which are activated by goal-directed and attention-demanding tasks (Fox et al., 2005; Sridharan, Levitin, & Menon, 2008). However, this earlier view of the default mode has since been revised continuously. While regions associated with the DMN indeed deactivate during many goal-directed tasks, several functions are now known to be associated with increased default mode activity. Some default mode areas are associated with the performance of memory functions (Schacter, Addis, & Buckner, 2007; Spreng, Mar, & Kim, 2009). Moreover, there are several default mode areas that subserve theory of mind tasks and are involved in social processing (Adams et al., 2010; Mars, Neubert, et al., 2012). Corbetta et al. (2002) found the right TPJ associated with attention, although in the past several authors emphasized deactivations in default mode areas during attentional tasks. Finally, delay discounting tasks are also accompanied by brain areas which are part of the default mode (Hoffman et al., 2008), just to mention a few. Hence, ”task-negative” network might not be the right term to conceptualize the default mode. Rather it seems that the DMN is de-activated during many experiments but at the same time may also be recruited by others.
It is further important to appreciate that the contention of anti-correlation between task-positive and task-negative brain regions (Fox et al., 2005) has recently been challenged. For example Murphy and colleagues (2009) suggested that particular correction techniques (e.g. global signal regression method) are the cause of anti-correlated functional networks. Finally, the synonymous use of “default mode” and “resting state” (RS) (Damoiseaux et al., 2006; Fransson, 2005) has added confusion to the field. While RS describes a method of functional brain imaging that is performed in the absence of an experimental paradigm, DMN denotes a neurobiological network.
Recent evidence then suggested that the DMN shows considerable overlap with regions involved in social, affective and introspective processes (Northoff et al., 2006; Schilbach et al., 2012). Several key areas of the DMN, such as the medial prefrontal and the posterior inferior parietal cortices are reliably activated by social cognition tasks, such as theory of mind paradigms, perspective taking and inter-personal inference (Amodio & Frith, 2006; Bzdok, Langner, Schilbach, Engemann, et al., 2013; Forbes & Grafman, 2010; Mars, Neubert, et al., 2012). Saxe and Kanwisher (2003) for example found the temporo-parietal junction to be involved in theory of mind tasks. Bzdok and colleagues (2012) found several default mode regions to be involved in theory of mind, moral cognition and empathy. Moreover, Amodio and Frith (2006) highlighted the role of the medial prefrontal cortex in social cognition and self-other referencing. Psychologically, such convergence would indicate that people, when allowed to let their mind wander in the absence of specific tasks, show a strong disposition to engage in thoughts about themselves and their fellow men (Schilbach, Eickhoff, Rotarska-Jagiela, Fink, & Vogeley, 2008). Likewise, the DMN has also been associated with emotional processing (Laird et al., 2009). A meta-analysis by Sergerie et al. (2008) revealed activation of amygdala (which sometimes is discussed as default mode region) during emotional processing, especially when face stimuli are presented (See also (Baas, Aleman, & Kahn, 2004). Rainville and colleagues (1997) further found the anterior cingulate cortex to be involved in pain affect. In spite of this evidence, it must be noted, that several default mode areas have also been implicated in tasks other than social cognition or emotional processing. For example, the right temporo-parietal junction is, apart from social cognition, consistently associated with attentional processes (Corbetta & Shulman, 2002) and its left homologue with language processes (Binder, Desai, Graves, & Conant, 2009). Moreover some other DMN regions are frequently associated with memory processes (Spreng et al., 2009) or delay discounting tasks (Hoffman et al., 2008). Taken together, literature indicates that a lot of default mode regions are routinely involved in social cognition and affective processing (Adams et al., 2010; Mars, Neubert, et al., 2012; Saxe & Kanwisher, 2003). However, the default mode has also been associated with several other mental functions. This can be understood as social cognition and affect related mental activities playing a central role within specific parts of the default mode network (but not within the DMN as a whole).
This view has previously been put forward by Schilbach and colleagues (2012). They took three different meta-analytic approaches to delineate regions that are (i) part of the DMN and regions consistently involved in social cognition, (ii) part of the DMN and associated with affective processing, (iii) part of the DMN and involved in EMO and SOC. Aside from underlining the commonalities between the DMN and social-affective processing, this analysis highlighted the insufficiencies of a purely “task-negative” view on the DMN. In particular, while the DMN has traditionally been conceptualized as “task-negative” network, this recent meta-analysis showed that at least parts of this “task-negative” network are reliably engaged by social-affective processing. Based on this evidence we here performed a conjunction analysis of the DMN and those regions that are consistently recruited by either social or affective tasks. This conjunction of regions involved in unconstrained (DMN) as well as in either social or affective processing, yielded a consistent set of seven brain regions: the amygdala, anterior cingulate cortex, precuneus, dorsomedial prefrontal cortex, subgenual cingulate cortex and bilateral temporo-parietal junction (Figure 1). Put differently, based on a large-scale meta-analysis involving many hundreds of neuroimaging experiments, it could be shown that several brain regions are both part of the default mode as well as being consistently associated with social cognition and emotional processes. In this context, it may be noted, that it is tempting to assign all of the DMN these functions. Still, we here opted for a more cautious approach. Hence, rather than associating “the DMN” with socio-affective processing, we specifically use this notion for those aforementioned regions where this association could be quantitatively shown. We therefore conceptualize the aforementioned brain regions as a social-affective default (SAD). That is, the SAD should reflect a (not purely “task-negative”) component within the whole default mode network. Even more so, it could be conceptualized as specialized sub-network strongly related to for social cognition and affective processing.
Figure 1. Seed Regions.
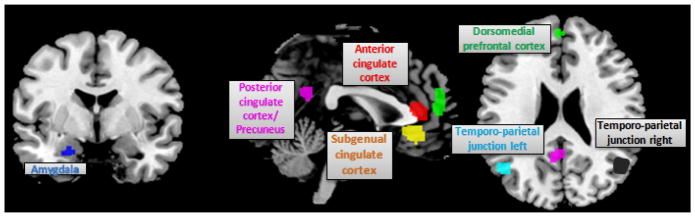
This figure illustrates the seeds derived meta-analytically by Schilbach et al (2012) as being part of the DMN and additionally are involved in either social cognition or emotion. Seeds have been normalized using BrainMap database. They are displayed on coronal, sagittal and axial sections of the MNI single subject template.
The DMN attracted a lot of attention due to the fact that aberrations in this network were observed in a number of psychiatric disorders such as schizophrenia, autism and Alzheimer’s disease (Buckner, Andrews-Hanna, & Schacter, 2008; Greicius, Srivastava, Reiss, & Menon, 2004; Menon, 2011). Orosz and colleagues (2012) moreover linked parts of the DMN, in particular the posterior inferior parietal lobe, the posterior cingulate cortex as well as amygdala and anterior cingulate to the pathophysiology of major depression (Hamani et al., 2011). Intriguingly, most of these disorders are related to disturbed social and affective processing. This fits the observation that aberrations are mostly found in default mode regions that are strongly associated with social and affective processing and points to a potential importance of the social-affective default (SAD) subsystem in the context of mental illnesses. In turn, understanding the characteristics of this network and the functions of its individual components should thus provide important to the pathophysiology of common mental disorders.
In spite of the relatively large body of work devoted to the brain’s “default mode”, the concept of the here proposed SAD network has surprisingly few precedents. The SAD can be conceptualized as network of brain regions that are part of the DMN and also consistently activated by socio-affective tasks. Given that somewhat hybrid nature of the SAD (as it deactivates in most cognitive tasks [“task-negative”] but is activated by social and affective processes [“task-positive”]), it remains elusive whether the SAD regions are robustly connected to each other in both task-based functional connectivity and resting state. Moreover, it may well be argued, that its components as outlined above may entertain robust connectivity with regions not (traditionally) associated with the DMN. Therefore, the aim of our study was to define an “extended” social-affective default (eSAD). This eSAD would then reflect a network of brain regions that are (i) part of the default mode and additionally involved in either social cognition or affective processing or (ii) strongly and consistently connected to these. In other words, we wanted to identify an extended SAD network comprising regions of the DMN that are likewise associated with socio-affective processing (SAD) as well as those regions that are intimately coupled with these. Moreover, the functional roles and specialization, beyond the expected but broad category of socio-affective processing are yet to be characterized. Finally, a key question is how connectivity and function of the different regions in the eSAD relate to each other. That is, are there subgroups of eSAD regions that are closer to each other, e.g., in terms of their sustained functions? The aim of the present study was thus to test whether SAD regions show consistent connectivity to each other or to other brain regions outside the SAD, and hereby provide a robust definition of the eSAD. Moreover, we wanted to functionally characterize the ensuing regions and to cluster them based on similarities in connectional and functional patterns.
To this end, we performed task-dependent and task-independent functional connectivity analyses from seed regions that are part of the default mode while also being involved in either social or affective processing (quantitative definition of SAD regions). In particular, we performed MACM analysis and resting state functional connectivity analysis for each of the SAD regions. Computing the conjunction across both approaches then allowed to define the consensus functional connectivity maps for each seed. The eSAD was then identified by testing for regions showing overlap between these consensus functional connectivity maps across seed regions, i.e., regions that showed robust connectivity with multiple SAD regions. To identify the eSAD, we only considered regions, in which at least two of these consensus functional connectivity maps overlapped. Resulting regions were then characterized by quantitative behavioral inference using the BrainMap database. Finally, similarities between the eSAD regions’ connections and functions were investigated by hierarchical and non-hierarchical cluster-analyses as well as multi-dimensional scaling.
Materials and Methods
Meta-analytic connectivity modeling
Functional connectivity (FC) of regions of interest during task performance was delineated by meta-analytical connectivity modeling (MACM) (Laird et al., 2009). The idea behind this approach is that FC reflects the correlation of activity in spatially distinct brain regions. That is, regions that are functionally connected with particular seed regions should coactivate above chance with those in neuroimaging studies. For mapping task-based coactivation of our seed regions, we used the neuroimaging findings stored in the BrainMap database (Laird et al., 2011). At the moment it contains the published coordinates from almost 2500 functional neuroimaging papers and covers about 25 % of the entire literature. Compared to other databases, BrainMap offers the highest number of available papers and the most extensive meta-data associated with these (See Derrfuss and Mar (2009)). For more than 15 years studies have been added to from all different fields of neuroimaging by people interested in all kinds of processes. Moreover there are no specific inclusion criteria beyond the reporting of activation coordinates in a standard stereotaxic space. Therefore this database provides the currently most comprehensive and broad coverage of the neuroimaging literature.
In the present work, we only considered normal mapping experiments (no between-group comparisons, no interventions) of healthy subjects whereas those on psychiatric or neurological disorders were excluded. This resulted in ~7500 eligible experiments. Using this pool of neuroimaging results, MACM can then be used to test for statistical relationships between activation probabilities of different areas. Importantly, this inference is performed independently of the paradigms used or other experimental factors but is solely based on the likelihood of observing activation in a target region given that activation is present within the seed area. This completely data-driven approach thus avoids selection biases that may result from adhering to current cognitive ontologies, which might not always overlap with the organizational modes of brain function. In practice, the first step of the MACM analysis was to identify those experiments, which activate each particular seed. That is, we searched for experiments in BrainMap that show activation within each particular seed region of interest. We provide the workspaces in the supplemental material, which allows to identify those experiments and to replicate our study (see supplementary online material). This approach resulted in a heterogeneous number of experiments for the different seeds, which potentially would have biased the analysis towards those that featured a high number of associated experiments. Consequently, we normalized the seeds by identifying experiments in BrainMap that show activation in voxel that surround the seed voxels. In particular, we successively added neighboring voxel to the seed-volumes until all seeds were activated by the same number of BrainMap experiments. In particular, we targeted for 164 experiments, which was the highest number observed for any individual seed. Put differently, all other seeds were normalized to this highest number in order to provide experiment pools of identical size and hence an unbiased analysis. After normalizing the seed regions, the initial step of the actual MACM analysis was to identify all those experiments in BrainMap database that were associated with the respective seed (which always yielded a set of 164 experiments given the normalization procedure). No additional constraints were applied, i.e., we filtered experiments only based on the location of the reported maxima, not on the investigated topic, their experimental design, analysis approach etc. The reason for this completely data-driven approach is, that any additional filter would represent a strong a priori hypothesis on how the eSAD network is organized that would bias the subsequent analysis. Subsequently, a quantitative meta-analysis was employed to test for convergence across all foci reported in these experiments. Given that experiments were identified by activation in the seed, highest convergence will evidently be found in this region itself. Significant convergence outside of it, in turn, then indicates consistent coactivation and hence task-based functional connectivity.
Meta-analysis was performed using the revised version (Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012; Eickhoff et al., 2009) of the ALE approach. The key idea behind ALE is to treat all foci in those experiments that activate the seed not as single points, but as centers for 3D Gaussian probability distributions that reflect the spatial uncertainty associated with neuroimaging results. For each experiment, the probability distributions are then combined into a modeled activation (MA) map using the recently proposed approach that prevents undue summation between foci (Turkeltaub et al., 2012). ALE scores were then calculated by taking the voxel-wise union of these individual MA maps. The aim of the next step was to identify those voxels where the convergence across experiments (the ALE score) was higher than expected under a null-distribution of spatial independence, i.e., to distinguish ‘true’ convergence between experiments from random overlap (i.e., noise). For statistical inference ALE results were thus assessed against a null-distribution of random spatial association between experiments, which was done by a recently proposed analytical method (Eickhoff et al., 2012). This method consists of non-linear integration of histograms to derive the null-distribution of ALE values under spatial independence. p-values were then calculated for each ALE score reflecting the probability of observing ALE scores with a more extreme value under this null- distribution. Finally, the ALE map reflecting the coactivation of each seed was thresholded at p<0.05 (cluster-level FWE corrected for multiple comparison; cluster-forming threshold p<0.001 at voxel-level).
Resting State connectivity
Resting state fMRI images of 153 healthy volunteers (mean age 41.1 ± 18.0 years; 92 males) from the NKI/Rockland sample were obtained through the 1000 functional connectomes project (www.nitrc.org/projects/fcon_1000/). The retrospective analysis of these (anonymized) datasets was approved by the local ethics committee of Heinrich-Heine university in Düsseldorf. During the resting state scans subjects were instructed to keep their eyes closed and to think about nothing in particular but not to fall asleep. For each subject 260 resting state EPI images were acquired on a Siemens TimTrio 3T scanner using blood-oxygen-level-dependent (BOLD) contrast [gradient-echo EPI pulse sequence, TR = 2.5s, TE = 30ms, flip angle = 80°, voxel size= 3.0 × 3.0 × 3.0 mm, 38 axial slices (3.0 mm thickness) covering the entire brain]. The first four scans were excluded from further processing analysis using SPM8 to allow for magnet saturation. The remaining EPI images were first corrected for movement artifacts by affine registration using a two pass procedure in which the images were first aligned to the initial volume and subsequently to the mean after the first pass. Then images were spatially normalized to the MNI single subject template using the unified segmentation approach (Ashburner & Friston, 2005). The ensuing deformation was applied to the individual EPI volumes. In order to improve signal-to noise ratio and for compensating residual anatomical variations, the normalized images were smoothed by a 5mm FWHM Gaussian kernel. In order to reduce spurious correlations, variance that could be explained by the following nuisance variables was removed from each voxel’s time series (Reetz et al., 2012; Satterthwaite et al., 2013; zu Eulenburg, Caspers, Roski, & Eickhoff, 2012): i) The six motion parameters derived from the image realignment, ii) the first derivative of the realignment parameters, and iii) mean gray matter, white matter and CSF signal per time-point as obtained by averaging across voxels attributed to the respective tissue class in the SPM8 segmentation. Data was then filtered preserving frequencies between 0.01 and 0.08 Hz, noting that meaningful resting state correlations will predominantly be found in these frequencies given that the hemodynamic response function acts as a band-pass filter on the observable neuronal activation-changes.
For the analysis of resting-state connectivity we used the same (normalized) seed regions as for the MACM analysis. The key idea behind resting state functional connectivity analysis is to extract the fMRI time series from each seed (represented by the first eigenvariate of the individual voxels’ time-series) and comparing it to time-courses of all other gray matter voxel in brain by computing linear correlation coefficients (Jakobs et al., 2012; Sommer, Clos, Meijering, Diederen, & Eickhoff, 2012). These voxel-wise correlation coefficients were then transformed into Fisher’s Z-scores and tested for consistency across subjects using the standard SPM 8 implementations (random-effects ANOVA including non-sphericity correction accommodating that resting-state connectivity maps for the different seeds are correlated measures per subject and allowing for unequal variance across subjects and seeds). Results were then thresholded using the same significance-criteria as for the MACM analysis (p<0.05 cluster-level FWE corrected for multiple comparisons, cluster-forming threshold p<0.001).
Identification of the extended SAD
The aim of our study was to establish a robust definition of the extended social-affective default (eSAD). This is, we wanted to identify those brain regions that are part of the default mode but at the same time also involved in social or affective processing as well as those that are strongly connected to multiple of these regions. We therefore sought to identify areas showing robust task-dependent as well as task-independent functional connectivity with more than one of the meta-analytically defined seed regions. To this end, we first performed a conjunction analysis of the task-dependent (MACM) and task-independent (resting-state) whole-brain functional connectivity maps for each seed individually by computing their intersection after family-wise error (FEW) corrected thresholding. This process resulted in one consensus functional connectivity map for each seed, reflecting areas consistently interacting with that seed across brain states. This, in turn, allowed identifying the extended“ SAD (eSAD) as those regions that are significantly connected with multiple of the seed regions, i.e., those regions in which the consensus (task-based and task-independent) functional connectivity maps of more than one seeds-regions overlapped. In this it must be noted, that the consensus connectivity maps themselves were derived by a statistical conjunction of the resting-state and MACM connectivity maps of a particular seed. That is, each location in the consensus functional connectivity map showed statistically significant resting-state and MACM connectivity to that seed. In turn, regions of the eSAD, which are defined by significant overlap between two or more consensus connectivity maps, thus needed to show statistically significant resting-state and MACM connectivity with more than one of the seeds, i.e., show up as significant in at least four different connectivity analyses (resting-state and MACM for at least two seeds). An additional extended threshold of k> 50 voxel was then applied to remove smaller areas of presumably spurious overlap.
Connectivity of the eSAD regions
The task-dependent (MACM) and task-independent (resting-state) whole-brain functional connectivity of each of the identified eSAD regions was computed using the same data and approach as described for the original seed (from the Schilbach et al., 2012 meta-analysis). That is, for each of the eSAD regions delineated in the above analysis, we computed the significant co-activation pattern using the BrainMap database as well as the significant resting-state correlations in 152 subjects. Again, all analyses were thresholded at p<0.05 (cluster-level FWE corrected for multiple comparisons, cluster-forming threshold p<0.001). In this context, it should be noted, that we sought statistical inference on these connectivity maps to display those regions showing significant functional connectivity with the eSAD regions. For the later cluster-analysis (cf. below), however, the features were given by the un-thresholded functional connectivity maps representing the voxel-wise strength of the co-activation and resting-state correlations with each particular eSAD seed, respectively.
Functional characterization
The functional characterization of the identified eSAD regions was based on the BrainMap database that describes the classes of mental processes isolated by the archived experiments’ statistical contrasts. The behavioral domains comprise the main categories cognition, action, perception, emotion, and interoception, as well as their related subcategories and denote the mental processes isolated by the respective contrast. In turn, paradigm classes categorize the specific task employed, e.g., Theory of Mind“ or paired associate recall“ (see http://brainmap.org/scribe/ for the complete BrainMap taxonomy). As a first step, we thus filtered the BrainMap database for those experiments that featured at least one focus of activation within the current region of interest (each region of the eSAD as defined above). We then analyzed the behavioral domain and paradigm class metadata of the retrieved BrainMap experiments, i.e., those that activated the current seed region, to determine the frequency of domain ‘hits’ relative to its likelihood across the entire database. The functional role of the eSAD areas were thus identified by significant overrepresentation of behavioral domains and paradigm classes (Cieslik et al., 2012). More precisely, for quantitative functional inference we tested whether the conditional probability of activation given a particular label [P(Activation|Task)] was significantly higher than the a priori probability of activation [P(Activation)] as assessed by a binomial test (p<0.05, FDR-corrected for multiple comparisons).
Clustering of ROIs
The last goal of the present study was to delineate the similarities and differences between the delineated eSAD regions’ with respect to their connectional and functional profiles. That is, we investigated, whether we could identify sub-groups or cliques within the above-defined eSAD network, which are formed by regions with similar connectivity patterns or functions. This question was addressed by different cluster-analytical approaches, which were applied to three sets of raw data (all un-thresholded to reflect the full information): i) the functional profiles ii) the whole-brain coactivation maps or iii) the whole-brain resting-state functional connectivity maps. In this context, “clustering” or “cluster-analysis” is the task of assigning objects (the eSAD regions) into groups or “clusters” so that objects within a cluster show similar features while features of objects in different clusters are more distinct of each other. In other words, we grouped the identified eSAD regions in such manner, that regions in the same group or cluster were as similar as possible with respect to their function or connectivity, whereas the function or connectivity was maximally different between the groups or clusters. Clustering the eSAD was performed based on three heterogeneous algorithms. First K-means clustering, which is a non-hierarchical clustering method that uses an iterative algorithm to separate the seed region into a previously selected number of K non-overlapping clusters (Forgy, 1965; Hartigan, 1979). K-means aims at minimizing the variance within clusters and maximizing the variance between clusters by first computing the centroid of each cluster and subsequently reassigning voxels to the clusters such that their difference from the centroid is minimal. Second, hierarchical clustering is an approach in which clusters are formed by first linking the seeds that were more similar to each other and then performing a complete linkage of the clusters (Eickhoff et al., 2011). Third, we used multidimensional scaling, an approach that allows to visualize the regions similarities in a 2D room with dissimilar regions being farther from each other than similar ones. This was done by using Sammon’s nonlinear mapping as the goodness-of-fit criterion. We used all three types of cluster analyses given of the fact that they all have different characteristics (hierarchical clustering is a local approach whereas k-means is more global and multidimensional scaling is particularly useful to represent the overall relational pattern). This allowed us to compare these different approaches with different advantages and identify convergent evidence. Taken together the aforementioned cluster analyses allowed us to identify subgroups or cliques of brain regions within the eSAD, which share similarities in function, MACM coactivation and resting state connectivity.
Results
Normalized Regions
The initial seed regions were provided by the work of Schilbach et al. (2012) as those, which deactivate during most (but not all) goal-directed cognitive tasks and which are additionally involved in either social cognition or emotion. These regions were: left amygdala (center of gravity: −22/−6/−24, in MNI space), anterior cingulate cortex (0/38/10), precuneus (−2/−52/26), dorsomedial prefrontal cortex (−2/52/14), subgenual cingulate cortex (−2/32/−8) and the bilateral temporo-parietal junction (left: −46/−64/18; right: 50/−60/18). They were then normalized to feature 164 activations in BrainMap by iteratively expanding regions containing less than that number of foci. The normalized seed regions entering the MACM and resting-state functional connectivity analyses are displayed in figure 1.
Resting State and MACM
To establish a functional connectivity map for each seed that is robustly present across task-dependent and task-free states, we first performed MACM and resting state functional connectivity analysis for each seed and subsequently computed the conjunction across these. This procedure resulted in one consensus functional connectivity map for each seed region as exemplified for dorsomedial prefrontal cortex in figure 2. The consensus functional connectivity maps of all seeds are provided in figure 3.
Figure 2. Workflow from normalized region to consensus functional connectivity map of seeds.
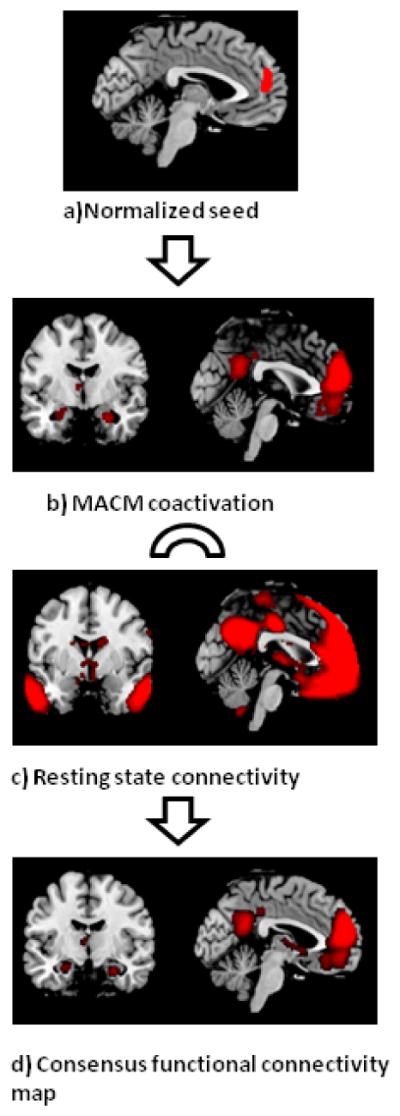
a Exemplary seed region in the dorso-medial prefrontal cortex
b) For MACM-analysis we first identified all experiments in BrainMap database that reported activation within the seed and then tested for convergence across all foci reported in these experiments by using the revised ALE approach. Brain regions that show consistent MACM- coactivation with the dorsomedial prefrontal seed comprise precuneus, posterior cingulate cortex, amygdala/hippocampus, left temporo-parietal junction, anterior cingulate cortex, left ventral basalganglia and ventromedial prefrontal cortex.
c) Significant resting-state functional connectivity with the dorsomedial prefrontal cortex was found extensively within the medial frontal lobe (including ventromedial prefrontal cortex, anterior cingulate cortex, subgenual cingulate cortex), as well as with the precuneus, posterior cingulum, thalamus, cerebellum, temporal pole and amygdala/hippocampus.
d) The conjunction analysis of the task-dependent (MACM) and task-independent (resting-state) functional connectivity analyses resulted in a consensus functional connectivity map comprising the anterior cingulate cortex, subgenual cingulate cortex, precuneus, ventral basalganglia left, amygdala/hippocampus right, ventromedial prefrontal cortex and left temporo-parietal junction.
Figure 3. Consensus functional connectivity maps.
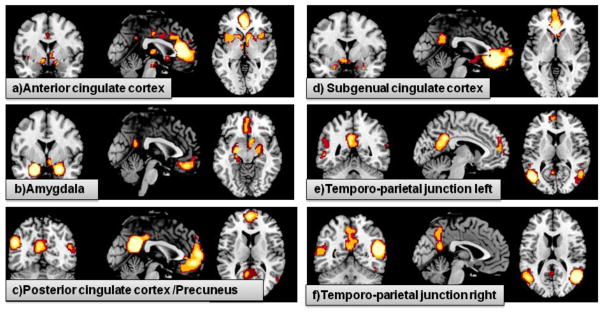
This figure illustrates the conjunction of MACM and resting state connectivity maps.
a) Consensus map of anterior cingulate cortex, comprises dorsomedial prefrontal cortex, gyrus cinguli, ventral basalganglia, ventromedial prefrontal cortex, precuneus, subgenual cingulate cortex
b) Consensus map of amygdale, comprises contralateral amygdala/hippocampus, precuneus, right ventral basal ganglia, ventromedial prefrontal cortex, subgenual cingulate cortex
c) Consensus map of posterior cingulate cortex/precuneus, comprises posterior cingulate cortex, amygdala/hippocampus, anterior cingulate cortex, subgenual cingulate cortex, dorsomedial prefrontal cortex, ventromedial prefrontal cortex, anterior middle temporal sulcus, temporo-parietal junction
d) Consensus map of subgenual cingulate cortex, comprises precuneus, amygdala/hippocampus, anterior cingulate cortex, ventromedial prefrontal cortex, ventral basalganglia
e) Consensus map of left temporo-parietal junction, comprises dorsomedial prefrontal cortex, precuneus, occipital lobe, right temporo-parietal junction, anterior middle temporal sulcus
f) Consensus map of right temporo-parietal junction, comprises left temporo-parietal junction and precuneus
Regions forming the eSAD
In total, we identified twelve distinct brain regions in which the consensus (task-based and task-independent) functional connectivity maps of multiple seeds-regions overlapped. More precisely, in all but one of these twelve regions (the exception being the aMTS/aMTG) we found an overlap of at least three consensus functional connectivity maps. The identified regions are thus considered to form the “extended” social-affective default (eSAD). The respective regions were located in the anterior cingulate cortex (ACC, center of gravity 0/38/10), subgenual cingulate cortex (SGC, −2/32/−8), precuneus/posterior cingulate cortex (PCC/PrC, −2/−52/26), dorsomedial prefrontal cortex (dmPFC, −2/52/14), bilateral temporo-parietal junction (TPJ, 50/−60/18 and −46/−66/18), bilateral ventral basal ganglia (vBG, −6/10/−8 and 6/10/−8), left anterior middle temporal sulcus/gyrus (aMTS/aMTG, −54/−10/−20), bilateral amygdala/hippocampus (Amy/Hippo, 24/−8/−22 and −24/−10/−20) and ventromedial prefrontal cortex (vmPFC; −2/50/−10). The location of these regions is illustrated in figure 4a, while figure 4b illustrates, which of the consensus functional connectivity maps of our seven (normalized) seed regions, i.e., the meta-analytically defined regions, contained the respective eSAD regions.
Figure 4. Constituent nodes of the eSAD.
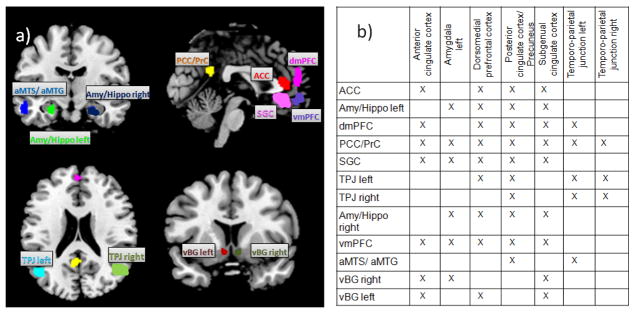
a) the 12 eSAD regions resulting from an overlap of multiple consensus maps are displayed on the MNI single subject template.
b) Summary of the definition of the eSAD regions (rows) from overlap in the consensus functional connectivity maps of the seed regions from Schilbach et al., 2012 (columns).
Functional characterization of the eSAD regions
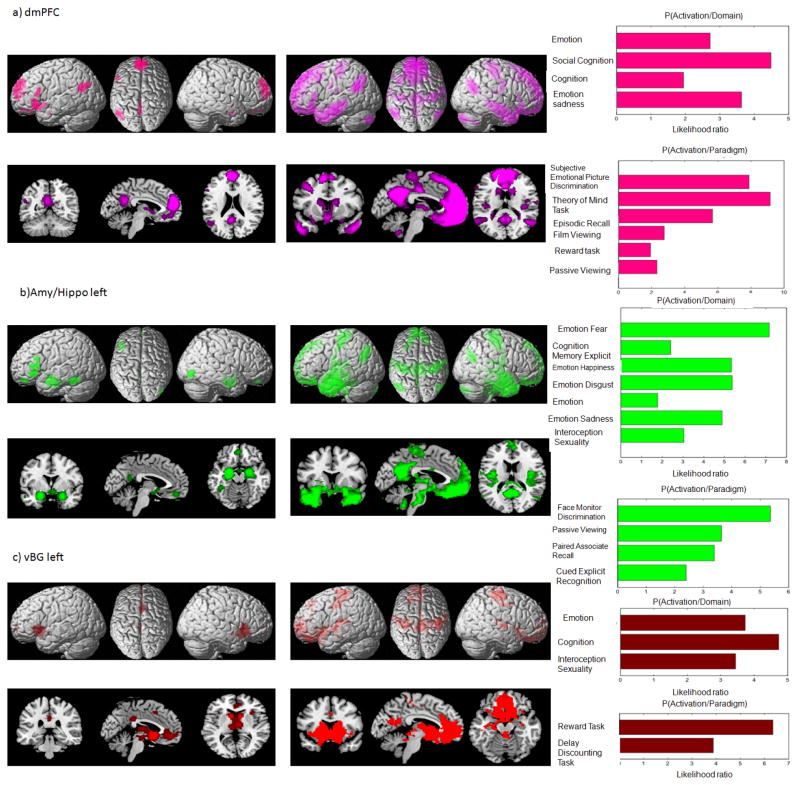
We characterized the functions associated with each of the eSAD regions outlined above by testing for significantly over-represented behavioral domain and paradigm classes among those experiments in BrainMap that activate the respective region. We found that the ACC was significantly associated with emotional processes, cognition, reward as well as interoception (gustation, sexuality). The SGC was associated with the same behavioral domains and paradigm classes as the ACC apart from a missing association with sexual interoception studies. Testing those experiments that activated the ventral basal ganglia revealed the behavioral domains on emotion, cognition and interoception as well as an association with reward delay discounting tasks. The key difference between right and left ventral basal ganglia was that this region was stronger associated with sexuality on the right and with gustation on the left hemisphere. The vmPFC was activated by experiments dealing with emotion (especially fear), cognition, reward and imagination. Both the PCC/PrC and the dmPFC were preferentially activated by studies probing social cognition, emotion, memory and theory of mind (TOM). The dmPFC was moreover associated with subjective emotional picture discrimination tasks and cognition, whereas PCC/PrC was associated with language processing. Left and right TPJ are activated by experiments on social cognition and TOM. In addition, the left TPJ was associated with language, the right with imagination and observation of objects and scenes as well as olfaction. The aMTS/aMTG showed a diverse functional profile comprising language, explicit memory, motor learning, reading, drawing, listening and TOM. Finally, the ROIs in the amygdala/hippocampus region were activated above chance by studies on emotional processes, explicit memory and face discrimination. In addition, the right side was associated with olfaction, reward and cognition.
MACM and Resting State functional connectivity of eSAD regions
In order to characterize the regions of the eSAD in more detail, we additionally performed MACM- and resting-state functional connectivity analyses of each area separately. Figure 5 illustrates the results (p<0.05, cluster-level FWE corrected for multiple comparisons) of these task-dependent and task-independent functional connectivity analyses as well as a graphical representation of the functional decoding using the BrainMap database (summarized in the last paragraph) for three exemplary eSAD regions, the left amygdala/hippocampus region the dorsomedial prefrontal cortex and the left ventral basal ganglia. The full details on all twelve regions are presented in the supplementary material.
Figure 5.
Figure 5a–c. Functional characterization, MACM and resting state analysis of the eSAD regions
The panel on left column presents results of MACM analysis on surface and orthogonal sections of the MNI template. The one in the middle column illustrates results of resting state analysis. The panel on right side illustrates the functional decoding of the regions. The behavioral domains are shown on top, whereas paradigm classes are provided below.
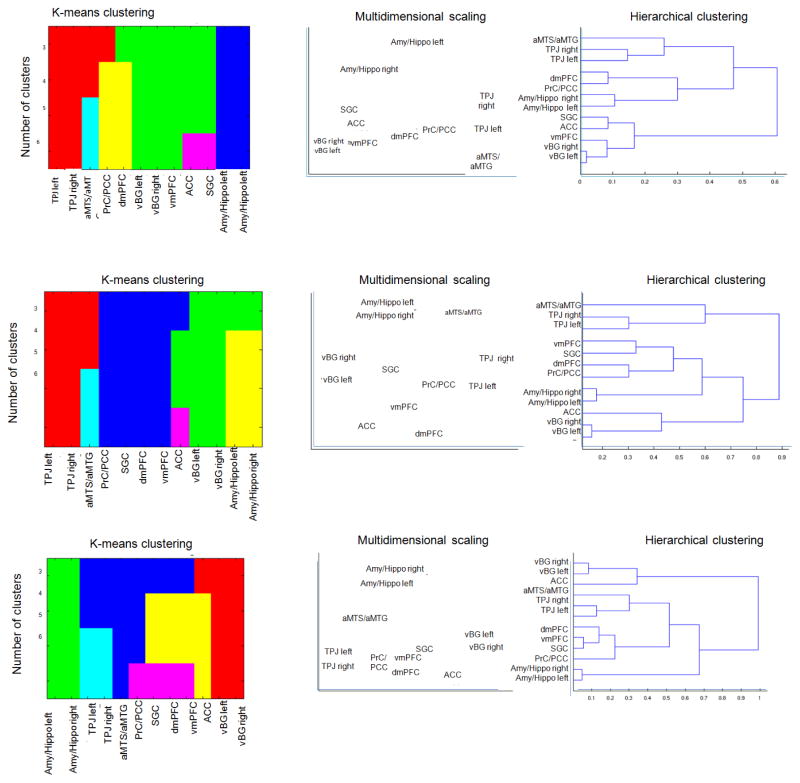
Clustering of the eSAD regions
Three clusters were highly consistently revealed across the different clustering algorithms (k-means, HC and MDS) and aspects (resting-state, MACM and function). Most conspicuously, the two amygdala/hippocampus regions were always clustered together as were the two (left/right) regions in the ventral basal ganglia and the two TPJ regions (again left/right). The last cluster moreover routinely also contained the left aMTS/aMTG. Among these three cluster, it is interesting to note, that in particular the cluster formed by the bilateral amygdala/hippocampus was rather isolated, i.e., showed connections and functions that were dissimilar to those of the other eSAD regions, while the same is, to a lesser extend, true for the cluster formed by the bilateral TPJ and the aMTS/aMTG, which is only related to one other region, namely the PCC/PrC, in terms of function and coactivations. The latter region, i.e., the PCC/PrC moreover features consistently strong similarities in function and connectivity to the dmPFC. The remaining medial regions on the frontal lobe, i.e., the vmPFC, the ACC and SGC formed a more heterogeneous cluster in which the similarity between elements way more dependent on the assessed feature (function, co-activation profiles or resting-state functional connectivity profiles). Among these, the vmPFC usually had the closest association with the dmPFC, whereas the ACC was most similar to the ventral basal ganglia. The latter finally, showed a rather isolated resting-state functional connectivity profile.
Discussion
Methodical considerations
To robustly establish the extended variant of the social-affective default (SAD) we performed individual task-dependent (MACM) and task-independent (resting state) analyses for each seed region derived from a neuroimaging meta-analysis on the default mode network by Schilbach et al. (2012). The authors performed three different conjunction analyses in order to identify brain regions that are (i) part of the default mode (DMN) and involved in social cognition (SOC), (ii) part of the DMN and involved in emotional processes (EMO), (iii) part of the DMN and involved in EMO and SOC. The conjunction analyses across the metaanalytic results related to DMN and SOC or DMN and EMO, i.e., regions involved in unconstrained (DMN) as well as in either social or affective processing, yielded the preliminary seeds. These were fed into analyses identifying the ‘extended’ social-affective default in human brain as those regions that were consistently connected to multiple of these original meta-analytically derived seeds across brain states, i.e., the presence (MACM) or absence (resting-state functional connectivity) of a task.
These two complementary analyses revealed that the functional connectivity of the seeds is not identical but depends on the brain state at hand, i.e., more introspective, unconstrained cognition on one hand and mind states induced by a specific task set on the other (see Figure 2). For the definition of the eSAD we thus only considered brain regions that were congruently connected to the seeds across both mental states. In particular, performing both MACM and resting state analyses allowed for creating consensus maps of each individual seed. The ensuing consensus maps illustrated the overlap of task-dependent coactivation and task-independent correlations of the seeds and hence those regions featuring robust functional interactions with the respective seed independently of the current state (Jakobs et al., 2012; Reetz et al., 2012). The set of regions that were identified in the consensus functional connectivity map of multiple seeds, i.e., that featured robust coupling independent of the current state with more than one seeds, then constituted the here proposed “extended social-affective default” (eSAD). It contains not only the regions of the default mode network that are at the same time also consistently recruited by social-affective tasks (Bzdok, Langner, et al., 2013b; Schilbach et al., 2012) but moreover also the brain areas which show a robust functional connectivity with these and may hence be considered part of the same network system. Interestingly, several of these regions have so far not been widely viewed to directly relate to the default mode network. Nevertheless, they were shown here to feature strong and consistent connectivity to social and affective default mode areas across disparate brain states, i.e., task and rest, highlighting the “hybrid” role of the SAD as a “default” and (social-affective) task network.
Importantly, the combined use of MACM and resting state analyses should not only provide a focus on the state-independent functional connectivity but moreover reduce the influence of random noise and systematic confounds on the computed connectivity maps. That is because potentially disturbing factors should differ in both methods (which are performed on independent datasets) and therefore their effect should be eliminated or at least minimized by performing two different types of analyses. The concurrent use of MACM and resting state therefore is a feasible and attractive approach for the establishment of a network formation consistent across mental states as well as less susceptible to potential noise and biases.
PCC/PrC & dmPFC
PCC/PrC and dmPFC feature a lot of similarities in connectivity and function, which is reflected by consistent allocation of these two regions into a common cluster. This cluster showed connectivity to a particularly high quantity of eSAD regions (see figure 4b), among these ACC and vmPFC. Especially the latter region showed strong connectivity to the dmPFC in resting state analyses (see figure 5) potentially mediated by dense axonal interconnections (Kringelbach & Rolls, 2004). In terms of function, this cluster was furthermore similar to the cluster formed by bilateral TPJ and aMTS/aMTG as all of these regions were associated with language processes. Behavioral inference revealed that both PCC/PrC and dmPFC were associated with social cognition, mindreading and memory functions. In addition, the dmPFC was associated with passive observation and viewing whereas the PCC/PrC was associated with semantic processing which could explain the similarities in function and connectivity to the cluster formed by TPJs and aMTS/aMTG.
As mentioned, the cluster formed by PCC/PrC and dmPFC showed strong connectivity to many other eSAD regions (See Figure 4b), which points to them as an important node of the (extended) social-affective default. This matches, using a different analytical approach, the conclusions from Schilbach et al. (2012). They showed the PCC/PrC and dmPFC to be the core regions of that part of the default mode which is related to social, affective and interospective processing. Indeed, there are also several studies indicating the critical role of the PCC/PrC in the default mode network (Buckner et al., 2008; Buckner et al., 2009; Fransson & Marrelec, 2008; Laird et al., 2009). Because of its important functional role (Cavanna & Trimble, 2006; Schilbach et al., 2012) and its diverse functional connectivity this region is discussed as ‘core node’ (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Margulies et al., 2009) within the DMN. In this context it is noteworthy that the PCC/PrC is supposed to be involved in a wide spectrum of highly integrated tasks (Cavanna & Trimble, 2006) which again supports the idea of PCC/PrC being central nodes within the DMN and potentially, as shown here, also the eSAD network.
Our analyses revealed an involvement of the cluster consisting of the PCC/PrC and dmPFC in social-cognitive abilities by association with theory of mind tasks. There are several hints in the neuroscience literature that suppose this assumption. Mar (2011) found a network consisting of mPFC, PCC/PrC and bilateral TPJ to be involved in theory of mind. Such a mentalizing network might be important for inferring other people’s thoughts as well as for empathy (Schnell, Bluschke, Konradt, & Walter, 2011). This also fits with findings from Saxe and Powell (2006) which implicated the PrC/PCC in attributing mental states to other individuals. Moreover, this cluster has been related to watching social interactions (Iacoboni et al., 2004). This fits with our findings that dmPFC is associated with passive observation. It seems that engaging in observation of social scenes makes people think about social relationships (Iacoboni et al., 2004). Another behavioral domain associated with this cluster seems to be moral cognition (Amodio & Frith, 2006). The authors link activation within mPFC to mentalizing and moral judgments. Bzdok et al. (2012) also related dmPFC to moral judgments and moral cognition i.e. functions that should rely on the aforementioned social-cognitive abilities including mentalizing. Given the present and previous involvement of PCC/PrC and dmPFC in theory of mind, empathy and moral judgments, it seems that this cluster is associated with other-related processing, i.e, thinking about other people’s thoughts, feelings and intentions. Our hypothesis is supported by Denny et al. (2012) who emphasize the role of dmPFC and vmPFC in both self- and other related judgments with the dmPFC preferentially being involved in making judgments about the external world. Mitchell and colleagues (2006) further assume that activation within the dmPFC is higher when people judge about opinions they do not identify themselves with. In line with our results, both dmPFC and PCC/PrC were frequently linked to social-cognitive abilities involving other-related processing.
Additionally, our analyses also indicated the involvement of this cluster in autobiographic memory, in line with a recent quantitative meta-analysis (Spreng et al., 2009). Schacter and colleagues (2007) consider parts of the DMN including PCC/PrC to be related to memory functions as well as thinking about the future (for further discussion see below). The involvement of PCC/PrC and dmPFC in autobiographical memory could indicate a possible role for this cluster in self-related processing, noting that autobiographic memory might be essential for self-reflection (Johnson et al., 2002).This fits findings from Cavanna and Trimble (2006) who stress the involvement of PCC/PrC in a wide spectrum of tasks, among these self-processing and self-awareness. All these aspects finally are supported by Qin and Northoff (2011) which consider cortical midline structures to be related to the self and, thus potentially, self-awareness (Northoff et al., 2006). Moreover, Vogt and Laureys (2005) link PCC/PrC and retrosplenial cortices to self-reflection and consciousness awareness in general. Finally it has to be mentioned that self-referential mental tasks seem to be associated with increases from baseline in dmPFC (Gusnard, Akbudak, Shulman, & Raichle, 2001), which again supports the involvement of this cluster in self-related processing. Taken together, we conclude that PCC/PrC and dmPFC are involved in both self- and other-related processing. In this context, it is noteworthy that Timmermans et al. (2012) suggested that social cognition and self-relevance rely on the same brain mechanisms which is activation in dmPFC and PCC/PrC. Interestingly, with their hybrid neural network approach, they hypothesize that learning about oneself results from learning about the external world including other person. The involvement of PCC/PrC and dmPFC in both self- and other related processing could further indicate a possible role of this cluster in self-other distinction which is supported by Lombardo et al. (2010) who suppose a shared neuronal network (including PCC/PrC) for both self-and other mentalizing. In this context, however, it must be acknowledged, that these functions were likewise attributed to the bilateral TPJ in the previous literature, i.e., those regions the most similar to the current cluster among the other eSAD regions.
In conclusion, PCC/PrC and dmPFC thus seem to be similarly involved in several shared functions, including theory of mind and autobiographical memory. Previous and present findings indicate that this cluster might therefore be relevant for self-and other-related processing. There is furthermore evidence that both are jointly important for successful simulation of future events (Buckner & Carroll, 2007; Iacoboni et al., 2004), based on the idea of shared networks between episodic remembering and envisioning the future. This is supported by Andrews-Hanna and colleagues (2010) who point out an increased activity in default mode areas during passive epochs, supposing that people spent a majority of their time thinking about their past and future (Bar, 2007; Schilbach et al., 2008). Considering the aforementioned aspects we thus assume that PCC/PrC and dmPFC are associated with self-projection, which combines autobiographic memory, imagining the future and perspective taking (Buckner & Carroll, 2007; Spreng et al., 2009) and might be regions within the eSAD relevant for successful (social) future behavior.
TPJ & aMTS/aMTG
Another subgroup is formed by bilateral TPJs and the aMTS/aMTG which showed similarities in connectivity and function. With respect to task-based functional connectivity (MACM) these three areas differed rather markedly from other eSAD regions. While this cluster showed few coactivation with frontal areas, bilateral TPJ and aMTS/aMTG all featured pronounced coactivation with areas of parietal and temporal lobes (cf. Figure 3). Whereas there is indirect evidence for anatomical connection between the posterior IPL/TPJ and prefrontal cortex in the literature (Caspers et al., 2011; Mars, Sallet, et al., 2012), these connections might be absent in non-human primates including macaques (see review by Mars, Sallet, et al. (2012)). This observation could indicate a growing importance of the respective connections in the evolution from non-human to human primates, although they were not picked up in our functional connectivity analyses. In terms of function and coactivation (MACM) the brain region featuring the most similarities to this cluster is the PCC/PrC.
The functional decoding of the bilateral TPJ regions characterized them as brain regions associated with social-cognition, especially theory of mind tasks such as mindreading, which matches the assumption that the TPJ is more specifically involved in the representation of other people’s mental states than in the visual appearance of human bodies in general (Saxe & Kanwisher, 2003). This fits findings from Decety and Lamm (2007) pointing out the relevance of inferior parietal lobule for theory of mind and empathy. Moreover, several studies indicate that the TPJ is functionally lateralized (Seghier & Price, 2012). This fits well with our finding of the left TPJ’s particularly involvement in language processes and semantic knowledge retrieval. Our results thus resonate well with findings from Binder et al. (2009) who suggest a left-lateralized network involved in language processes and highlights the role of left inferior parietal lobule. Considering the involvement of left TPJ in both social cognition and language processes, we would thus propose that this region might be (among other processes) involved in communication. This idea is supported by Stephens et al. (2010) who suggest TPJ to be involved in speaker-listener neural coupling during communication. In contrast, the right TPJ was (apart from involvement in social processes) here additionally associated with imagination and action observation and therefore seems to play a role for attentional processes and perhaps even impression formation. Mende-Siedlecki and colleagues (2012) underline the latter aspect by stressing the involvement of this region in updating impression in the context of evaluating behavior of other individuals. In a non-social context, the importance of right TPJ for attention and reorientation has likewise been emphasized (Corbetta & Shulman, 1998; Langner & Eickhoff, 2012; Mitchell, 2008). Also a recent connectivity-based parcellation of the right TPJ demonstrated its distinct involvement in both social and attentional classes of neural processes (Bzdok, Langner, et al., 2013a). We therefore conclude that the right TPJ is not exclusively related to theory of mind and other types of social processing but is moreover associated to attention. Taken together bilateral TPJ are associated with social cognitive processes such as theory of mind tasks, whereas the left TPJ is also involved in language processing and the right TPJ in attention, suggesting a hemispheric lateralization.
Several previous studies also pointed to a particular role of TPJ in self-processing and self-other distinction. Blanke and Arzy (2005), for example, analyzed TPJ activation during out-of-body-experiences and came to the conclusion that TPJ is an important node of self-processing and spatial unity of self and body. Self-processing moreover includes the identification of self-relevant information compared to non-self-reverential information. The findings of a meta-analysis by Decety and Lamm (2007) in which they indicated the right TPJ in self-processing and self-awareness as well as self-other distinction highlights the role of this brain region in the discrimination between self-relevant and other-related information. This also fits with the findings of Northoff et al. (2006) as well as Qin and Northoff (2011). As mentioned above the TPJ was shown to be involved in theory of mind tasks. Such tasks e.g. attributing feeling, beliefs and thoughts to another person probably requires self-reflection, comparing one’s own mental states to those of another person as well as autobiographical memory (Dimaggio, Lysaker, Carcione, Nicolo, & Semerari, 2008). This aspect again supports the possible importance of this cluster for self-referential processing.
The aMTS/aMTG is a rather distinct eSAD region showing less connectivity to other brain regions of this network (only precuneus and left TPJ, see Figure 4b). This is also reflected by the fact that only two consensus maps of the original seeds overlapped in the aMTS/aMTG, whereas at least three consensus maps overlapped in all other eSAD regions. We nevertheless considered the aMTS/aMTG to be part of the eSAD region as it still was a brain region that showed overlap of multiple consensus maps. Although the aMTS/aMTG might be considered rather distinct given its connectivity and functional profile, it shares some similarities to the cluster formed by TPJs, e.g., the involvement of this area in language-related processes. This fits with findings from Binder et al. (2009) who suppose a role for middle temporal gyrus in semantic processes. Our analyses further showed that aMTS/aMTG is associated to many other functions. Apart from the involvement of this region in theory of mind and semantics, it is noteworthy that aMTS/aMTG is further associated with memory processing and auditory perception. These findings could indicate the role of this brain region in speech perception as well as storing these pieces of information on language and speech in memory, i.e., auditory memory and potentially the ensuing semantic knowledge.
Taken together the cluster formed by TPJs and aMTS/aMTG seems to be involved in several aspects of social cognition (theory of mind, perspective taking) and social interaction such as communication, the latter mainly based on the associations of the left-hemispheric regions with language processing. In other words, this cluster may be associated with processing and, thus perhaps even, exchanging social relevant information, pointing to a potentially important role in communication for this eSAD region. The right TPJ moreover might be related to attention. We further assume a possible role for these brain regions in self-related processing including self-awareness and self-other distinction, albeit noting that the role for TPJ in self-processing is still a matter of conjecture.
Amygdala/Hippocampus
Bilateral amygdala and hippocampus form another cluster with distinct connectivity and function. In fact, given the observed (i) similarities in connectivity and (ii) involvement in emotion and memory processes (Figure 5 a and b), the only other eSAD-regions featuring similarity to this cluster are PCC/PrC and dmPFC. The functional characterization revealed that this cluster is involved in more basic emotional processes such as fear but also sexual interoception and is further activated during explicit memory and the face processing. The right side moreover is involved in olfaction, perception of gustation, reward and cognition. Some of the functions identified indicate that this group of regions is important for the processing of biologically relevant information from the environment (Sander, Grafman, & Zalla, 2003; Sergerie et al., 2008). This matches the findings from Bzdok and colleagues (2012) who especially linked the laterobasal nuclei group of human amygdala with higher-level input processing, whereas superficial nuclei were more linked to social information processing. Moreover, the association with affective processing may not be surprising given the critical role for this region in emotional processes (Baas et al., 2004). Sander et al. (2003) have further stressed the role of amygdala in the general appraisal of self-relevant environmental stimuli, including positive and negative emotional stimuli. Our finding of the amygdala’s association with emotion, including fear (cf. figure 5b), thus matches the current view on the functions of the amygdala and adjacent anterior hippocampus. It is important to note that Adolphs et al. (1997) moreover suppose an important role for this region in long-term memory, particularly when the subject matter is highly emotional (cf. Chase et al., 2011 for a clinical extension of these findings to drug-cue reactivity). This resonates well with memory related processes identified in this region in the current and former studies (cf. Figure 5b as well as Sergerie et al. (2008)). It must be pointed out, however, the amygdala might subserve memory functions, while episodic memory processing were mainly being attributed to its close interactions with the hippocampus (Burgess, Maguire, & O’Keefe, 2002). That is, there is evidence that the amygdala has substantial influences on memory processing mediated by the hippocampus (Adolphs et al., 1997). Consequently, Phelps (2004) described amygdala and hippocampus as two distinct memory systems which interact and influence each other. That is, emotional processes have influence on episodic memory formation in the adjacent anterior hippocampus and hippocampal information in turn influence processes in amygdala.
We would thus conclude that the cluster formed by amygdala and anterior hippocampus seems to be important in extracting affective information from the environment and potentially subsequent memory formation. Within the eSAD, it seems that the amygdala plays a critical role for higher-level input processing and filtering of emotionally or behaviorally salient information. This information in turn may interact with memory processes mediated by hippocampus. Importantly, there should be a close interaction between both processes, as emotional processing seems to rely on hippocampal feedback to amygdala (Phelps, 2004) while salience signals from the latter may influence storage of information in long-term memory (Adolphs et al., 1997).
VmPFC, ACC, SGC & vBG
vmPFC, ACC, SGC and vBG are regions within the eSAD which were not as tightly or consistently grouped into distinct clusters as the ones discussed up to now. Rather, these regions form a kind of aggregation in which the similarity between them is much dependent on the assessed feature of function or connectivity as well as on the clustering approach. Behavioral inference revealed that all of the regions within this loose subgroup, i.e., the ACC, SGC, vmPFC as well as the vBG, were functionally associated with emotional processes, reward and cognition. Additionally, most of these regions were involved in sensory processes. ACC and SGC were related to gustation, as are the left vBG. Bilateral vBG in contrast were associated with sexual interoception. The vmPFC is the most distinct region in that ‘group’ with a distinct relationship to fear processing.
Our finding that this ‘group’ is involved in reward receives wide support from the literature. Paus (2001) emphasized the role of ACC in translations to actions as well as the involvement in reward. Moreover, there are several studies implicating the role of ventral striatum in reward-related processing (Schultz, 2006). Schilbach et al. (2010), for example, reported the activation of reward-related areas, among these ventral striatum, during self- initiated attention. Another study by Grabenhorst and colleagues (2010) suggests a relation between activation of this brain region and the reward value/hedonic value of food. They investigated that activity within ventral striatum directly correlates with fattiness-ratings of subjects consuming food and indirectly with the pleasantness of it. The aspect that ventral striatum is associated with reward processing further is supported by Liu et al. (2007) who investigated reward-seeking behavior during a monetary decision-making task. Furthermore, there are several hints in neuroscience literature that the vmPFC is another brain region associated to reward (Schilbach et al., 2010). Xue et al. (2009) support this assumption by claiming that vmPFC plays an important role in making risky decisions in order to win money. All the aforementioned studies could indicate that the heterogeneous group formed by vBG, ACC, SGC and vmPFC is associated with motivation considering that reward is an important determinant which influences and maintains motivation. The assumption that ventral basal ganglia encode expected reward value to modulate actions and therefore represent motivation signals (Tachibana & Hikosaka, 2012) fits well to our hypothesis. Moreover, processes related to motivation seem to recruit the ACC, another region within this heterogeneous cluster (Pessoa, 2009). We therefore conclude that the regions within this somewhat loose cluster may play an important role in reward and motivation, behavioral domains, which together enable goal-directed behavior. In this context, we would also like to point to the relationship between motivation and, rewardingly perceived, social interactions.
Apart from that, many studies indicate an important role for several of the aforementioned brain regions for the regulation of emotional processes. This fits well with our findings implicating this ‘group’ in both emotional and cognitive processes. We would take this to indicate a potential locus of cognitive modulation of affect, i.e. the ability to control and reappraise emotions and in particular negative affect. This notion resonates well with the assumption that in particular the ACC is involved in emotional self-control and problem solving and therefore enables intelligent behavior (Allman, Hakeem, Erwin, Nimchinsky, & Hof, 2001). Other studies in turn implicate the role of SGC in the maintenance of emotional homoeostasis, a process that may be particularly disturbed in depression (Mayberg et al., 1999). Drevets and colleagues (2008) further stress the role of SGC in the regulation of mood and emotional behavior and furthermore assume a network involved in emotional regulation including other brain regions such as amygdala. Kellermann et al. (2013), finally, underline this hypothesis by emphasizing the functional coupling of SGC with the orbitofrontal cortex and the amygdala as two brain regions in which affective processing in attenuated by concurrent cognitive demand.
In summary the ‘group’ formed by vmPFC, SGC, ACC and vBG potentially represents brain regions within the eSAD that are involved in functions at the interface between affective and executive processes such as reward, motivation as well as cognitive modulation of affect, behavioral domains which together might be relevant for successful emotional behavior.
Summary and conclusions
The aim of the current study was to establish a robust definition of the social-affective default mode and to identify brain areas that are strongly connected to the seeds described by Schilbach et al. (2012) in task-dependent and task-independent brain states. We then characterized the connectivity of these regions and performed quantitative functional decoding using the BrainMap database. Moreover, we wanted to investigate the presence of sub-groups of regions within the eSAD which feature similarities in connectivity and function.
Cluster-analysis and behavioral inference allowed us to demonstrate sub-groups among the eSAD regions that share stronger similarities in connectivity and function. Among these, PCC/PrC and dmPFC seem to play a critical role in social cognition and more generally self-and other-related processing as well as self-projection. A cluster formed by bilateral TPJs and aMTS/aMTG is involved in social-cognitive abilities such as mentalizing, and may play a role in self-awareness and self-other distinction. Moreover, the additional association of this cluster with language processing may provide a link between social cognition and possibly communication. These aspects confirm the importance of the two clusters within the social aspects of the eSAD. Bilateral amygdala and anterior hippocampus as a further cluster should relate to the extraction of biologically relevant information from the environment and the bidirectional relationship between affective processing and episodic memory. The remaining regions, vmPFC, ACC, SGC and vBG, finally formed a heterogeneous ‘group’ involved in several functions at the interface between affective and executive processes such as reward, motivation as well as cognitive modulation of affect. These two clusters might form the more affective part of the eSAD. According to the concept of social-affective processing as a ‘physiological’ baseline (Schilbach et al, 2008), we would argue that the eSAD may provide the link between the traditional (but frequently questioned) “task-negative” role of the DMN and its consistent activation during several social-affective tasks. In summary, we would thus conclude that the delineated eSAD may be conceptualized as a closely coupled set of regions that form a social-affective network related to self- and other-related mental processes that people maintain during unconstrained cognition. Considering the aspect that the default mode attracted a lot of attention because of aberrations in patients with psychiatric disorders, it would be interesting to investigate to what extent the individual regions and sub-groups of the eSAD are affected in such patients.
Supplementary Material
Figure 6. Clustering of eSAD regions.
Illustration of the clustering results based on disparate approaches: non-hierarchical k-means clustering performed at different levels, hierarchical cluster analysis (HC, correlation-distance and complete linkage criteria) as well as multidimensional scaling for the projection of inter-regional differences in 2D space. Top row illustrates clustering referring to function
Middle row shows clustering referring to similarities in whole-brain co-activation
Bottom row illustrates clustering based on resting state connectivity.
Acknowledgments
This study was supported by the Human Brain Project (R01-MH074457-01A1, PTF, ARL, SBE), the Helmholtz Initiative on Systems-Biology “The Human Brain Model” (SBE), and the German National Academic Foundation (DB).
Footnotes
The authors declare no conflict of interest.
References
- Adams RB, Jr, Rule NO, Franklin RG, Jr, Wang E, Stevenson MT, Yoshikawa S, Ambady N. Cross-cultural reading the mind in the eyes: an fMRI investigation. J Cogn Neurosci. 2010;22(1):97–108. doi: 10.1162/jocn.2009.21187. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learn Mem. 1997;4(3):291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;935:107–117. [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 2010;104(1):322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev. 2004;45(2):96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bar M. The proactive brain: using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11(7):280–289. doi: 10.1016/j.tics.2007.05.005. S1364-6613(07)00129-5 [pii] [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Arzy S. The out-of-body experience: disturbed self-processing at the temporo-parietal junction. Neuroscientist. 2005;11(1):16–24. doi: 10.1177/1073858404270885. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Engemann DA, Laird AR, Fox PT, Eickhoff SB. Segregation of the human medial prefrontal cortex in social cognition. Front Hum Neurosci. 2013;7:232. doi: 10.3389/fnhum.2013.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Jakobs O, Roski C, Caspers S, Eickhoff SB. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage. 2013a doi: 10.1016/j.neuroimage.2013.05.046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Jakobs O, Roski C, Caspers S, Eickhoff SB. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage. 2013b;81:381–392. doi: 10.1016/j.neuroimage.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct. 2012;217(4):783–796. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Rick T, von Kapri A, Kuhlen T, Huang R, Zilles K. Probabilistic fibre tract analysis of cytoarchitectonically defined human inferior parietal lobule areas reveals similarities to macaques. Neuroimage. 2011;58(2):362–380. doi: 10.1016/j.neuroimage.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Eickhoff SB. Is There “One” DLPFC in Cognitive Action Control? Evidence for Heterogeneity From Co-Activation-Based Parcellation. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Human cortical mechanisms of visual attention during orienting and search. Philos Trans R Soc Lond B Biol Sci. 1998;353(1373):1353–1362. doi: 10.1098/rstb.1998.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13(6):580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 2012;24(8):1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Mar RA. Lost in localization: the need for a universal coordinate database. Neuroimage. 2009;48(1):1–7. doi: 10.1016/j.neuroimage.2009.01.053. [DOI] [PubMed] [Google Scholar]
- Dimaggio G, Lysaker PH, Carcione A, Nicolo G, Semerari A. Know yourself and you shall know the other... to a certain extent: multiple paths of influence of self-reflection on mindreading. Conscious Cogn. 2008;17(3):778–789. doi: 10.1016/j.concog.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59(3):2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage. 2011;57(3):938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu Rev Neurosci. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- Forgy EW. Cluster analysis of multivariate data : efficiency versus interpretability of classifications. Biometrics. 1965;21:768–769. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Parris BA, d’Souza AA. How the brain represents the reward value of fat in the mouth. Cereb Cortex. 2010;20(5):1082–1091. doi: 10.1093/cercor/bhp169. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69(4):301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Wong MA. Algorithm AS 136: A k-means clustering algorithm. Journal of the Royal Statistical Society. Series C (Applied Statistics. 1979;28(1):100–108. [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (Berl) 2008;201(2):183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, Molnar-Szakacs I, Moritz M, Throop CJ, Fiske AP. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage. 2004;21(3):1167–1173. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, Eickhoff SB. Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus-context integration. Neuroimage. 2012;60(4):2389–2398. doi: 10.1016/j.neuroimage.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(Pt 8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kellermann TS, Caspers S, Fox PT, Zilles K, Roski C, Laird AR, Eickhoff SB. Task- and resting-state functional connectivity of brain regions related to affection and susceptible to concurrent cognitive demand. Neuroimage. 2013;72:69–82. doi: 10.1016/j.neuroimage.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, Jr, Fox PT. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes. 2011;4:349. doi: 10.1186/1756-0500-4-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29(46):14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining Attention to Simple Tasks: A Meta-Analytic Review of the Neural Mechanisms of Vigilant Attention. Psychol Bull. 2012 doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27(17):4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, Baron-Cohen S. Shared neural circuits for mentalizing about the self and others. J Cogn Neurosci. 2010;22(7):1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Mar RA. The neural bases of social cognition and story comprehension. Annu Rev Psychol. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci U S A. 2009;106(47):20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MF. On the relationship between the “default mode network” and the “social brain”. Front Hum Neurosci. 2012;6:189. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schuffelgen U, Jbabdi S, Toni I, Rushworth MF. Connectivity-based subdivisions of the human right “temporoparietal junction area”: evidence for different areas participating in different cortical networks. Cereb Cortex. 2012;22(8):1894–1903. doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Cai Y, Todorov A. The neural dynamics of updating person impressions. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2008;18(2):262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Orosz A, Jann K, Federspiel A, Horn H, Hofle O, Dierks T, Walther S. Reduced cerebral blood flow within the default-mode network and within total gray matter in major depression. Brain Connect. 2012;2(6):303–310. doi: 10.1089/brain.2012.0101. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2(6):417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14(2):198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Reetz K, Dogan I, Rolfs A, Binkofski F, Schulz JB, Laird AR, Eickhoff SB. Investigating function and connectivity of morphometric findings--exemplified on cerebellar atrophy in spinocerebellar ataxia 17 (SCA17) Neuroimage. 2012;62(3):1354–1366. doi: 10.1016/j.neuroimage.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14(4):303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19(4):1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 2006;17(8):692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8(9):657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One. 2012;7(2):e30920. doi: 10.1371/journal.pone.0030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn. 2008;17(2):457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, Vogeley K. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. J Cogn Neurosci. 2010;22(12):2702–2715. doi: 10.1162/jocn.2009.21401. [DOI] [PubMed] [Google Scholar]
- Schnell K, Bluschke S, Konradt B, Walter H. Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. Neuroimage. 2011;54(2):1743–1754. doi: 10.1016/j.neuroimage.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Price CJ. Functional Heterogeneity within the Default Network during Semantic Processing and Speech Production. Front Psychol. 2012;3:281. doi: 10.3389/fpsyg.2012.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32(4):811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Clos M, Meijering AL, Diederen KM, Eickhoff SB. Resting state functional connectivity in patients with chronic hallucinations. PLoS One. 2012;7(9):e43516. doi: 10.1371/journal.pone.0043516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens GJ, Silbert LJ, Hasson U. Speaker-listener neural coupling underlies successful communication. Proc Natl Acad Sci U S A. 2010;107(32):14425–14430. doi: 10.1073/pnas.1008662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana Y, Hikosaka O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron. 2012;76(4):826–837. doi: 10.1016/j.neuron.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans B, Schilbach L, Pasquali A, Cleeremans A. Higher order thoughts in action: consciousness as an unconscious re-description process. Philos Trans R Soc Lond B Biol Sci. 2012;367(1594):1412–1423. doi: 10.1098/rstb.2011.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp. 2012;33(1):1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cereb Cortex. 2009;19(5):1019–1027. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zu Eulenburg P, Caspers S, Roski C, Eickhoff SB. Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage. 2012;60(1):162–169. doi: 10.1016/j.neuroimage.2011.12.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.