Abstract
Introduction
An option for active surveillance is not currently offered to patients with ductal carcinoma in situ (DCIS); however a small number of women decline standard surgical treatment for noninvasive cancer. The purpose of this study was to assess outcomes in a cohort of 14 well-informed women who elected non-surgical active surveillance with endocrine treatment alone for estrogen receptor-positive DCIS.
Methods
Retrospective review of 14 women, 12 of whom were enrolled in an IRB-approved single-arm study of 3 months of neoadjuvant endocrine therapy prior to definitive surgical management. The patients in this report withdrew from the parent study opting instead for active surveillance with endocrine treatment and imaging.
Results
8 women had surgery at a median follow up of 28.3 months (range 10.1–70 months), 5 had stage I IDC at surgical excision, and 3 had DCIS alone. 6 women remain on surveillance without evidence of invasive disease for a median of 31.8 months (range 11.8–80.8 months).
Conclusion
Long-term active surveillance for DCIS is feasible in a well-informed patient population, but is associated with risk of invasive cancer at surgical excision.
Keywords: DCIS, ER-positive, Surveillance, Breast MRI, Non-surgical management
Introduction
Ductal carcinoma in situ (DCIS) represents 25–30% of breast cancers mammographically diagnosed in the United States and is thought to be a non-obligate precursor to invasive breast cancer.1–3 Since treatment is undertaken without reliable prognostic markers to predict which women with DCIS will progress to invasive cancer in the absence of surgical excision, there is increased interest in whether an “active surveillance” option, as is commonly offered to patients with prostate cancer, may be feasible for some women with DCIS.
Most DCIS is nonpalpable; thus a safe surveillance strategy for non-surgical management of DCIS must rely on radiologic findings. Although mammography remains the “gold standard” for imaging of DCIS recent studies suggest that MRI may be superior to mammography for higher grade DCIS and for detection of occult invasive disease despite limitations of MRI in detection of lesions <5 mm.4–8 The purpose of this study was to evaluate clinical outcomes in a cohort of women diagnosed with ER-positive DCIS who declined surgery and were followed with close radiographic surveillance including MRI.
Materials and methods
Between 2003 and 2008, 52 women enrolled in an IRB-approved single-arm study of 3 months of neoadjuvant endocrine therapy for ER-positive DCIS, which included definitive surgical excision at the end of the study. However, 12 women withdrew from the parent study upon completion of 3 months of endocrine therapy and declined surgery, opting instead for active surveillance with the intent to consider surgery in the event of clinical progression. 2 additional women were not enrolled in the clinical study but elected a similar course of management. Each patient was extensively counseled and informed of the current standard treatment guidelines for DCIS, as well as the increased future probability for invasive cancer in the absence of surgical treatment for DCIS. Despite this, all 14 women in this report declined surgery and strongly indicated preference for active surveillance. However, all women were amenable to imaging follow up and planned to continue endocrine therapy, despite unproven benefit in this setting. All women had at least 6 months of follow up.
For those enrolled in the parent study, a baseline mammogram and MRI were obtained at study entry and after 3 months of therapy. All patients electing active surveillance were compliant with follow up which consisted of MRI, mammogram, and clinical exam biannually. Biopsies were recommended if imaging findings were suspicious for progression. Mammographic criteria for progression included increase in number and extent of microcalcifications or suspicion of mass or architectural distortion. MRI progression was defined as increase in extent of clumped ductal enhancement or development of mass-like enhancement measuring at least 5 mm. All imaging studies were reviewed by a fellowship-trained breast radiologist, and all patients were followed closely by a breast surgeon. At every follow-up visit, imaging studies and treatment options were reviewed with the patient.
Results
14 women who declined surgery for ER-positive DCIS are included in this review. Patient characteristics are presented in Table 1. The median age was 49 years (range 41–71 years) at presentation. Of these, nine women were premenopausal and took tamoxifen; five were postmenopausal and took aromatase inhibitors. The average length of follow up is 42.3 months (range 11.8–80.8 months). Three women elected to discontinue endocrine therapy due to side effects at 1.4 months, 8.7 months, and 13 months from treatment initiation. The remainder elected to continue endocrine therapy as part of active surveillance.
Table 1.
Patient demographics: At initiation of surveillance all women had biopsy proven ER(+) DCIS. Premenopausal women were prescribed tamoxifen. Postmenopausal women were prescribed an aromatase inhibitor. For the three women who discontinued endocrine therapy due to side effects, total months on therapy for each patient were: Patient 5–13 months; Patient 8–1.4 months; Patient 9–8.7 months.
| Study ID | Age at diagnosis | Menopausal Status | Endocrine therapy | Initial DCIS Grade | Radiographic extent of DCIS at diagnosis |
|---|---|---|---|---|---|
| 1 | 41 | Pre | Tamoxifen | Int | MMGa: No findings MRI: multifocal 5–9 mm enhancing lesions |
| 2 | 42 | Pre | Tamoxifen | Low | MMG: 7 cm Ca2+ |
| 3 | 46 | Pre | Tamoxifen | Int | MMG: 4.4 cm Ca2+ |
| 4 | 47 | Pre | Tamoxifen | Low | MMG: 6 cm Ca2+ |
| 5 | 47 | Pre | Tamoxifen: discontinued | Int | MMG: 4 cm Ca2+ |
| 6 | 48 | Pre | Tamoxifen | Low | MMG: 8 cm Ca2+ |
| 7 | 48 | Pre | Tamoxifen | High | MMG: Multifocal up to 3 cm Ca2+ |
| 8 | 50 | Pre | Tamoxifen: discontinued | Low | MMG: no MMG available MRI: up to 4.4 cm abnormal enhancement |
| 9 | 52 | Pre | Tamoxifen: discontinued | Int | MMG: 7 cm Ca2+ |
| 10 | 55 | Post | Letrozole | Int | MMG: 9 cm Ca2+ |
| 11 | 56 | Post | Letrozole | Int | MMG: 2 cm Ca2+ |
| 12 | 58 | Post | Letrozole | High | MMG: 2.5 cmCa2+ |
| 13 | 60 | Post | Arimidex | Int | MMG: Multicentric in 2 quadrants; largest focus 2.5 cm |
| 14 | 71 | Post | Letrozole | Int | MMG: 1.6 cm Ca2+ |
Surgery subset
Eight patients in the cohort underwent definitive surgery during the follow up period (Table 2). Median time to surgery was 22.1 months (range 10.1–70 months). The indications for surgery were: imaging progression,6 limited imaging response,1 and patient choice at 17.4 months due to plans for pregnancy.1 Examples of radiographic findings suggestive of disease progression prompting surgery are shown in Figs. 1–3.
Table 2.
Final Pathologic Findings on Surgery Cohort: 6 of 8 patients underwent surgery for progression on follow-up MMG and MRI. 5 of 8 patients had stage I IDC at surgical intervention, including 3 of whom showed increasing mass-like enhancement, one showed increasing extent of disease, and one showed extensive disease that did not continue to respond to endocrine therapy. One patient developed a contralateral mammographically occult finding during surveillance that showed DCIS on MRI-directed core biopsy (patient 9).
| Study ID |
Time to Surgery (mos) |
Initial DCIS Grade |
Surgical Recommendation at Presentation |
Surgical Indication | Outcome | Pathology | Stage |
|---|---|---|---|---|---|---|---|
| 1 | 17.4 | Intermediate | Mastectomy | Elective | Lumpectomy | 0.7 cm low grade DCIS ER+/PR+ | 0 |
| 2 | 15.9 | Low | Mastectomy | Imaging progression | Left: Lumpectomy | 1.2 cm low grade DCIS | 0 |
| 5 | 70 | Intermediate/Low | Mastectomy | Imaging Progression | Mastectomy with sentinel node bx | 7 mm multifocal intermediate grade IDC ER+/PR+, Her2neu− with 2.1 cm low-int grade DCIS | I |
| 6 | 10.1 | Low | Mastectomy | Limited imaging response | Mastectomy with sentinel node bx | multi focal IBC up to 4 mm ER+/PR−, Her2Neu− and 6 cm high grade DCIS | I |
| 9 | 29.8 | Low/Intermediate | Right: Mastectomy Left: Lumpectomy | Imaging Progression | Right: Lumpectomy with sentinel node bx Left: Lumpectomy | Right: 1.7 cm IDC ER+/PR+, Hcr2Neu− and 2.6 cm int to high grade DCIS; Left: 2.4 cm intermediate grade DCIS | Right: Stage I Left: Stage 0 |
| 10 | 16.5 | Intermediate | Mastectomy | Imaging Progression | Lumpectomy with sentinel node biopsy | 6 cm high grade DCIS ER+/PR+ | 0 |
| 11 | 39.4 | Intermediate | Lumpectomy | Imaging Progression | Lumpectomy with sentinel node bx | 5 mm intermediate grade IDC ER+/PR, Her2Neu + and extensive high grade DCIS | I |
| 12 | 26.9 | High | Mastectomy | Imaging Progression | Lumpectomy with sentinel node bx | 8 mm high grade IDC ER−/PR−, Her2Neu + and multifocal microscopic high grade comedo DCIS | I |
Fig. 1.
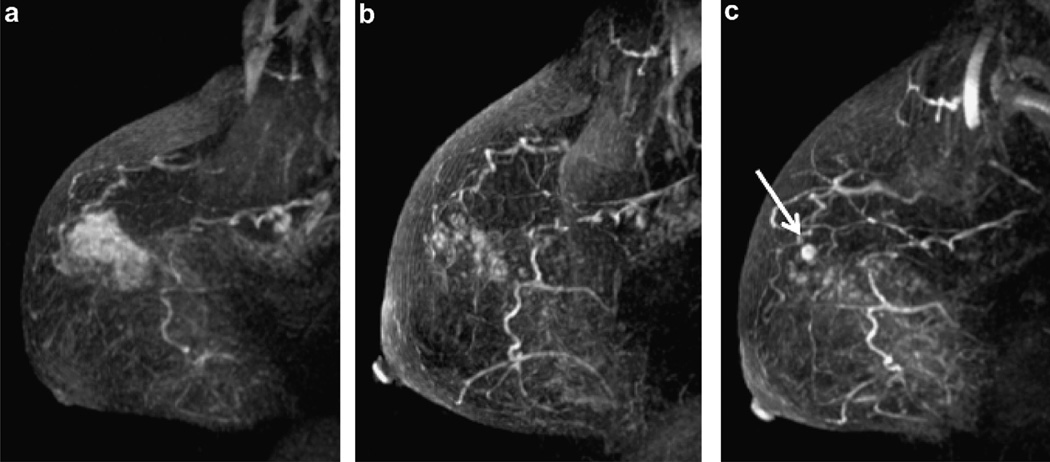
58 year old woman with high grade DCIS (patient 12). (a) Baseline MRI shows extensive abnormal clumped ductal enhancement in the upper breast. (b) Breast MRI at 19.3 months on aromatase inhibitor therapy demonstrates improved appearance of abnormal enhancement in the right breast. (c) Breast MRI at 25.5 months since diagnosis demonstrated continual improvement in clumped ductal enhancement but a new 6 mm mass enhancement (white arrow) representing 8 mm of Grade 3 ER−/PR−/Her2neu + IDC at surgery. (Sagittal T1 post gadolinium subtracted maximum intensity projections).
Fig. 3.
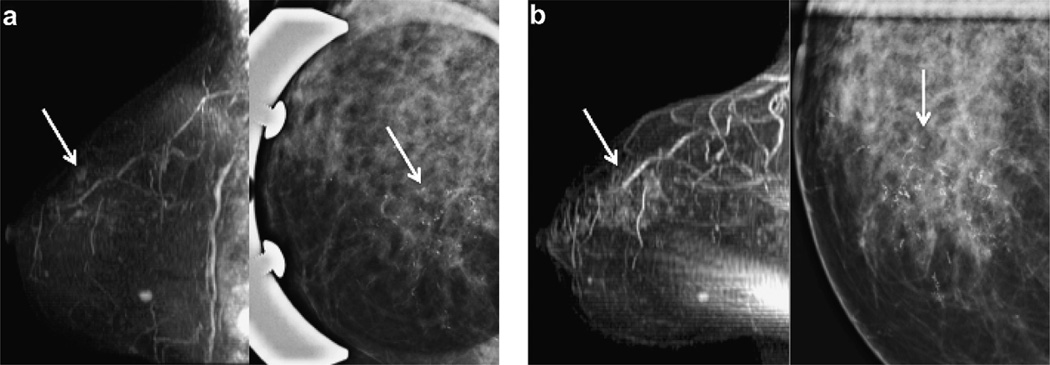
55 year old woman with intermediate grade DCIS (patient 10). (a) Baseline MRI with upper inner clumped ductal enhancement (arrow, left image) and pleomorphic microcalcifications at mammography (arrow, right image). (b) 14.5 months after diagnosis breast MRI shows increasing clumped ductal enhancement in a segmental distribution (arrow, left image) and increasing pleomorphic microcalcifications at mammography (arrow right image). Lumpectomy specimen revealed 6 cm of high grade weakly ER+/PR+ and Her2neu + DCIS. (Sagittal T1 post gadolinium subtracted maximum intensity projections (MIP) and CC spot compression magnification mammography).
A total of 8 women (9 breasts) underwent surgery consisting of 7 mastectomies and 2 lumpectomies. Surgical treatment was altered from mastectomy to lumpectomy in 5 women. Both women initially recommended lumpectomy successfully avoided mastectomy. Final pathology at surgical excision showed 4 pure DCIS lesions and 5 Stage I IDC (Table 2). 4 of 5 invasive cancers were hormone-receptor-positive; all invasive cancers were node-negative.
Surveillance subset
Six patients remain on active surveillance; four remain on hormonal therapy. One woman discontinued tamoxifen at 80 months and another discontinued AI at 60 months (Table 3). At a median follow up of 31.8 months (range 11.8–80.8 months), all have shown continued radiographic improvement or imaging stabilization of their known disease. In 2 of 6 patients, core biopsy has been performed to confirm absence of invasive progression based in imaging findings. All of these additional core biopsies have shown either persistent DCIS or atypical ductal hyperplasia (ADH), but no invasive cancer.
Table 3.
Surveillance Cohort: 6 women with unresected DCIS. All women remain under active surveillance with regular clinical examination, MMG, and MRI. 5 of 6 women remain on endocrine therapy.
| Study ID |
Drug | Time to Surgery (mos) |
Initial DCIS Grade |
Surgical Recommendation at Presentation |
Time on Hormone Therapy |
# of Core Biopsies after Diagnosis |
# of Core Biopsies with DCIS |
|---|---|---|---|---|---|---|---|
| 3 | Tamoxifen | 43.3 | Intermediate | Mastectomy | 24 | 3 | 1 |
| 4 | Tamoxifen | 39.2 | Low | Mastectomy | 23.6 | 0 | 0 |
| 7 | Tamoxifen | 97.6 | Intermediate/High | Mastectomy | 80.6 | 6 | 3 |
| 8 | Tamoxifen | 52.1 | Low | Mastectomy | 1.4 | 0 | 0 |
| 13 | Arimidex | 94.7 | Intermediate | Mastectomy | 66.6 | 0 | 0 |
| 14 | Letrozole | 30 | Intermediate | Lumpectomy | 8.9 | 0 | 0 |
Discussion
The incidence of DCIS has markedly increased since the widespread uptake of screening mammography.1,9,10 The frequency of developing IDC from DCIS in the absence of surgical therapy has been estimated to be between 14 and 75% with long-term follow up,11–13 which suggests that many patients treated for DCIS do not derive a benefit in breast cancer specific survival. However, if patients decline surgery for DCIS, it is imperative that there be an effective follow-up strategy to follow these patients safely. An analogous patient population is that of that of BRCA mutation carriers who have for many years been offered routine annual MRI surveillance and clinical examination as a safe and acceptable alternative to prophylactic surgery.14–17 However, to our knowledge, this is the first report in the literature to document such an approach for patients with DCIS.
Our report is limited to patients with hormone receptor-positive DCIS treated with endocrine therapy. In invasive cancer, tamoxifen use has consistently been associated with a significant benefit in reduction of local, distant and contralateral disease in both postmenopausal and premenopausal women.18–20 In DCIS, when tamoxifen is given in conjunction with surgery and radiation, ipsilateral breast recurrence was reduced by 30% and contralateral events reduced by 50%; it is likely that this effect is seen primarily in ER(+) disease.19–22 Tamoxifen has been shown to stabilize IBC in the setting of locally advanced breast cancer and severe comorbidities that preclude surgery.23 Thus it is conceivable that hormonal treatment may also prevent progression in some ER-positive DCIS. One short-term preoperative trial has confirmed that hormonal intervention reduces proliferation in DCIS24; more long-term studies of hormonal therapy alone will determine which subsets of DCIS may benefit most from such an approach. In the present report, is possible that for the women who successfully avoided surgery endocrine therapy may have played a role, although this cannot be substantiated.
When recommending treatment for DCIS, it is important to determine how the decision to forego surgery at presentation impacts patient outcome. If one models patient outcome on Adjuvant! Online (adjuvantonline.com) for a 60 year old woman in perfect health with a grade 2, 7 mm, node negative invasive cancer, the likelihood of death from breast cancer is 2.9% in 10 years, reduced to 2.1% with adjuvant tamoxifen. This same woman would have more than double this risk for non-breast cancer mortality. For all women with DCIS, the breast cancer specific mortality has been estimated to range from 1 to 2%. Thus, for women who follow a plan of close observation and are subsequently found to have invasive cancer, there is likely minimal impact on breast cancer mortality, provided the invasive cancer is detected at an early stage.
Such data must be weighed against the potential morbidity of standard treatment. The present study shows that the impact of active surveillance on subsequent treatment is likely to be small or may even be beneficial for some patients; in this cohort of women, most of whom were on hormonal therapy, 11/14 patients were initially recommended to have mastectomy and to date, 2 have had mastectomy, 6 have had lumpectomy, and 6 remain without surgical treatment. In this cohort, effective breast imaging played an important role in follow up, and our findings support that the combination of mammography, ultrasound, and MRI was effective at diagnosis of early invasive disease in women undergoing surveillance for DCIS. However, cost of surveillance will also be a key consideration.
Critically important to the success of an expectant management approach is patient and provider education. A detailed discussion with an informed patient, fully outlining possible tradeoffs resulting from such a plan is absolutely essential. Areas of consensus, controversy, and standard treatment recommendations must be carefully reviewed in order for a patient to make decisions that take into account what is known about DCIS, likelihood of invasive progression, and impact on breast cancer outcome, as well as patient comorbidities.
This study provides early insight into possible outcomes resulting from expectant management for DCIS. Clearly, the report is limited by its small size, variability of patient and DCIS characteristics, and selection bias, making it difficult to draw definitive conclusions. However, we found that there are a few well-informed, highly motivated patients who make the decision to forego surgery in favor of a surveillance strategy for management of DCIS. The future challenge to the medical community is to render this option an increasingly safe one by further refining optimal patient selection, education and follow up for this approach.
Fig. 2.
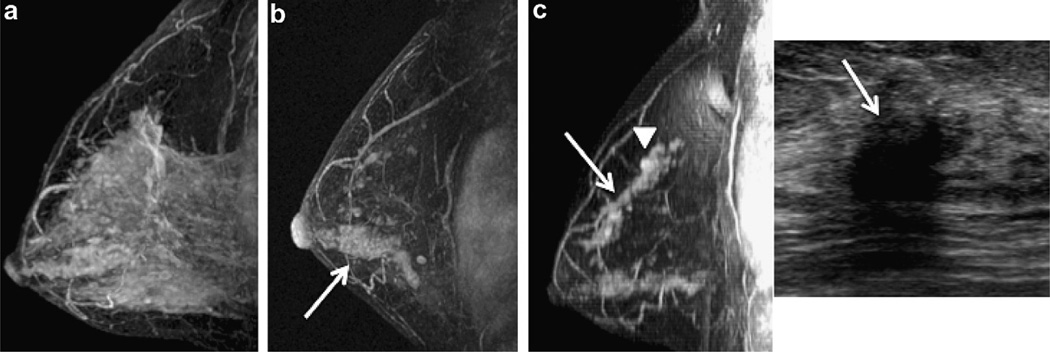
47 year old woman with intermediate grade DCIS who discontinued endocrine therapy after 13 months (patient 5). (a) Baseline MRI revealed extensive background enhancement which was dramatically reduced at 3 month follow up where extensive clumped ductal enhancement is seen in the lower breast (arrow) and scattered clumped ductal enhancement is seen in the upper breast (b). (c) 65.5 months from diagnosis, breast MRI showed increasing clumped ductal (arrow, left image) and mass-like (arrowhead, left image) enhancement in the upper breast with a corresponding suspicious hypoechoic mass seen at ultrasound (arrow, right image). Mastectomy revealed multifocal grade 2 IDC (ER+/weakly PR+/Her2neu−) and low to intermediate grade DCIS. (Sagittal T1 post gadolinium subtracted maximum intensity projections (MIP) and targeted ultrasound).
Acknowledgements
To the patients and the staff of the University of California San Francisco Breast Care Center who dedicate themselves to the compassionate care and improved quality of life for our patients.
Funding source
This work was supported by the K23 CA0977181 (Hwang) and R01 CA116182 (Hylton).
Footnotes
Ethical approval
This study was reviewed and approved by the UCSF IRB (H10367-19345).
Conflicts of interest statement
There are no conflicts of interest to disclose.
References
- 1.Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002 Oct 16;94(20):1546–1554. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 2.Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004 Apr 1;350(14):1430–1441. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 3.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010 Feb 3;102(3):170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 4.Kuhl CK, Schrading S, Bieling HB, Wardelmann E, Leutner CC, Koenig R, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007 Aug 11;370(9586):485–492. doi: 10.1016/S0140-6736(07)61232-X. [DOI] [PubMed] [Google Scholar]
- 5.Hwang ES, Kinkel K, Esserman LJ, Lu Y, Weidner N, Hylton NM. Magnetic resonance imaging in patients diagnosed with ductal carcinoma-in-situ: value in the diagnosis of residual disease, occult invasion, and multicentricity. Ann Surg Oncol. 2003 May;10(4):381–388. doi: 10.1245/aso.2003.03.085. [DOI] [PubMed] [Google Scholar]
- 6.Marcotte-Bloch C, Balu-Maestro C, Chamorey E, Ettore F, Raoust I, Flipo B, et al. MRI for the size assessment of pure ductal carcinoma in situ (DCIS): a prospective study of 33 patients. Eur J Radiol. 2009 Nov 5; doi: 10.1016/j.ejrad.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Jansen SA, Paunesku T, Fan X, Woloschak GE, Vogt S, Conzen SD, et al. Ductal carcinoma in situ: X-ray fluorescence microscopy and dynamic contrastenhanced MR imaging reveals gadolinium uptake within neoplastic mammary ducts in a murine model. Radiology. 2009 Nov;253(2):399–406. doi: 10.1148/radiol.2533082026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang ES, Esserman L. Neoadjuvant hormonal therapy for ductal carcinoma insitu: trial design and preliminary results. Ann Surg Oncol. 2004 Jan;11(1 Suppl):37S–43S. doi: 10.1007/BF02524794. [DOI] [PubMed] [Google Scholar]
- 9.Li CI, Daling JR, Malone KE. Age-specific incidence rates of in situ breast carcinomas by histologic type, 1980 to 2001. Cancer Epidemiol Biomarkers Prev. 2005 Apr;14(4):1008–1011. doi: 10.1158/1055-9965.EPI-04-0849. [DOI] [PubMed] [Google Scholar]
- 10.Allegra CJ, Aberle DR, Ganschow P, Hahn SM, Lee CN, Millon-Underwood S, et al. National institutes of health State-of-the-science conference statement: diagnosis and management of ductal carcinoma. In Situ September 22–24. J Natl Cancer Inst. 2009 Feb 3;102(3):161–169. doi: 10.1093/jnci/djp485. [DOI] [PubMed] [Google Scholar]
- 11.Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005 Jun 15;103(12):2481–2484. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 12.Collins LC, Tamimi RM, Baer HJ, Connolly JL, Colditz GA, Schnitt SJ. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the nurses’ health study. Cancer. 2005 May 1;103(9):1778–1784. doi: 10.1002/cncr.20979. [DOI] [PubMed] [Google Scholar]
- 13.Eusebi V, Feudale E, Foschini MP, Micheli A, Conti A, Riva C, et al. Long-term follow-up of in situ carcinoma of the breast. Semin Diagn Pathol. 1994 Aug;11(3):223–235. [PubMed] [Google Scholar]
- 14.Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005 May 21–27;365(9473):1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 15.Dent R, Warner E. Screening for hereditary breast cancer. Semin Oncol. 2007 Oct;34(5):392–400. doi: 10.1053/j.seminoncol.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005 Nov 20;23(33):8469–8476. doi: 10.1200/JCO.2004.00.4960. [DOI] [PubMed] [Google Scholar]
- 17.Causer PA, Jong RA, Warner E, Hill K, Wong JW, Curpen BN, et al. Breast cancers detected with imaging screening in the BRCA population: emphasis on MR imaging with histopathologic correlation. Radiographics. 2007 Oct 27;(Suppl 1):S165–S182. doi: 10.1148/rg.27si075503. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 1998 Sep 16;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Dignam J, Bryant J, DeCillis A, Wickerham DL, Wolmark N, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996 Nov 6;88(21):1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 2005 Nov 16;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 21.Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, et al. Tamoxifen in treatment of intraductal breast cancer: National surgical adjuvant breast and bowel project B-24 randomised controlled trial. Lancet. 1999 Jun 12;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 22.Allred DCBJ, Land S, Paik S, Fisher E, Julian T, Margolese RSR, et al. Estrogen receptor expression as a predictive marker of the effectiveness of tamoxifen in the treatment of DCIS: findings from NSABP protocol B-24. Breast Cancer Res Treat. 2002;76(Suppl. 1):S36. [Abstract]. 2002. [Google Scholar]
- 23.Hoff PM, Valero V, Buzdar AU, Singletary SE, Theriault RL, Booser D, et al. Combined modality treatment of locally advanced breast carcinoma in elderly patients or patients with severe comorbid conditions using tamoxifen as the primary therapy. Cancer. 2000 May 1;88(9):2054–2060. doi: 10.1002/(sici)1097-0142(20000501)88:9<2054::aid-cncr11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Chen YY, DeVries S, Anderson J, Lessing J, Swain R, Chin K, et al. Pathologic and biologic response to preoperative endocrine therapy in patients with ER-positive ductal carcinoma in situ. BMC Cancer. 2009;9:285. doi: 10.1186/1471-2407-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]