Abstract
Purpose
This study aimed to investigate the role of CYP1B1 mutations in primary congenital glaucoma (PCG) in Pakistani patients.
Methods
After consent was received, 20 families with at least more than one member affected with primary congenital glaucoma were enrolled in the study. The disease was confirmed with standard ophthalmological investigations. Genomic DNA was extracted from whole blood for localization of linkage and sequencing. Bioinformatics tools were used to assess the predicted pathological role of novel variants.
Results
Ten out of 20 families (50%, 10/20) showed homozygosity with CYP1B1-linked short tandem repeat (STR) markers. On direct sequencing of the CYP1B1 gene in the linked families, six mutations, including two novel pathogenic variants, were identified. p. R390H was the most frequently found mutation in five families (50%, 5/10), whereas c.868_869insC, p.E229K, and p.A115P were found once in three families. Two novel mutations, a missense mutation (p.G36D) and an in-frame deletion mutation (p.G67-A70del), were segregated with disease phenotype in two families. Age of disease onset was congenital in all mutations; however, disease severity and response to clinical interventions varied among the mutations and families. Haplotype analysis using five polymorphisms revealed a distinct haplotype for a common mutation.
Conclusions
This is the largest cohort of Pakistani patients with PCG to be genetically screened for CYP1B1 mutations. Identifying common mutation and genotype-phenotype correlations may help in genetic testing and better prognosis for the disease. Novel mutations identified in the study may help in better understanding the pathophysiology of CYP1B1-associated glaucoma.
Introduction
Primary congenital glaucoma (PCG, OMIM 231300) is a rare form of glaucoma, inherited as an autosomal recessive trait [1]. PCG is associated with developmental anomalies of the anterior chamber as well as the trabecular meshwork and is characterized by elevated intraocular pressure (IOP), increased corneal diameter, and optic disc damage [2]. PCG is the most common form of glaucoma in infants. The disease manifests at birth or in the first year of life and usually leads to permanent vision impairment. The incidence however varies among different populations: 1 in 1,250 in Slovakian gypsies, 1 in 2,500 in Saudi Arabians, 1 in 3,300 in southern Indians [3], and from 1:10,000 to 1:20,000 in Western populations [4].
Three chromosomal locations have been mapped for PCG: GLC3A at 2p21 [5], GLC3B at 1p36.2 [6], and GLC3C at 14q24.3 [7]. Mutations in only two genes, CYP1B1 (ID: 1545, OMIM: 601771) at GLC3A and LTBP2 (ID: 4053, OMIM: 602091) at GLC3C, have been associated with PCG [7]. Although contribution of cytochrome P4501B1 (CYP1B1) varies in different populations, worldwide CYP1B1 accounts for 50% of PCG cases [8].CYP1B1 consists of two coding exons and encodes the cytochrome P450 superfamily, subfamily B, polypeptide 1, a 543 amino acids long protein. It is expressed in the trabecular meshwork and in the posterior segment of the eye [9].
More than 150 PCG-associated mutations in CYP1B1 have been described [10,11]. The prevalence of CYP1B1 mutations varies significantly across different populations and geographic locations [12]. Notably, CYP1B1 mutations show incomplete penetrance and variable expressivity among populations of different ethnic origin [13,14].
The spectrum of CYP1B1 mutations in Pakistani patients with PCG is not well understood. Previously, only three familial cases with CYP1B1 mutations had been reported [15]. This study, therefore, was aimed to screen 20 familial cases of PCG for CYP1B1, to identify and determine common mutations, and to understand its penetrance and prevalence in the area.
Methods
Patient enrollment and clinical investigations
Twenty families affected with PCG were enrolled from Liaquat University Tertiary Care Hospital after obtaining written informed consent. The study followed the tenets of the Declaration of Helsinki and was approved by the Ethical Review Committee of Liaquat University of Medical and Health Sciences, Jamshoro. Detailed medical history of all participating families was recorded, and pedigrees were drawn to conform the inheritance pattern (Figure 1, Figure 2, Figure 3). Ethnicity-matched controls with no history of ocular disorders were enrolled in the study. All available affected individuals underwent detailed clinical investigations. IOP was assessed with Schiotz indentation tonometry. Angles of anterior chamber were examined with Goldmann gonioscope, and the field of vision was attempted by Humphrey perimetry wherever possible. The fundus was examined by using slit-lamp biomicroscopy with a +78 diopter lens.
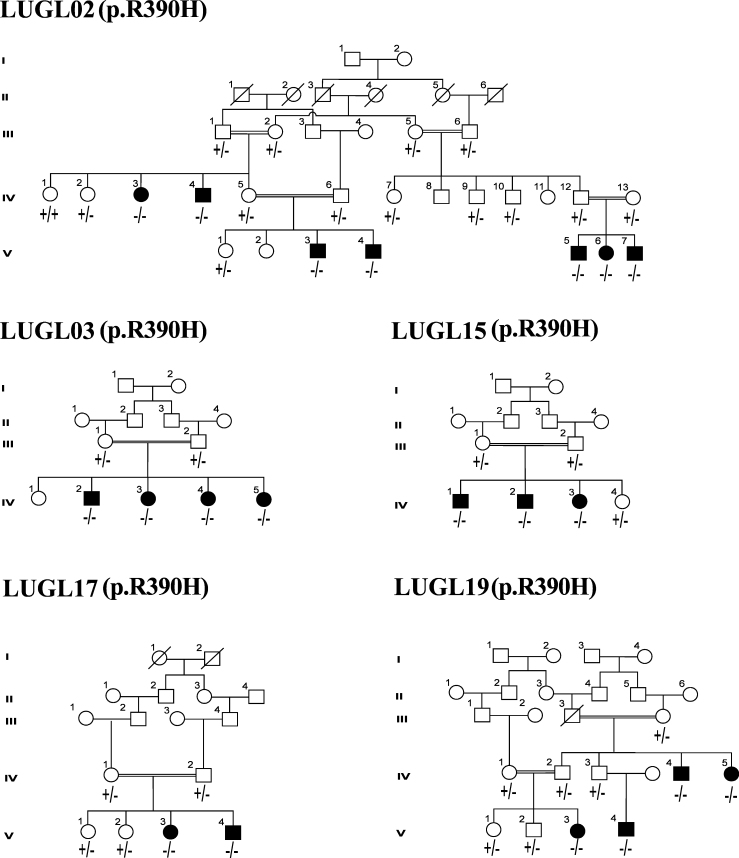
Figure 1.
Pedigrees mutated with p.R390H mutation. Male individuals are denoted by squares and females by circles. The filled squares and circles denote the affected individuals. The double line between individuals represents consanguineous marriage.
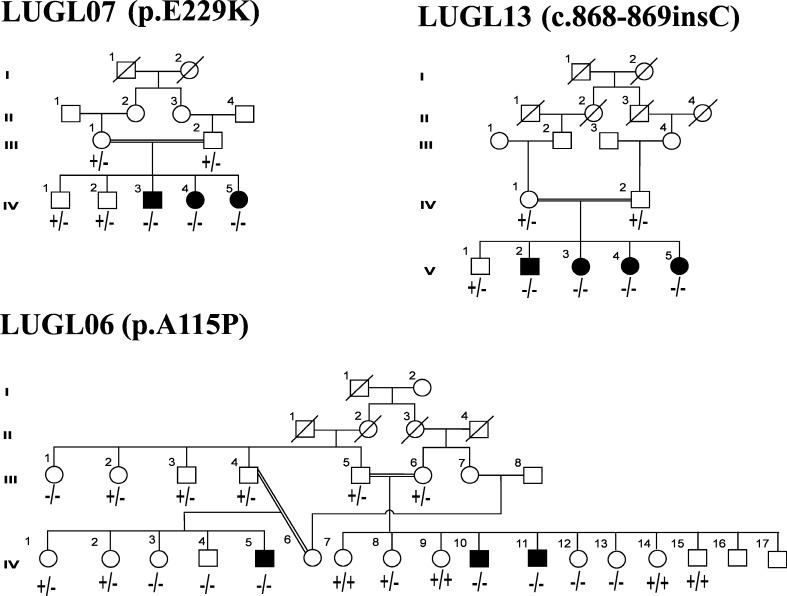
Figure 2.
Pedigrees with three reported mutations. Male individuals are denoted by squares and females by circles. The filled squares and circles denote the affected individuals. The double line between individuals represents consanguineous marriage.
Figure 3.
Pedigrees with novel mutations showing electropherograms with representative mutated, heterozygous carrier, and corresponding normal sequences. Male individuals are denoted by squares and females by circles. The filled squares and circles denote the affected individuals. The double line between individuals represents consanguineous marriage.
Linkage analysis and genotyping
Peripheral blood was collected in EDTA filled tubes and genomic DNA was extracted from leukocytes as described previously [16]. Four fluorescently labeled microsatellite markers, D2S1346, D2S177, D2S2163, and D2S2331, closely linked to the GLC3A locus, were genotyped for linkage analysis. PCR was performed for each marker in a 10 μl reaction mixture containing 50 ng DNA, 1 mM dNTPs, 0.4 U Taq polymerase, and 2 mM MgCl2. Amplification was performed on the GeneAmp PCR 2720 (Applied Biosystems, Foster City, CA) and analyzed on an ABI Prism 3130 Genetic Analyzer. Alleles were determined with Genotyper version 4 software (Applied Biosystems). Logarithm of the odds (LOD) scores were calculated by using the Easy Linkage 5.02 v graphical user interface. An autosomal recessive mode of inheritance with 100% penetrance and a disease allele frequency of 0.001 were used.
Mutation screening
Five overlapping pairs of sequencing primers for CYP1B1 were designed from the flanking sequence of each exon by using the Primer3 web tool. Amplification reaction was performed by using 50 ng DNA, 1 mM dNTPs, 1.5 pM primer, 0.3 U Taq polymerase, and 1.5 mM MgCl2 in the GeneAmp PCR 2720 (Applied Biosystems). PCR thermal conditions were optimized by denaturation at 95 °C for 5 min followed by 35 cycles, each 20 s at 95 °C, 45 s at 58 °C, and 10 s at 72 °C, and final extension 72 °C for 10 min. The amplified product was resolved on 2% agarose gel and purified with ethanol precipitation. The sequencing reaction was performed by using the Big Dye Terminator v3.1 (Applied Biosystems) as described previously [17]. Sequencing was performed on the ABI 3130 Genetic Analyzer, and the electropherograms were analyzed by using Chromas v 1.45.
Bioinformatics analysis
PolyPhen2 was used to predict pathogenic role of the novel substitution mutation [18]. The effect of the in-frame deletion on protein was predicted by using PROVIEN software [19] whereas CYP1B1 sequences of seven species were aligned with the help of the Clustal W tool to find the conservation status of the substituted and deleted amino acids. The biochemical properties of the wild-type and mutant amino acids and possible functional changes were studied by using the HOPE web tool [20].
Results
Twenty consanguineous families affected with congenital glaucoma who had more than one patient were enrolled from a tertiary care eye hospital in Hyderabad. Pedigree analysis suggested the autosomal recessive mode of inheritance. Linkage analysis showed homozygosity with CYP1B1-associated short tandem repeat (STR) markers in ten families (50%, 10/20). On direct sequencing of two coding exons of CYP1B1, six distinct homozygous mutations, segregating with disease phenotype, were found in all linked families. Among the six variants, three missense mutations, p. R390H, p.E229K, and p.A115P, and an insertion mutation, c.868_869insC, had previously been reported; while one missense variant, p.G36D, and a 12 bp in-frame deletion mutation, p.Gly67-Ala70del, were novel. The clinical features of all patients who have CYP1B1 mutations are summarized in Table 1.
Table 1. Clinical features of PCG patients with CYP1B1 mutations.
| Family ID (mutation) patient No | Current age |
Corneal diameter |
Corneal edema |
Corneal opacity |
Haabs’ Striae |
IOP (mmHg) |
Visual acuity |
C/D ratio |
Surgery | |
|---|---|---|---|---|---|---|---|---|---|---|
| Years | OS/OD (mm) | OS/OD | OS/OD | OS/OD | OS/OD | OS/OD | OS/OD | |||
| LUGL02 (p.R390H) |
||||||||||
| IV:4 |
25 |
NR/NR |
NR/NR |
++/++ |
−/− |
NR/15 |
NPL/NPL |
NR/NR |
OS Trab |
|
| V:3 |
5 |
14/11.5 |
-/NR |
-/++ |
−/− |
17/29 |
6/36/NR |
NR/0.3 |
OS Trab |
|
| V:4 |
6 |
14/14 |
−/− |
−/− |
−/− |
17/29 |
6/12;6/24 |
0.2/0.5 |
Bilateral Trab |
|
| V:5 |
1 |
15/14 |
+/NR |
-/++ |
+/NR |
17/40 |
NR/NR |
NR/NR |
OS Trab |
|
| V:6 |
1.5 |
14/14 |
+/NR |
-/++ |
−/− |
35/35 |
FF/NR |
0.4/NR |
OS Trab |
|
| V:7 |
2.5 |
13/11.5 |
NR/NR |
++/++ |
−/− |
40/40 |
NR/NR |
NR/NR |
Bilateral Trab |
|
| LUGL03 (p.R390H) |
||||||||||
| IV:2 |
2.5 |
13/11.5 |
NR/NR |
++/++ |
−/− |
40/40 |
NR/NR |
NR/NR |
Bilateral Trab |
|
| LUGL15 (p.R390H) |
||||||||||
| IV:1 |
16 |
16/15.5 |
NR/NR |
+/+ |
NR/NR |
30/35 |
NPL/NPL |
NR/NR |
Not Done |
|
| IV:2 |
7 |
NR/NR |
NR/NR |
++/++ |
NR/NR |
NR/NR |
NPL/NPL |
NR/NR |
Not Done |
|
| IV:3 |
19 |
15/15 |
+/+ |
+/+ |
+/+ |
18/20 |
6/36;6/36 |
0.7/0.8 |
Bilateral Trab |
|
| LUGL17 (p.R390H) |
||||||||||
| V:4 |
11 |
10.3/10.4 |
−/− |
−/− |
−/− |
14/17 |
6/24;6/18 |
0.8/0.9 |
Bilateral Trab |
|
| LUGL19 (p.R390H) |
||||||||||
| IV:4 |
18 |
NR/NR |
NR/NR |
NR/NR |
++/++ |
40/35 |
NPL/NPL |
NR/NR |
Not Done |
|
| IV:5 |
33 |
NR/NR |
NR/NR |
NR/NR |
NR/NR |
NR/NR |
NR/NR |
NR/NR |
Bilateral |
|
| V:3 |
7 |
15/15 |
++/++ |
−/− |
−/− |
38/32 |
HM/HM |
NR/NR |
Bilateral Trab |
|
| V:4 |
2 |
15/12 |
++/++ |
−/− |
−/− |
40/35 |
FF/FF |
NR/NR |
Bilateral Trab |
|
| LUGL06 (p.A115P) |
||||||||||
| IV:5 |
2.5 |
15.5/15 |
+/+ |
+/+ |
−/− |
16/25 |
FF/FF |
0.7/0.5 |
OS Trab |
|
| IV:10 |
30 |
15.5/15 |
NR/+ |
+/++ |
NR/NR |
40/16 |
NPL/CF |
TC/0.2 |
OD Trab |
|
| IV:11 |
16 |
15.5/15 |
+/NR |
+/++ |
+/NR |
24/40 |
NPL/NPL |
NR/NR |
OS Trab |
|
| LUGL07 (p.E229K) |
||||||||||
| IV:3 |
19 |
15/15 |
NR/NR |
++/++ |
NR/NR |
41.5/NR |
NPL/NPL |
NR/NR |
OS Trab |
|
| IV:4 |
20 |
15/15 |
NR/NR |
++/++ |
NR/NR |
26.6/24 |
PL/PL |
NR/NR |
Bilateral Trab |
|
| IV:5 |
16 |
15/15 |
NR/NR |
++/++ |
NR/NR |
24.4/NR |
NPL /NPL |
NR/NR |
OD Trab |
|
| LUGL08 (p.G36D) |
||||||||||
| 4 |
16/11 |
++/++ |
++/++ |
+/NR |
35/29 |
NPL/FF |
NR/NR |
Bilateral Trab |
||
| IV:3 |
3 |
41,980 |
++/NR |
++/++ |
NR/NR |
29/5 |
FF/NPL |
NR/NR |
OS Trab |
|
| IV:5 |
23 |
NR |
NR |
++/++ |
NR/NR |
NR/NR |
NPL/NPL |
NR/NR |
Not Done |
|
| IV:6 |
38 |
16/15 |
+/+ |
−/− |
−/− |
29/35 |
NPL/NPL |
TC/TC |
Not Done |
|
| LUGL09 (p.G67-A70del) |
||||||||||
| 2 |
41,987 |
+/++ |
+/−- |
−/− |
40/40 |
FF/NPL |
0.4/TC |
|||
| IV:3 |
11 |
14/11.5 |
−/− |
–/– |
−/− |
17/19 |
6/12;6/24 |
0.5/0.6 |
Bilateral Trab |
|
| LUGL13 (c.868_869InsC) |
||||||||||
| 32 |
NR/NR |
-/++ |
-/++ |
−/− |
41,922 |
NPL/NPL |
NR/NR |
OS Trab |
||
| V:3 |
17 |
41,986 |
+/+ |
+/+ |
-/+ |
41,988 |
1/60;1/60 |
0.9/0.9 |
Bilateral Trab |
|
| V:4 |
11 |
41,986 |
++/− |
+/− |
−/− |
41,933 |
NPL/6/12 |
NR/0.7 |
Not Done |
|
| V:5 | 19 | 16/NR | ++/++ | +/+ | NR/NR | 42/10 | NPL/NPL | NR/NR | Not Done | |
NPL: No Perception of Light. PL: Perception of Light. FF: Fixation and Follow. HM: Hand Movement TC: Total Cupping. NR: Not Recordable. IOP: Intraocular Pressure
p.R390H, the predominant mutation, was found in 50% (5/10) of the families with CYP1B1-linked PCG, totaling 18 patients (Figure 1). All patients with the p.R390H mutation showed bilateral congenital glaucoma with variable severity and clinical features (Table 1). The maximum bilateral elevated IOP, 40 mmHg, was recorded in the patients of families LUGL02 and LUGL19. All patients had enlarged cornea with maximum diameter up to 15 mm except a few physical eyes. Congenital bilateral corneal opacity was observed in the patients of families LUGL02 and LUGL15. Three patients in family LUGL02, V:3, V:5, and V:6, who underwent early left trabeculectomy, showed corneal opacity in the right eye only. The cornea was clear in the patients of families LUGL03, LUGL17, and LUGL19. Two patients in families LUGL15 and LUGL17 showed restored visual acuity after undergoing bilateral trabeculectomy.
p.A115P was found in the homozygous state in three patients of family LUGL06. The patients were affected with congenital glaucoma with maximum IOP of 40 mmHg. The age of the patients ranged from 2.5 to 30 years. One patient, IV:11, had no perception of light, while patient IV:10 had reduced visual acuity up to counting of fingers. The patients underwent left and right eye trabeculectomy, respectively (Table 1). Five clinically normal individuals, III:1, IV:3, IV:4, IV:12, and IV:13, in two generations of family LUGL06 were also homozygous for p.A115P (Figure 2 and Table 2). Bioinformatics tools predicted this substitution was pathogenic and was not observed in 120 control chromosomes.
Table 2. Clinical characteristics of normal individuals of LUGL06 homozygous for p.A115P.
| Family ID (Mutation) Patient No | Disease status | Current age | Corneal diameter |
Corneal edema |
Corneal opacity |
Habbs’ Striae |
IOP (mmHg) |
Visual acuity |
C/D ratio |
|---|---|---|---|---|---|---|---|---|---|
| OS/OD (mm) | OS/OD | OS/OD | OS/OD | OS/OD | OS/OD | ||||
| LUGL06 (p.A115P) | |||||||||
| III:1 |
Normal |
25 |
11/10 |
−/− |
−/− |
−/− |
12/14 |
6/6 |
0.2/0.3 |
| IV:3 |
Normal |
32 |
11/10.5 |
−/− |
−/− |
−/− |
13/12 |
6/6 |
0.2/0.2 |
| IV:4 |
Normal |
20 |
12/11 |
−/− |
−/− |
−/− |
14/13 |
6/6 |
0.3/0.2 |
| IV:12 |
Normal |
29 |
12/12 |
−/− |
−/− |
−/− |
14/14 |
6/6 |
0.3/0.2 |
| IV13 | Normal | 40 | 11/11.5 | −/− | −/− | −/− | 12/12 | 6/6 | 0.3/0.3 |
Homozygous p.E229K segregated with disease phenotype in three patients of family LUGL07 (Figure 2). Two patients, IV:3 and IV:4, age 16 and 19 years currently, respectively, exhibited no perception of light in both eyes, while the third patient, IV:5, at the age of 20 years had reduced visual acuity to the perception of light and underwent bilateral trabeculectomy. The maximum IOP recorded was 41.5 mmHg, and all patients had severe corneal opacity (Table 1).
Affected members of family LUGL13 were homozygous for an insertion c.868_869insC. The mutation segregated with disease phenotype in all patients (Figure 2). The maximum IOP, 42 mmHg, was recorded in the left eye of patient V:5. Normal corneal diameter was observed bilaterally in patients V:3 and V:4 while patient V:4 had megalocornea with the diameter measuring 16 mm in the left eye. The presence of corneal opacity was not consistent in all patients; two patients, V:3 and V:5, had bilateral corneal opacity, whereas V:2 and V:4 had corneal opacity only in the right and left eye, respectively. Patients V:3 and V:4, however, exhibited visual acuity up to 1/60 and 6/12 (Table 1).
Two novel alleles were identified in families LUGL09 and LUGL08 (Figure 3). The affected members of LUGL09 were diagnosed with bilateral congenital glaucoma, with maximum IOP, 40 mmHg, recorded in patient IV:01. The patient di not undergo surgical intervention, and she had a C/D ratio of 0.4 in the left eye (Table 1). Visual acuity was recorded to fixation and follow in the left eye. Patient IV:3 underwent bilateral trabeculectomy and had normal intraocular pressure. CYP1B1 sequencing in both patients revealed a homozygous deletion of 12 bp, GGGCCAGGCGGC, c.198–209del 12, resulting in deletion of four amino acids, p.G67-A70del; glycine at 67, glutamine at 68, and alanine at 69 and 70 from the CYP1B1 protein (Figure 3 and Figure 4). Both parents and one normal sibling in the family were heterozygous.
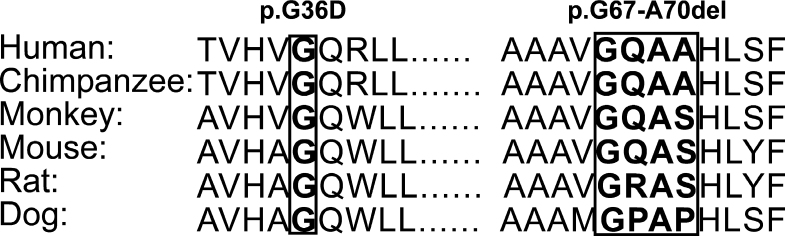
Figure 4.
Multiple sequence alignment of CYP1B1 proteins from various species. Boxed amino acids are conserved in all species and cause primary congenital glaucoma (PCG) when substituted or deleted.
The family LUGL08 has four affected members diagnosed with bilateral congenital glaucoma. The maximum IOP recorded was 35 mmHg in patient IV:01. The current ages of the patients ranged from 3 to 35 years (Table 1). The affected individuals revealed bilateral corneal opacity except patient IV:6. Visual acuity in patients IV:1 and IV:2 was from fixation and follow to no perception of light; patients IV:5 and IV:6, however, had no perception of light (Table 1). On sequencing a novel missense mutation, substituting glycine at the 107th nucleotide to aspartic acid, resulting in the replacement of glycine 36 with aspartic acid was identified (Figure 3). All patients were homozygous while six normal parents and three siblings were heterozygous. Both variants were predicted to be pathogenic by bioinformatics tools and were not found in the 120 ethnically matched control chromosomes.
Six previously reported SNPs, rs2617266, rs10012, rs1056827, rs1056836, rs1056837, and rs1800440, were identified in this cohort. These SNPs were used to generate haplotypes for the CYP1B1 mutations, and three different haplotypes were obtained in these patients. Five families harboring the p.R390H mutation and a novel mutation p.G36D shared the same haplotype, C-C-G-C-C-G. Two families harboring p.A115P and c.868_869insC mutations had the same haplotype, C-C-G-G-T-A; the remaining two families with p.E229K and novel deletion p.Gly67-Ala70del shared the same haplotype, T-G-T-C-C-A (Table 3).
Table 3. Haplotypes associated with CYP1B1 mutations in Pakistani PCG patients.
| Family | Mutation | rs2617266 | rs10012 | rs1056827 | rs1056836 | rs1056837 | rs1800440 |
|---|---|---|---|---|---|---|---|
| LUGL02 |
p.R390H |
C |
C |
G |
C |
C |
G |
| LUGL03 |
p.R390H |
C |
C |
G |
C |
C |
G |
| LUGL15 |
p.R390H |
C |
C |
G |
C |
C |
G |
| LUGL17 |
p.R390H |
C |
C |
G |
C |
C |
G |
| LUGL19 |
p.R390H |
C |
C |
G |
C |
C |
G |
| LUGL06 |
p.A115P |
C |
C |
G |
G |
T |
A |
| LUGL07 |
p.E229K |
T |
G |
T |
C |
C |
A |
| LUGL08 |
p.G67-A70del |
T |
G |
T |
C |
C |
A |
| LUGL09 |
p.G36D |
C |
C |
G |
C |
C |
G |
| LUGL13 | c.868_869insC | C | C | G | G | T | A |
Discussion
Primary congenital glaucoma is a rare form of glaucoma leading to irreversible blindness and follows a recessive mode of inheritance [21]. CYP1B1 is major contributing gene for PCG, and its prevalence ranges from 70% to 100% in consanguineous and inbred populations [22]. In this study, we screened 20 consanguineous Pakistani families affected with congenital glaucoma and found CYP1B1 mutations segregating with disease phenotype in 50% (10/20) of the families, which is comparable with the prevalence of CYP1B1 mutations (44%) in the Indian population and is higher than Chinese (17.2%) [23] but lower than Iranians (70%) [24].
The p.R390H mutation was detected first in a patient of Pakistani origin [8]. Since then, this mutation has been identified as the second most common mutation in Indian and Iranian patients affected with congenital glaucoma, accounting for 16% and 19.2%, respectively [24,25]. This study revealed p.R390H was the most common mutation in the population accounting for 50% (5/10) of the CYP1B1 alleles and 25% of (5/20) of the families with PCG. This is the highest frequency of p.R390H reported thus far. All patients who harbor the p.R390H mutation were homozygous and showed variable interfamilial and intrafamilial disease severity, clinical features, and surgical success rates (Table1). Patient V:7 in family LUGL02 age 2.5 years showed uncontrollable IOP and severe corneal opacity despite undergoing bilateral trabeculectomy, while his sibling age 6 years exhibited clear cornea, controlled IOP, and a lower C/D ratio after bilateral trabeculectomy (Table 1). Surgical intervention was successful in patients LUGL15:VI:3 and LUGL17:V:4 but failed to control the IOP in patients in family LUGL19. p.R390H has been reported to show variable phenotypes in different populations. Homozygous p.R390H has been found to segregate with juvenile open angle glaucoma (JOAG) in Taiwanese patients [21]. These variable clinical manifestations of the mutation among ethnically similar and different patients may be attributed to unknown genetic and environmental factors.
Homozygous p.A115P segregated with the PCG phenotype in three affected individuals of family LUGL06. Previously, it has been reported in a patient of Indian origin with non-familial PCG [26]. This mutation is near the heme-binding region of the CYP1B1 protein and may cause conformational changes and disturb hydrogen bonding interactions of the protein [27]. Bioinformatics tools predicted this substitution was probably damaging with deleterious effects on protein function. In this study, five clinically normal individuals in family LUGL06 were also found to be homozygous for p.A115P (Figure 2 and Table 2). This could be due to either non-penetrance or variable expression of CYP1B1 mutations, which has been described in glaucoma patients in Saudi Arabia and Iran [13,14]. Previously, there has been no report of non-penetrance and variable expressivity for p.A115P. The ages of the five unaffected individuals with homozygous p.A115P ranged from 20 to 40 years; thus, variable expression of p.A115P with late disease onset may not be excluded. These individuals may require ophthalmological follow-up to detect early disease symptoms. Non-penetrance or variable expressivity of p.A115P in five unaffected individuals suggests the presence of a modifier locus that may be responsible for suppressing the disease phenotype.
In earlier studies, homozygous p.E229K has been reported segregating with PCG in various populations including Pakistani population [15]. This study found three patients were homozygous for p.E229K in family LUGL07 with severe phenotype. All patients had uncontrollable IOP and bilateral corneal opacity with no perception of light. Surgical intervention was not successful in these patients. Heterozygous p.E229K has also been reported to segregate in patients with PCG and late onset primary open angle glaucoma [14,28,29]. In this study, all the carriers of p.E229K were phenotypically normal. This severe phenotype of p.E229K homozygous mutants and clinically normal carriers is in agreement with previously reported findings from Pakistan [15].
No frameshift mutation in CYP1B1, segregating with PCG has been reported from Pakistan before this study. The present study revealed an insertion of cytosine at c.868_869insC, causing frameshift and truncation of the protein (p.R290fs*37) in four affected members of family LUGL13. This was one of the first mutations reported in CYP1B1 in two American families [8]. The detailed phenotype of this mutation has not been described thus far. The patients who were homozygous for c.868_869ins C showed less severe phenotype than the individuals affected with other mutations reported in this study. Patient V:3 had controlled IOP and visual acuity of 1/60 in both eyes after bilateral trabeculectomy. Patient V:4 did not undergo any surgical intervention and had bilaterally normal IOP and 6/12 visual acuity in the right eye (Table 1).
A novel missense mutation, c.107 G to A, substituting glycine at the 36th position with aspartic acid, segregated with disease in family LUGL08. The structure of the first 49 residues from the NH2 terminal end of the CYP1B1 protein has not been determined [25]. Glycine is conserved in the CYP1B1 protein among different species (Figure 4). The wild-type residue is located in the predicted trans-membrane domain and differs in size and charge from the mutant residue. This substitution may affect the hydrophobic interactions, conformation, and functions of the protein [20]. The PolyPhen web tool predicted this substitution was probably damaging with a score of −1. All patients in family LUGL08 have bilateral corneal opacity and uncontrollable IOP after surgical interventions.
The second novel allele was a deletion of 12 bp, GGGCCAGGCGGC, c.198–209del12, found in family LUGL09. This mutation resulted in the deletion of four amino acids, p.G67-A70del (Figure 3). Glycine at 67 and alanine at 69 are conserved among CYP1B1 proteins of different species (Figure 4). The PROVIEN web tool was used to predict the possible effect of this deletion. The mutation, with score below −2.5, is pathogenic. This in-frame deletion scored −15.8 and was predicted to be deleterious [19]. Previously, a heterozygous frameshift mutation, which deleted eight nucleotides, c.199–206delGGCCAGGC, was reported in German patients with PCG [30]. Patient LUGL09:IV:3, homozygous for this deletion, showed controlled IOP and maintained visual acuity after bilateral trabeculectomy.
Six SNPs, rs2617266, rs10012, rs1056827, rs1056836, rs1056837, and rs1800440, were extensively used to construct haplotypes of the CYP1B1 mutations in ethnically different populations [31,32]. The most common haplotype is C-C-G-G-T-A, which is associated with 50% of CYP1B1 mutations whereas T-G-T-C-C-A and C-C-G-C-C-G are associated with 9.7% and 7% of CYP1B1 mutations, respectively [15]. In this study, the most common haplotype was C-C-G-C-C-G, which was found in six families who harbored p.R390H and p.G36D mutations (Table 3). p.R390H has been reported in the same haplotype background in Indian patients with PCG; although it has been associated with three haplotypes in Iranian patients, including C-C-G-C-C-G [24,32,33]. This indicates that Pakistani and Indian patients with PCG have a common founder for p.R390H; whereas p.R390H may have multiple origins in Iranian patients with PCG. Previously, C-C-G-C-C-G haplotype has been associated with a frameshift mutation in American and Brazilian patients [34]. p.A115P and c.868_869insC were associated with C-C-G-G-T-A, a haplotype frequently found in other populations. Novel deletions p.G67-A70del and p.E229K were associated with T-G-T-C-C-A. p.E229K has been associated with the same haplotype in ethnically different populations [15]
The results of the study reflect that CYP1B1 mutations are the predominant cause of primary congenital glaucoma in Pakistani patients. Identification of p.R390H as a frequent mutation on distinct haplotype may help to adopt economic genetic testing services for patients with PCG, and the genotype-phenotype correlation will help and assist in better prognosis for the disease. Non-penetrance shown by p.A115P requires further study to identify factors that may be responsible for suppressing the disease phenotype. Identification of the novel variants indicates the genetic heterogeneity of our population and may lead to better understanding of the disease mechanism.
Acknowledgments
The authors are thankful to all the family members for their participation in the study. We are thankful to Dr. Muhammad Yaqoob Shahani and Mr.Ameer Ali Bohiyo for their assistance in enrollment of families. The study was supported by Pakistan Science Foundation Grant No: LUMHS/PSF/Biotech/101
References
- 1.Sarfarazi M, Stoilov I. Molecular genetics of primary congenital glaucoma. Eye (Lond) 2000;14(Pt 3B):422–8. doi: 10.1038/eye.2000.126. [DOI] [PubMed] [Google Scholar]
- 2.Kim HJ, Suh W, Park SC, Kim CY, Park KH, Kook MS, Kim YY, Kim CS, Park CK, Ki CS, Kee C. Mutation spectrum of CYP1B1 and MYOC genes in Korean patients with primary congenital glaucoma. Mol Vis. 2011;17:2093–101. [PMC free article] [PubMed] [Google Scholar]
- 3.Dandona L, Williams JD, Williams BC, Rao GN. Population-based assessment of childhood blindness in southern India. Arch Ophthalmol. 1998;116:545–6. [PubMed] [Google Scholar]
- 4.Tanwar M, Dada T, Sihota R, Dada R. Identification of four novel cytochrome P4501B1 mutations (p.I94X, p.H279D, p.Q340H, and p.K433K) in primary congenital glaucoma patients. Mol Vis. 2009;15:2926–37. [PMC free article] [PubMed] [Google Scholar]
- 5.Sarfarazi M, Akarsu AN, Hossain A, Turacli ME, Aktan SG, Barsoum-Homsy M, Chevrette L, Sayli BS. Assignment of a locus (GLC3A) for primary congenital glaucoma (Buphthalmos) to 2p21 and evidence for genetic heterogeneity. Genomics. 1995;30:171–7. doi: 10.1006/geno.1995.9888. [DOI] [PubMed] [Google Scholar]
- 6.Akarsu AN, Turacli ME, Aktan SG, Barsoum-Homsy M, Chevrette L, Sayli BS, Sarfarazi M. A second locus (GLC3B) for primary congenital glaucoma (Buphthalmos) maps to the 1p36 region. Hum Mol Genet. 1996;5:1199–203. doi: 10.1093/hmg/5.8.1199. [DOI] [PubMed] [Google Scholar]
- 7.Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA, Hejtmancik JF, Khan SN, Firasat S, Shires M, Gilmour DF, Towns K, Murphy AL, Azmanov D, Tournev I, Cherninkova S, Jafri H, Raashid Y, Toomes C, Craig J, Mackey DA, Kalaydjieva L, Riazuddin S, Inglehearn CF. Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet. 2009;84:664–71. doi: 10.1016/j.ajhg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoilov I, Akarsu AN, Alozie I, Child A, Barsoum-Homsy M, Turacli ME, Or M, Lewis RA, Ozdemir N, Brice G, Aktan SG, Chevrette L, Coca-Prados M, Sarfarazi M. Sequence analysis and homology modeling suggest that primary congenital glaucoma on 2p21 results from mutations disrupting either the hinge region or the conserved core structures of cytochrome P4501B1. Am J Hum Genet. 1998;62:573–84. doi: 10.1086/301764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang M, Guo X, Liu X, Shen H, Jia X, Xiao X, Li S, Fang S, Zhang Q. Investigation of CYP1B1 mutations in Chinese patients with primary congenital glaucoma. Mol Vis. 2009;15:432–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014;133:1–9. doi: 10.1007/s00439-013-1358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N, Zhou Y, Du L, Wei M, Chen X. Overview of Cytochrome P450 1B1 gene mutations in patients with primary congenital glaucoma. Exp Eye Res. 2011;93:572–9. doi: 10.1016/j.exer.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Kaur K, Mandal AK, Chakrabarti S. Primary Congenital Glaucoma and the Involvement of CYP1B1. Middle East African Journal of Ophthalmology. 2011;18:7–16. doi: 10.4103/0974-9233.75878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bejjani BA, Stockton DW, Lewis RA, Tomey KF, Dueker DK, Jabak M, Astle WF, Lupski JR. Multiple CYP1B1 mutations and incomplete penetrance in an inbred population segregating primary congenital glaucoma suggest frequent de novo events and a dominant modifier locus. Hum Mol Genet. 2000;9:367–74. doi: 10.1093/hmg/9.3.367. [DOI] [PubMed] [Google Scholar]
- 14.Suri F, Yazdani S, Narooie-Nejhad M, Zargar SJ, Paylakhi SH, Zeinali S, Pakravan M, Elahi E. Variable expressivity and high penetrance of CYP1B1 mutations associated with primary congenital glaucoma. Ophthalmology. 2009;116:2101–9. doi: 10.1016/j.ophtha.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 15.Firasat S, Riazuddin SA, Khan SN, Riazuddin S. Novel CYP1B1 mutations in consanguineous Pakistani families with primary congenital glaucoma. Mol Vis. 2008;14:2002–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Grimberg J, Nawoschik S, Belluscio L, McKee R, Turck A, Eisenberg A. A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res. 1989;17:8390. doi: 10.1093/nar/17.20.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waryah AM, Narsani AK, Sheikh SA, Shaikh H, Shahani MY. The novel heterozygous Thr377Arg MYOC mutation causes severe Juvenile Open Angle Glaucoma in a large Pakistani family. Gene. 2013;528:356–9. doi: 10.1016/j.gene.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. Predicting the functional effect of amino acid substitutions and indels. PLoS ONE. 2012;7:e46688. doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venselaar H, Te Beek TA, Kuipers RK, Hekkelman ML, Vriend G. Protein structure analysis of mutations causing inheritable diseases. An e-Science approach with life scientist friendly interfaces. BMC Bioinformatics. 2010;11:548. doi: 10.1186/1471-2105-11-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su CC, Liu YF, Li SY, Yang JJ, Yen YC. Mutations in the CYP1B1 gene may contribute to juvenile-onset open-angle glaucoma. Eye (Lond) 2012;26:1369–77. doi: 10.1038/eye.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu-Amero KK, Osman EA, Mousa A, Wheeler J, Whigham B, Allingham RR, Hauser MA, Al-Obeidan SA. Screening of CYP1B1 and LTBP2 genes in Saudi families with primary congenital glaucoma: genotype-phenotype correlation. Mol Vis. 2011;17:2911–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Jiang D, Yu L, Katz B, Zhang K, Wan B, Sun X. CYP1B1 and MYOC mutations in 116 Chinese patients with primary congenital glaucoma. Arch Ophthalmol. 2008;126:1443–7. doi: 10.1001/archopht.126.10.1443. [DOI] [PubMed] [Google Scholar]
- 24.Chitsazian F, Tusi BK, Elahi E, Saroei HA, Sanati MH, Yazdani S, Pakravan M, Nilforooshan N, Eslami Y, Mehrjerdi MA, Zareei R, Jabbarvand M, Abdolahi A, Lasheyee AR, Etemadi A, Bayat B, Sadeghi M, Banoei MM, Ghafarzadeh B, Rohani MR, Rismanchian A, Thorstenson Y, Sarfarazi M. CYP1B1 mutation profile of Iranian primary congenital glaucoma patients and associated haplotypes. The Journal of molecular diagnostics. JMD. 2007;9:382–93. doi: 10.2353/jmoldx.2007.060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanwar M, Dada T, Sihota R, Das TK, Yadav U, Dada R. Mutation spectrum of CYP1B1 in North Indian congenital glaucoma patients. Mol Vis. 2009;15:1200–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy AB, Kaur K, Mandal AK, Panicker SG, Thomas R, Hasnain SE, Balasubramanian D, Chakrabarti S. Mutation spectrum of the CYP1B1 gene in Indian primary congenital glaucoma patients. Mol Vis. 2004;10:696–702. [PubMed] [Google Scholar]
- 27.Achary MS, Reddy AB, Chakrabarti S, Panicker SG, Mandal AK, Ahmed N, Balasubramanian D, Hasnain SE, Nagarajaram HA. Disease-causing mutations in proteins: structural analysis of the CYP1B1 mutations causing primary congenital glaucoma in humans. Biophys J. 2006;91:4329–39. doi: 10.1529/biophysj.106.085498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melki R, Colomb E, Lefort N, Brezin AP, Garchon HJ. CYP1B1 mutations in French patients with early-onset primary open-angle glaucoma. J Med Genet. 2004;41:647–51. doi: 10.1136/jmg.2004.020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López-Garrido MP, Sanchez-Sanchez F, Lopez-Martinez F, Aroca-Aguilar JD, Blanco-Marchite C, Coca-Prados M, Escribano J. Heterozygous CYP1B1 gene mutations in Spanish patients with primary open-angle glaucoma. Mol Vis. 2006;12:748–55. [PubMed] [Google Scholar]
- 30.Weisschuh N, Wolf C, Wissinger B, Gramer E. A clinical and molecular genetic study of German patients with primary congenital glaucoma. Am J Ophthalmol. 2009;147:744–53. doi: 10.1016/j.ajo.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Bagiyeva S, Marfany G, Gonzalez-Angulo O, Gonzalez-Duarte R. Mutational screening of CYP1B1 in Turkish PCG families and functional analyses of newly detected mutations. Mol Vis. 2007;13:1458–68. [PubMed] [Google Scholar]
- 32.Chakrabarti S, Kaur K, Kaur I, Mandal AK, Parikh RS, Thomas R, Majumder PP. Globally, CYP1B1 mutations in primary congenital glaucoma are strongly structured by geographic and haplotype backgrounds. Invest Ophthalmol Vis Sci. 2006;47:43–7. doi: 10.1167/iovs.05-0912. [DOI] [PubMed] [Google Scholar]
- 33.Suri F, Kalhor R, Zargar SJ, Nilforooshan N, Yazdani S, Nezari H, Paylakhi SH, Narooie-Nejhad M, Bayat B, Sedaghati T, Ahmadian A, Elahi E. Screening of common CYP1B1 mutations in Iranian POAG patients using a microarray-based PrASE protocol. Mol Vis. 2008;14:2349–56. [PMC free article] [PubMed] [Google Scholar]
- 34.Sena DF, Finzi S, Rodgers K, Del Bono E, Haines JL, Wiggs JL. Founder mutations of CYP1B1 gene in patients with congenital glaucoma from the United States and Brazil. J Med Genet. 2004;41:e6. doi: 10.1136/jmg.2003.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]