Abstract
Background
Extensive evidence has accumulated regarding the role of mesenchymal stromal cells (MSCs) in tumor progression, but the exact effects and mechanisms underlying this role remain unclear. We investigated the effects of MSC-associated tumor progression in MSC-sarcoma models and a gastric cancer metastatic model.
Methods
We conducted an in vitro growth kinetics assay and an in vivo tumor progression assay for sarcoma cells and gastric cancer cells in the presence or absence of MSCs.
Results
MSC-cocultured human fibrosarcoma cells (HT1080) showed accelerated growth compared with HT1080 alone (79- vs 37-fold change, p<.050). For HT1080, human MSC-coinjected tumors showed significantly greater and highly infiltrative growth compared to those of HT1080 alone (p=.035). For mouse fibrosarcoma cells (WEHI164), mouse MSC-coinjected tumors had greater volume than those of WEHI164 alone (p=.141). For rat sarcoma cells (RR1022), rat MSC-coinjected tumors exhibited greater volume and infiltrative growth than those of RR1022 alone (p=.050). For human gastric cancer cells (5FU), tumors of 5FU alone were compact, nodular in shape, and expansile with good demarcation and no definite lung metastatic nodules, whereas tumors grown in the presence of human MSCs showed highly desmoplastic and infiltrative growth and multiple lung metastasis.
Conclusions
We observed morphological evidence for MSC-associated tumor progression of fibrosarcomas and gastric cancer cells.
Keywords: Mesenchymal stromal cells, Tumor progression, Fibrosarcoma, Gastric cancer cells
Mesenchymal stromal cells (MSCs) and their derivatives localize to tumor sites and are major cellular compartments of tumor microenvironments.1,2 Despite extensive studies, the effects and mechanisms of MSCs in tumor invasion and metastasis are not fully understood. Many studies have shown that MSCs promote tumor progression and metastasis, while other studies have reported that MSCs suppress tumor growth.3,4,5,6,7 This discrepancy might be associated with variation in experimental conditions, including cancer cell types, MSC resources, injected cell numbers/timing, host animal models, and other factors.3
Most studies of MSC-cancer interactions have focused on epithelial malignancies, including breast cancer,4,6,7,8 while few studies of MSC-sarcoma interactions are found in the literature.9,10 Tsukamoto et al.9 reported MSC-associated tumor progression in a rat osteosarcoma model, and Xu et al.10 described the effects of human MSCs (hMSCs) on human osteosarcoma progression. Osteosarcoma, defined as malignant neoplasm showing evidence of malignant bone formation, is very heterogeneous in terms of differentiation, genetic profiles, and clinical behavior.11,12 Fibrosarcoma is the least differentiated type of mesenchymal malignancy and is defined as a spindle cell malignant neoplasm lacking any line of specific differentiation,13 which implies that it has the least heterogeneous characteristics among the sarcomas.
The purpose of the current study was to investigate the effects of MSCs on the growth and progression of fibrosarcomas. We observed morphological evidence for MSC-associated tumor progression in human, mouse, and rat sarcoma models, and in a gastric cancer cell metastatic model.
MATERIALS AND METHODS
This is an institutional review board-approved study (CUMC-2012-0026-02 and KC10SNSI0689).
Cell lines and cell culture
A human fibrosarcoma cell line (HT1080), mouse fibrosarcoma cell line (WEHI164), and rat Schmidt-Ruppin sarcoma cell line (RR1022) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The human gastric cancer cell line SNU-620-5FU/1000 (5FU) was obtained from the Korean Cell Line Bank (Seoul, Korea). The hMSCs used in this study were human bone marrow-derived MSCs (Catholic MASTER Cells) obtained from the Catholic Institute of Cell Therapy (CIC, Seoul, Korea). Mouse MSCs (mMSCs) were isolated from C57BL/6 mice, and rat MSCs (rMSCs) were isolated from Sprague-Dawley rats, as described previously.14 In brief, femora and tibiae were harvested and carefully cleaned of adherent soft tissue, the epiphyses were removed, and the bone was flushed with phosphate buffered saline (PBS; Welgene, Daegu, Korea). Bones were dissected into fragments of 1-3 mm3 and digested in Dulbecco's modified Eagle's medium (DMEM; Welgene) supplemented with collagenase type I and dispase (Gibco, Grand Island, NY, USA) for 1-2 hours in an incubator. The bone fragments were washed thoroughly with PBS and then cultivated in a 100-mm dish in DMEM supplemented with 10% fetal bovine serum (FBS; Welgene), 100 IU/mL penicillin, and 100 µg/mL streptomycin (Gibco) at 37℃ in a 5% CO2 humidified incubator. Non-adherent cells were removed after 72-96 hours, and when the adherent cells reached 90% confluence, they were harvested with trypsin/EDTA (Gibco) and replated. MSCs, passages 5-9, were used after characterization by fluorescence activated cell sorting analysis (data not shown). HT1080 was maintained in DMEM, and WEHI164 and RR1022 were maintained in RPMI1640 with supplements of 10% vol/vol FBS, 2 mM L-glutamine, 100 IU/mL penicillin, and 100 µg/mL streptomycin. We cultivated hMSCs, mMSCs, and rMSCs in DMEM containing 10% vol/vol FBS, 2 mM L-glutamine, 100 IU/mL penicillin, and 100 µg/mL streptomycin. All cells were incubated at 37℃ in a humidified atmosphere containing 5% CO2.
Animals and immunosuppression
All experiments using mice and rats were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the Catholic University (Seoul, Korea). Nonobese diabetic/severe combined immunodeficient (NOD/SCID)/IL-2Rγ-/- (NOG/SCID) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Female Sprague-Dawley rats and C57BL/6 mice were purchased from Orient Bio (Seongnam, Korea). Animals were housed under pathogen-free conditions and were given autoclaved food and water.
To establish immunosuppression in the Sprague-Dawley rats and C57BL/6 mice, FK-506 (tacrolimus) and dexamethasone (Cayman Chemical, Ann Arbor, MI, USA) were dissolved in saline at a concentration of 1 mg/mL and sterilized with a 0.22-µm filter (Millipore Co., Billerica, MA, USA). Both immunosuppressants were intraperitoneally administered 1 mg/kg/day each for 3 days before cell injection.
In vitro growth kinetics assay
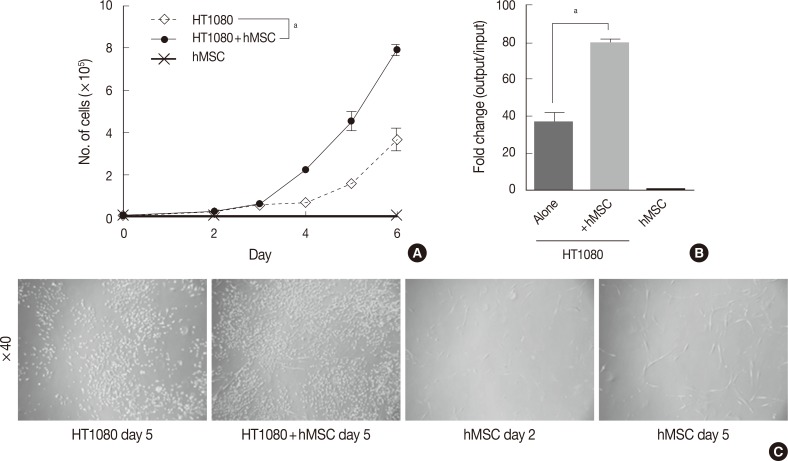
The in vitro growth kinetics assay was performed in triplicate in 6-well cell culture plates (Falcon, BD Bioscience, San Jose, CA, USA). For the co-culture assay, hMSCs were irradiated with 40 Gy for growth inhibition. HT1080 alone, hMSCs alone, and HT1080 co-cultured hMSCs were cultured at a starting cell number of 1.0×104 cells each. The proliferation status (i.e., cell number) was evaluated with a LUNA automated cell counter (Logos Biosystems, Annandale, VA, USA) on days 2-6 (Fig. 1).
Fig. 1.
Effects of mesenchymal stromal cells (MSC) on in vitro proliferation of human fibrosarcoma cells (HT1080). (A) In vitro growth kinetics of HT1080 cells in the presence of human-MSC (hMSC) are significantly enhanced compared to cells grown in the absence of hMSC. (B) The change is about 80-fold for hMSC-cocultures HT1080, but 40-fold for HT1080 alone. (C) Representative cell densities of cultured cells. Irradiated hMSCs show little change between cultures on days 2 and 5. ap<.05 by student's t-test.
Modified colony-forming cell assay
Colony-forming cell (CFC) assays are used to quantify progenitors via a simple in vitro assay.15 We adopted and modified a previously published method to determine the appropriate cell dose for in vivo inoculation. In brief, serially diluted cells (5, 10, 50, 100, 400, and 1,000 cells) for HT1080, WEHI164, and RR1022 were plated in a 24-well culture plate (Falcon, BD Bioscience). After 7 or 10 days of incubation, the culture dishes were washed gently, air dried, and then stained with hematoxylin. Under an inverted microscope, the numbers of demarcated areas of cell proliferation (colony formation) were counted. The mean number of colonies/plated cell number was referred to as the CFC frequency. All experiments were performed in triplicate.
In vivo tumor formation and growth in the presence or absence of MSCs
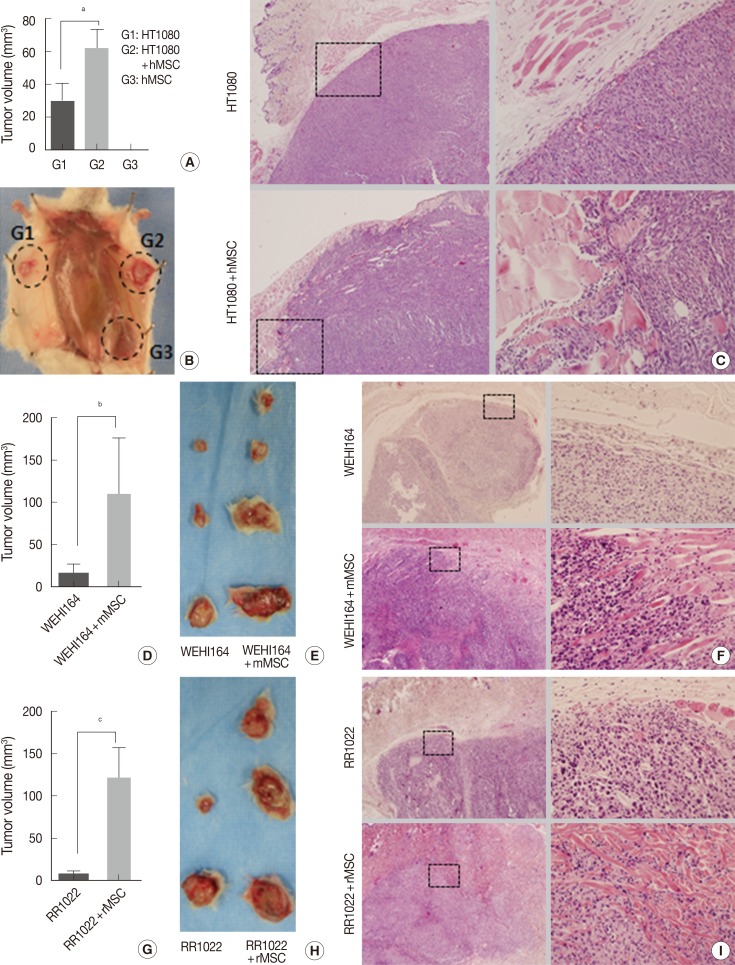
Experiment I (Fig. 2A-C): HT1080 alone (group 1), HT1080 combined with hMSCs (group 2), and hMSCs alone (group 3) in 100 µL of PBS were inoculated into the subcutaneous tissue of the abdomens of 6- to 8-week-old NOG/SCID mice, 5×104 cells for HT1080 and 1×105 cells for hMSCs. Each group consisted of six mice and was evaluated for tumor formation, tumor volume, microscopic growth pattern, and lung metastatic nodules on day 14.
Fig. 2.
Effects of mesenchymal stromal cells (MSC) on in vivo proliferation and invasion of various sarcoma cell types. Tumors of the MSC-treated groups of human sarcoma cells in NOG/SCID mice (A-C), mouse sarcoma cells in Sprague-Dawley rats (D-F), and rat sarcoma cells in C57BL/6 mice (G-I) show significantly increased and highly infiltrative growth compared with controls. Human MSC alone does not form tumors. HT1080, human sarcoma cell; WEHI164, mouse sarcoma cell; RR1022, rat sarcoma cell; hMSC, human mesenchymal stromal cell; mMSC, mouse mesenchymal stromal cell; rMSC, rat mesenchymal stromal cell. ap=.035, bp=.14, and cp=.050 by Student's t-test.
Experiment II (Fig. 2D-F): WEHI164 alone (group 1) and WEHI164 combined with mMSCs (group 2) in 100 µL PBS were inoculated into the subcutaneous tissue of the backs of 6- to 8-week-old Sprague-Dawley rats, 4×104 cells for WEHI164 and 1×105 cells for mMSCs. Each group consisted of six rats and was evaluated for tumor formation on day 21.
Experiment III (Fig. 2G-I): RR1022 alone (group 1) and RR1022 combined with rMSCs (group 2) in 100 µL PBS were inoculated into the subcutaneous tissue of the abdomens of 6- to 8-week-old C57BL/6 mice, 1.5×104 cells for RR1022 and 1×105 cells for rMSCs. Each group consisted of six mice and was evaluated for tumor formation on day 14.
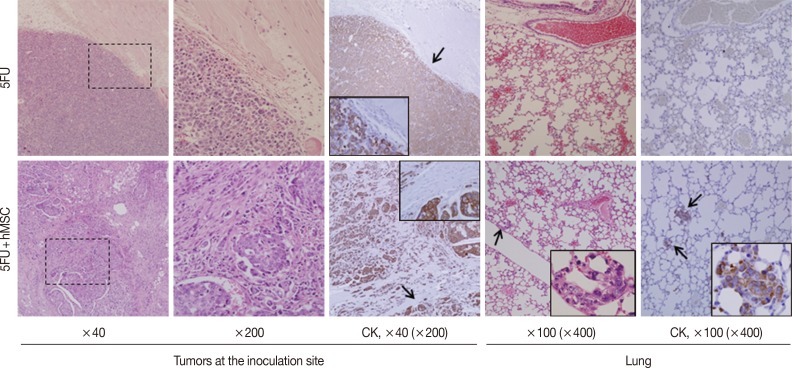
Experiment IV (Fig. 3): For 5FU alone (group 1) and 5FU combined with hMSCs (group 2), 1×105 cells in 100 µL PBS, were inoculated into the subcutaneous tissue of the backs of 6-week-old NOG/SCID mice. Each group consisted of three mice and was evaluated for tumor formation, microscopic growth characteristics, and lung metastasis at 10 weeks.
Fig. 3.
Effects of mesenchymal stromal cells (MSC) on invasion and metastasis in NOG/SCID mice bearing 5FU cells, a human gastric cancer cell line. Subcutaneous inoculation of 5FU alone results in compact expansile growth without lung metastasis (upper panels), whereas 5FU combined with human MSC (hMSC) shows marked desmoplastic and infiltrative growth and lung metastasis, highlighted by immunostaining for cytokeratin (lower panels). CK, cytokeratin.
After sacrifice, tumor tissues were measured by caliper, and volume was calculated according to the following formula: volume=0.2618×L×W×(L+W).16 The number of lung metastatic nodules for each animal was counted macroscopically and microscopically. Samples from subcutaneous tumors and lung metastatic lesions in each group were fixed in 3.7% formaldehyde neutral buffer solution and then processed routinely for histology, stained with hematoxylin and eosin, and examined under light microscopy.
Immunohistochemical staining
Immunohistochemical staining was performed according to conventional protocols. In brief, 4-µm-thick formalin-fixed paraffin embedded tissue sections were processed for deparaffinization, antigen retrieval, and endo-blocking and then incubated with primary antibody cytokeratin AE1/AE3 (1:100, Dako, Cambridgeshire, UK). After washing, the slides were incubated with secondary antibody (K5007, Dako Real Envision/HRP) for 30 minutes at room temperature and were then visualized with DAB (K5007, Dako Real DAB+Chromogen) and counterstained with hematoxylin.
Statistical analysis
Data were statistically analyzed for significant differences using the Graph Pad Prism ver. 5.0 statistical package (Graphpad, La Jolla, CA, USA). Student's t-test was used to assess differences in means and to assess statistical significance. Results were expressed as means±standard deviation, and a value of p<.05 was considered statistically significant.
RESULTS
MSC co-culture promotes in vitro growth of fibrosarcoma cells
To investigate the effect of hMSCs on the proliferation of human fibro sarcoma cells, we performed a co-culture assay with HT1080 in the presence or absence of hMSCs. There was little difference in the cell numbers of both groups by day 3. However, during the exponential growth phase after day 4, the hMSC-co-cultured group showed significantly more rapid growth compared with HT1080 alone (Fig. 1A). On day 6, hMSC-co-cultured HT1080 showed 2.14 times more rapid growth compared with HT1080 alone (79- vs 37-fold change, p<0.05) (Fig. 1B). The cell density of hMSC-co-cultured HT1080 was higher than that of HT1080 alone, consistent with the increase in cell number (Fig. 1C). Irradiated hMSCs showed significant increases in cell size and cell processes (Fig. 1C) but not in cell number (10,667 cells at day 6/10,000 cells when initially plated).
CFC assay
To determine the inoculation cell number for in vivo experiments, we assessed the CFC frequencies of the sarcoma cells of human, mouse, and rat origins. The CFC frequencies were calculated as 1/5 (20%), 1/4 (25%), and 1/1.5 (66.6%) for HT1080, WEHI164, and RR1022, respectively. RR1022 was the most colony-genic (Table 1). To induce rapid in vivo tumor development and avoid cell dose-dependent variables, 10,000 CFC equivalent cells were inoculated for experiments to test the effects of MSCs on fibrosarcoma.
Table 1.
Colony-forming cell (CFC) assay
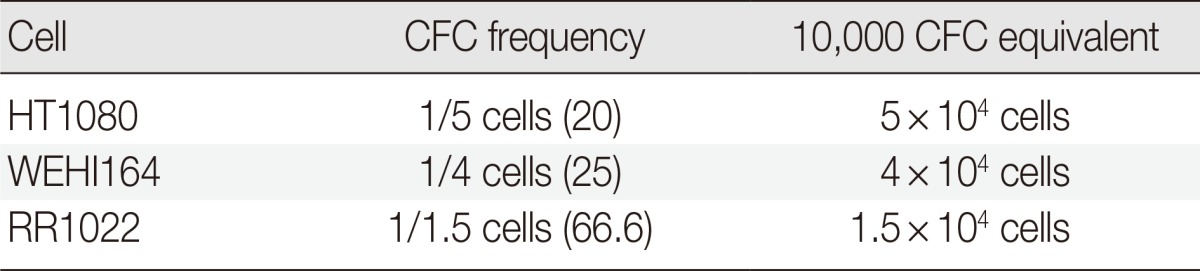
Values are presented as number (%).
HT1080, human fibrosarcoma cells; WEHI164, mice fibrosarcoma cells; RR1022, rat sarcoma cells.
Effects of MSCs on in vivo growth of human fibrosarcoma cells in NOG/SCID mice
The 10,000-CFC equivalent for HT1080 cells was 5×104 cells. HT1080 alone (group 1). HT1080 combined with hMSCs (group 2), and hMSCs alone (group 3) were inoculated into the subcutaneous tissues of NOG/SCID mice (n=6). Tumor formation began to be detected on day 7 in group 1 and group 2, but no tumor formation was detected in group 3 by the end of the experiment. To investigate early stage growth characteristics in the presence or absence of hMSCs, mice were sacrificed on day 14. The number of tumors in the hMSC-co-injected group was significantly greater than that of HT1080 alone (62.01±28.83 mm3 vs 30.08±25.74 mm3, p=.035) (Table 2, Fig. 2A, B). All HT1080 tumors showed expansile growth with good demarcation in the absence of hMSCs, whereas all tumors in the presence of hMSCs showed highly infiltrative growth (Fig. 2C).
Table 2.
The effects of MSCs on in vivo growth characteristics of various sarcoma cells
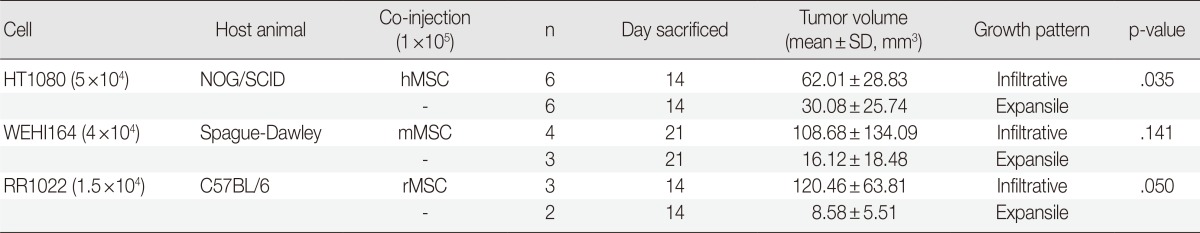
Sprague-Dawley rats and C57BL/6 mice are immunosuppressed with FK-506 and dexamethasone, 1 mg/kg/day, each.
HT1080, human fibrosarcoma cells; WEHI164, mice fibrosarcoma cells; RR1022, rat sarcoma cells; hMSC, human mesenchymal stromal cell; mMSC, mouse mesenchymal stromal cell; rMSC, rat mesenchymal stromal cells.
Effects of MSCs on in vivo growth of mouse and rat sarcoma cells in xeno-environment animal models
The 10,000-CFC equivalent for WEHI164 cells was 4×104 cells. WEHI164 alone and WEHI164 combined with mMSCs were inoculated into the subcutaneous tissue of Sprague-Dawley rats (n=6). Five rats died of infection before the detection of tumor formation, which began at day 10. All live rats were sacrificed on day 21. Tumors in the mMSC-co-injected group (n=4) showed greater volume than those of WEHI164 alone (n=3) (108.68±134.09 mm3 vs 16.12±18.48 mm3, p=.141) (Table 2, Fig. 2D, E). All WEHI164 tumors showed nodular, compact, and expansile growth with relatively good demarcation in the absence of mMSCs, whereas all tumors in the presence of mMSCs showed infiltrative growth (Fig. 2F).
The 10,000-CFC equivalent for RR1022 cells was 1.5×104 cells. RR1022 alone and RR1022 combined with rMSCs were inoculated into the subcutaneous tissue of C57BL/6 mice (n=6). Seven mice died of infection before the detection of tumor formation, which began on day 8. All live mice showed tumor formation and were sacrificed on day 14. Tumors of the mMSC-co-injected group (n=3) showed greater volume than those of RR 1022 alone (n=2) (120.46±63.81 mm3 vs 8.58±5.51 mm3, p=.050) (Table 2, Fig. 2G, H). All RR1022 tumors showed nodular and expansile growth with relatively good demarcation in the absence of mMSCs, whereas all tumors in the presence of mMSCs showed infiltrative growth dissecting the adjust collagen bundles (Fig. 2I).
Effects of MSCs on in vivo tumor progression of human gastric cancer cells in NOG/SCID mice
To assess the impact of MSCs on late stages of tumor progression, relatively slow in vivo growing cancer cells, 5FU, were inoculated into the subcutaneous tissue of NOG/SCID mice in the presence or absence of hMSCs (n=6). Mice began to form tiny but palpable nodules earlier than week 5, and they were maintained with monitoring twice a week. By week 10, the largest tumors had grown to more than 1.0 cm in diameter, so the mice were sacrificed. The tumors were multi-nodular or irregular, which made it difficult to measure their sizes exactly. However, the shapes and demarcation of the tumors were quite different between the groups. All tumors of 5FU alone were nodular in shape and showed compact expansile growth with good demarcation and no definite lung metastatic nodule (Fig. 3, upper panels), whereas tumors grown in the presence of hMSCs showed highly desmoplastic and infiltrative growth and multiple lung metastasis (Fig. 3, lower panels). Irregular-sized nesting and infiltrating tumor cells were positive for cytokeratin, but desmoplastic spindle cells were negative. The multiple lung metastatic nodules were strongly positive for cytokeratin immunostaining.
DISCUSSION
A great deal of evidence has accumulated concerning the role of MSCs in tumor progression, in contexts such as cancer cell proliferation, invasion, and metastasis. However, the exact effects and mechanisms underlying these observations have not yet been clearly explained. This is likely due to the intrinsic attributes of cancer types, which explains the variable effects of MSCs on tumor progression.3 Fibrosarcoma, in lacking any specific differentiation characteristics, is a useful cancer model because of its limited heterogeneity. The purpose of the current study was to investigate the effects of MSCs on the growth and progression of fibrosarcomas and to evaluate the similarity of these effects in various MSC-fibrosarcoma xeno-environment models.
Fibrosarcoma is a malignant neoplasm that maintains mesenchymal characteristics in terms of phenotype and gene-expression profile.13 Although many studies of cancer-microenvironment interactions have focused on epithelial malignancies,1,4,8 it has been suggested that some sarcoma cells require interactions with their microenvironments for growth and metastasis.9 In the current study, we demonstrated the advantages of hMSC-co-cultured conditions, as sarcoma proliferation increased more than two times in conditions with hMSCs compared to those without hMSCs. The proliferation of hMSCs themselves is significantly reduced by irradiation with doses up to 20 Gy.17 Moreover, in the current study, hMSCs were irradiated with 40 Gy and showed little change between initially plated cells and cultured cells in terms of morphology and cell number. MSCs are relatively radioresistant, and maintain their differentiation potential without an increase of irradiation-induced apoptosis at a dose range of up to 20 Gy.18,19 Taken together, these findings suggest that hMSCs promote fibrosarcoma cell proliferation not as proliferating bodies themselves but as supportive elements.
To investigate whether the supportive effects of hMSCs on fibrosarcoma cells works in vivo, we conducted co-injection experiments using hMSCs and HT1080 and found that in vivo, tumor growth is enhanced in the presence of hMSCs. Moreover, hMSC-co-injected tumors showed aggressive tumor growth characteristics. Infiltrative growth was previously regarded as pro-metastatic behavior. To counter concerns that increased tumor volume could be attributed to the proliferation of co-injected hMSCs, studies have found that inoculated MSCs alone become undetectable within two weeks.2 In addition, in the current study, no definite nodule formation was detected at the inoculation sites of hMSCs alone at two weeks. Taken together, these findings suggest that hMSCs not only promote in vivo tumor growth but also favor pro-metastatic infiltrative growth of human fibrosarcoma cells.
To explore the significance of this phenomenon, MSCs and fibrosarcoma cells of nonhuman origin were investigated. To provide experimental conditions similar to those of the xeno-environment, mouse fibrosarcoma cells and mMSCs were injected into Sprague-Dawley rats, and rat sarcoma cells and rMSCs were injected into C57BL/6 mice, under induced immunosuppressed conditions. Mouse and rat sarcoma cells co-injected with mMSCs and rMSCs, respectively, resulted in greater tumor formation in comparison with those without matched MSCs, similar to the results of previous studies. Unfortunately, the loss of some mice and rats due to infection resulted in failure to achieve statistical significance. Infiltrative growth patterns, however, were apparent in all MSC-co-injected groups for both sarcoma cells. Infiltrative growth has been regarded as an unfavorable tumor behavior and a pro-metastatic factor.17
Few studies have focused on MSCs as a tumor microenvironment of sarcomas.9,10 The hMSCs have been reported to migrate to the osteosarcoma site, integrate into the tumor stroma, and thereby promote tumor growth and metastasis.10 Osteosarcoma is a specialized committed type of malignant mesenchymal tumor with the ability to form osteoids, which is what differentiates it from fibrosarcoma lacking osteoblastic differentiation. To the best of our knowledge, our results are the first regarding the effects of MSCs on fibrosarcoma cells.
Due to the rapid growth of fibrosarcoma cells and the infection risk of induced immunosuppressed animal models, our MSCs-fibrosarcoma in xeno-environment models were useful only to assess MSC effects in the early stages of tumor progression. To evaluate the late stages of metastasis, a more stable model is required.
To investigate the effects of hMSCs on tumor progression and metastasis, relatively slow growing gastric cancer cells, 5FU, were injected into the stable immunocompromised animal model of NOG/SCID mice. As expected, hMSCs promoted desmoplastic and highly infiltrative tumor growth and metastasis to the lung. These results are compatible with those of earlier studies.20,21 For example, Nomoto-Kojima et al.20 reported that adipose tissue stromal cells promote the progression of gastric cancer cells in vitro. Bone marrow-derived MSCs were recruited into the inflammatory gastric mucosa and contributed to tumorigenesis and tumor progression via a cytokine-mediated interaction.21 However, to the best of our knowledge, our results are the first demonstrating morphological evidence for MSC-associated gastric cancer progression in an animal model.
In conclusion, the results of the current study constitute morphological evidence for MSC-associated tumor progression of fibrosarcomas and gastric cancer cells. Understanding the relationships between MSCs and tumor progression will lead to further insights into tumor progression mechanisms and eventually to targeted treatment strategies.
Acknowledgments
This research was supported by a grant (10172MFDS993) from the Ministry of Food and Drug Safety in 2013. The Catholic MASTER Cells supplied by the Catholic Institute of Cell Therapy (CIC, Seoul, Korea) were derived from human bone marrow donated by healthy donors after informed consent. This research was supported partly by Seoul St. Mary's Clinical Medicine Research Program in 2009 through the Catholic University of Korea.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Artacho-Cordón A, Artacho-Cordón F, Ríos-Arrabal S, Calvente I, Núñez MI. Tumor microenvironment and breast cancer progression: a complex scenario. Cancer Biol Ther. 2012;13:14–24. doi: 10.4161/cbt.13.1.18869. [DOI] [PubMed] [Google Scholar]
- 2.Kidd S, Spaeth E, Dembinski JL, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F., 3rd Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 5.Secchiero P, Zorzet S, Tripodo C, et al. Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin's lymphoma xenografts. PLoS One. 2010;5:e11140. doi: 10.1371/journal.pone.0011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke MR, Imhoff FM, Baird SK. Mesenchymal stem cells inhibit breast cancer cell migration and invasion through secretion of tissue inhibitor of metalloproteinase-1 and -2. Mol Carcinog. 2014 May 13; doi: 10.1002/mc.22178. [Epub]. http://dx.doi.org/10.1002/mc.22178. [DOI] [PubMed] [Google Scholar]
- 7.Shinagawa K, Kitadai Y, Tanaka M, et al. Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int J Cancer. 2010;127:2323–2333. doi: 10.1002/ijc.25440. [DOI] [PubMed] [Google Scholar]
- 8.Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukamoto S, Honoki K, Fujii H, et al. Mesenchymal stem cells promote tumor engraftment and metastatic colonization in rat osteosarcoma model. Int J Oncol. 2012;40:163–169. doi: 10.3892/ijo.2011.1220. [DOI] [PubMed] [Google Scholar]
- 10.Xu WT, Bian ZY, Fan QM, Li G, Tang TT. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 2009;281:32–41. doi: 10.1016/j.canlet.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Ottaviani G, Jaffe N. The etiology of osteosarcoma. Cancer Treat Res. 2009;152:15–32. doi: 10.1007/978-1-4419-0284-9_2. [DOI] [PubMed] [Google Scholar]
- 12.Fox MG, Trotta BM. Osteosarcoma: review of the various types with emphasis on recent advancements in imaging. Semin Musculoskelet Radiol. 2013;17:123–136. doi: 10.1055/s-0033-1342969. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher CD, Bridge JA, Hogendoorn PC, Mertens F. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013. pp. 299–300. [Google Scholar]
- 14.Zhu H, Guo ZK, Jiang XX, et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550–560. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
- 15.Pereira C, Clarke E, Damen J. Hematopoietic colony-forming cell assays. Methods Mol Biol. 2007;407:177–208. doi: 10.1007/978-1-59745-536-7_14. [DOI] [PubMed] [Google Scholar]
- 16.Luu HH, Kang Q, Park JK, et al. An orthotopic model of human osteosarcoma growth and spontaneous pulmonary metastasis. Clin Exp Metastasis. 2005;22:319–329. doi: 10.1007/s10585-005-0365-9. [DOI] [PubMed] [Google Scholar]
- 17.Koelzer VH, Lugli A. The tumor border configuration of colorectal cancer as a histomorphological prognostic indicator. Front Oncol. 2014;4:29. doi: 10.3389/fonc.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cmielova J, Havelek R, Soukup T, et al. Gamma radiation induces senescence in human adult mesenchymal stem cells from bone marrow and periodontal ligaments. Int J Radiat Biol. 2012;88:393–404. doi: 10.3109/09553002.2012.666001. [DOI] [PubMed] [Google Scholar]
- 19.Nicolay NH, Sommer E, Lopez R, et al. Mesenchymal stem cells retain their defining stem cell characteristics after exposure to ionizing radiation. Int J Radiat Oncol Biol Phys. 2013;87:1171–1178. doi: 10.1016/j.ijrobp.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Nomoto-Kojima N, Aoki S, Uchihashi K, et al. Interaction between adipose tissue stromal cells and gastric cancer cells in vitro. Cell Tissue Res. 2011;344:287–298. doi: 10.1007/s00441-011-1144-3. [DOI] [PubMed] [Google Scholar]
- 21.Martin J, Donnelly JM, Houghton J, Zavros Y. The role of sonic hedgehog reemergence during gastric cancer. Dig Dis Sci. 2010;55:1516–1524. doi: 10.1007/s10620-010-1252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]