Solitary fibrous tumor (SFT) is a mesenchymal tumor characterized by fibroblast-like tumor cells, thick collagen bands, a hemangiopericytoma-like branching vascular pattern and CD34 expression of tumor cells.1 Although some cases show malignant behavior, most of the cases are benign and have histologically bland-looking tumor cells. Rarely, highly pleomorphic sarcoma arises within a primary or recurrent SFT having typical histologic features. This is a phenomenon similar to that seen in dedifferentiated liposarcoma. Dedifferentiated SFT (DSFT) is rare, with less than 30 cases reported worldwide,2,3,4,5,6 and it has not been previously reported in Korea. Here, we present a case of DSFT.
CASE REPORT
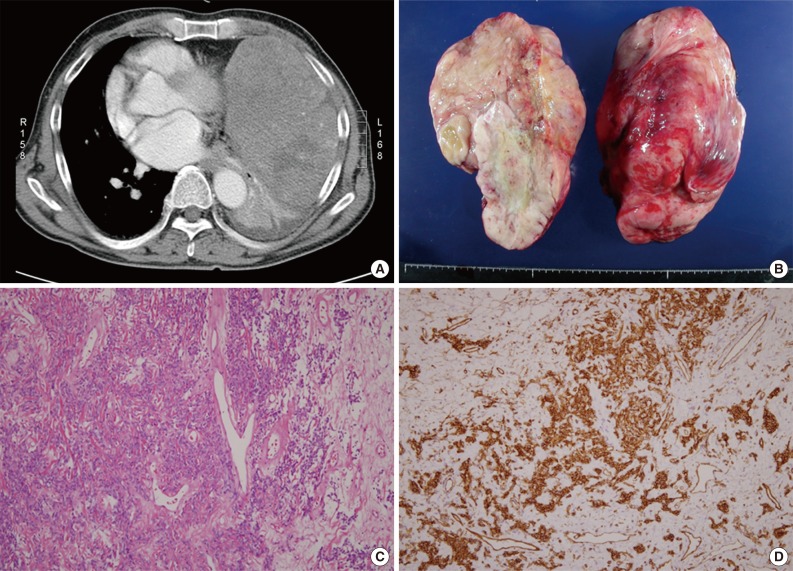
A 73-year-old male presented with dyspnea for one month. On computerized tomography, a large mass involving the left thoracic cavity and mediastinum was found (Fig. 1A). Sarcoma was suspected upon needle biopsy, based on the large size and mesenchymal features of the tumor cells. During surgery, a 30×25×20-cm-sized well-encapsulated multinodular solid tumor was found. The tumor was divided surgically into two parts and removed (Fig. 1B). Microscopically, the tumor was composed of fibroblast-like tumor cells, collagen fibers and many thin-walled blood vessels, which are characteristics of a SFT (Fig. 1C). The tumor cells were diffusely and strongly positive for CD34 (Fig. 1D) and showed moderate nuclear atypism in focal areas. The mitotic index was generally low, but it was up to 4/10 high-power fields (HPF) in a few areas. Necrosis was absent, and the diagnosis of SFT with malignant potential was made.
Fig. 1.
Solitary fibrous tumor. (A) A large solid mass involves the mediastinum and left thoracic cavity. (B) A portion of the tumor shows a smooth-surfaced multinodular appearance and a white cut surface with myxoid change in some areas. (C) The tumor is composed of fibroblast-like tumor cells, collagen fibers and thin-walled blood vessels, which are characteristics of a solitary fibrous tumor. (D) The tumor cells are diffusely and strongly positive for CD34.
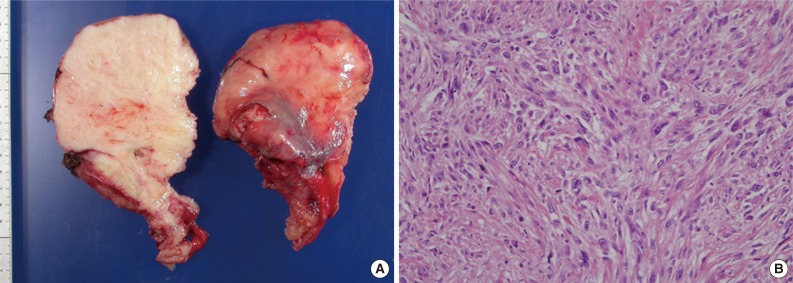
After 38 months, a computerized tomography showed a 20-cm-sized recurrent mass involving the mediastinum and left thoracic cavity and a separate 4.5-cm-sized mass in the mediastinum. The tumor appeared to have invaded rib bones. The tumor was divided surgically into several parts and removed along with three segments of rib bones (Fig. 2A). The larger mass was composed of highly pleomorphic spindle cells with a mitotic index up to 21/10 HPFs (Fig. 2B). It showed necrosis and invasion to rib bones. The tumor cells were nondistinctive and showed no muscular or osteoid differentiation. They were arranged in a storiform pattern in some areas and were negative for CD34, desmin, S-100, and CD99. Conversely, the smaller mass showed typical microscopic findings of SFT, similar to those seen in the primary tumor. This part of the tumor did not contain any dedifferentiated areas and was positive for CD34.
Fig. 2.
Dedifferentiated solitary fibrous tumor. (A) A portion of the tumor shows a white solid cut surface with a tough trabeculated appearance. (B) The tumor is composed of pleomorphic spindle cells arranged in a storiform pattern.
DISCUSSION
Although most cases of SFT are benign, about 10% are aggressive.1 Most, but not all, tumors that show malignant behavior (malignant SFT, MSFT) are hypercellular and show cytological atypia, necrosis, numerous mitoses and infiltrative margins.7 Rarely, SFT is accompanied by an overgrowth of a high-grade component, morphologically mimicking a high-grade pleomorphic spindle or small cell sarcoma, as seen in dedifferentiated liposarcoma.
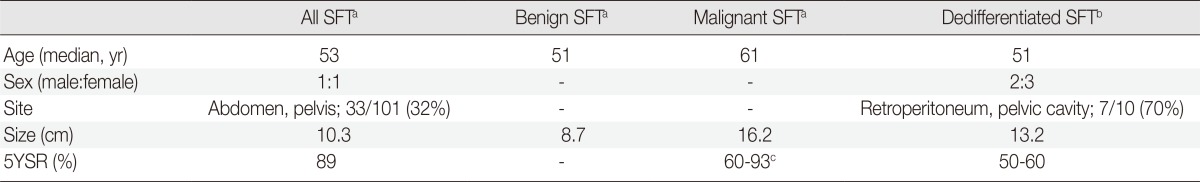
Patients of DSFT are middle-to-old-aged (Table 1), and males and females are equally affected. These age and sex distributions are similar to those of typical SFT. However, the sites of DSFT seem to be slightly different from those of SFT. Although our case occurred in the thoracic cavity, DSFT occurs more frequently in the retroperitoneum or pelvic cavity than in the pleura, in comparison to SFT. In the report by Collini et al.,2 7 out of 10 DSFT cases occurred in the retroperitoneum or pelvic cavity. The tumor size of DSFT appears to be larger than that of typical SFT and similar to that of MSFT.
Table 1.
Clinical differences among benign, malignant, and dedifferentiated solitary fibrous tumor (SFT)
Histologically, DSFT is composed of two portions; one is an area of typical SFT, and the other is a high-grade sarcomatous dedifferentiated area. The dedifferentiated area shows a very high-mitotic rate, with an average of 30/10 HPFs. In the dedifferentiated area, there are no morphologic features that would suggest a diagnosis of SFT. This is in contrast to MSFT, in which even the atypical portion contains some features of typical SFT. The dedifferentiated area in our case was a nondistinctive undifferentiated high-grade pleomorphic sarcoma. However, in other reports,2,3 Ewing-like undifferentiated small round cell sarcomas were seen more frequently. In several cases, rhabdomyosarcomatous2 or osteosarcomatous4 features were seen in the dedifferentiated areas. As in our case, the dedifferentiated area is usually negative for CD34,2,3 in contrast to the area of typical SFT, which is positive for CD34.
The five-year survival rate of DSFT is approximately 50% to 60%.2 The prognosis of DSFT is worse than that of SFT or even that of MSFT, although an accurate evaluation is difficult because of the limited number of cases.
Collini et al.2 demonstrated that activated (phosphorylated) ribosomal protein 6 (S6) and activated eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) are strongly expressed in the dedifferentiated area. In contrast to DSFT, MSFT weakly expresses these proteins, while typical SFT does not. S6 and 4E-BP1 control transcription and protein synthesis and have been reported to be associated with malignant progression.
Collini et al.2 used the term "evoluted SFT" to describe the cases showing a gradual transition from classic SFT/MSFT to a high-grade sarcomatous component or when the tumor consisted of a high-grade sarcomatous component without any trace of SFT/MSFT at a site of local relapse or metastasis. They used the term DSFT only to describe cases with evidence of an abrupt synchronous transition from the classic SFT/MSFT to the high-grade sarcomatous component. According to their criteria, our case corresponds to DSFT because the recurrent tumor was composed of two separate masses, with one showing classic SFT component only and the other showing high-grade sarcomatous component only. However, we do not think this distinction between evoluted and DSFT has a significant meaning because there seems to be no difference in clinical significance, including prognosis, between the two subtypes.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Fletcher CD, Bridge JA, Hogendoorn P, Mertens F. WHO classification of tumours of soft tissue and bone. Lyon: IARC Press; 2013. pp. 80–82. [Google Scholar]
- 2.Collini P, Negri T, Barisella M, et al. High-grade sarcomatous overgrowth in solitary fibrous tumors: a clinicopathologic study of 10 cases. Am J Surg Pathol. 2012;36:1202–1215. doi: 10.1097/PAS.0b013e31825748f0. [DOI] [PubMed] [Google Scholar]
- 3.Mosquera JM, Fletcher CD. Expanding the spectrum of malignant progression in solitary fibrous tumors: a study of 8 cases with a discrete anaplastic component: is this dedifferentiated SFT? Am J Surg Pathol. 2009;33:1314–1321. doi: 10.1097/pas.0b013e3181a6cd33. [DOI] [PubMed] [Google Scholar]
- 4.Thway K, Hayes A, Ieremia E, Fisher C. Heterologous osteosarcomatous and rhabdomyosarcomatous elements in dedifferentiated solitary fibrous tumor: further support for the concept of dedifferentiation in solitary fibrous tumor. Ann Diagn Pathol. 2013;17:457–463. doi: 10.1016/j.anndiagpath.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Subramaniam MM, Lim XY, Venkateswaran K, Shuen CS, Soong R, Petersson F. Dedifferentiated solitary fibrous tumour of the nasal cavity: the first case reported with molecular characterization of a TP53 mutation. Histopathology. 2011;59:1269–1274. doi: 10.1111/j.1365-2559.2011.03997.x. [DOI] [PubMed] [Google Scholar]
- 6.Moritani S, Ichihara S, Hasegawa M, et al. Dedifferentiation and progression of an intracranial solitary fibrous tumor: autopsy case of a Japanese woman with a history of radiation therapy of the head during infancy. Pathol Int. 2011;61:143–149. doi: 10.1111/j.1440-1827.2010.02627.x. [DOI] [PubMed] [Google Scholar]
- 7.Demicco EG, Park MS, Araujo DM, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25:1298–1306. doi: 10.1038/modpathol.2012.83. [DOI] [PubMed] [Google Scholar]