Abstract
Neuroinflammation plays a main role in neurological deficits in rats with minimal hepatic encephalopathy (MHE) due to portacaval shunt (PCS). Treating PCS rats with SB239063, an inhibitor of MAP-kinase-p38, reduces microglial activation and brain inflammatory markers and restores cognitive and motor function. The translocator protein-(18-kDa) (TSPO) is considered a biomarker of neuro-inflammation. TSPO is increased in brain of PCS rats and of cirrhotic patients that died in hepatic coma. Rats with MHE show strong microglial activation in cerebellum and milder in other areas when assessed by MHC-II immunohistochemistry. This work aims were assessing: 1) whether binding of TSPO ligands is selectively increased in cerebellum in PCS rats; 2) whether treatment with SB239063 reduces binding of TSPO ligands in PCS rats; 3) which cell type (microglia, astrocytes) increases TSPO expression. Quantitative autoradiography was used to assess TSPO-selective 3H-(R)-PK11195 binding to different brain areas. TSPO expression increased differentially in PCS rats, reaching mild expression in striatum or thalamus and very high levels in cerebellum. TSPO was expressed in astrocytes and microglia. Treatment with SB239063 did not reduces 3[H]-PK11195 binding in PCS rats. SB239063 reduces microglial activation and levels of inflammatory markers, but not binding of TSPO ligands. This indicates that SB239063-induced neuroinflammation reduction in PCS rats is not mediated by effects on TSPO. Also, enhanced TSPO expression is not always associated with cognitive or motor deficits. If enhanced TSPO expression plays a role in mechanisms leading to neurological alterations in MHE, SB239063 would interfere these mechanisms at a later step.
Keywords: Minimal hepatic encephalopathy, TSPO, p38 inhibitor, Hyperammonemia, Cerebellum, Microglial activation
Introduction
Patients with chronic liver disease (e.g. cirrhosis) often show hepatic encephalopathy (HE), a complex neuropsychiatric syndrome. In the early phases, known as minimal hepatic encephalopathy (MHE), patients do not show evident symptoms of encephalopathy, but show mild cognitive impairment, attention deficits, psychomotor slowing and impaired bimanual and visuomotor coordination which can be unveiled using psychometric tests (Ferenci et al. 2002; Amodio et al. 2004; Montoliu et al. 2007). MHE can progress to clinical HE, showing evident, more serious alterations in motor activity and coordination (hypokinesia, asterixis, ..), in intellectual and cognitive functions and alterations in consciousness which, in the worst cases, can progress to coma and death. Both MHE and clinical HE reduce quality of life and life span of the patients (Groeneweg et al. 1998; Romero-Gómez et al. 2001).
The main factor responsible for the neurological alterations in HE is hyperammonemia. It is becoming increasingly clear that inflammation acts synergistically with hyperammonemia to induce the neurological alterations both in patients with minimal or clinical HE (Odeh et al. 2004, 2005; Shawcross et al. 2004; Montoliu et al. 2009; Felipo et al. 2012).
Studies in animal models of chronic hyperammonemia and HE also support the synergic effects of hyperammonemia and inflammation in the cognitive and motor alterations.
A main animal model of MHE are rats with porta-caval shunt (PCS), recommended by the International Society for Hepatic Encephalopathy (ISHEN) for the study of the mechanisms and treatment of HE because it reproduces cognitive and motor alterations present in patients with MHE (Butterworth et al. 2009).
Rats with PCS show neuroinflammation, with increased microglial activation and levels of inflammatory markers in brain (Cauli et al. 2007; Agusti et al. 2011). Neuroinflammation is also present in other models of HE such as rats with bile duct ligation or of chronic hyperammonemia without liver failure (Rodrigo et al. 2010).
Neuroinflammation plays a main role in the neurological deficits in rats with MHE and it is possible to restore their cognitive and motor function with different anti-inflammatory treatments. Ibuprofen, a non-steroidal anti-inflammatory drug (NSAID), reduces neuroinflammation and restores the cognitive and motor function in PCS rats (Cauli et al. 2007, 2009a, b), in rats with bile-duct ligation (BDL) or with chronic hyperammonemia (Rodrigo et al. 2010). However, ibuprofen and other NSAIDS are not recommended in patients with liver disease because they may induce kidney damage. As an alternative treatment to reduce microglial activation and neuroinflammation without affecting kidney, we used a chronic treatment with a p38 MAPK inhibitor, SB239063, which reduces microglial activation and neuroinflammation in PCS rats and restores cognitive and motor function without affecting renal function (Agusti et al. 2011).
The translocator protein (18 kDa) (TSPO) formerly known as peripheral benzodiazepine receptor (PBR or PTBR), is a protein expressed in steroid-synthesizing tissues, including the brain (Jorda et al. 2005; Jayakumar et al. 2002; Delavoie et al. 2003; Chen and Guilarte. 2008). TSPO is present in the outer mitochondrial membrane where it mediates the transport of cholesterol into mitochondria (Papadopoulos et al. 1997; Chen and Guilarte. 2008). This is the rate limiting step in steroidogenesis. Under normal conditions TSPO is poorly expressed in brain and limited to glial cells (astrocytes and microglia). TSPO expression increases in glial cells in response to inflammatory stimuli or brain damage (Chen and Guilarte 2006, 2008; Chen et al. 2004; Guilarte et al. 1995, 2003; Kuhlmann and Guilarte 1997, 1999, 2000). By this reason TSPO is being used as a biomarker of neuroinflammation, especially in vivo studies by positron emission tomography (PET) using the binding of TSPO ligands as a measure of neuroinflammation.
TSPO is increased in brain of PCS rats (Desjardins et al. 1997) and in animal models of acute liver failure due to liver ischemia (Desjardins and Butterworth. 2002). TSPO expression is also increased in the brain of cirrhotic patients that died in hepatic coma (Lavoie et al. 1990; Desjardins et al. 1997).
Studies in vivo in patients with HE show an increased binding of 11[C]-PK11195, a selective TSPO antagonist, in different brain areas (Cagnin et al. 2006).
It is considered that increased binding of TSPO ligands reflects mainly activation of microglia (Garvey et al. 2013). We have shown that animal models of chronic hyperammonemia and HE show strong activation of microglia in cerebellum and milder activation in other brain areas when assessed by immunohistochemistry using an antibody for MHC-II to detect activated microglia (Rodrigo et al. 2010; Agusti et al. 2011). The first aim of the present work was to assess whether binding of ligands to TSPO is also increased selectively in cerebellum or affects most brain areas in PCS rats. This was assessed by quantitative autoradiography using the TSPO-selective radioligand 1-(2-chlorophenyl)-N-methyl-N-(1-methyl-propyl)-3-isoquinoline carboxamide 3H-(R)-PK11195.
We have shown that treatment with p38 inhibitors (SB239063) reduces activation of microglia and neuroinflammation in PCS rats (Agusti et al. 2011). The second aim of this work was to assess whether treatment with the p38 inhibitor SB239063 reduces the binding of ligands to TSPO in different brain areas of PCS rats. The third aim was to assess in which cell type (microglia, astrocytes) is expression of TSPO increased in PCS rats.
Material and methods
Porta-caval shunt
Male Wistar rats (180–210 g) were anaesthetized and an endto-side porta-caval shunt was constructed as described by Lee and Fisher 1961. Control rats were sham-operated. All experiments were approved by the Center and met the guidelines of the European Union for care and management of experimental animals.
Chronic intracerebral administration of SB239063
Two weeks after PCS surgery, rats were anaesthetized with isoflurane and miniosmotic pumps (Alzet 2004, Palo Alto, USA) were implanted subcutaneously and connected to a cannula for cerebral infusion (Brain infusion KIT II) implanted stereotaxically in the lateral ventricle (0.9 mm previous, 1.3 mm to the right of bregma and 4.5 mm below Dura). The pumps released 0.25 μl/h during 28 days. Four groups of rats were used. One PCS and one sham group were implanted with pumps filled (250 μL) with SB239063 (2 mg/ml in 50 % dimethylsulfoxide). One PCS and one sham group were implanted with pumps filled with 50 % dimethylsulfoxide. Control experiments showed that this dose of dimethylsulfoxide released at 0.25 μl/h does not affect motor activity or learning ability. SB239063 was from Tocris (Bristol, UK). SB239063 was administered intracerebrally because peripheral administrations would require large amounts of the inhibitor which will make this chronic treatment study prohibitively expensive.
Quantitative receptor autoradiography
The TSPO-selective radioligand 1-(2-chlorophenyl)-N-methyl-N-(1-methyl-propyl)-3-isoquinoline carboxamide 3H-(R)-PK11195 (85.5 Ci/mmol) was radiolabeled and purified by NEN Life Science Products (Boston, MA). Twenty-six days after implantation of osmotic pumps, rats were perfused transcardially under deep anesthesia (pentobarbital, 100 mg/ Kg, i.p.) with 0.9 % saline followed by 4 % paraformalde-hyde. Brains were dissected out and stored in 30 % sucrose. Brains were sectioned (20 μm) on a freezing cryostat (Microm HM505E) in the horizontal plane. Brain sections were thaw mounted onto poly-L-lysine–coated slides (Sigma) and stored at −20 °C until used. 3H- (R)-PK11195 autoradiography to measure PBR levels was performed on adjacent brain sections using the same procedures. Slides were thawed and dried at 37 °C for 30 min and prewashed in 50 mM Tris–HCl buffer (pH 7.4) for 5 min at room temperature. Sections were then incubated with 1nM 3H-(R) - PK11195 in buffer solution for 30 min at room temperature. For nonspecific binding, adjacent sections were incubated in the presence of 10 μM racemic PK11195. The reaction was terminated by two 3-min washes in cold buffer (4 °C) and two dips in cold, deionized water (4 °C). Sections incubated with 3H-(R)-PK11195 were air-dried and apposed to Hyperfilm-[3H] (Amersham, Arlington Heights, IL) with 3[H]-Microscales (Amersham) for 3 weeks. Images were captured and digitized using MCID Images Systems, QICAM 12 BIT digital camera and the Northern Light Precision Illuminator (InterFocus Imaging Ltd, Cambridge, England) and quantified using MCID densitometry software (MCID™ Core Densitometry System) (InterFocus Imaging Ltd, Cambridge, England).
Immunofluorescence
Twenty-six days after implantation of osmotic pumps, 5–6 rats per group were perfused transcardially under deep anesthesia (pentobarbital, 100 mg/Kg, i.p.) with 0.9 % saline followed by 4 % paraformaldehyde. Brains were dissected out and postfixed in the same fixative and subsequently, transferred to phosphate buffer (PB) with 0.1 sodium azide. Coronal sections (40 μm) were cut on a vibratome (Leica VT 1000S) and were stored at 4 °C in PB with 0.1 % azide until further processing. Free-floating sections were washed and sequential incubations with blocking serum and primary antibodies (overnight 4° C) were performed. We stained cerebellum sections with antiserum raised against TSPO (Rabbit polyclonal anti-TSPO, 1:200, Trevigen, USA) GFAP (1:400, Sigma- Aldrich, USA) and iba-1 (1:200, Abcam, UK). Finally, the following secondary antibodies: Alexa fluor 488 Goat anti-mouse, Alexa fluor 488 Donkey anti-goat, and Alexa fluor 647 Donkey anti-rabbit (all of them, 1:400, Invitrogen, USA), were incubated for 1 h at room temperature. To assess the co-expression of TSPO with astrocytes and microglial cells we analyzed the double inmunofluorescence stained sections with a confocal microscope (Leica TCS SP2AOBS).
Statistical analysis
Results are expressed as a mean ± SEM (Standard error of the mean). An ANOVA analysis was performed followed by post-hoc Newman-Keuls Multiple Comparison Test (GraphPAD Prism 5.03).
Results
Binding of 3[H]-PK11195 to TSPO in different regions of the brain
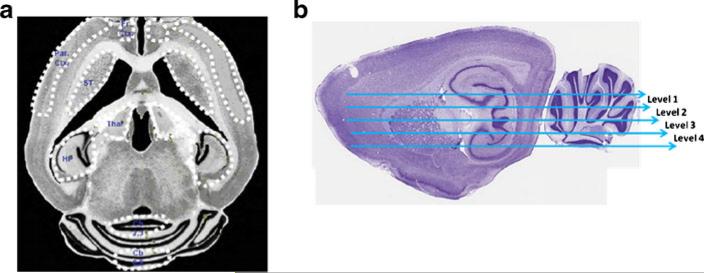
Quantitative film autoradiography of binding of the TSPO-selective ligand 3[H]-PK11195 was used to measure TSPO levels in the striatum (STR), frontal cortex (FrCx), parietal cortex (ParCx), thalamus (Tal), hippocampus (HP) and regions 2,3 (CB 2,3) and 8,9 of cerebellum (CB 8,9). The areas quantified are shown in Fig. 1a.
Fig. 1.
Brain regions in which binding of the TSPO-selective ligand 3[H]-PK11195 was quantified. Binding of the TSPO-selective ligand 3[H]-PK11195 was quantified by autoradiography in striatum (STR), frontal cortex (FrCx), parietal cortex (ParCx), thalamus (Tal), hippocampus (HP) and regions 2,3 (CB 2,3) and 8,9 of cerebellum (CB 8,9). The areas quantified are shown in (a). To analyze the 3[H]-PK11195 binding to TSPO the brain was divided in the 4 neuroanatomical levels shown in (b). Level 1 is the most dorsal and the level 4 is the most ventral
To analyze the 3[H]-PK11195 binding to TSPO the brain was divided in 4 neuroanatomical levels (Fig. 1b). The brain was sectioned in axial slices. Level 1 is the most dorsal and the level 4 is the most ventral. The results for level 1 are shown in Fig. 2. The results for levels 2–4 are shown in supplementary Figs. 1, 2, and 3. All results are summarized in Table 1.
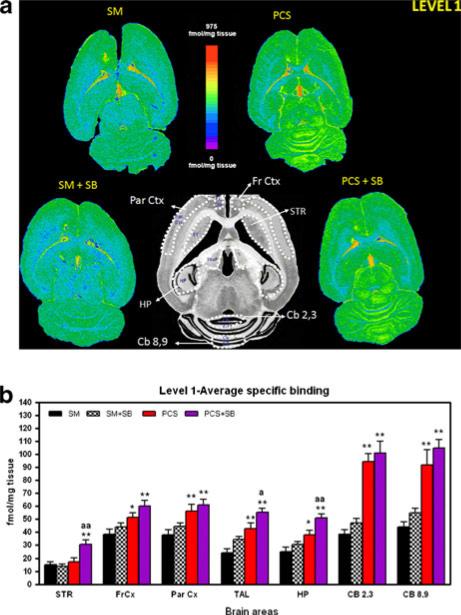
Fig. 2.
Binding of 3[H]-PK11195 in different regions of neuroanatomical level 1 of control and PCS rats treated or not with the inhibitor of p38 SB239063. a Shows representative autoradiography images for each group. b Shows the quantification of the binding of 3[H]-PK11195 to cerebellar region 2,3 (Cb 2,3) and 8,9 (Cb 8,9), hippocampus (HP), striatum (STR), thalamus (Tal), Frontal cortex (Fr Ctx) and Parietal cortex (Par Ctx). Values are the mean ± SEM of the number of rats indicated in Table 1 for each group. SM = control rats with “sham” operation treated with vehicle, SM + SB = control rats with “sham” operation and treated with SB239063, PCS = PCS rats treated with vehicle, PCS + SB = PCS rats treated with SB239063
Fig. 3.
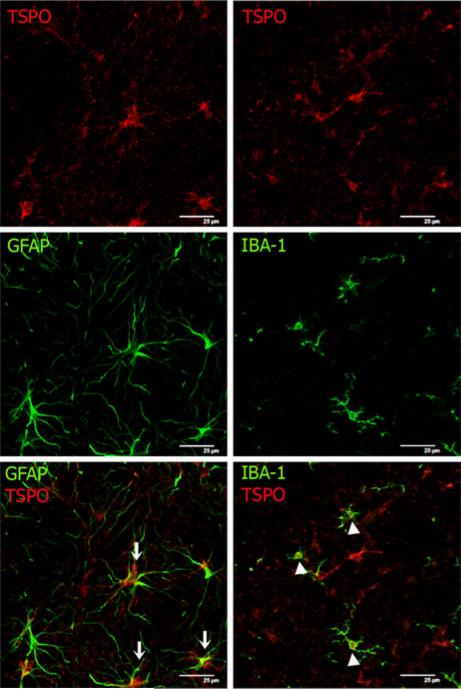
TSPO is expressed in astrocytes and microglia in the cerebellum. Co-expression of TSPO and markers for astrocytes (GFAP) or microglia (Iba-1) was analyzed by double immunofluorescence. All of the experimental groups co-expressed both GFAP and Iba-1 (green) with TSPO (red). Co-expression of GFAP positive cells (astrocytes) with TSPO (arrows) and of the iba-1 positive cells (microglia) with TSPO (arrow heads) is illustrated in the bottom pannels for PCS rats. Scale bar: 25 μm
Table 1.
Effects of chronic HE caused by PCS and the treatment with MAPK p38 inhibitor on the 3[H]-PK11195- binding in different brain regions
| Levels | Control (SM) | SM + SB | PCS | PCS+SB |
|---|---|---|---|---|
| 3[H]-PK11195- binding foiols/mg tissue | ||||
| Striatum | ||||
| Level 1 | 15±2 (8) | 14±2 (12) | 17±3 (13) | 31±3 **aa(14) |
| Level 2 | 14±2 (10) | 11±1 (10) | 29±4 * (13) | 25±4 (14) |
| Level 3 | 15±2 (6) | 16±2 (13) | 24±5 (13) | 35±4 ** (13) |
| Level 4 | 15±2 (12) | 19±2 (15) | 36±3 ** (16) | 32±4 ** (16) |
| Frontal cortex | ||||
| Level 1 | 38±4 (15) | 44±3 (15) | 52±3 * (16) | 60±4 ** (16) |
| Level 2 | 34±3 (12) | 34±2 (16) | 41±6 (16) | 64±7 ** aa (13) |
| Level 3 | 26±3 (12) | 35±3 (16) | 44±4 * (16) | 61±6 ** a (14) |
| Level 4 | 27±2 (13) | 38±2 (16) | 46±5 ** (16) | 62±3 ** aa (16) |
| Parietal cortex | ||||
| Level 1 | 38±4 (16) | 45±3 (16) | 56±5 ** (15) | 61±4 ** (16) |
| Level 2 | 35±3 (12) | 35±3 (16) | 49±6 (16) | 52±5 * (14) |
| Level 3 | 27±3 (11) | 32±2 (16) | 43±5 * (15) | 51±4 ** (15) |
| Level 4 | 28±3 (16) | 36±2 (16) | 45±4 ** (16) | 47±4 ** (16) |
| Thalamus | ||||
| Level 1 | 24±3 (15) | 34±2 (16) | 43±4 ** (16) | 55±3 ** a (16) |
| Level 2 | 12±2 (10) | 10±2 (9) | 43±6 ** (15) | 42±7 ** (11) |
| Level 3 | 17±3 (6) | 12±2 (11) | 37±6 (14) | 44±5 ** (15) |
| Level 4 | 13±2 (9) | 14±2 (14) | 40±5 ** (15) | 45±5 ** (13) |
| Hippocampus | ||||
| Level 1 | 25±3 (12) | 31±2 (16) | 38±3 * (14) | 51±3 ** aa (16) |
| Level 2 | 24±4 (12) | 21±3 (15) | 36±5 (16) | 33±4 (11) |
| Level 3 | 24±3 (11) | 25±2 (15) | 31±5 (15) | 44±5 ** (16) |
| Level 4 | 22±2 (16) | 28±3 (15) | 38±4 ** (16) | 43±4 ** (15) |
| Cerebellum (2, 3) | ||||
| Level 1 | 39±3 (8) | 47±3 (8) | 94±6 ** (7) | 101±9 ** (8) |
| Level 2 | 33±6 (6) | 33±3 (8) | 86±9 ** (8) | 93±10 ** (7) |
| Level 3 | 29±7 (4) | 41±7 (7) | 80±10**(6) | 89±9 ** (6) |
| Level 4 | 39±7 (6) | 46±8 (5) | 94±4 ** (6) | 94±16 ** (7) |
| Cerebellum (8, 9) | ||||
| Level 1 | 44±4 (7) | 55±3 (8) | 92±12**(8) | 105±6 ** (8) |
| Level 2 | 44±5 (6) | 51 ±4 (8) | 91±10 ** (7) | 106±8 ** (5) |
| Level 3 | 41 ±4 (5) | 51±6(7) | 92±12**(7) | 105±3 ** (6) |
| Level 4 | 57±6 (6) | 58±6 (5) | 91±14* (7) | 84±12 (7) |
Each value represents the mean ± SEM of the number of animals indicated in parenthesis. Values significantly different from sham rats are indicated by asterisk.
p <0,05,
p <0.01.
Values significantly different from PCS rats are indicated by “a”. a p <0.05, aa p <0.01 SM = control rats with “sham” operation treated with vehicle, SM + SB = control rats with “sham” operation and treated with SB239063, PCS = PCS rats treated with vehicle, PCS + SB = PCS rats treated with SB239063 STR striatum, FrCx frontal cortex, ParCx parietal cortex, Tal thalamus, HP hippocampus, CB 2,3 2,3 cerebellum regiuon, CB 8,9 8,9 cerebellum region
Binding of 3[H]-PK11195 to TSPO in striatum (STR)
In level 1, binding of 3[H]-PK11195 in striatum was not different in PCS (17±2 fmols/mg) than in control rats (15± 2 fmols/mg). In levels 2, 3 and 4, PCS rats showed an increase in 3[H]-PK11195 binding compared sham rats. The increase was statistically significant in levels 2 and 4 and did not reach statistical significance in level 3 (Table 1).
Treatment with SB239063 did not affect binding to TSPO in sham rats in any of the anatomical levels. SB239063 increased the binding to TSPO in PCS rats in levels 1 and 3 but not in levels 2 and 4 (Table 1).
Binding of3[H]-PK11195 to TSPO in the frontal cortex (FrCx)
In the frontal cortex (FrCx) 3[H]-PK11195 binding increased in PCS rats compared to control (SM) rats in all anatomical levels. The increase was statistically significant in levels 1, 3 and 4 and did not reach statistical significance in level 2 (Table 1).
Treatment with SB239063 did not affect binding to TSPO in sham rats in any of the anatomical levels and increased the binding to TSPO in PCS rats in all anatomical levels (Table 1).
Binding of 3[H]-PK11195 to TSPO in the parietal cortex (ParCx)
As occurs in frontal cortex, in parietal cortex 3[H]-PK11195 binding increased in PCS rats compared to control (SM) rats in all anatomical levels and the increase was statistically significant in levels 1, 3 and 4 and did not reach statistical significance in level 2 (Table 1).
Treatment with SB239063 did not affect binding to TSPO in sham or PCS rats in any of the anatomical levels.
Binding of 3[H]-PK11195 to TSPO in the thalamus (Tal)
In the thalamus there is a very strong increase in the binding of 3[H]-PK11195 in all anatomical levels of PCS rats compared to control (SM) rats (Table 1).
Treatment with SB239063 did not affect binding to TSPO in sham or PCS rats in any of the anatomical levels except for anatomical level 1, in which binding is increased in PCS rats (Table 1, Fig. 2b).
Binding of 3[H]-PK11195 to TSPO in the hipocamppus (HP)
In hippocampus 3[H]-PK11195 binding increased in PCS rats compared to control (SM) rats in all anatomical levels. The increase was statistically significant in levels 1 and 4 and did not reach statistical significance in levels 2 and 3 (Table 1).
Treatment with SB239063 did not affect binding to TSPO in sham rats in any of the anatomical levels and increased the binding to TSPO in PCS rats in anatomical levels 1 and 3 (Table 1).
Binding of 3[H]-PK11195 to TSPO in the cerebellum (CB)
Cerebellum was the brain region where the increase of 3[H]-PK11195 binding to TSPO was more intense in PCS rats. There was a very strong increase in the binding both in cerebellar regions 2–3 (CB 2, 3) and 8–9 (CB 8, 9).
Treatment with SB239063 did not affect to the 3[H]-PK11195 binding in sham or PCS rats in cerebellum. (Table 1, Fig. 1).
TSPO is expressed in astrocytes and microglial cells
To assess in which cell types occurs the increase in expression of TSPO, we analyzed the co-expression of TSPO with markers of astrocytes (GFAP) and microglia (Iba-1) using confocal microscopy. We choose the cerebellum because it was the anatomical region where TSPO expression was highest. The analysis of the confocal images shows that the TSPO positive cells expressed also astrocyte and microglia markers (Fig. 3) in all the experimental group. This supports that TSPO increases both in astrocytes and microglia.
Discussion
Under normal conditions TSPO expression in brain is low but increases following brain injury. Although it was initially postulated that TSPO is expressed mainly in activated microglia (Myers et al. 1991; Stephenson et al. 1995; Banati 2002), it has been shown that reactive astrocytes also express TSPO (Chen et al. 2004; Maeda et al. 2007; Rojas et al. 2007). Our results confirm expression of TSPO in both types of glial cells.
In the context of hepatic encephalopathy it has been described that TSPO (known as peripheral benzodiazepine receptor, PBR or PTBR in earliest studies) expression increases in hyperammonia and in PCS rats. This increase was not then related with neuroinflammation, mainly because it was reported before TSPO was identified as a neuroinflammation marker. Giguère et al. (1992) and Raghavendra Rao et al. (1994) observed increased TSPO expression in PCS rats.
The increase of TSPO expression in PCS rats must be consequence of hyperammonemia because hyperammonemia per se enhances TSPO expression, which is also increased in cultured astrocytes exposed to ammonia (Ducis et al. 1989; Itzhak and Norenberg. 1994).
The data reported here (Table 1) show that expression of TSPO (binding of 3[H]-PK11195) is very different in different brain areas both in normal rats and in PCS rats. In normal rats, binding in striatum and thalamus is low (around 15 fmols/mg, depending on the neuroanatomical level) in all neuroanatomical levels. Hippocampus show a slightly higher level (around 24 fmols/mg) and cortex around 30 fmols/mg. The highest expression is in cerebellum, around 40 fmols/mg.
Binding of 3[H]-PK11195 is increased in general in all areas and levels of PCS rats. The difference is statistically significant in most of them. For some areas the increase in some anatomical levels does not reach statistical significance, mainly due to the variability of the results. It may be therefore considered that, in general, binding of 3[H]-PK11195 is increased in general in PCS rats. However the increase is larger in some areas than in others. In PCS rats, binding of 3[H]-PK11195 is only slightly increased in striatum (17-36 fmols/ mg depending on the neuroanatomical level and in hippocampus (31–38 fmols/mg). In cortex the increase is around 50 %, to reach 41–56 fmols/mg. The strongest expression in PCS is also in cerebellum, where binding of 3[H]-PK11195 increases more than 2-fold to reach 80–94 fmols/mg.
The larger expression of TSPO in cerebellum in PCS rats is in agreement with the stronger activation of microglia found in this area by immunohistochemistry in models of chronic hyperammonemia and MHE (Rodrigo et al. 2010; Agusti et al. 2011). The present work also shows that TSPO is expressed in microglia, but also in astrocytes, both in PCS and normal rats.
Portacaval shunt affects all types of glial cells: astrocytes and microglia and oligodendrocytes .
There is a good number of studies on the effects of PCS on astrocytes, showing changes that are different in depending on the brain region (Bodega et al. 1991; Suárez et al. 1997) and on the time after PCS surgery (Ma et al. 1991). In general these studies show enlarged nuclear size (Diemer and Laursen 1977), increased astrocyte cell and cytoplasmic area (Laursen and Diemer 1980) and no evidence of Alzheimer type II cells (Pilbeam et al. 1983). In cerebellum there is a down-regulation of astroglial proteins (Suárez et al. 1992). Bergmann glia accumulated GFAP in response to PCS, whereas astrocytes decreased GFAP immunoreactivity when compared to control rats (Suárez et al. 2005). Although there are very few studies, changes in oligodendrocytes have been also reported (Diemer et al. 1977). Microglia is activated mainly in cerebellum of PCS rats (Agusti et al. 2011). The data reported here show that TSPO is increased in PCS rats, and both microglia and astrocytes could contribute to this increase.
TSPO transports cholesterol from the outer to the inner mitochondria membrane to produce pregnenolone, the parent compound of all steroids (Papadopoulos et al. 1997). Therefore TSPO plays an important role in the neurosteroids synthesis. The concentration of some neurosteroids is increased in brain of cirrhotic patients died in hepatic encephalopathy (Ahboucha et al. 2006), in rats with chronic hyperammonemia (Cauli et al. 2009b) and in rats with hepatic encephalopathy due to portacaval shunt (Ahboucha and Butterworth 2008). This also supports that hyperammonemia would be the main contributor to changes in neurosteroids in HE. Hyperamonemia also alters the modulation by different neurosteroids of NMDA- GABAA- or sigma receptors in cerebellum in vivo (González-Usano et al. 2013). It has been proposed that the neurosteroid system plays a role in the pathophysiology and may be a therapeutic target for the treatment of hepatic encephalopathy (Ahboucha and Butterworth 2007, 2008). The increase in TSPO may be involved in the increased levels of neurosteroids in hyperammonemia and HE.
Recently, we have identified a new therapeutic target for HE, MAP kinase p38. Inhibition of p38 by SB239063 reduces microglial activation as well as inflammatory markers in brain (prostaglandin E2, cyclooxygenase activity, iNOS, IL-1β,TNFα) and restores cognitive and motor function in PCS rats (Agusti et al. 2011). We show here that treatment with SB239063 does not affect binding of 3[H]-PK11195 in normal rats but enhances it in most brain areas of PCS rats. However, the increase is larger in some areas (e.g. cerebellum) than in others (e.g. striatum or frontal cortex), as shown in Fig. 2b, and supplementary Figures 1B, 2b and 3B. Treatment with the p38 inhibitor did not affect the binding of 3[H]-PK11195 in those brain areas (e.g. cerebellum) which already showed a strong increase in PCS rats. However, the p38 inhibitor enhanced binding of 3[H]-PK11195 in those areas showing no or low binding increase due to PCS.
As mentioned above, binding of ligands to TSPO is considered a marker of neuroinflammation. However, inflammation may occur independently of TSPO activation. For example, it has been shown that the TSPO ligand Ro5-4864 did not affect the response of microglia to LPS. Moreover, neither PK11195 nor Ro5-4864 affected the LPS-mediated increase in the number of vimentin-immunoreactive astrocytes (Veiga et al. 2007). The same authors showed later that PK11195 is unable to reduce reactive gliosis induced by kainic acid (Veiga et al. 2005). This TSPO-independent inflammation could be reduced by p38 without reducing TSPO expression. This could explain why SB239063 reduces microglial activation and levels of some inflammatory markers (Agusti et al. 2011), without reducing binding of ligands to TSPO. It is also possible that SB239063-induced reduction of neuroinflammation in PCS rats is not mediated by an effect on TSPO but on a later step in the neuroinflammatory chain.
These data also show that enhanced expression of TSPO is not always associated with cognitive or motor deficits or with enhanced levels of some inflammatory markers. If enhanced TSPO expression plays a role in the mechanisms leading to neurological alterations in chronic hyperammonemia and HE, SB239063 would interfere these mechanisms at a later step.
Supplementary Material
Acknowledgments
Supported in part by grants from Ministerio de Ciencia Innovacion Spain (SAF2011-23051; CSD2008-00005); Consellería Educación, (PROMETEO-2009-027; ACOMP/2012/066; ACOMP/2013/101) and by NIEHS grant number ES007062 to TRG.
Contributor Information
Ana Agusti, Laboratory of Neurobiology, Centro Investigación Príncipe Felipe, Eduardo Primo Yufera, 3, 46012 Valencia, Spain.
Jennifer L. Dziedzic, Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, NY 10027, USA
Vicente Hernandez-Rabaza, Laboratory of Neurobiology, Centro Investigación Príncipe Felipe, Eduardo Primo Yufera, 3, 46012 Valencia, Spain.
Tomas R. Guilarte, Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, NY 10027, USA
Vicente Felipo, Laboratory of Neurobiology, Centro Investigación Príncipe Felipe, Eduardo Primo Yufera, 3, 46012 Valencia, Spain.
References
- Agusti A, Cauli O, Rodrigo R, Llansola M, Hernandez-Rabaza V, Felipo V. p38 MAP kinase is a therapeutic target for hepatic encephalopathy in rats with portacaval shunts. Gut. 2011;60:1572–1579. doi: 10.1136/gut.2010.236083. [DOI] [PubMed] [Google Scholar]
- Ahboucha S, Butterworth RF. The neurosteroid system: an emerging therapeutic target for hepatic encephalopathy. Metab Brain Dis. 2007;22(3–4):291–308. doi: 10.1007/s11011-007-9065-2. [DOI] [PubMed] [Google Scholar]
- Ahboucha S, Butterworth RF. The neurosteroid system: implication in the pathophysiology of hepatic encephalopathy. Neurochem Int. 2008;52(4–5):575–587. doi: 10.1016/j.neuint.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Ahboucha S, Pomier-Layrargues G, Mamer O, Butterworth RF. Increased levels of pregnenolone and its neuroactive metabolite allopregnanolone in autopsied brain tissue from cirrhotic patients who died in hepatic coma. Neurochem Int. 2006;49:372–378. doi: 10.1016/j.neuint.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Amodio P, Montagnese S, Gatta A, Morgan MY. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis. 2004;19:253–267. doi: 10.1023/b:mebr.0000043975.01841.de. [DOI] [PubMed] [Google Scholar]
- Banati R. Visualising microglial activation in vivo. Glia. 2002;40:206–217. doi: 10.1002/glia.10144. [DOI] [PubMed] [Google Scholar]
- Bodega G, Suárez I, Arilla E, Rubio M, Fernández B. Heterogeneous astroglial response in the rat spinal cord to long-term portacaval shunt: an immunohistochemical study. Glia. 1991;4:400–407. doi: 10.1002/glia.440040408. [DOI] [PubMed] [Google Scholar]
- Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 2009;29:783–788. doi: 10.1111/j.1478-3231.2009.02034.x. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Taylor-Robinson SD, Forton MD, Banati RB. In vivo imaging of cerebral “peripheral benzodiazepine binding sites” in patients with hepatic encephalopathy. Gut. 2006;55:547–553. doi: 10.1136/gut.2005.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli O, Rodrigo R, Piedrafita B, Boix J, Felipo V. Inflammation and hepatic encephalopathy: ibuprofen restores learning ability in rats with portacaval shunts. Hepatology. 2007;46:514–519. doi: 10.1002/hep.21734. [DOI] [PubMed] [Google Scholar]
- Cauli O, Rodrigo R, Llansola M, Montoliu C, Monfort P, Piedrafita B, El Mlili N, Boix J, Agustí A, Felipo V. Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab Brain Dis. 2009a;24(1):69–80. doi: 10.1007/s11011-008-9115-4. [DOI] [PubMed] [Google Scholar]
- Cauli O, Mansouri MT, Agusti A, Felipo V. Hyperammonemia increases GABAergic tone in the cerebellum but decreases it in the rat cortex. Gastroenterology. 2009b;136:1359–1367. doi: 10.1053/j.gastro.2008.12.057. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR. Imaging the peripheral benzodiazepine receptor response in central nervous system demyelination and remyelination. Toxicol Sci. 2006;91:532–539. doi: 10.1093/toxsci/kfj172. [DOI] [PubMed] [Google Scholar]
- Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Baidoo K, Verina T, Guilarte TR. Peripheral benzodiazepine receptor imaging in CNS demyelination: functional implications of anatomical and cellular localization. Brain Res Mol Brain Res. 2004;127:1379–1392. doi: 10.1093/brain/awh161. [DOI] [PubMed] [Google Scholar]
- Delavoie F, Li H, Hardwick M, Robert JC, Giatzakis C, Peranzi G, Yao ZX, Maccario J, Lacapere JJ, Papadopoulos V. In vivo and in vitro peripheral-type benzodiazepine receptor polymerization: functional significance in drug ligand and cholesterol binding. Biochemistry. 2003;42:4506–4519. doi: 10.1021/bi0267487. [DOI] [PubMed] [Google Scholar]
- Desjardins P, Butterworth RF. The “peripheral-type” benzodiazepine(omega 3) receptor in hyperammonemic disorders. Neurochem Int. 2002;41:109–114. doi: 10.1016/s0197-0186(02)00031-1. [DOI] [PubMed] [Google Scholar]
- Desjardins P, Bandeira P, Raghavendra Rao VL, Ledoux S, Butterworth RF. Increased expression of the peripheral-type benzodiaze-pine receptor-isoquinoline carboxamide binding protein mRNA in brain following portacaval anastomosis. Brain Res Brain Res Rev. 1997;758(1–2):255–258. doi: 10.1016/s0006-8993(97)00339-9. [DOI] [PubMed] [Google Scholar]
- Diemer NH, Laursen H. Glial cell reactions in rats with hyperammoniemia induced by urease or porto-caval anastomosis. Acta Neurol Scand. 1977;55:425–442. doi: 10.1111/j.1600-0404.1977.tb07623.x. [DOI] [PubMed] [Google Scholar]
- Diemer NH, Klee J, Schröder H, Klinken L. Glial and nerve cell changes in rats with porto-caval anastomosis. Acta Neuropathol. 1977;39:59–68. doi: 10.1007/BF00690386. [DOI] [PubMed] [Google Scholar]
- Ducis I, Norenberg NL, Norenberg MD. Effect of ammonium chloride on the astrocyte benzodiazepine receptor. Brain Res. 1989;493(2):362–5. doi: 10.1016/0006-8993(89)91171-2. [DOI] [PubMed] [Google Scholar]
- Felipo V, Ordoño JF, Urios A, El Mlili N, Giménez-Garzó C, Aguado C, González-Lopez O, Giner-Duran R, Serra MA, Wassel A, Rodrigo JM, Salazar J, Montoliu C. Patients with minimal hepatic encephalopathy show impaired mismatch negativity correlating with reduced performance in attention tests. Hepatology. 2012;55(2):530–9. doi: 10.1002/hep.24704. [DOI] [PubMed] [Google Scholar]
- Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy. Definition, nomenclature, diagnosis and quantification: final report of the working party at the 11Word congress gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, Winston A. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART; An 11C-PK11195 PET study. AIDS. 2013 2013 Jul 24; doi: 10.1097/01.aids.0000432467.54003.f7. [DOI] [PubMed] [Google Scholar]
- Giguère JF, Hamel E, Butterworth RF. Increased densities of binding sites for the “peripheral-type” benzodiazepine receptor ligand [3H]PK11195 in rat brain following portacaval anastomosis. Brain Res. 1992;585:295–298. doi: 10.1016/0006-8993(92)91222-z. [DOI] [PubMed] [Google Scholar]
- González-Usano A, Cauli O, Agustí A, Felipo V. Hyperamonemia alters the modulation by different neurosteroids of the glutamate-nitric oxide-cyclic GMP pathway through NMDA- GABAA- or sigma receptors in cerebellum in vivo. J Neurochem. 2013;125(1):133–43. doi: 10.1111/jnc.12119. [DOI] [PubMed] [Google Scholar]
- Groeneweg M, Quero JC, De Bruijn I, Hartmann IJ, Essink-bot ML, Hop WC, et al. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28:45–49. doi: 10.1002/hep.510280108. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Kuhlmann AC, O'Callaghan JP, Miceli RC. Enhanced expression of peripheral benzodiazepine receptors in trimethyltin-exposed rat brain: a biomarker of neurotoxicity. Neurotoxicology. 1995;16(3):441–50. [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS. Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neuroscience. 2003;122(2):499–513. doi: 10.1016/s0306-4522(03)00476-7. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Norenberg M. Attenuation of ammonia toxicity in mice by PK 11195 and pregnenolone sulfate. Neurosci Lett. 1994;182(2):251–4. doi: 10.1016/0304-3940(94)90809-5. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Panickar KS, Norenberg MD. Effects on free radical generation by ligands of the peripheral benzodiazepine receptor in cultured neural cells. J Neurochem. 2002;83:1226–1234. doi: 10.1046/j.1471-4159.2002.01261.x. [DOI] [PubMed] [Google Scholar]
- Jorda EG, Jimenez A, Verdaguer E, Canudas AM, Folch J, Sureda FX, Camins A, Pallas M. Evidence in favour of a role for peripheral-type benzodiazepine receptor ligands in amplification of neuronal apoptosis. Apoptosis. 2005;10:91–104. doi: 10.1007/s10495-005-6064-9. [DOI] [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte TR. The peripheral benzodiazepine receptor is a sensitive indicator of domoic acid neurotoxicity. Brain Res. 1997;751:281–288. doi: 10.1016/s0006-8993(96)01409-6. [DOI] [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte TR. Regional and temporal expression of the peripheral benzodiazepine receptor in MPTP neurotoxicity. Toxicol Sci. 1999;48:107–116. doi: 10.1093/toxsci/48.1.107. [DOI] [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte T. Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J Neurochem. 2000;74(4):1694–704. doi: 10.1046/j.1471-4159.2000.0741694.x. [DOI] [PubMed] [Google Scholar]
- Laursen H, Diemer NH. Morphometry of astrocyte and oligodendrocyte ultrastructure after portocaval anastomosis in the rat. Acta Neuropathol. 1980;51:65–70. doi: 10.1007/BF00688851. [DOI] [PubMed] [Google Scholar]
- Lavoie J, Layrargues GP, Butterworth RF. Increased densities of peripheral-type benzodiazepine receptors in brain autopsy samples from cirrhotic patients with hepatic encephalopathy. Hepatology. 1990;11(5):874–878. doi: 10.1002/hep.1840110524. [DOI] [PubMed] [Google Scholar]
- Lee SH, Fisher B. Portacaval shunt in the rat. Surgery. 1961;50:668–672. [PubMed] [Google Scholar]
- Ma KC, Chang ZH, Shih H, Zhu JH, Wu JY. The compensatory ‘rebound’ of reactive astrogliosis: glial fibrillary acidic protein immunohistochemical analysis of reactive astrogliosis after a puncture wound to the brain of rats with portocaval anastomosis. Acta Neuropathol. 1991;82:72–77. doi: 10.1007/BF00310926. [DOI] [PubMed] [Google Scholar]
- Maeda J, Higuchi M, Inaji M, Ji B, Haneda E, Okauchi T, Zhang MR, Suzuki K, Suhara T. Phase-dependent roles of reactive microglia and astrocytes in nervous system injury as delineated by imaging of peripheral benzodiazepine receptor. Brain Res Mol. 2007;1157:100–111. doi: 10.1016/j.brainres.2007.04.054. [DOI] [PubMed] [Google Scholar]
- Montoliu C, Piedrafita B, Serra MA, del Olmo JA, Ferrandez A, Rodrigo JM, et al. Activation of soluble guanylate cyclase by nitric oxide in lymphocytes correlates with minimal hepatic encephalopathy in cirrhotic patients. J Mol Med. 2007;85:233–241. doi: 10.1007/s00109-006-0149-y. [DOI] [PubMed] [Google Scholar]
- Montoliu C, Serra MA, del Olmo JA, Urios A, Rodrigo JM, Felipo V. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J Clin Gastroenterol. 2009;43(3):272–279. doi: 10.1097/MCG.0b013e31815e7f58. [DOI] [PubMed] [Google Scholar]
- Myers R, Manjil LG, Cullen BM, Price GW, Frackowiak RS, Cremer JE. Macrophage and astrocyte populations in relation to [3H]PK11195 binding in rat cerebral cortex following a local ischaemic lesion. J Cereb Blood Flow Metab. 1991;11:314–322. doi: 10.1038/jcbfm.1991.64. [DOI] [PubMed] [Google Scholar]
- Odeh M, Sabo E, Srugo I, Oliven A. Serum levels of tumor necrosis factor-alpha correlate with severity of hepatic encephalopathy due to chronic liver failure. Liver Int. 2004;24(2):110–116. doi: 10.1111/j.1478-3231.2004.0894.x. [DOI] [PubMed] [Google Scholar]
- Odeh M, Sabo E, Srugo I, Oliven A. Relationship between tumor necrosis factor-alpha and ammonia in patients with hepatic encephalopathy due to chronic liver failure. Ann Med. 2005;37:603–612. doi: 10.1080/07853890500317414. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, Hardwick M, Li H, Vidic B, Brown AS, Reversa JL, Bernassau JM, Drieu K. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62(1):21–8. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- Pilbeam CM, Anderson RM, Bhathal PS. The brain in experimental portal-systemic encephalopathy. I. Morphological changes in three animal models. J Pathol. 1983;140:331–345. doi: 10.1002/path.1711400403. [DOI] [PubMed] [Google Scholar]
- Raghavendra Rao VL, Audet R, Therrien G, Butterworth RF. Tissue specific alterations of binding sites for peripheral-type benzodiazepine receptor ligand [3H]-PK11195 in rats following portacaval anastomosis. Dig Dis Sci. 1994;39:1055–1063. doi: 10.1007/BF02087558. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Cauli O, Gomez-Pinedo U, Agusti A, Hernandez-Rabaza V, Garcia-Verdugo JM, Felipo V. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology. 2010;139(2):675–684. doi: 10.1053/j.gastro.2010.03.040. [DOI] [PubMed] [Google Scholar]
- Rojas S, Martín A, Arranz MJ, Pareto D, Purroy J, Verdaguer E, Llop J, et al. Imaging brain inflammation with [(11)C]PK11195 by PET and induction of the peripheral-type benzodiazepine receptor after transient focal ischemia in rats. J Cereb Blood Flow Metab. 2007;27:1975–1986. doi: 10.1038/sj.jcbfm.9600500. [DOI] [PubMed] [Google Scholar]
- Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96:2718–2723. doi: 10.1111/j.1572-0241.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- Shawcross DL, Davies N, Williams R, Jalan R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol. 2004;40:247–254. doi: 10.1016/j.jhep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Stephenson DT, Schober D, Smalstig EB, Mincy RE, Gehlert DR, Clemens Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J Neurosci Res. 1995;15:5263–5274. doi: 10.1523/JNEUROSCI.15-07-05263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez I, Bodega G, Arilla E, Rubio M, Villalba R, Fernández B. Different response of astrocytes and Bergmann glial cells to portacaval shunt: an immunohistochemical study in the rat cerebellum. Glia. 1992;6:172–179. doi: 10.1002/glia.440060304. [DOI] [PubMed] [Google Scholar]
- Suárez I, Bodega G, Arilla E, Fernández B. Region-selective glutamine synthetase expression in the rat central nervous system following portocaval anastomosis. Neuropathol Appl Neurobiol. 1997;23:254–261. [PubMed] [Google Scholar]
- Suárez I, Bodega G, Rubio M, Fernández B. Down-regulation of astroglial proteins in the rat cerebellum after portacaval anastomosis. Neuropathol Appl Neurobiol. 2005;31:163–169. doi: 10.1111/j.1365-2990.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- Veiga S, Azcoitia I, Garcia-Segura LM. Ro5-4864, a peripheral benzodiazepine receptor ligand, reduces reactive gliosis and protects hippocampal hilar neurons from kainic acid excitotoxicity. J Neurosci Res. 2005;80:129–137. doi: 10.1002/jnr.20430. [DOI] [PubMed] [Google Scholar]
- Veiga S, Carrero P, Pernia O, Azcoitia I, Garcia-Segura LM. Translocator protein 18 kDa is involved in the regulation of reactive gliosis. Glia. 2007;55:1426–1436. doi: 10.1002/glia.20558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.