Abstract
Insulin resistance is a major underlying mechanism for the “metabolic syndrome”, which is also known as insulin resistance syndrome. Metabolic syndrome is increasing at an alarming rate, becoming a major public and clinical problem worldwide. Metabolic syndrome is represented by a group of interrelated disorders, including obesity, hyperglycemia, hyperlipidemia, and hypertension. It is also a significant risk factor for cardiovascular disease and increased morbidity and mortality. Animal studies demonstrate that insulin and its signaling cascade normally control cell growth, metabolism and survival through activation of mitogen-activated protein kinases (MAPKs) and phosphotidylinositide-3-kinase (PI3K), of which activation of PI-3K-associated with insulin receptor substrate-1 and -2 (IRS1, 2) and subsequent Akt→Foxo1 phosphorylation cascade has a central role in control of nutrient homeostasis and organ survival. Inactivation of Akt and activation of Foxo1, through suppression IRS1 and IRS2 in different organs following hyperinsulinemia, metabolic inflammation, and over nutrition may provide the underlying mechanisms for metabolic syndrome in humans. Targeting the IRS→Akt→Foxo1 signaling cascade will likely provide a strategy for therapeutic intervention in the treatment of type 2 diabetes and its complications. This review discusses the basis of insulin signaling, insulin resistance in different mouse models, and how a deficiency of insulin signaling components in different organs contributes to the feature of the metabolic syndrome. Emphasis will be placed on the role of IRS1, IRS2, and associated signaling pathways that couple to Akt and the forkhead/winged helix transcription factor Foxo1.
Introduction
Obesity, hyperglycemia, hyperlipidemia, and hypertension cluster together described as “Insulin resistance syndrome” or “Syndrome X” by Reaven and others (Moller and Kaufman 2005; Reaven 1988). The constellation of metabolic abnormalities tightly correlates with cardiovascular dysfunction, resulting in high morbidity and mortality rates (Reaven 2005a). The term “metabolic syndrome” has been adopted (DeFronzo and Ferrannini 1991; Kahn, et al. 2005; Reaven 1988) and clinical features for the syndrome established (Table 1) (2002; Alberti, et al. 2005; Alberti and Zimmet 1998; Grundy, et al. 2005; Simmons, et al. 2010). Metabolic syndrome is a major risk factor for type 2 diabetes mellitus, which afflicts 8% of Americans and 11% of Chinese and threatens public health worldwide (Alberti et al. 2005; Cornier, et al. 2008; Eckel, et al. 2005; Roger, et al. 2011). An estimated 366 million people had diabetes worldwide in 2011 and this number is predicted to rise to 522 million by 2030, with a high economic cost for disease management (Whiting, et al. 2011).
Table 1. Clinical criteria for diagnosis of the metabolic syndrome.
| Metabolic Parameters | ATP III | WHO | IDF | Diabetes |
|---|---|---|---|---|
| Abdominal obesity (cm) | ||||
| Men: waist circumference | > 102 | > 102 | > 94 | |
| Women: waist circumference | > 88 | > 88 | > 80 | |
| Fasting glucose (mg/dl) | > 110, <126 | > 110 | > 100 | > 130 |
| Blood pressure (mm Hg) | > 130/85 | 140/90 | >130/85 | |
| Triglyceride (mg/dl) | 150 | 150 | 150 | |
| HDL cholesterol (mg/dl) | ||||
| Men | <40 | <35 | <40 | |
| Women | <50 | <39 | <50 | |
| References | 2002, Circulation | 2004, Lancet | Alberti, K, 1998 |
Abbreviation: ATPIII: Adult Treatment Panel III based on the National Education Program (NCEP); WHO: World Health Organization; IDF: International Diabetes Foundation
Patients with type 1 diabetes suffer from insulin deficiency, owing to pancreatic β-cell failure and insulin is a primary and effective therapy to lower hyperglycemia and reduce the risk of cardiovascular dysfunction, as demonstrated by the Diabetes Control and Complications trial (DCCT) (Nathan, et al. 2005; Wilson 2011). However, patients with type 2 diabetes are non-insulin dependent, in which intensive insulin therapy lowers blood glucose, but increases body weight and cardiovascular risk, as demonstrated in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (Wilson 2011). Intensive insulin therapy dose not provided much cardio-protective benefit in adults and two-thirds of patients with type 2 diabetes die of heart failure. Understanding the action of insulin and finding an effective management of metabolic syndrome, type 2 diabetes mellitus and associated cardiovascular dysfunction have important clinical implications.
Hyperinsulinemia, a major characteristic of the metabolic syndrome, results from over secretion of insulin from pancreatic β-cells and is recognized as a primary contributor to the development of type 2 diabetes and cardiovascular dysfunction (Battiprolu, et al. 2010; Cao, et al. 2010; Qi, et al. 2013; Reaven 2005b). Understanding the mechanisms responsible for insulin action and resistance will be critical for the management of metabolic syndrome and development of therapeutic interventions to prevent or treat type 2 diabetes. In this review, we provide mechanistic insights from animal studies, as to how insulin resistance in different organs contributes to the metabolic syndrome at a molecular, biochemical, and physiological level.
Part 1: Molecular basis of insulin signaling
Insulin and signal transduction studies have resulted in breakthroughs in the area of diabetes and biomedical research. Innovative attempts on insulin purification from the pancreas of animals, DNA and protein sequencing, crystallography, and radioimmunoassay led by Banting, Sanger, Hodgkin, and Yalow, who all received Nobel prizes in 1923, 1958, 1969, and 1977, respectively (Yalow and Berson 1960). With the advent of molecular cloning technology in 1980, the genes encoding insulin receptor and insulin receptor substrate proteins were identified and sequenced (Kasuga, et al. 1983; Sun, et al. 1991; White and Kahn 1994; White, et al. 1985).
1.1 Insulin receptor substrate-1 and -2
Insulin receptor, a glycoprotein consisting of an extracellular α-subunit (135 kDa) and a transmembrane β-subunit (95 kDa), is an allosteric enzyme in which the α-subunit inhibits tyrosine kinase activity of the β-subunits. Insulin binding to the α-subunit results in receptor dimerization to form the α2β2 complex in the cell membrane and autophosphorylation of the β-subunit at Tyr1158, Try1162, and Tyr1163, the first step of insulin receptor activation. The insulin receptor tyrosine kinase activation recruits and phosphorylates several substrates, including IRS1-4, Shc, Grb-2-assocated protein (Gab1), Dock1, Cbl, and APS adaptor proteins, all of which provide specific docking sites for recruitment of other downstream signaling proteins, leading to activation of both Ras→MAP kinases and PI-3K→Akt signaling cascades (White 2003).
Insulin receptor and its homologous insulin-like growth factor-1 receptor (IGF-1R), can also form heterodimers (IR/IGF1R) that modulate the selectivity and affinity for insulin and IGF-1 in activating downstream signaling molecules (White 2003). Moreover, a recent report indicates that insulin receptor forms a hybrid complex with Met, a transmembrane tyrosine kinase cell surface receptor for hepatocyte growth factor (HGF) and structurally related to the insulin receptor (Fafalios, et al. 2011). The IR/Met hybrid complex results in robust signal output, by activating insulin receptor downstream signaling cascades and mediates metabolic effects of insulin (Fafalios et al. 2011).
IRS protein and the docking proteins for insulin receptor provide interfaces that insulin, IGF-1, or HGF signaling propagates and engages in similar intracellular signaling components. IRS proteins are characterized by the presence of an NH2-terminal pleckstrin homology (PH) domain adjacent to a phosphotyrosine binding (PTB) domain, followed by a COOH-terminal tail that contains numerous tyrosine and serine/threonine phosphorylation sites (Copps and White 2012). The PH domain mediates cell membrane interactions and PTB domain binds to the phosphorylated NPXpY-motif (Asn-Pro-Xaa-Try (pi): X-any amino acid; p: inorganic phosphate) of activated insulin receptor. The COOH-terminal of each IRS protein has about 20 potential tyrosine phosphorylation sites that act as on/off switches to transduce insulin action, recruiting downstream signaling proteins, including PI3K subunit, phosphotyrosine phosphatase SHP-2, and adaptor molecules such as Grb-2, SOCS3, Nck, Crk, SH2B and others (Sun and Liu 2009; White 2003).
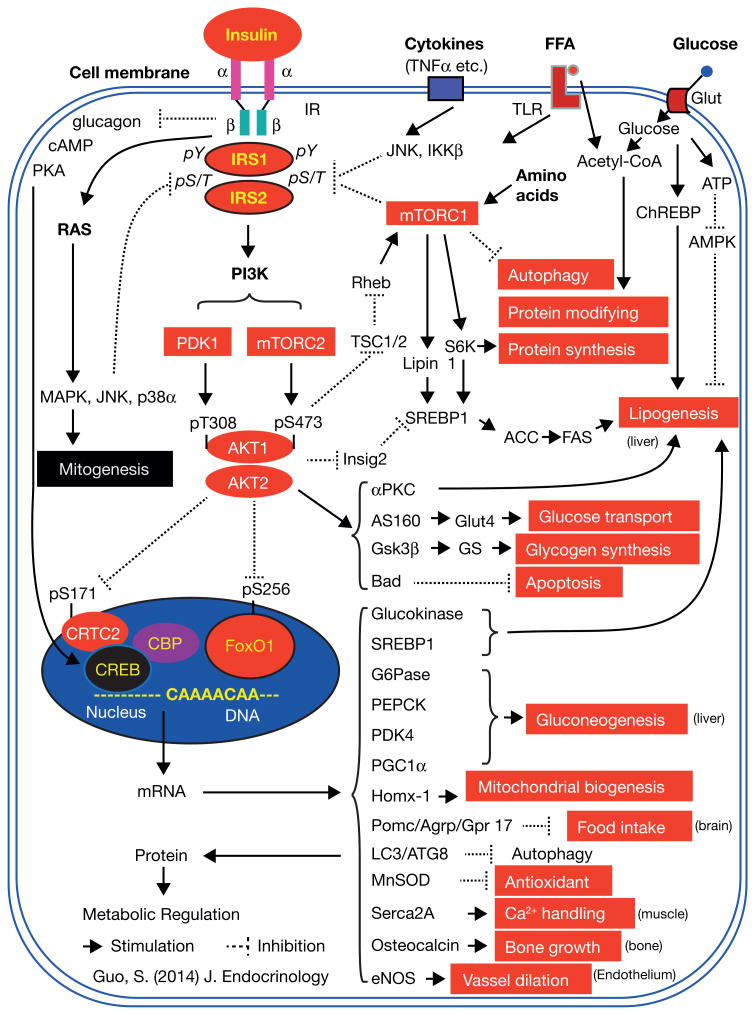
Activation of Ras→MAP kinases mediates the effect of insulin on mitogenesis and cellular growth; however, activation of PI-3K generates phosphatidylinositol (3,4,5)-triphosphate (PIP3), a second messenger activating 3-phosphoinositide dependent protein kinase-1 and -2 (PDK1 and PDK2), which mediate the effect of insulin on metabolism and pro-survival. PDK1 and PDK2 in turn activate the protein kinase Akt (PKB), by inducing phosphorylation at T308 and S473, respectively, and both PDK1 and PDK2 are crucial for Akt activation (Figure 1).
Figure 1.
1.2 PDK1 and TORC2→Akt→TORC1 signaling cascades
Although PDK1 phosphorylates the T308 of Akt resulting in Akt activation and has a profound effect on cellular survival and metabolism (Alessi, et al. 1997; Kikani, et al. 2005; Williams, et al. 2000), understanding the action of PDK2 remain more of an enigma (Dong and Liu 2005). mTORC2, which interacts with rictor adaptor protein, is a rapamycin-insensitive companion of mTOR, has been identified to be PDK2 that phosphorylates the S473 of Akt (Alessi et al. 1997; Sarbassov, et al. 2006; Sarbassov, et al. 2005). mTOR (mammalian Target of Rapamycin) is a highly conserved protein kinase that controls cell growth and metabolism in response to nutrients, growth factors and energy status, and which exists in two distinct complexes called complex 1 (mTORC1) and complex 2 (mTORC2) (Sengupta, et al. 2010).
mTROC2 phosphorylates and activates Akt and other protein kinases, such as PKC, controlling cell survival and energy homeostasis (Hagiwara, et al. 2012; Sarbassov et al. 2006). mTROC2, through Akt, promotes expression and activation of the Srebp1 transcription factor, a family member of the sterol regulatory element binding proteins (SREBPs) that promote lipid and cholesterol synthesis (Yecies, et al. 2011). Moreover, mTORC2 and PDK1 suppress the Foxo1 forkhead transcription factor that promotes gluconeogenesis, mediating the effect of insulin on suppression of hepatic glucose production (Hagiwara et al. 2012) (Figure 1).
mTORC1 is the mTOR interacting with the raptor adaptor protein which is rampamycin-senstive and is activated by RhebGTPase, via suppression of TSC2 following Akt activation (Sengupta et al. 2010). mTROC1, which is not required for hepatic gluocoenogenesis (Li, et al. 2010), has as its substrates ribosomal protein S6 kinase (S6K) and eukaryotic initiation factor 4E-binding protein (4E-BP), both of which control protein synthesis. Recent data indicate that mTROC1 promotes lipogenenesis via phosphorylating a phosphatidic acid phosphatase Lipin 1, and nuclear translocation of Lipin 1 stimulating SREBP1c and lipogenesis (Li et al. 2010; Peterson, et al. 2011). S6K is required for stimulating SREBP1c in rat hepatocytes (Owen, et al. 2012). Additionally, mTORC1 is also activated by nutrients, such as amino acids, suppressing cellular autophagy. Autophagy is a basic catabolic mechanism that involves cell degradation of unnecessary or dysfunctional cellular components through lysosomal machinery and expression of a number of autophagy genes (Klionsky 2007). The breakdown of cellular components ensures cellular survival during starvation by maintaining cellular energy levels (Liu, et al. 2009b). Thus, TORC1 and TORC2 serve as sensors and mediators for the action of both nutrients and hormones in cells.
1.3 Targets of Akt in metabolic control
Akt phosphorylates a number of downstream targets, including inhibitors of macromolecular synthesis as follows: 1) it phosphorylates and inhibits glycogen synthase kinase-3β (Gsk3β), which in turn dephosphorylates and activates glycogen synthase (GS); 2) inhibits tuberous sclerosis protein-2 (TSC-2), thereby activating RhebGTPase for mTORC1 and S6K activation, which promote protein synthesis (Inoki, et al. 2002). Akt also phosphorylates many other mediators in control of numerous biological responses, including AS160 for Rab10GTPase activation and Glut4 translocation; Bad for inhibition of apoptosis; and PDE3B for cAMP degradation. Akt phosphorylates and inhibits CRTC2 (cAMP response element binding protein (CREB)-regulated transcription coactivator-2), a CREB coactivator that increases hepatic gluoconeogenesis (Wang, et al. 2010). Most importantly, Akt regulates metabolism and survival by controlling expression of a number of genes through transcription factors, such as SREBP1c and Foxo1.
Akt phosphorylates and stimulates Srepb1c, promoting liver lipogenesis through suppression of insig2, a protein of the endoplasmic reticulum that blocks processing of SREBP1c activation, by binding to SCAP (SREBP cleavage-activating protein) and preventing it from escorting SREBPs to the Golgi (Yabe, et al. 2002). By contrast, Akt phosphorylates Foxo1 at S256 and inhibits Foxo1 transcriptional activity, suppressing hepatic glucose production in liver and promoting cell survival in heart (Battiprolu, et al. 2012; Evans-Anderson, et al. 2008; Guo, et al. 1999; Hannenhalli, et al. 2006; Matsumoto, et al. 2007; Zhang, et al. 2012). Many of these phosphorylation events are indicators of insulin signaling, and Akt→Foxo1 phosphorylation serve as powerful indicators for insulin sensitivity on metabolic regulation in a variety of cells and tissues (Gonzalez, et al. 2011; Guo, et al. 2009; Guo, et al. 2006; Qi et al. 2013) (Figure 1).
1.4 Forkhead transcription factor Foxo1 signaling
Foxo1, a member of the O-class of forkhead/winged helix transcription factors (Foxo), was first identified as an Akt substrate in insulin signaling (Guo et al. 1999; Rena, et al. 1999). Insulin suppresses gene expression of IGF-binding protein-1 (IGFBP-1) through a conserved insulin response element (IRE: CAAAACAA), located on the IGFBP-1 promoter region (Cichy, et al. 1998; Guo et al. 1999). A similar sequence is present in the promoter regions of a number of genes, including phosphoenolpyruvate carboxykinase (Pepck) and glucose-6-phosphatase (G6pc), two rate-limiting enzymes for gluconeogenesis (Schmoll, et al. 2000; Yeagley, et al. 2001). We demonstrated that Foxo1 serves as the endogenous transcription factor interacting with the insulin response element for activation of target gene expression (Guo et al. 1999; Zhang et al. 2012). Foxo1 has three Akt phosphorylation sites at T24, S256 and S319 (Rena et al. 1999) and phosphorylation of these residues, by insulin, promotes Foxo1 cytoplasmic translocation from the nucleus and interaction with Skp2, a subunit of the Skip1/Cul1/-F-box protein for Foxo1 ubiquitination, and inhibits Foxo1-mediated gene transcription, by removing Foxo1 from gene transcriptional machinery (Biggs, et al. 1999; Huang, et al. 2005; Matsuzaki, et al. 2003; Nakae, et al. 1999; Rena, et al. 2001; Rena, et al. 2002; Woods, et al. 2001). This provides a molecular link by which Foxo1 integrates cell surface receptor signaling into gene transcriptional profiling (Guo et al. 1999). Other members of O-class of forkhead family include Foxo3, Foxo4, and Foxo6, sharing the conserved Akt phosphorylation motif -RXRXXS/T (R-arginine, X-any amino acid, and S/T, Akt phosphorylation site of serine or threonine). Mice lacking Foxo1 were embryonic lethal and failed to complete embryonic angiogenesis, while mice lacking Foxo3 or Foxo4 survived beyond parturition (Hosaka, et al. 2004). Mice lacking hepatic Foxo1, rather than Foxo3 or Foxo4, had lower hepatic glucose production and blood glucose, and mice lacking both Foxo1 and Foxo3 or all three Foxo1, 3, and 4 exhibited a further reduction in blood glucose (Estall 2012; Haeusler, et al. 2010; Zhang et al. 2012). Similarly, mice lacking Foxo6 also had impaired hepatic glucose production (Kim, et al. 2011; Kim, et al. 2013). Thus, each of the Foxo members has redundant, as well as distinct roles in regulating physiological functions, the mechanisms of which are incompletely understood; but, inhibiting Foxo transcription factors mediates many of the metabolic effects of insulin (Figure 1).
Part 2: Mechanisms for insulin resistance
During the postprandial state, insulin secretion from the pancreatic β-cells controls systemic nutrient homeostasis by promoting anabolic processes in a variety of tissues. Insulin stimulates glucose influx into muscle and fat, protein and glycogen synthesis in muscle and liver, lipid synthesis and storage in liver and fat, while it inhibits fatty acid oxidation, glycogenolysis, and gluconeogenesis, as well apoptosis and autophagy in insulin responsive tissues. During the fasting state, insulin secretion decreases, and tissues coordinate with counter-regulatory hormones, such as glucagon in liver and fat, in favor of using fatty acids largely derived from adipocyte lipolysis for ATP generation and maintenance of glucose homeostasis. The substrate preferences for metabolic adaptation, during the transit from fasting to the postprandial state, are tightly controlled by insulin under physiological conditions (Randle, et al. 1963). This adaptive transition reflects the action of insulin in insulin responsive organs, while it is largely blunted in organs with insulin resistance preceding the development of type 2 diabetes (Johnson and Olefsky 2013).
2.1 Loss of IRS1 and IRS2 results in insulin resistance
Gene knockout experiments in mice have helped to elucidate the role of the insulin receptor, IRS1 and IRS2, in control of growth and nutrient homeostasis (Guo 2013). Mice lacking the insulin receptor gene were born with slight growth retardation but rapidly developed hyperglycemia and hyperinsulinemia, followed by diabetic ketoacidosis and early postnatal death (Accili, et al. 1996; Joshi, et al. 1996). Although both IRS1 and IRS2 null mice were embryonic lethal (Withers, et al. 1999), systemic IRS1 null mice displayed growth retardation and peripheral resistance to insulin and IGF-1, mainly in skeletal muscle; but, avoided developing diabetes because of IRS2-dependent pancreatic β-cell growth and compensatory insulin secretion (Araki, et al. 1994). Systemic IRS2 null mice displayed metabolic defects in liver, muscle, and adipose tissues; but, developed diabetes secondary to pancreatic β-cell failure (Withers, et al. 1998).
Tissue-specific gene knockout studies in mice provided new insights into the action of the insulin receptor and control of glucose homeostasis and body weight (Biddinger and Kahn 2006; Nandi, et al. 2004; Rask-Madsen and Kahn 2012). Mice lacking insulin receptor in liver, pancreatic β-cells, fat, or brain developed hyperglycemia, hyperlipidemia, hyperinsulinemia, and obesity (Boucher and Kahn 2013; Bruning, et al. 2000; Kulkarni, et al. 1999; Michael, et al. 2000). Deficiency of insulin receptor in skeletal muscle also impaired glucose tolerance, even though circulating blood glucose levels were normal (Bruning, et al. 1998; Katic, et al. 2007; Kulkarni et al. 1999). Moreover, reconstitution of insulin receptor in liver, β-cells, and brain rescued diabetes in mice lacking the insulin receptor and prevented premature postnatal death (Lin and Accili 2011; Okamoto, et al. 2004), suggesting that liver, pancreatic β-cells, and brain are crucial for maintenance of glucose homeostasis.
Recently, we demonstrated that deletion of both IRS1 and IRS2 genes in the liver of mice, designated as L-DKO mice (Liver Double IRS1 and IRS2 gene Knockout mice), prevented activation of hepatic Akt→Foxo1 phosphorylation and resulted in the development of hyperglycemia, hyperinsulinemia, insulin resistance, and hypolipidemia (Dong, et al. 2008; Guo et al. 2009). Deletion of both IRS1 and IRS2 in cardiac muscle, diminished Akt phosphorylation (T308 and S473) and Foxo1 phosphorylation (S253), and caused sudden death of male animals at the age of 6 to 8 weeks (Qi et al. 2013) (Table 2). These results suggest that loss of IRS1 and IRS2 may serve as a key component for insulin resistance and cardiac failure.
Table 2. Phenotypes of conditional IRS knockout and Foxo knockout mice using the Cre-LoxP genetic approaches.
| Tissue-specific IRS or Foxo null mice genotype | Phenotype | Cre- mice | Ref. |
|---|---|---|---|
| Hypothalamic & β-cell IRS2-/- | Obesity; hyperglycemia; insulin resistance | Rip-cre | Lin X |
| Hypothalamic (AGRP neuron) Foxo1-/- | Leanness, reduced food intake; increase insulin and leptin sensitivity | Agrp-cre | Ren H |
| Hypothalamic (POMC neuron) Foxo1-/- | Leanness, reduced food intake; increase insulin and leptin sensitivity | Pomc-cre | Plum L |
| Leptin receptor neuron IRS2-/- | Obesity; hyperglycemia; insulin resistance | Lep-R-cre | Sadagur |
| Leptin receptor neuron Foxo1-/-∷IRS2-/- | Leanness; protect obesity and hyperglycemia from IRS2 deficiency | Lep-R-cre | Sadagur |
| Liver IRS1-/- | Normal glucose; severe insulin resistance on high-fat diet | Alb-cre | Guo S |
| Liver IRS2-/- | Normal glucose | Alb-cre | Guo S |
| Liver IRS1-/-∷IRS2-/- | Hyperglycemia; insulin resistance | Alb-cre | Guo S Kubota |
| Liver Foxo1-/- | Reduced blood glucose | Alb-cre | Zhang K |
| Liver Foxo3-/- | Normal glucose | Alb-cre | Zhang K |
| Liver Foxo4-/- | Normal glucose | Alb-cre | Zhang K |
| Liver Foxo1-/-∷ Foxo3-/-∷ Foxo4-/- | Reduced blood glucose; increased triglycerides and hepatic steatosis | Alb-cre | Zhang K Haeusler |
| Liver Foxo1-/-∷ IRS1/-∷ IRS2-/- | Protect hyperglycemia from hepatic IRS1 and IRS2 deficiency | Alb-cre | Dong X |
| Skeletal&Cardiac muscle IRS1-/-∷IRS2-/- | Normal glucose; normal insulin; die 2 weeks after birth | MCK-cre | Long Y |
| Cardiac IRS1-/-∷IRS2-/- | Male die of heart failure at the age of 7 weeks; hyperlipidemia | αMhc-cre | Qi Y |
| Cardiac Foxo1-/- | Prevent heart failure from high-fat diet | αMhc-cre | Battipr K |
| Cardiac Foxo3-/- | No prevent heart failure from high-fat diet | αMhc-cre | Battipr K |
| Pancreatic β-cell Foxo1-/- | Reduced β-cell regeneration; β-cell dedifferentiated into progenitor-like cells or α-cells; hyperglucagonemia; hyperglycemia | Ins2-cre | Talchaic |
| Endothelium IRS1-/-∷IRS2-/- | Reduced Akt and eNOS phosphorylation; impaired skeletal muscle glucose uptake and insulin resistance | Tie2-cre | Kubota T |
| Endothelium Foxo1-/-∷Foxo3-/-∷Foxo4-/- | Increased eNOS phosphorylation; reduced inflammation and oxidative stress of endothelium; prevented atherosclerosis | Tie2-cre | Tsuchiya |
| Bone osteoblasts Foxo1-/- | Increased osteocalcin and insulin production; reduced blood glucose concentration | Collagen I-cre | Rached |
Abbreviation of promoters driving cre expression: RIP- rat insulin promoter; Agrp- agouti-regulated peptide; Pomc- pro-opiomelanocortin; Lep R- leptin receptor; Alb- albumin; MCK- muscle creatine kinase; αMHC- myosin heavy chain α; Ins2- insulin 2; Tie2- Angiopoietin 2 receptor.
2.2 Loss of IRS1 and IRS2 links to inactivation of PI3K and Akt
IRS1 and IRS2 are tightly associated with PI3K and Akt activation and minimally with MAP kinase activity. Deficiency of IRS1 and IRS2 causes biased PI3K inactivation and sustained MAP kinase activation in liver and heart of mice (Chatrchyan, et al. 2013; Dong et al. 2008; Guo et al. 2009; Qi et al. 2013). Differential PI3K inactivation and MAPK activation by loss of IRS1 and IRS2 in vivo, may provide a fundamental mechanism elucidate the prevalence of insulin resistance and association with type 2 diabetes, obesity and cardiovascular dysfunction. Inhibition of IRS1 and IRS2 inactivates PI3K, disrupting nutrient homeostasis and prolongs activation of MAP kinases (ERK1/2, p38, & JNK), promoting mitogenesis and overgrowth, resulting in obesity. Supporting this concept, mice lacking either the PI3K catalytic subunit or Akt2 exhibited insulin resistance and type 2 diabetes (Brachmann, et al. 2005; Cho, et al. 2001), while mice lacking ERK1 prevented growth of adipocytes and improve insulin resistance following a high-fat diet treatment (Bost, et al. 2005). Furthermore, mice lacking Gab1, which is an ERK activator, enhanced insulin sensitivity with elevated hepatic Akt activity (Bard-Chapeau, et al. 2005).
2.3 Inactivation of PI3K→Akt→Foxo1 signaling causes diabetes and heart failure
Activation of PI3K and Akt plays a central role in metabolic regulation, which is supported by the studies in animals and humans. Hepatic inactivation of PI3K, PDK1, mTORC2, or both Akt1 and Akt2 is sufficient for induction of hyperglycemia, hyperinsulinemia and hypolipidemia (Hagiwara et al. 2012; Lu, et al. 2012; Miyake, et al. 2002; Mora, et al. 2005). Mice lacking Akt2 developed type 2 diabetes mellitus (Cho et al. 2001), and Akt2 mutation has also been described in patients with type 2 diabetes mellitus (George, et al. 2004). Expression of constitutively active Foxo1, when three Akt sites were mutated to alanine, blocking phosphorylation in either liver causing insulin resistance (Zhang, et al. 2002) or in hearts, resulting in embryonic lethality in mice (Evans-Anderson et al. 2008); Conversely, Foxo1 inactivation in either the liver of mice with type 2 diabetes reversing hyperglycemia (Lu et al. 2012) or the heart of animals with type 2 diabetes preventing heart failure (Battiprolu et al. 2012), suggests that Foxo1 activation is both sufficient and necessary for induction of hyperglycemia and organ failure following insulin resistance or type 2 diabetes.
2.4 Mechanism of insulin resistance by hyperinsulinemia
Insulin resistance occurs at multiple levels in cells, from the cell surface to the nucleus, including insulin receptor desensitization, suppression of IRS protein and functionality, inhibition of PI3K cascades, and failure to restrain Foxo1-activated gene transcriptional profiling, all of which can result from inhibition of IRS1 and IRS2.
IRS1 and IRS2 each contain 40 potential serine/threonine sites, which are phosphorylated by p38α MAP kinase, JNK, mTOR, and protein kinase C (PKC), stimulating IRS protein degradation or inhibiting IRS-associated PI3K activation under pathological conditions (Copps and White 2012; Guo 2013; Qi et al. 2013; Sun and Liu 2009). Even under physiological conditions, there is a 50% reduction in hepatic IRS2 protein during feeding, compared to the fasting condition (Ide, et al. 2004). This observation suggests that liver is likely more insulin resistant during feeding than a fasting state, in which serine/threonine phosphorylation of IRS2 may decrease IRS2 protein expression and function. Of note is that PI3K→Akt signaling serves as a common platform for multiple hormone and growth factor signaling events (Hirsch, et al. 2007; Sussman, et al. 2011). Our recent studies demonstrated that IRS1 and IRS2 are the major endogenous mediators activating the PI3K→Akt signaling cascade in liver and heart of animals (Guo et al. 2009; Qi et al. 2013). Normal expression and functionality of IRS activating PI3K and Akt signaling pathway is essential for animals to maintain nutrient homeostasis and cardiac function, while many factors can result in insulin resistance.
Hyperinsulinemia has profound effects on inducing insulin resistance, which is supported by several lines of recent evidence: 1) prolonged insulin treatment is sufficient for preventing acute insulin action on Foxo1 phosphorylation or Glut4 cellualr membrane trafficking, in myocardium and adipocytes (Gonzalez et al. 2011; Qi et al. 2013); 2) insulin inhibits IRS2 gene transcription in liver (Zhang, et al. 2001) and promotes IRS2 ubiquitination or degradation in murine embryonic fibroblasts (Guo et al. 2006; Rui, et al. 2001). mTORC1 activation following insulin stimulation is a major pathway resulting in IRS2 ubiquitination and the mTORC1 inhibitor rapamycin, completely prevented insulin or IGF-1-induced IRS2 degradation (Guo et al. 2006; Rui et al. 2001). Moreover, deletion of hepatic S6K, a downstream target of mTORC1, improved insulin resistance, enhancing IRS1 and IRS2 gene expression and preventing diabetes in mice (Bae, et al. 2012; Um, et al. 2004). By contrast, deletion of mTORC2 in liver of mice resulted in a diabetic phenotype, similar to L-DKO mice lacking both IRS1 and IRS2 in liver (Guo et al. 2009; Hagiwara et al. 2012). Of note is that long-term treatment with rapamycin blocks mTORC2-mediated Akt phosphorylation/activation and the use of rapamycin for the treatment type 2 diabetes is a clinical challenge (Sarbassov et al. 2005); 3) hyperinsulinemic treatment induces insulin resistance and is associated with oxidative stress and mitochondrial dysfunction in skeletal muscle and liver of mice with type 1 diabetes (Liu, et al. 2009a); 4) decreased IRS1 and IRS2 expression levels are present in tissues of animals and patients with hyperinsulinemia or type2 diabetes (Kerouz, et al. 1997; Qi et al. 2013; Rondinone, et al. 1997); 5) p38α MAP kinase activation following prolonged insulin treatment in cardiomyocytes mediates insulin resistance by increasing IRS1 and IRS2 serine/threonine phosphorylation and degradation, as demonstrated in our recent studies (Qi et al. 2013); 6) p38 MAP kinase also mediates induction of inflammatory cytokines that promote insulin resistance (Li, et al. 2005; Shoelson, et al. 2006); and 7) many, if not all, MAP kinases can induce IRS serine/threonine phosphorylation and degradation, particularly when animals are fed a high fed-diet. JNK activation induces IRS1 phosphorylation at S307 and desensitizes insulin action in liver and other tissues, providing a mechanism for insulin resistance (Lee, et al. 2003). Deletion of JNK1, in mice, reduced blood glucose and improved insulin sensitivity following a high-fat diet treatment (Tuncman, et al. 2006). Although ERK1/2 was thought to have a minor effect on metabolic regulation (Gabbay, et al. 1996), recent data suggest that ERK1/2 mediated upstream MEK activation, reduced hepatic Akt phosphorylation, and contributed to insulin resistance (Jager, et al. 2011; Jiao, et al. 2013). It is likely that activation of MKP-3 (MAP kinase phosphatase-3) or phosphatase 2A (PP2A) following ERK1/2 activation may result in Foxo1 dephosphorylation at S253, promoting gluconeogenesis. Indeed, either MKP3 or PP2A interact with Foxo1 and contribute to Foxo1 dephosphorylation at S253 and activation (Wu, et al. 2010; Yan, et al. 2008). Additionally, some PKC isoforms, such as PKCδ and PCKθ, also have important roles in inducing IRS serine/threonine phosphorylation, resulting in insulin resistance in tissues following high-fed diet treatment (Bezy, et al. 2011; Gao, et al. 2007). Currently, there are about 1100 protein kinases found in mouse or human genome sequences. It will be important to identify these kinases and activation mechanisms under different cellular and environmental conditions for induction of IRS serine/threonine phosphorylation and inactivation of insulin signaling.
2.5. Foxo1 activation following insulin resistance
During the development of insulin resistance and diabetes mellitus, following loss of IRS and inactivation of the PI3K→Akt signaling pathway, the inhibitory mechanism of Foxo1, by Akt activation upon feeding or insulin stimulation is uncontrolled. Thus, dephosphorylation of Foxo1 at the conserved Akt phosphorylation sites (T24, S256, and S319), enhances Foxo1 stability and transcriptional activity, stimulating gluconeogenesis and resulting in hyperglycemia. An increase in nuclear dephosphorylated Foxo1-S253 was detected in liver and heart of animals with type 2 diabetes (Altomonte, et al. 2003; Battiprolu et al. 2012). Deletion of Foxo1 in the liver of L-DKO mice and db/db mice, reduced hepatic glucose production and ameliorated diabetes (Dong et al. 2008; Zhang et al. 2012) and deletion of Foxo1 in the heart of HFD-mice prevented heart failure (Battiprolu et al. 2012). These results suggest that IRS→Akt→Foxo1 signaling cascades are critical in nutrient homeostasis and organ survival.
Aberrant Foxo1 activation disrupts metabolic homeostasis and promotes organ failure, through regulating expression of a number of target genes (Figure 1). Foxo1 promotes hepatic glucose production via expression of Pepck and G6Pase and inhibits lipogenesis, resulting from suppression of Srebp1c, glucokinase and fatty acid synthase (Deng, et al. 2012; Zhang et al. 2012; Zhang, et al. 2006). Recently, we identified a novel Foxo1 target gene - hemeoxygenase-1 (Hmox-1), an enzyme catalyzing degradation of heme to produce biliverdin, iron, and carbon monoxide. Heme is a component of the mitochondrial electron transport chain complex III and IV and constitutive Foxo1 activation, following the loss of IRS1 and IRS2, is a key component for heme degradation and impairment of mitochondrial biosynthesis and function (Cheng, et al. 2009; Qi et al. 2013). This impairment results in reduced fatty acid oxidation and ATP generation, significantly contributing to triglyceride accumulation, resulting in organ steatosis or energy deficiency, as often observed in type 2 diabetes mellitus.
2.6 Activation of Foxo1 by multiple signaling mechanisms
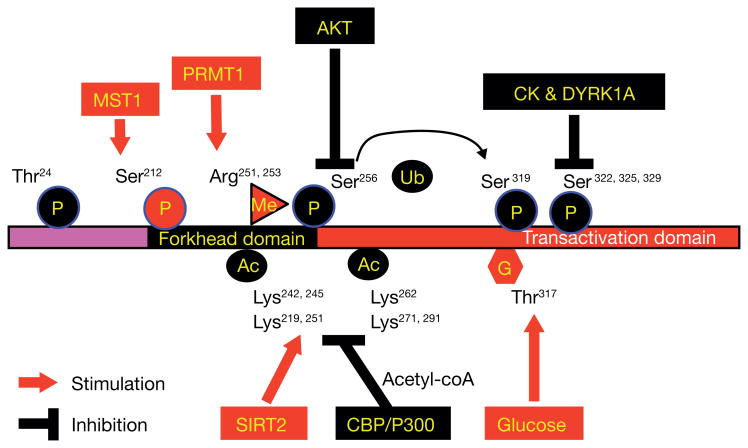
Phosphorylation of Foxo1 at S253 by Akt promotes Foxo1 cytoplasmic retention and ubiquitination, which serves as a central mechanism controlling Foxo1 stability and activity (Guo 2013). However, Foxo1 can also be phosphorylated at different serine or threonine residues by other protein kinases, enhancing transcriptional activity. For example, mammalian sterile 20-like kinase-1 (MST1) promotes Foxo1 phosphorylation at S212, which promotes neuronal cell apoptosis (Yuan, et al. 2009) or anti-oxidative stress responses, extending lifespan in C. elegans (Lehtinen, et al. 2006). In addition to the phosphorylation-based pathway, Foxo1 activity can also be regulated by other post-translational modifications, including methylation, glycosylation, and acetylation (Figure 2).
Figure 2.
Methylation of Foxo1 at arginine R251 and R253 by protein arginine methyltransferase (PRMT1) at the Akt consensus motif RXRXXS/T, blocks Akt-mediated phosphorylation of Foxo1 at S253, resulting in long-lasting Foxo1 retention in the nucleus and activation of Foxo1 transcriptional activity (Takahashi, et al. 2011; Yamagata, et al. 2008). However, whether PRMT1 expression and Foxo1 methylation are altered in diabetics is unclear.
Glycosylation of Foxo1 at threonine T317 via O-GlcNac modification in response to glucose, increased Foxo1 transcriptional activation for expression of gluconeogenic genes (Pepck and G6Pase) and anti-oxidative stress genes (MnSOD and Catalase) (Housley, et al. 2008). The flux of glucose through the hexosamine biosynthetic pathway (HBP) provides a substrate for the glucosamine-6-phosphate forming UDP-GlcNAc (UDP-N-acetylglucosamine). O-GlcNAc modification of proteins results in an enzymatic addition of the N-acetyl glucosamine (GlcNAc) moiety of UDP-GlcNAc on the hydroxyl oxygen of serines and threonines (Kuo, et al. 2008). Foxo1-T317 is GlcNAcylated in liver and a modification which is increased in diabetic animals (Housley et al. 2008), suggesting that hyperglycemia further enhances Foxo1 activity in the absence Foxo1-S253 phosphorylation following insulin resistance.
Acetylation of Foxo1 at several lysine residues have been identified, including K242, K245, and K262, and the reversible acetylation is regulated by histone acetyltransferase CBP/p300 and NAD+-dependent histone deacetylase SIRT2 (Matsuzaki, et al. 2005). Early studies indicate that p300 acetylates Foxo1 and enhances Foxo1-induced transcription (Perrot and Rechler 2005), which may also involve histone acetylation by p300 for activation of basal transcriptional machinery, while deacetylation of Foxo1 by SIRT1 represses Foxo1 (Motta, et al. 2004; Yang, et al. 2005). By contrast, recent studies suggest that acetylation suppresses, while deacetylation of Foxo1 by SIRT1 increases Foxo1 activity (Jing, et al. 2007; Matsuzaki et al. 2005), which is supported by a report that mutations of the lysines to glutamines (Q) in Foxo1 mimicking acetylation resulted in loss-of Foxo1 function and embryonic lethality, while mutations of the lysines to arginines (R) prevented acetylation and potentiated Foxo1 activity (Banks, et al. 2011). Moreover, Foxo1 is deacetylated and activated by the class IIa histone deacetylases (HDACs), promoting hepatic glucose production (Mihaylova, et al. 2011). Nuclear HDAC4, 5, and 7 are phosphorylated and excluded from the nucleus by AMPK; but, fasting hormone glucagon rapidly dephosphorylated and translocated the HDACs to the nucleus, where they associate with promoters of gluconeogenic enzymes, such as Pepck and G6Pase. In turn, HDAC4 and 5 recruit HDAC3, which results in acute transcriptional induction of these genes via deacetylation and activation of Foxo transcription factors. Loss of the class IIa HDACs in murine liver resulted in inhibition of Foxo target genes and lowered blood glucose (Mihaylova et al. 2011). Thus, suppression of class IIa HDACs in mouse models of type 2 diabetes ameliorates hyperglycemia, suggesting that inhibitors of class I/II HDACs may provide a potential therapeutic modality for the metabolic syndrome. Moreover, with food intake, cells accumulate acetyl-CoA from glucose oxidation, providing substrate for Foxo1 acetylation and suppression of Foxo1 activity, in addition to insulin-induced inhibitory phosphorylation. Thus, Foxo1 merges the nutritional and hormonal signaling into a well-controlled metabolic regulation (Figure 2).
Of note is that Foxo1 stimulates expression of MnSOD and catalase and enhances antioxidant responses. In rodents, Foxo1 activation following IRS2 deficiency, in the brain enhanced longevity, but promoted obesity and diabetes (Taguchi, et al. 2007). Also, Foxo1 activation enhances myocardial survival upon induction of oxidative stress (Sengupta, et al. 2012; Sengupta, et al. 2011; Sengupta, et al. 2009), and autophagy, for control of cell size following serum starvation (Sengupta et al. 2009). Mice lacking systemic Foxo1 are embryonic lethal, since Foxo1 is required for endothelial cell lineage during cardiovascular development (Hosaka et al. 2004; Sengupta et al. 2012). In C. elegans, the Foxo1 ortholog Daf-16 enhances longevity when insulin/IGF-1 receptor signaling is inactivated, and potentially increases expression of anti-oxidative genes (MnSOD), and also stimulates lipid droplet accumulation (Ogg, et al. 1997). Together, these data suggest that Foxo1 activation is required for maintenance of the life cycle with stressful conditions, such as prolonged fasting in liver for hepatic glucose production and activation of anti-oxidative mechanisms promoting survival in C. elegans. However, Foxo1 is activated through multiple layers of regulatory mechanisms, contributing to the development of type 2 diabetes mellitus and organ failure, following insulin resistance.
Part 3: Insulin resistance differentially contributes to the metabolic syndrome phenotype
3.1 Central nervous system (CNS) insulin resistance causes obesity
Human appetite is tightly controlled by insulin action in the central nervous system (CNS). The hypothalamus at the base of the forebrain, is comprised of numerous small nuclei, each with distinct connections and neurochemistry, which regulate food intake, hormone release, sleep and wake cycles, and other biological functions. When an action potential, traveling along an axon, arrives at a neuronal synapse, it causes neurotransmitter release triggering biological responses in target cells (Myers and Olson 2012). A low dose of insulin delivery by the intracerebroventricular infusion, decreased both food intake and hepatic glucose production, effects which were blocked by PI3K inhibitors (Obici, et al. 2002; Woods, et al. 1979). Combined with evidence from mice with neuron-specific insulin receptor deletion are overweight and insulin resistant (Bruning et al. 2000), current data indicate that neuronal insulin signaling is required for both body weight control and glucose homeostasis.
The functional significance of brain insulin signaling is further evidenced by deletion of IRS2 in the hypothalamus, resulting in hyperglycemia and obesity in mice (Lin, et al. 2004; Taguchi et al. 2007). Deletion of IRS1 in the hypothalamus did not disrupt glucose homeostasis and obesity did not develop in young mice (Table 2, Guo and White unpublished data). Similar to the action of leptin, an adipocyte-derived hormone that inhibits food intake through central nervous system leptin receptor neurons activating the Jak2→Stat3 signaling cascade (Bates, et al. 2003; Myers and Olson 2012), brain insulin signaling reduced food intake by activation of PI3K via IRS2 and inactivation of Foxo1, which can be independent of the Jak2→Stat3 pathway (Taguchi et al. 2007). However, both leptin and insulin promoted IRS2 tyrosine phosphorylation and PI3K activation in the brain (Warne, et al. 2011) and IRS2 deletion in leptin receptor-expressing neurons caused diabetes and obesity, in which Foxo1 inactivation completely reversed the metabolic dysfunction (Sadagurski, et al. 2012).
Hypothalamic neurons expressing Agouti—regulated peptide (Agrp) stimulate food intake (orexigenic: appetite stimulant) during the fasting state. Foxo1 stimulates orexigenic Agrp expression, an effect reversed by leptin delivery, of which activation of Stat3 abrogates Foxo1 occupancy on the Agrp promoter region (Kitamura, et al. 2006). Foxo1 deletion in Agrp neurons of mice resulted in reduced food intake, leanness and decreased hepatic glucose production, involving suppression of a G-protein-coupled receptor Gpr17, a Foxo1 target gene in Agrp neurons (Ren, et al. 2012). By antagonizing the effect of Agrp, hypothalamic neurons expressing pro-opiomelanocortin (Pomc) inhibit food intake during feeding (anorexic: lack of appetite). Foxo1 deletion in Pomc neurons resulted in reduced food intake and body weight, by increasing the obesity susceptibility gene, carboxypeptidase E (Cpe) and subsequent β-endorphin production that mediates anorexigenic effects in mice (Plum, et al. 2009).
3.2 Insulin resistance in adipose tissue, hyperlipidemia, and the role of inflammation
A key feature of the metabolic syndrome is hyperlipidemia, which likely results from insulin resistance in adipose tissues. Insulin promotes fat cell differentiation, enhances adipocyte glucose uptake and inhibits adipocyte lipolysis. Mice lacking adipocyte TORC2, exhibited hyperglycemia, hyperinsulinemia, failure to suppress lipolysis in response to insulin, elevated circulating fatty acids and glycerol, insulin resistance in skeletal muscle and liver (Kumar, et al. 2010). Recent studies from mice lacking insulin receptor in adipose tissue, created by the adiponectin promoter-driven Cre/loxP system, developed severe lipoatrophic diabetes, a 95% reduction of white adipose tissue, hyperglycemia, hyperinsulinemia, hyperlipidemia, and liver steatosis (Boucher and Kahn 2013). These data suggest that when insulin action fails in fat, adipocyte development is retarded and lipids are unable to convert from carbohydrates for storage. Thus, both glucose and lipids will redistribute to the circulation and organs, resulting in hyperlipidemia and fatty organs. These studies significantly underscore the contribution of insulin resistance in adipose tissue, via inactivation of Akt signaling, in control of systemic nutrient homeostasis.
Adipose tissue is also an endocrine organ secreting cytokines and hormones, including TNFα, IL6, leptin, adiponectin, and many others, influencing food intake, systemic insulin sensitivity and nutrient homeostasis. However, obesity from fat expansion disrupts a proper balance of cytokine and hormone generation, promoting insulin resistance. For example, TNFα, IL-6, and leptin are pro-inflammation and are markedly increased in obesity, where adiponectin that has anti-inflammatory effect on enhancing insulin sensitivity is markedly reduced (Hotamisligil and Erbay 2008; Hotamisligil, et al. 1993; Romeo, et al. 2012; Shoelson et al. 2006). Overexpression of IKKb for NFҡB activation (a key player in control of pro-inflammatory responses) in the liver of mice, is sufficient for inducing insulin resistance and type 2 diabetes (Cai, et al. 2005). TNFα reduces IRS1 protein by activation of JNK or S6K, resulting in insulin resistance (Gao, et al. 2002; Zhang, et al. 2008). Thus, suppression of inflammation increases insulin sensitivity and improves metabolic dysfunction in type 2 diabetes mellitus (Hotamisligil, et al. 1996). However, the outcome of anti-inflammatory therapy in treating insulin resistance deserves a cautionary note for several reasons as follows: 1) inflammation is involved in deploying and mobilizing immune cell leukocytes to defend against infections or toxins. Many inflammatory actors, such as TNFα, reduce body weight and increase energy expenditure (Ye and McGuinness 2013). Overexpression of IL6, in the liver, increased energy expenditure and insulin sensitivity in mice (Sadagurski, et al. 2010); 2) during physical exercise, inflammatory factors, such as TNFα and IL6, are secreted resulting in inhibition of anabolic metabolism (insulin action) and promoting catabolic metabolism (fat lipolysis) to meet fuel requirements for muscle; 3) NFҡB is essential for hepatocyte proliferation and survival, and mice lacking the p65 subunit of NFҡB died of liver failure (Geisler, et al. 2007; Malato, et al. 2012); and 4) inflammation not only triggers pro-inflammatory responses, but also activates anti-inflammatory processes. Together, the data suggest that a balance between inflammation and anti-inflammation is required for proper insulin actions and nutrient homeostasis. Thus, correcting the imbalance of hormones, nutrients, and inflammation may provide opportunities and challenges for prevention and treatment of the metabolic syndrome and type 2 diabetes.
In general, excess energy storage in tissues, particular lipids, is now believed to be a primary factor contributing to the metabolic syndrome (Reaven 2005a). Free fatty acids derived from nutritional intake or conversion from carbohydrates, not only provide an important energy source, but also act as signaling molecules in modulating intracellular protein kinases (PKC and JNK etc) for inactivation of insulin signaling (Holzer, et al. 2011; Oh, et al. 2010). Excess lipid accumulation in several organs, including adipose tissue, liver, muscle, heart, and blood vessels resulted in insulin resistance and triggered metabolic inflammation, a low grade and chronic inflammatory response (Samuel, et al. 2010; Samuel and Shulman 2012). An acute lipid or fatty acid infusion or chronic high-fat diet, directly induces insulin resistance in mice via activation of protein kinase Cθ (Boden 2011; Griffin, et al. 1999). Saturated fatty acids also interact with a liver-secreted glycoprotein fetuin A that binds and activates toll-like receptor 4 (TLR4) resulting in NҡB activation (Pal, et al. 2012) and c-SRC recruitment for JNK activation and inhibition of insulin action (Holzer et al. 2011). Moreover, saturated fatty acids induce apoptosis in hepatocytes and pancreatic β-cells, by activating PKCξ, JNK, and oxidative stress inhibiting IRS1/2 tyrosine phosphorylation and blocking insulin signaling (Figure 1) (Galbo, et al. 2013; Malhi, et al. 2006; Wong, et al. 2009; Wrede, et al. 2002). By contrast, unsaturated fatty acids interact with the G-protein coupled receptor GRP120 inhibiting inflammation and obesity and increasing insulin sensitivity (Ichimura, et al. 2012). In the liver, lipid accumulation (hepatic steatosis) is a risk factor for nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and liver cancer (Kumashiro, et al. 2011; Samuel and Shulman 2012).
3.3 Hepatic insulin resistance results in hyperglycemia
Hyerglycemia is caused by insulin resistance not only in the brain and fat, but also from liver, which is a central organ controlling blood glucose and lipid homeostasis. Insulin promotes synthesis of the macromolecules glycogen, lipids and protein in liver and suppresses hepatic glucose production via inhibiting gluconeogenesis. Deletion of either IRS1 or IRS2 in liver maintained glucose homeostasis; but deletion of both IRS1 and IRS2 (L-DKO mice) blocked insulin or feeding induction on Akt and Foxo1 phosphorylation and resulted in unrestrained gluconeogenesis for hepatic glucose production, resulting in hyperglycemia, with a reduction in hepatic lipogenesis and blood lipids (Guo et al. 2009; Kubota, et al. 2008). Moreover, a high-fat diet severely impaired IRS2 expression and tyrosine phosphorylation in hepatocytes of liver-specific IRS1 null mice and the mice developed severe diabetes (Guo et al. 2009). Over nutrition or a high fat-diet can modify intracellular signaling, affecting IRS2 expression and functionality, altering metabolic gene expression and impairing glucose homeostasis.
Hepatic insulin resistance also results in insulin resistance in other tissues, which is demonstrated in L-DKO mice. The L-DKO mice not only demonstrated inhibition of the hepatic Akt signaling cascade, but also blunted brain intracerebroventricular (ICV) insulin action on reducing hepatic glucose production in ICV clamp experiments (Guo et al. 2009). Moreover, L-DKO mice exhibited features of heart failure, likely secondary to hyperinsulinemia, resulting in cardiac IRS1 and IRS2 suppression (Qi et al. 2013). Likewise, mice lacking hepatic insulin receptor displayed pro-atherogenic lipoprotein profiles with reduced high-density lipoprotein (HDL) cholesterol and very low-density lipoprotein (VLDL) particles, and within 12 weeks of being placed on an atherogenic diet, developed severe hypercholesterolemia (Biddinger, et al. 2008). These data suggest that hepatic insulin resistance is sufficient to produce the dyslipidemia and increased risk of atherosclerosis and cardiac dysfunction.
The role of Foxo1 activation in control of the development of diabetes is supported by findings in L-TKO mice, which lack IRS1, IRS2, and Foxo1 genes in the liver. L-TKO mice demonstrated significant reversal of elevated blood glucose, glucose intolerance and the fasting-feeding effect on hepatic gene expression, which were observed in L-DKO mice (Dong et al. 2008). Similarly, mice lacking both Akt1 and Akt2 in the liver (Akt-DLKO), or lacking PDK1 or mTORC2 (blocks Akt activation) developed a similar diabetic phenotype as seen in L-DKO mice (Guo et al. 2009; Hagiwara et al. 2012; Lu et al. 2012; Mora et al. 2005). Moreover, mice lacking Akt1, Akt2, and Foxo1(TLKO) rescued diabetes in the Akt-DLKO mice (Lu et al. 2012). Of interest, L-TKO and TLKO mice had normal glucose tolerance and responses to the fasting-feeding challenge and suppressed Pepck and G6Pase gene expression at a degree similar to control mice (Chai, et al. 2008; Lu et al. 2012), suggesting that there is an Akt and Foxo1-independent pathway regulating blood glucose homeostasis, the mechanism of which is unclear. It is likely that hepatic Foxo1 deletion may sensitize brain insulin signaling to reduce hepatic glucose production, even though Akt activity is not controlled.
3.4 Cardiac insulin resistance promotes heart failure
Loss of IRS1 and IRS2 in liver and brain resulted in hyperglycemia, while loss in other tissues, such as heart and pancreas, resulted in organ failure. Thus, it is likely that diabetes may provide a link to the development of heart failure via loss of IRS proteins. The heart is an insulin-responsive and energy consuming organ that requires a constant fuel supply to maintain intracellular ATP for myocardial contraction. Deletion of both cardiac IRS1 and IRS2 (H-DKO mice: Heart-specific Double IRS1 and IRS2 gene knockout) diminished cardiac Akt and Foxo1 phosphorylation and resulted in heart failure and death of male animals at 7 to 8 weeks of age (Qi et al. 2013). Deletion of both IRS1 and IRS2 in skeletal and cardiac muscle caused heart failure and diminished Akt and Foxo1 phosphorylation in skeletal muscle; but, the mice had normal blood glucose and insulin sensitivity (Long, et al. 2011), suggesting that insulin resistance in skeletal muscle is not necessary for a disruption of glucose homeostasis in mice. In contrast, cardiac muscle requires either IRS1 or IRS2 for maintenance of endogenous Akt activity and Foxo1 inactivation, to promote cardiac function and survival. Cardiac Foxo1 overexpression, which caused heart failure in mice (Evans-Anderson et al. 2008), was also observed in failing human hearts (Hannenhalli et al. 2006).
Loss of IRS1 and IRS2 following chronic insulin stimulation and p38 MAK activation contributes to insulin resistance in the heart (Qi et al. 2013). Based on our recent studies, we proposed that regulation of IRS1 and IRS2 have major roles in control of cardiac homeostasis, metabolism, and function. This concept was based on the following observations: 1) metabolic adaptation during physiological conditions (phase I); 2) metabolic remodeling following the development of insulin resistance and mild cardiac dysfunction (phase II); and 3) maladaptive metabolic and cardiac remodeling, leading to cardiac failure and sudden death (phase III).
During phase I in the postprandial setting, insulin stimulates glucose transport and oxidation, resulting in effective cardiac utilizing glucose, as a substrate for the supply of ATP. A 20-40% reduction of IRS2 protein was found in mouse livers and hearts, compared to those in the fasting state (Guo et al. 2009). In phase II when insulin resistance occurs, the heart undergoes adaptive responses to limit glucose utilization (insulin-dependent) and respond to lipid oxidation (less insulin-dependent). The heart is capable of generating ATP for myocardial contraction and changes in gene expression patterns, with unaltered cardiac morphology. During this period, the metabolic adaptation or remodeling, compensates for cardiac energy demand, even without overt indications of heart failure. With continued insulin resistance resulting from hyperinsulinemia and/or other metabolic and mechanical stresses, cardiac dysfunction develops, as exhibited by L-DKO mice, which have a 60-70% reduction of cardiac IRS1 and IRS2 in the hearts in association with cardiac dysfunction (Qi et al. 2013). During phase III in H-DKO mice, when maladaptive metabolic remodeling occurs, there is a lack of compensation for cardiac energy demand, secondary to loss of IRS1 and IRS2, with Akt inactivation, utilization of both glucose and fatty acids being restrained, resulting in hyperlipidemia and cardiac ATP deficiency and sudden death (Qi et al. 2013). In this phase, the failing heart may exhibit a loss of mitochondrial biogenesis, a process required for fatty acid and glucose utilization via mitochondrial oxidative phosphorylation. In addition, unknown myocardial factors, which are derived from the loss of IRS1 and IRS2 and released to cardiofibroblasts, may also contribute to the onset of interstitial fibrosis. Thus, sensitizing myocardial Akt→Foxo1 signaling, by integrating insulin therapy and blocking the p38→IRS1/2 signaling cascade, may provide new treatment modality for heart failure, during insulin resistance, type 2 diabetes mellitus, and other chronic physiologic stresses (Guo 2013; Qi et al. 2013).
3.5 Insulin resistance in pancreas impairs β-cell regeneration
Pancreatic β-cell failure is essential for the development of hyperglycemia in type 1 diabetes; but, β-cell failure is also present in patients with type 2 diabetes (Rhodes 2005; Rhodes, et al. 2013). The β-cells secret insulin reducing blood glucose and the α-cells secret glucagon increasing the blood glucose level to meet bodily metabolic requirements. Recent studies showed that insulin enhances glucose-stimulated insulin secretion in healthy humans (Bouche, et al. 2010), and mice lacking insulin receptor in β-cells had impaired insulin secretion (Kulkarni et al. 1999). However, whether insulin has a direct autocrine action on β-cells in promoting insulin secretion is unclear (Rhodes et al. 2013).
Deletion of whole body IRS2 in mice resulted in diabetes owing to pancreatic β-cell failure (Withers et al. 1998), while Foxo1 inactivation in IRS2 null mice prevented β-cell apoptosis and diabetes (Nakae, et al. 2002), indicating that IRS2→Foxo1 signaling or Foxo1 inactivation is required for β-cell survival. On the other hand, deletion of IRS2 in β-cells triggered β-cell repopulation or regeneration, leading to a restoration of insulin secretion and resolution of diabetes in aged mice (Lin et al. 2004), suggesting that Foxo1 activation following IRS2 inactivation in β-cells promotes β-cell regeneration or differentiation. Conversely, Foxo1 inactivation in β-cells resulted in reduced β-cell mass, hyperglycemia, and hyperglucagonemia, owing to dedifferentiation of β cells into progenitor-like cells or pancreatic α-cells (Kitamura 2013; Talchai, et al. 2012).
Insulin resistance and/or hyperinsulinemia is the main cause of type 2 diabetes, but more recently, there is evidence for a failure of functional β-cell mass to meet metabolic demand, the mechanism of which is unclear (Kahn, et al. 2006; Rhodes 2005). On the other hand, antagonizing glucagon receptor action, in type 1 diabetes induced by streptozotocin and type 2 diabetes mellitus in mice. markedly reduced blood glucose and completely rescued diabetes (Ali and Drucker 2009; Lee, et al. 2011; Liang, et al. 2004; Sorensen, et al. 2006). Thus, an abnormality at the level of pancreas is critical for development of diabetes and correcting the imbalance of hormones between insulin (β-cells) and glucagon (α-cells) may provide a potential strategy to prevent diabetes.
3.6 Insulin resistance in skeletal muscle shortens lifespan
Skeletal muscle is an important fuel storage tissue for glucose uptake, converting to glycogen and triglycerides, a process stimulated by insulin. Skeletal muscle demonstrates remarkable metabolic flexibility to consume and store glucose and lipids. Mice lacking muscular insulin receptor display elevated fat mass, serum triglycerides, and free fatty acids; but, blood glucose, serum insulin, and glucose tolerance are normal. Thus, insulin resistance in muscle contributes to the altered fat metabolism associated with type 2 diabetes; but, tissues other than muscle appear to be more involved in insulin-regulated glucose disposal than previously recognized (Bruning et al. 1998). Mice lacking mTORC2 exhibited decreased insulin-stimulated phosphorylation of Akt-S473 and glucose uptake, and mild glucose intolerance (Kumar, et al. 2008), while mice lacking mTRORC1 developed dystrophic muscle, mild glucose intolerance, and shortened lifespan (Bentzinger, et al. 2008). Mice lacking both IRS1 and IRS2 in skeletal and cardiac muscle, died at 3 weeks of age, with a much shorter lifespan than mice lacking both IRS1 and IRS2 in only cardiac muscle (H-DKO mice), the latter which died at 7 weeks of age (Qi et al. 2013), suggesting that insulin action in skeletal muscle has a key and unrecognized role in control of lifespan, and mTORC1 may also contribute to this observed effect.
Mice lacking both IRS1 and IRS2 in skeletal and cardiac muscle did not develop hyperglycemia or hyperinsulinemia, though insulin-induced glucose uptake was diminished. However, AMP was elevated in skeletal muscle, resulting in activation of AMP-dependent protein kinase (AMPK) (Long et al. 2011). AMPK stimulates glucose uptake in an insulin-independent manner, by phosphorylating and activating the Rab GAP family member AS160, which promotes Glut4 translocation (Pehmoller, et al. 2009; Taylor, et al. 2008). AMPK also induces acetyl CoA carboxylase (ACC) phosphorylation and inhibits ACC activity, preventing the conversion of Acetyl-CoA to malonyl-CoA, disrupting lipid synthesis and enhancing fatty acid oxidation (Hoehn, et al. 2010). Together, these studies underscore the flexibility of skeletal muscle in control of glucose homeostasis and longevity. Since skeletal muscle actively secretes hormones (myokines), such as irisin, a hormone that systemically regulates glucose homeostasis and obesity (Bostrom, et al. 2012; Muoio and Neufer 2012), it would be of interest to determine if a skeletal muscle derived-hormone affects longevity in animals.
3.7 Insulin resistance in vascular endothelium promotes hypertension and disrupts glucose homeostasis
Vasodilator actions of insulin are mediated by phosphatidylinositol 3-kinase (PI3K)-dependent signaling pathways that stimulate production of nitric oxide from vascular endothelium (Muniyappa, et al. 2008; Xu and Zou 2009). Insulin resistance in vascular endothelium stimulates vasoconstriction, promotes hypertension and atherosclerosis, and impairs systemic insulin sensitivity and glucose homeostasis. Inactivation of insulin receptor in vascular endothelium diminished insulin-induced eNOS phosphorylation and blunted aortic vasorelaxant responses to acetylcholine and calcium ionophore in normal mice (Duncan, et al. 2008), and accelerated atherosclerosis in apolipoprotein E null mice (Rask-Madsen, et al. 2010). Vascular endothelium-deficient in IRS2 or both IRS1 and IRS2, reduced endothelial Akt and eNOS phosphorylation and impaired skeletal muscle glucose uptake, resulting in systemic insulin resistance (Kubota, et al. 2011). Foxo activation following deficiency of IRS2 or both IRS1 and IRS2, may play a key role in stimulating endothelial cell dysfunction. In fact, deletion of Foxo1, Foxo3, and Foxo4 in endothelium enhanced eNOS phosphorylation, reduced inflammation and oxidative stress of endothelial cells, and prevented atherosclerosis in high-diet fat or low-density lipoprotein receptor null mice (Tsuchiya, et al. 2012). Endothelium-targeted deletion of insulin receptor or Foxo genes in mice barely disrupted glucose homeostasis (Duncan et al. 2008; Rask-Madsen et al. 2010; Tsuchiya et al. 2012); however, we recently showed that endothelium-targeted deletion of the transcription factor related transcriptional enhancer factor-1 (RTEF-1) increased blood glucose levels and insulin resistance. RTEF-1 has the potential for interaction with the insulin response element and Foxo1 in cells (Messmer-Blust, et al. 2012). Thus, vascular endothelium serves as an organ that potentially regulates glucose homeostasis.
3.8 Insulin resistance in bone impairs glucose homeostasis
Insulin promotes bone formation and differentiation of osteoblasts that synthesize osteocalcin, a bone-derived insulin secretagogue which regulates pancreatic insulin secretion and systemically controls glucose homeostasis. Mice lacking insulin receptor in osteoblasts, exhibited reduced bone formation, increased peripheral adiposity, insulin resistance, primarily by reduced gene expression and activity of osteocalcin (Ferron, et al. 2010; Fulzele, et al. 2010). These studies suggest that in osteoblasts insulin may stimulate osteocalcin by suppressing Foxo1, which affects bone remodeling and control of glucose homeostasis. Foxo1 inhibits osteocalcin expression and activity by increasing expression of Esp, a protein tyrosine phosphatase, that inhibits bioactivity of osteocalcin by favoring its carboxylation. Moreover, osteoblast-specific Foxo1 null mice have increased osteocalcin expression and insulin production, and reduced blood glucose (Rached, et al. 2010). Collectively, these data suggest that bone serves as an endocrine organ in control of glucose homeostasis, through bone-pancreas crosstalk, in which Foxo1 plays a key role in insulin action regulating osteocalcin expression and activity in osteoblasts.
Part 4: Other considerations
4.1 Mouse models
A large body of evidence related to the mechanisms of diabetes, obesity and cardiovascular diseases has been derived from mouse studies. However, mice have a high heart rate: 600 beats/min versus 70 beats/min in humans; mouse brain glucose intake is much less than humans, 15% versus 65%, respectively; and mice are nocturnal animals and inactive during daytime when many data are often collected for analysis. Also, experimental mice have divergent immune gene transcriptional programs from humans (Shay, et al. 2013). Humans live in a mobile environment. Recent studies indicate gastrointestinal microbiota may trigger inflammation and insulin resistance (Johnson and Olefsky 2013; Kau, et al. 2011; Nicholson, et al. 2012), and increased levels of circulating bacteria or bacterial products derived from microbiota, such as lipopolysaccharides, can initiate infection and metabolic inflammation that induce insulin resistance and promote metabolic syndrome (Burcelin 2012). Genetic approaches often rely on the Cre/LoxP system. Since tissue-specific deletion of a gene of interest is dependent on the tissue-specificity and intensity of Cre-recombinase expression, a tissue-specific promoter that drives Cre-recombinase is critical to achieve a partial or complete deletion of the target gene, to affect the phenotype observed in animals. For example, MHC (myosin heavy chain)-cre driven IRS1 and IRS2 deletion is almost complete and the heart failure phenotype striking, while MEF (myocyte enhancer factor)-Cre-driven IRS1 and IRS2 deletion is partial and there is no observed phenotype. Likewise, adiponectin-Cre driven insulin receptor gene deletion is much stronger than aP2-cre driven insulin receptor gene deletion and a diabetic phenotype is evident. Interpretation of the role of insulin in adipose tissue and contribution to nutrient homeostasis may be affected. For example, RIP-cre is a rat insulin promoter-driven Cre transgenic mouse model; but, the Cre has leaky expression in the hypothalamus of the brain (Lin et al. 2004). Thus, deletion of IRS2 by the RIP-Cre system resulted in a phenotype that is derived not only from pancreatic β-cells, but also from the brain hypothalamus (Rhodes et al. 2013). Thus, tissue-specificity and intensity of Cre-recombinase expression though advancing our understanding of mouse genetical engineering, also has a significant role in analysis of gene function.
4.2 Integrative physiology of insulin resistance and hyperlipidema
Insulin inhibits hepatic glucose production and stimulates lipid synthesis, and deletion of insulin receptor or both IRS1 and IRS2 in the liver of mice resulted in hyperglycemia, hyperinsulinemia, and hypolipidemia (Guo et al. 2009; Michael et al. 2000). A valid question is whether the disease mouse models created by genetic engineering accurately reflect the clinical features of the metabolic syndrome and type 2 diabetes. Many patients with the metabolic syndrome and type 2 diabetes have hyperglycemia, hyperinsulinemia, and hyperlipidemia (Brown and Goldstein 2008). Given that the IRS→PI3K→PDK1/2→Akt→Foxo1 branch of the insulin signaling pathway has a central role in control of glucose homeostasis and organ survival, suppression will result in unchecked hepatic glucose production and hyperglycemia. Although inhibiting this signaling branch also limits hepatic TOCR2 or Akt-stimulated lipogenesis, suppression in adipose tissue may block the insulin inhibitory effect on fat lipolysis, contributing to hyperlipidemia in patients with type 2 diabetes mellitus where other alternative pathways promoting lipogenesis remain active. For example, insulin-independent mTORC1 activation and carbohydrate-activated lipogenic gene expression profiles via Chrebp and AMPK facilitate progression of lipogenesis in patients with metabolic syndrome and type 2 diabetes mellitus (Figure 1). Identifying these and other novel mediators in the control of lipid homeostasis is important to understand disease mechanisms and develop interventions for control of metabolic syndrome, type 2 diabetes mellitus and its complications.
4.3 Bariatric and metabolic surgery
More than 60% of patients with type 2 diabetes are obese, thus body weight loss is an attractive but challenging therapeutic option (Dixon, et al. 2012; Zimmet, et al. 2011). Bariatric surgery, designed to achieve and sustain substantial weight loss and reduce food intake, effectively prevents and remediates type 2 diabetes (Sjostrom, et al. 2012). Moreover, gastric bypass surgery reduces adverse cardiovascular events, not only in obese adults (Sjostrom et al. 2012), but also in patients suffering from type 2 diabetes without severe obesity (Cohen, et al. 2012). The mechanisms of metabolic surgery on metabolic control are unclear (Rubino, et al. 2010), but It is likely that the surgery resets metabolic parameters in a balanced way, such that energy intake and expenditure is controlled.
Part 5: Conclusion
Mouse studies demonstrated that Akt inactivation and Foxo1 activation following suppression of IRS1 and IRS2 provide a fundamental mechanism for insulin resistance, which occurs in insulin responsive tissues, impairing systemic glucose and lipid homeostasis and body weight control and serving as important mechanism for development of the metabolic syndrome. The metabolic syndrome includes insulin resistance in different organs of the body, such as brain, liver, pancreas, fat, muscle, and the cardiovascular system. The IRS→Akt→Foxo1 signaling cascade and its regulatory network, requires further exploration under different cellular and environmental contexts. Hyperinsulinemia, pro-inflammation, and over nutrition are important environmental factors that affect this system, contributing to type 2 diabetes and cardiovascular dysfunction.
Although genome-wide association analyses have identified a number of genes that control the development of diabetes and obesity (Doria, et al. 2008; Wagner, et al. 2013), the metabolic syndrome is a result of complex interactions between genetic and environmental factors, of which protein modifications by environmental stimuli, such as over nutrition through phosphorylation (hormones), ubiquitination, acetylation (excess acetyl-coA) and glycosylation (hyperglycemia), all of which modify the IRS→Akt→Foxo1 branch. Current anti-diabetic therapeutics, such as glucagon-like peptide, pioglitazone and metformin, as well as metabolic surgery, may affect this pathway directly or indirectly, helping to correct the imbalance of hormones, nutrients, and inflammation. Targeting IRS1 and IRS2 by activating the Akt→Foxo1 signaling cascade, associated protein kinases and gene expression profiles may provide important therapeutic modalities in the pursuit of a balanced action at the level of hormones, nutrients, and inflammation for treatment or prevention of metabolic syndrome, type 2 diabetes mellitus and cardiovascular dysfunction.
Acknowledgments
Dr. Guo's research is supported by Grants from the American Diabetes Association (JF-7-07-27), American Heart Association (BGIA-7880040), Faculty Start-up from Texas A&M University Health Science Center College of Medicine, and National Institutes of Health (RO1 DK095118). This material is also supported by resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas, USA. The author would like to thank Drs. Kenneth M. Baker and Yajuan Qi for reading/editing the manuscript.
References
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, Asico LD, Jose PA, Taylor SI, Westphal H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Ali S, Drucker DJ. Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. Am J Physiol Endocrinol Metab. 2009;296:E415–421. doi: 10.1152/ajpendo.90887.2008. [DOI] [PubMed] [Google Scholar]
- Altomonte J, Richter A, Harbaran S, Suriawinata J, Nakae J, Thung SN, Meseck M, Accili D, Dong H. Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. Am J Physiol Endocrinol Metab. 2003;285:E718–728. doi: 10.1152/ajpendo.00156.2003. [DOI] [PubMed] [Google Scholar]
- Araki E, Lipes MA, Patti ME, Bruning JC, Haag B, 3rd, Johnson RS, Kahn CR. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- Bae EJ, Xu J, Oh DY, Bandyopadhyay G, Lagakos WS, Keshwani M, Olefsky JM. Liver-specific p70 S6 kinase depletion protects against hepatic steatosis and systemic insulin resistance. J Biol Chem. 2012;287:18769–18780. doi: 10.1074/jbc.M112.365544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks AS, Kim-Muller JY, Mastracci TL, Kofler NM, Qiang L, Haeusler RA, Jurczak MJ, Laznik D, Heinrich G, Samuel VT, et al. Dissociation of the glucose and lipid regulatory functions of FoxO1 by targeted knockin of acetylation-defective alleles in mice. Cell Metab. 2011;14:587–597. doi: 10.1016/j.cmet.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard-Chapeau EA, Hevener AL, Long S, Zhang EE, Olefsky JM, Feng GS. Deletion of Gab1 in the liver leads to enhanced glucose tolerance and improved hepatic insulin action. Nat Med. 2005;11:567–571. doi: 10.1038/nm1227. [DOI] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- Battiprolu PK, Gillette TG, Wang ZV, Lavandero S, Hill JA. Diabetic Cardiomyopathy: Mechanisms and Therapeutic Targets. Drug Discov Today Dis Mech. 2010;7:e135–e143. doi: 10.1016/j.ddmec.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, et al. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Bezy O, Tran TT, Pihlajamaki J, Suzuki R, Emanuelli B, Winnay J, Mori MA, Haas J, Biddinger SB, Leitges M, et al. PKCdelta regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. J Clin Invest. 2011;121:2504–2517. doi: 10.1172/JCI46045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost F, Aouadi M, Caron L, Even P, Belmonte N, Prot M, Dani C, Hofman P, Pages G, Pouyssegur J, et al. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes. 2005;54:402–411. doi: 10.2337/diabetes.54.2.402. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche C, Lopez X, Fleischman A, Cypess AM, O'Shea S, Stefanovski D, Bergman RN, Rogatsky E, Stein DT, Kahn CR, et al. Insulin enhances glucose-stimulated insulin secretion in healthy humans. Proc Natl Acad Sci U S A. 2010;107:4770–4775. doi: 10.1073/pnas.1000002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J, Kahn CR. Differential role of insulin and IGF-1 receptors in brown and white adipose tissue and development of lipoatrophic diabetes. Diabetes. 2013;62:A37. doi: 10.2337/db16-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol Cell Biol. 2005;25:1596–1607. doi: 10.1128/MCB.25.5.1596-1607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- Burcelin R. Regulation of metabolism: a cross talk between gut microbiota and its human host. Physiology (Bethesda) 2012;27:300–307. doi: 10.1152/physiol.00023.2012. [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Liu HY, Hong T, Liu Z. Excess exposure to insulin may be the primary cause of insulin resistance. Am J Physiol Endocrinol Metab. 2010;298:E372. doi: 10.1152/ajpendo.00677.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Wu Y, Li G, Cao W, Yang Z, Liu Z. Activation of p38 mitogen-activated protein kinase abolishes insulin-mediated myocardial protection against ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2008;294:E183–189. doi: 10.1152/ajpendo.00571.2007. [DOI] [PubMed] [Google Scholar]
- Chatrchyan S, Khachatryan V, Sirunyan AM, Tumasyan A, Adam W, Aguilo E, Bergauer T, Dragicevic M, Ero J, Fabjan C, et al. Inclusive Search for Supersymmetry Using Razor Variables in pp Collisions at sqrt[s]=7 TeV. Phys Rev Lett. 2013;111:081802. doi: 10.1103/PhysRevLett.111.081802. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med. 2009;15:1307–1311. doi: 10.1038/nm.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Cichy SB, Uddin S, Danilkovich A, Guo S, Klippel A, Unterman TG. Protein kinase B/Akt mediates effects of insulin on hepatic insulin-like growth factor-binding protein-1 gene expression through a conserved insulin response sequence. J Biol Chem. 1998;273:6482–6487. doi: 10.1074/jbc.273.11.6482. [DOI] [PubMed] [Google Scholar]
- Cohen RV, Pinheiro JC, Schiavon CA, Salles JE, Wajchenberg BL, Cummings DE. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35:1420–1428. doi: 10.2337/dc11-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- Deng X, Zhang W, I OS, Williams JB, Dong Q, Park EA, Raghow R, Unterman TG, Elam MB. FoxO1 inhibits sterol regulatory element-binding protein-1c (SREBP-1c) gene expression via transcription factors Sp1 and SREBP-1c. J Biol Chem. 2012;287:20132–20143. doi: 10.1074/jbc.M112.347211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB, le Roux CW, Rubino F, Zimmet P. Bariatric surgery for type 2 diabetes. Lancet. 2012;379:2300–2311. doi: 10.1016/S0140-6736(12)60401-2. [DOI] [PubMed] [Google Scholar]
- Dong LQ, Liu F. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab. 2005;289:E187–196. doi: 10.1152/ajpendo.00011.2005. [DOI] [PubMed] [Google Scholar]
- Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8:65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria A, Patti ME, Kahn CR. The emerging genetic architecture of type 2 diabetes. Cell Metab. 2008;8:186–200. doi: 10.1016/j.cmet.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan ER, Crossey PA, Walker S, Anilkumar N, Poston L, Douglas G, Ezzat VA, Wheatcroft SB, Shah AM, Kearney MT. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes. 2008;57:3307–3314. doi: 10.2337/db07-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Estall JL. The Foxo family: partners in crime or silent heroes. Endocrinology. 2012;153:549–551. doi: 10.1210/en.2011-2080. [DOI] [PubMed] [Google Scholar]
- Evans-Anderson HJ, Alfieri CM, Yutzey KE. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res. 2008;102:686–694. doi: 10.1161/CIRCRESAHA.107.163428. [DOI] [PubMed] [Google Scholar]
- Fafalios A, Ma J, Tan X, Stoops J, Luo J, Defrances MC, Zarnegar R. A hepatocyte growth factor receptor (Met)-insulin receptor hybrid governs hepatic glucose metabolism. Nat Med. 2011;17:1577–1584. doi: 10.1038/nm.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Bruning JC, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay RA, Sutherland C, Gnudi L, Kahn BB, O'Brien RM, Granner DK, Flier JS. Insulin regulation of phosphoenolpyruvate carboxykinase gene expression does not require activation of the Ras/mitogen-activated protein kinase signaling pathway. J Biol Chem. 1996;271:1890–1897. doi: 10.1074/jbc.271.4.1890. [DOI] [PubMed] [Google Scholar]
- Galbo T, Perry RJ, Jurczak MJ, Camporez JP, Alves TC, Kahn M, Guigni BA, Serr J, Zhang D, Bhanot S, et al. Saturated and unsaturated fat induce hepatic insulin resistance independently of TLR-4 signaling and ceramide synthesis in vivo. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1311176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- Gao Z, Wang Z, Zhang X, Butler AA, Zuberi A, Gawronska-Kozak B, Lefevre M, York D, Ravussin E, Berthoud HR, et al. Inactivation of PKCtheta leads to increased susceptibility to obesity and dietary insulin resistance in mice. Am J Physiol Endocrinol Metab. 2007;292:E84–91. doi: 10.1152/ajpendo.00178.2006. [DOI] [PubMed] [Google Scholar]
- Geisler F, Algul H, Paxian S, Schmid RM. Genetic inactivation of RelA/p65 sensitizes adult mouse hepatocytes to TNF-induced apoptosis in vivo and in vitro. Gastroenterology. 2007;132:2489–2503. doi: 10.1053/j.gastro.2007.03.033. [DOI] [PubMed] [Google Scholar]
- George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, Flier E, Molle D, Accili D, McGraw TE. Hyperinsulinemia leads to uncoupled insulin regulation of the GLUT4 glucose transporter and the FoxO1 transcription factor. Proc Natl Acad Sci U S A. 2011;108:10162–10167. doi: 10.1073/pnas.1019268108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Guo S. Molecular Basis of insulin resistance: the role of IRS and Foxo1 in the control of diabetes mellitus and its complications. Drug Discov Today Dis Mech. 2013;10:e27–33. doi: 10.1016/j.ddmec.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Copps KD, Dong X, Park S, Cheng Z, Pocai A, Rossetti L, Sajan M, Farese RV, White MF. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol. 2009;29:5070–5083. doi: 10.1128/MCB.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Dunn SL, White MF. The reciprocal stability of FOXO1 and IRS2 creates a regulatory circuit that controls insulin signaling. Mol Endocrinol. 2006;20:3389–3399. doi: 10.1210/me.2006-0092. [DOI] [PubMed] [Google Scholar]
- Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- Haeusler R, Han S, Accili D. Hepatic FOXO1 ablation exacerbates lipid abnormalities during hyperglycemia. J Biol Chem. 2010 doi: 10.1074/jbc.M110.134023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Ruegg MA, Hall MN. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012;15:725–738. doi: 10.1016/j.cmet.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Hannenhalli S, Putt ME, Gilmore JM, Wang J, Parmacek MS, Epstein JA, Morrisey EE, Margulies KB, Cappola TP. Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation. 2006;114:1269–1276. doi: 10.1161/CIRCULATIONAHA.106.632430. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Costa C, Ciraolo E. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J Endocrinol. 2007;194:243–256. doi: 10.1677/JOE-07-0097. [DOI] [PubMed] [Google Scholar]
- Hoehn KL, Turner N, Swarbrick MM, Wilks D, Preston E, Phua Y, Joshi H, Furler SM, Larance M, Hegarty BD, et al. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab. 2010;11:70–76. doi: 10.1016/j.cmet.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, Solinas G, Karin M. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell. 2011;147:173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka T, Biggs WH, 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem. 2008;283:16283–16292. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci U S A. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483:350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- Ide T, Shimano H, Yahagi N, Matsuzaka T, Nakakuki M, Yamamoto T, Nakagawa Y, Takahashi A, Suzuki H, Sone H, et al. SREBPs suppress IRS-2-mediated insulin signalling in the liver. Nat Cell Biol. 2004;6:351–357. doi: 10.1038/ncb1111. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Jager J, Corcelle V, Gremeaux T, Laurent K, Waget A, Pages G, Binetruy B, Le Marchand-Brustel Y, Burcelin R, Bost F, et al. Deficiency in the extracellular signal-regulated kinase 1 (ERK1) protects leptin-deficient mice from insulin resistance without affecting obesity. Diabetologia. 2011;54:180–189. doi: 10.1007/s00125-010-1944-0. [DOI] [PubMed] [Google Scholar]
- Jiao P, Feng B, Li Y, He Q, Xu H. Hepatic ERK activity plays a role in energy metabolism. Mol Cell Endocrinol. 2013;375:157–166. doi: 10.1016/j.mce.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007;6:105–114. doi: 10.1016/j.cmet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152:673–684. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- Joshi RL, Lamothe B, Cordonnier N, Mesbah K, Monthioux E, Jami J, Bucchini D. Targeted disruption of the insulin receptor gene in the mouse results in neonatal lethality. EMBO J. 1996;15:1542–1547. [PMC free article] [PubMed] [Google Scholar]
- Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kasuga M, Fujita-Yamaguchi Y, Blithe DL, White MF, Kahn CR. Characterization of the insulin receptor kinase purified from human placental membranes. J Biol Chem. 1983;258:10973–10980. [PubMed] [Google Scholar]
- Katic M, Kennedy AR, Leykin I, Norris A, McGettrick A, Gesta S, Russell SJ, Bluher M, Maratos-Flier E, Kahn CR. Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat-specific insulin receptor knock-out mice. Aging Cell. 2007;6:827–839. doi: 10.1111/j.1474-9726.2007.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerouz NJ, Horsch D, Pons S, Kahn CR. Differential regulation of insulin receptor substrates-1 and -2 (IRS-1 and IRS-2) and phosphatidylinositol 3-kinase isoforms in liver and muscle of the obese diabetic (ob/ob) mouse. J Clin Invest. 1997;100:3164–3172. doi: 10.1172/JCI119872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikani CK, Dong LQ, Liu F. “New”-clear functions of PDK1: beyond a master kinase in the cytosol? J Cell Biochem. 2005;96:1157–1162. doi: 10.1002/jcb.20651. [DOI] [PubMed] [Google Scholar]
- Kim DH, Perdomo G, Zhang T, Slusher S, Lee S, Phillips BE, Fan Y, Giannoukakis N, Gramignoli R, Strom S, et al. FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes. 2011;60:2763–2774. doi: 10.2337/db11-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Zhang T, Lee S, Dong HH. FoxO6 in glucose metabolism (FoxO6) J Diabetes. 2013;5:233–240. doi: 10.1111/1753-0407.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T. The role of FOXO1 in beta-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013;9:615–623. doi: 10.1038/nrendo.2013.157. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- Kubota N, Kubota T, Itoh S, Kumagai H, Kozono H, Takamoto I, Mineyama T, Ogata H, Tokuyama K, Ohsugi M, et al. Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding. Cell Metab. 2008;8:49–64. doi: 10.1016/j.cmet.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13:294–307. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- Kumar A, Harris TE, Keller SR, Choi KM, Magnuson MA, Lawrence JC., Jr Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances Basal glycogen synthase activity. Mol Cell Biol. 2008;28:61–70. doi: 10.1128/MCB.01405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Lawrence JC, Jr, Jung DY, Ko HJ, Keller SR, Kim JK, Magnuson MA, Harris TE. Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes. 2010;59:1397–1406. doi: 10.2337/db09-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M, Zilberfarb V, Gangneux N, Christeff N, Issad T. O-GlcNAc modification of FoxO1 increases its transcriptional activity: a role in the glucotoxicity phenomenon? Biochimie. 2008;90:679–685. doi: 10.1016/j.biochi.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60:391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem. 2003;278:2896–2902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Li M, Georgakopoulos D, Lu G, Hester L, Kass DA, Hasday J, Wang Y. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation. 2005;111:2494–2502. doi: 10.1161/01.CIR.0000165117.71483.0C. [DOI] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, She P, Jetton TL, Demarest KT. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes. 2004;53:410–417. doi: 10.2337/diabetes.53.2.410. [DOI] [PubMed] [Google Scholar]
- Lin HV, Accili D. Reconstitution of insulin action in muscle, white adipose tissue, and brain of insulin receptor knock-out mice fails to rescue diabetes. J Biol Chem. 2011;286:9797–9804. doi: 10.1074/jbc.M110.210807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J Clin Invest. 2004;114:908–916. doi: 10.1172/JCI22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Cao SY, Hong T, Han J, Liu Z, Cao W. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM) J Biol Chem. 2009a;284:27090–27100. doi: 10.1074/jbc.M109.016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi J, Liu Z, Cao W. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem. 2009b;284:31484–31492. doi: 10.1074/jbc.M109.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long YC, Cheng Z, Copps KD, White MF. Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Mol Cell Biol. 2011;31:430–441. doi: 10.1128/MCB.00983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, Ahima RS, Ueki K, Kahn CR, Birnbaum MJ. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18:388–395. doi: 10.1038/nm.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malato Y, Ehedego H, Al-Masaoudi M, Cubero FJ, Bornemann J, Gassler N, Liedtke C, Beraza N, Trautwein C. NF-kappaB essential modifier is required for hepatocyte proliferation and the oval cell reaction after partial hepatectomy in mice. Gastroenterology. 2012;143:1597–1608. e1511. doi: 10.1053/j.gastro.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci U S A. 2005;102:11278–11283. doi: 10.1073/pnas.0502738102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci U S A. 2003;100:11285–11290. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer-Blust AF, Philbrick MJ, Guo S, Wu J, He P, Li J. RTEF-1 attenuates blood glucose levels by regulating insulin-like growth factor binding protein-1 in the endothelium. Circ Res. 2012;111:991–1001. doi: 10.1161/CIRCRESAHA.112.268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Ogawa W, Matsumoto M, Nakamura T, Sakaue H, Kasuga M. Hyperinsulinemia, glucose intolerance, and dyslipidemia induced by acute inhibition of phosphoinositide 3-kinase signaling in the liver. J Clin Invest. 2002;110:1483–1491. doi: 10.1172/JCI15880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- Mora A, Lipina C, Tronche F, Sutherland C, Alessi DR. Deficiency of PDK1 in liver results in glucose intolerance, impairment of insulin-regulated gene expression and liver failure. Biochem J. 2005;385:639–648. doi: 10.1042/BJ20041782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin North Am. 2008;37:685–711. ix–x. doi: 10.1016/j.ecl.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab. 2012;15:595–605. doi: 10.1016/j.cmet.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Jr, Olson DP. Central nervous system control of metabolism. Nature. 2012;491:357–363. doi: 10.1038/nature11705. [DOI] [PubMed] [Google Scholar]
- Nakae J, Biggs WH, 3rd, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- Nandi A, Kitamura Y, Kahn CR, Accili D. Mouse models of insulin resistance. Physiol Rev. 2004;84:623–647. doi: 10.1152/physrev.00032.2003. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Nakae J, Kitamura T, Park BC, Dragatsis I, Accili D. Transgenic rescue of insulin receptor-deficient mice. J Clin Invest. 2004;114:214–223. doi: 10.1172/JCI21645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JL, Zhang Y, Bae SH, Farooqi MS, Liang G, Hammer RE, Goldstein JL, Brown MS. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc Natl Acad Sci U S A. 2012;109:16184–16189. doi: 10.1073/pnas.1213343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012 doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- Pehmoller C, Treebak JT, Birk JB, Chen S, Mackintosh C, Hardie DG, Richter EA, Wojtaszewski JF. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14-3-3 binding in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E665–675. doi: 10.1152/ajpendo.00115.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot V, Rechler MM. The coactivator p300 directly acetylates the forkhead transcription factor Foxo1 and stimulates Foxo1-induced transcription. Mol Endocrinol. 2005;19:2283–2298. doi: 10.1210/me.2004-0292. [DOI] [PubMed] [Google Scholar]
- Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Xu Z, Zhu Q, Thomas C, Kumar R, Feng H, Dostal DE, White MF, Baker KM, Guo S. Myocardial Loss of IRS1 and IRS2 Causes Heart Failure and Is Controlled by p38alpha MAPK During Insulin Resistance. Diabetes. 2013;62:3887–3900. doi: 10.2337/db13-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rached MT, Kode A, Silva BC, Jung DY, Gray S, Ong H, Paik JH, DePinho RA, Kim JK, Karsenty G, et al. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J Clin Invest. 2010;120:357–368. doi: 10.1172/JCI39901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen C, Kahn CR. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2012;32:2052–2059. doi: 10.1161/ATVBAHA.111.241919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, et al. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005a;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Why Syndrome X? From Harold Himsworth to the insulin resistance syndrome. Cell Metab. 2005b;1:9–14. doi: 10.1016/j.cmet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Ren H, Orozco IJ, Su Y, Suyama S, Gutierrez-Juarez R, Horvath TL, Wardlaw SL, Plum L, Arancio O, Accili D. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell. 2012;149:1314–1326. doi: 10.1016/j.cell.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- Rena G, Prescott AR, Guo S, Cohen P, Unterman TG. Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14-3-3 binding, transactivation and nuclear targetting. Biochem J. 2001;354:605–612. doi: 10.1042/0264-6021:3540605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G, Woods YL, Prescott AR, Peggie M, Unterman TG, Williams MR, Cohen P. Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion. EMBO J. 2002;21:2263–2271. doi: 10.1093/emboj/21.9.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- Rhodes CJ, White MF, Leahy JL, Kahn SE. Direct Autocrine Action of Insulin on beta-Cells: Does It Make Physiological Sense? Diabetes. 2013;62:2157–2163. doi: 10.2337/db13-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012;32:1771–1776. doi: 10.1161/ATVBAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondinone CM, Wang LM, Lonnroth P, Wesslau C, Pierce JH, Smith U. Insulin receptor substrate (IRS) 1 is reduced and IRS-2 is the main docking protein for phosphatidylinositol 3-kinase in adipocytes from subjects with non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1997;94:4171–4175. doi: 10.1073/pnas.94.8.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411. doi: 10.1146/annurev.med.051308.105148. [DOI] [PubMed] [Google Scholar]
- Rui L, Fisher TL, Thomas J, White MF. Regulation of insulin/insulin-like growth factor-1 signaling by proteasome-mediated degradation of insulin receptor substrate-2. J Biol Chem. 2001;276:40362–40367. doi: 10.1074/jbc.M105332200. [DOI] [PubMed] [Google Scholar]
- Sadagurski M, Leshan RL, Patterson C, Rozzo A, Kuznetsova A, Skorupski J, Jones JC, Depinho RA, Myers MG, Jr, White MF. IRS2 signaling in LepR-b neurons suppresses FoxO1 to control energy balance independently of leptin action. Cell Metab. 2012;15:703–712. doi: 10.1016/j.cmet.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M, Norquay L, Farhang J, D'Aquino K, Copps K, White MF. Human IL6 enhances leptin action in mice. Diabetologia. 2010;53:525–535. doi: 10.1007/s00125-009-1580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG. Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J Biol Chem. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Chakraborty S, Paik J, Yutzey KE, Evans-Anderson HJ. FoxO1 is required in endothelial but not myocardial cell lineages during cardiovascular development. Dev Dyn. 2012;241:803–813. doi: 10.1002/dvdy.23759. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–7478. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284:28319–28331. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay T, Jojic V, Zuk O, Rothamel K, Puyraimond-Zemmour D, Feng T, Wakamatsu E, Benoist C, Koller D, Regev A. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc Natl Acad Sci U S A. 2013;110:2946–2951. doi: 10.1073/pnas.1222738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RK, Alberti KG, Gale EA, Colagiuri S, Tuomilehto J, Qiao Q, Ramachandran A, Tajima N, Brajkovich-Mirchov I, Ben-Nakhi A, et al. The metabolic syndrome: useful concept or clinical tool? Report of a WHO Expert Consultation. Diabetologia. 2010;53:600–605. doi: 10.1007/s00125-009-1620-4. [DOI] [PubMed] [Google Scholar]
- Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- Sorensen H, Brand CL, Neschen S, Holst JJ, Fosgerau K, Nishimura E, Shulman GI. Immunoneutralization of endogenous glucagon reduces hepatic glucose output and improves long-term glycemic control in diabetic ob/ob mice. Diabetes. 2006;55:2843–2848. doi: 10.2337/db06-0222. [DOI] [PubMed] [Google Scholar]
- Sun X, Liu F. Phosphorylation of IRS proteins: Yin-Yang regulation of insulin signaling. Vitamins and Hormones. 2009;80:351–387. doi: 10.1016/S0083-6729(08)00613-4. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, Gude N, Avitabile D, Alvarez R, Sundararaman B, et al. Myocardial AKT: the omnipresent nexus. Physiol Rev. 2011;91:1023–1070. doi: 10.1152/physrev.00024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Daitoku H, Hirota K, Tamiya H, Yokoyama A, Kako K, Nagashima Y, Nakamura A, Shimada T, Watanabe S, et al. Asymmetric arginine dimethylation determines life span in C. elegans by regulating forkhead transcription factor DAF-16. Cell Metab. 2011;13:505–516. doi: 10.1016/j.cmet.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, et al. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem. 2008;283:9787–9796. doi: 10.1074/jbc.M708839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, DePinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15:372–381. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Wagner R, Machicao F, Fritsche A, Stefan N, Haring H, Staiger H. The genetic influence on body fat distribution. Drug Discov Today Dis Mech. 2013;10:e5–e13. [Google Scholar]
- Wang Y, Inoue H, Ravnskjaer K, Viste K, Miller N, Liu Y, Hedrick S, Vera L, Montminy M. Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107:3087–3092. doi: 10.1073/pnas.0914897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne JP, Alemi F, Reed AS, Varonin JM, Chan H, Piper ML, Mullin ME, Myers MG, Jr, Corvera CU, Xu AW. Impairment of central leptin-mediated PI3K signaling manifested as hepatic steatosis independent of hyperphagia and obesity. Cell Metab. 2011;14:791–803. doi: 10.1016/j.cmet.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- White MF, Maron R, Kahn CR. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985;318:183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Williams MR, Arthur JS, Balendran A, van der Kaay J, Poli V, Cohen P, Alessi DR. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10:439–448. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- Wilson C. Diabetes: ACCORD: 5-year outcomes of intensive glycemic control. Nat Rev Endocrinol. 2011;7:314. doi: 10.1038/nrendo.2011.67. [DOI] [PubMed] [Google Scholar]
- Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet. 1999;23:32–40. doi: 10.1038/12631. [DOI] [PubMed] [Google Scholar]
- Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- Woods YL, Rena G, Morrice N, Barthel A, Becker W, Guo S, Unterman TG, Cohen P. The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem J. 2001;355:597–607. doi: 10.1042/bj3550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1) J Biol Chem. 2002;277:49676–49684. doi: 10.1074/jbc.M208756200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Jiao P, Huang X, Feng B, Feng Y, Yang S, Hwang P, Du J, Nie Y, Xiao G, et al. MAPK phosphatase-3 promotes hepatic gluconeogenesis through dephosphorylation of forkhead box O1 in mice. J Clin Invest. 2010;120:3901–3911. doi: 10.1172/JCI43250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe D, Brown MS, Goldstein JL. Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc Natl Acad Sci U S A. 2002;99:12753–12758. doi: 10.1073/pnas.162488899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K, Mukai H, Kasuya Y, Fukamizu A. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol Cell. 2008;32:221–231. doi: 10.1016/j.molcel.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Yan L, Lavin VA, Moser LR, Cui Q, Kanies C, Yang E. PP2A regulates the pro-apoptotic activity of FOXO1. J Biol Chem. 2008;283:7411–7420. doi: 10.1074/jbc.M708083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005;24:1021–1032. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, McGuinness OP. Inflammation during obesity is not all bad: evidence from animal and human studies. Am J Physiol Endocrinol Metab. 2013;304:E466–477. doi: 10.1152/ajpendo.00266.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagley D, Guo S, Unterman T, Quinn PG. Gene- and activation-specific mechanisms for insulin inhibition of basal and glucocorticoid-induced insulin-like growth factor binding protein-1 and phosphoenolpyruvate carboxykinase transcription. Roles of forkhead and insulin response sequences. J Biol Chem. 2001;276:33705–33710. doi: 10.1074/jbc.M101215200. [DOI] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Lehtinen MK, Merlo P, Villen J, Gygi S, Bonni A. Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem. 2009;284:11285–11292. doi: 10.1074/jbc.M900461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Gao Z, Yin J, Quon MJ, Ye J. S6K directly phosphorylates IRS-1 on Ser-270 to promote insulin resistance in response to TNF-(alpha) signaling through IKK2. J Biol Chem. 2008;283:35375–35382. doi: 10.1074/jbc.M806480200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ou J, Bashmakov Y, Horton JD, Brown MS, Goldstein JL. Insulin inhibits transcription of IRS-2 gene in rat liver through an insulin response element (IRE) that resembles IREs of other insulin-repressed genes. Proc Natl Acad Sci U S A. 2001;98:3756–3761. doi: 10.1073/pnas.071054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Li L, Qi Y, Zhu X, Gan B, DePinho RA, Averitt T, Guo S. Hepatic suppression of Foxo1 and Foxo3 causes hypoglycemia and hyperlipidemia in mice. Endocrinology. 2012;153:631–646. doi: 10.1210/en.2011-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gan L, Pan H, Guo S, He X, Olson ST, Mesecar A, Adam S, Unterman TG. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J Biol Chem. 2002;277:45276–45284. doi: 10.1074/jbc.M208063200. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Rubino F, Dixon JB. IDF's view of bariatric surgery in type 2 diabetes. Lancet. 2011;378:108–110. doi: 10.1016/S0140-6736(11)61027-1. [DOI] [PubMed] [Google Scholar]