Abstract
Aims
Diabetic nephropathy (DN) is a major diabetic complication characterized by mesangial proliferation and glomerular hypertrophy. MicroRNAs might play an important role in these pathological processes. The aim of this study is to examine the possible association of miR-34a as one of the microRNAs with DN and underlying mechanisms in vitro and in vivo.
Methods
According to previous results of microarray which compared the different microRNAs between diabetic and normal control mice, miR-34a was chosen and its expression was detected by qRT-PCR. Cell viability was then assessed using Cell Counting Kit-8 (CCK8) and 5-ethynyl-20-deoxyuridine (EDU) incorporation. Antagomir was injected in db/db mice to down regulate miR-34a. Average diameter of glomeruli was analyzed by periodic acid-Schiff (PAS) stain of kidney. Luciferase gene report assay was then performed to identify the target gene of miR-34a. Additional immunoblotting and immunohistochemical analyses were implemented to verify the expression level of growth arrest-specific 1 (GAS1).
Results
MiR-34a expression level was increased under high glucose condition in vitro and in vivo. Down-regulation of miR-34a inhibits mice mesangial cells (MMCs) proliferation in vitro and alleviates glomerular hypertrophy in vivo. GAS1 was proved to be the target of miR-34a through luciferase report. Moreover, up-regulation of GAS1 expression was observed in the presence of miR-34a antagomir as compared withmiR-34a antagomir-NC in high-glucose-treated MMCs and db/db mice, respectively.
Conclusions
MiR-34a regulated mesangial proliferation and glomerular hypertrophy by directly inhibiting GAS1 in early DN.
Keywords: Diabetic nephropathy, Glomerular hypertrophy, miR-34a, GAS1
1. Introduction
Diabetic nephropathy (DN) is one of the severe complications of diabetes. It is characterized by mesangial cell proliferation, extracellular matrix (ECM) accumulation, glomerulosclerosis and always leads to end-stage kidney diseases (ESKDs) (Eid et al., 2013; Sun, Su, Li, & Wang, 2013). Evidences suggest that proliferation of mesangial cells is critical in the initiation and progression of DN (Liu et al., 2012; Wolf & Ziyadeh, 1999). In recent years, several studies have identified the regulatory roles of some factors in proliferation of mesangial cells (Onozaki et al., 2004; Zhang et al., 2012), but the detailed mechanisms involved in the progression of DN have not been fully understood.
MicroRNAs (miRNAs) are a group of small non-coding single-stranded RNAs which have about 17–24 nucleotides in length. It is estimated that more than two thirds of total human mRNAs are targeted by miRNAs (Fernandez-Hernando, Ramirez, Goedeke, & Suarez, 2013). Recently, it has been indicated that miRNAs are highly involved in pathology of diabetes and its relevant renal injuries (Conserva, Pontrelli, Accetturo, & Gesualdo, 2013; Zhang et al., 2009; Zhang et al., 2012). Previous microarray results suggested that miR-34a was up-regulated in DN (Chen et al., 2012). In some other studies, miR-34a is usually closely related with cell proliferation (Chen et al., 2011; Dang, Luo, Rong, & Chen, 2013). So, we hypothesize that miR-34a might affect mesangial cells proliferation in the pathological process of DN. Subsequently, we examine the mechanism of miR-34a regulation in DN in this study.
Growth arrest-specific 1 (GAS1) is identified as a putative tumor suppressor gene. GAS1 protein is a glycosyl-phosphatidyl-inositol (GPI)-anchored protein, which is overexpressed in growth-arrested embryonic mouse NIH/3 T3 fibroblasts and inhibits their proliferation by blocking the cell cycle G0/S transition (Evdokiou & Cowled, 1998; Stebel et al., 2000). GAS1 shows high homology to the glial cell=derived neurotrophic factor (GDNF) family receptor-α (Cabrera et al., 2006). GAS1 is also expressed in the kidney during development and under pathological conditions, but the function of GAS1 in the adult kidney is still not completely known.
In the present study, we aimed to investigate the critical roles of miR-34a in proliferation of mesangial cell under hyperglycemia and glomerular hypertrophy during early DN and to clarify the underlying mechanisms.
2. Materials and methods
2.1. Cell culture
Isolation of mice mesangial cells (MMCs) was performed according to the method described previously (Kim, Reddy, Lanting, Adler, & Natarajan, 2003). Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum(FBS) and were kept in a humidified incubator that was maintained at 37 °C and supplied with 5% CO2 and 95% air. Passages between 3 and 8 were used in all experiments.
2.2. Transient transfection
All of the transient transfections were performed with Lipofecta-mine 2000 Reagent (Invitrogen, USA) according to the manufacturer’s recommendations. miRNA oligonucleotide transfections used in loss of-function experiment were performed according to an established protocol. Briefly, cells were seeded onto 6-well plates and were grown to 80% confluency. Next, a miRNA antagomir, or a matched miRNA negative control (Genepharma, Shanghai, China) was added to the culture media at a final concentration of 100 nM. After 6 h of transfection, the medium was replaced with RPMI-1640 containing 5.6 or 30 mM glucose, and cells were incubated for 48 h. Transfection efficiency (>90%) was measured by qRT-PCR.
2.3. In vitro cell proliferation assay
Cell viability was assayed by a colorimetric procedure, using the Cell Counting Kit-8 (Dojindo, Shanghai, China) according to the manufacturer’s protocol. The absorbance at 450 nm was determined with a microplate reader. For each group, 5 duplicate wells were detected per experiment. To detect the exact proliferation rates of MMCs, an EDU (5-ethynyl-20-deoxyuridine) incorporation assay was executed with the Cell-Light™ EdU In Vitro Imaging Kit (Ribobio, Guangzhou, China) according to the producer’s instructions. Briefly, cells treated as mentioned above were incubated with 10 µM EDU in RPMI-1640 medium for 2 h before fixation in 4% paraformaldehyde. After EDU staining, cell nuclei were stained with Hoechst 33342 and visualized under fluorescence microscopy (Leica, Germany). For each group, 3 random fields were photographed. The proliferation rate was calculated by the number of EDU-stained cells normalized by the number of Hoechst 33342-stained cells.
2.4. Glomerular morphological analysis
The sections of renal glomeruli from mice of each group were stained with Periodic acid-Schiff (PAS) and observed under microscope. The average diameter of glomeruli was measured with image analysis software (Image-pro plus, Version 5.1.0.20, Media Cybernetics, Inc). Glomerular tufts in a group were counted in nine different visual fields.
2.5. Luciferase reporter gene assay
Using target scan software, GAS1was predicted to be a target of miR-34a. The full length 3’-UTR of GAS1 was amplified by PCR from mice genomic DNA and cloned at the SacI and XhoI sites into luciferase reporter vector (Promega, Madison, WI, USA). The mutant or WT construct of GAS1 3’UTR was generated by GuangZhou Ribobio Company in China. 293T cells were co-transfected with a reporter construct (pmiR-null Report plasmid, pmiR-GAS1 3’-UTR, pmiR-GAS1 3’-UTR-Mut) and miR-34amimic or miR-negative control. After 24 h of incubation, the luciferase activities were measured in a luminometer with the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s recommendations.
2.6. Real-time quantitative PCR (qRT-PCR)
Total RNA, including microRNA, was extracted from cultured cells and tissues using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. miRNA qRT-PCR was performed using SYBR Premix ExTaq TM(Takara, Tokyo, Japan) according to the manufacturer’s instructions and an Applied Biosystems 7500 Fast real-time PCR system, as reported previously (Bernardo et al., 2012). The PCR cycling conditions were as follows: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 35 s. U6 snRNA (Applied Biosystems) was used as an endogenous normalization control. Individual samples were run in triplicate, and each experiment was repeated at least 3 times. Data analyses were performed using the comparative CT (ΔΔCT)method for calculating relative gene expression.
2.7. Western blot analysis
Western blotting was performed as described previously (van Roeyen et al., 2013). The following antibodies were used: anti-GAS1 antibody (1:100; Santa Cruz), anti-Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) Antibody (1:3000; Sigma) was used as a loading control. The results were analyzed using Quantity One software (Bio-Rad, Richmond, Calif., USA).
2.8. Animals experiments
Male 4-week-old C57BL/KsJ type 2 diabetic db/db mice and heterozygote age-matched db/m mice were originally purchased from Model Animal Research Center of Nanjing University (Nanjing, China). Mice were kept in the Laboratory Animal Center of the Third Military Medical University under controlled temperature (20–22 °C), light (12-/12-h light/dark cycle) and humidity (50%–60%) and received food and water ad libitum. Db/db and db/m mice that were 8 weeks old were randomly subdivided into four experimental groups of 8 animals each: (1) control (untreated db/m mice) group, (2) untreated db/db group, (3) antagomir-34a-Negative Control (antagomir NC)-treated db/db group, and (4) antagomir-34a-treated db/db group. Db/db mice were injected with miR-34a antagomir or antagomir NC (80 mg/kg/day) (Genepharma, Shanghai, China) via the tail vein for three consecutive days. The mice injected with miR-34a antagomir or antagomir NC behave as the normal ones and did not exhibit any signs of discomfort. Four weeks later, the mice were sacrificed and the kidneys from the mice were collected as described previously (Park et al., 2007). One piece of kidney tissues was fixed by neutral formalin for immunohistochemical analysis, and the remaining tissues were stored at −70 °C for later use. All experiments were approved by the Animal Care and Use Committee of the Third Military Medical University and complied with the Declaration of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.9. Immunohistochemical studies
Sections of kidney tissues from mice of each group were examined by immunocytochemical staining of GAS1 with anti-GAS1 antibodies (1:50; Santa Cruz) using a previously reported method (Lee et al., 2001).
2.10. Statistical analysis
All data are presented as mean ± SEM. Differences between experimental groups were evaluated using Independent-Samples t test or a one-way ANOVA followed by Tukey’s multiple-comparisons test with SPSS 18.0 package (SPSS Inc., Chicago, Ill., USA). A P value < 0.05 was considered to be statistically significant.
3. Results
3.1. Over-expression of miR-34a in high glucose-treated cells and db/db mice
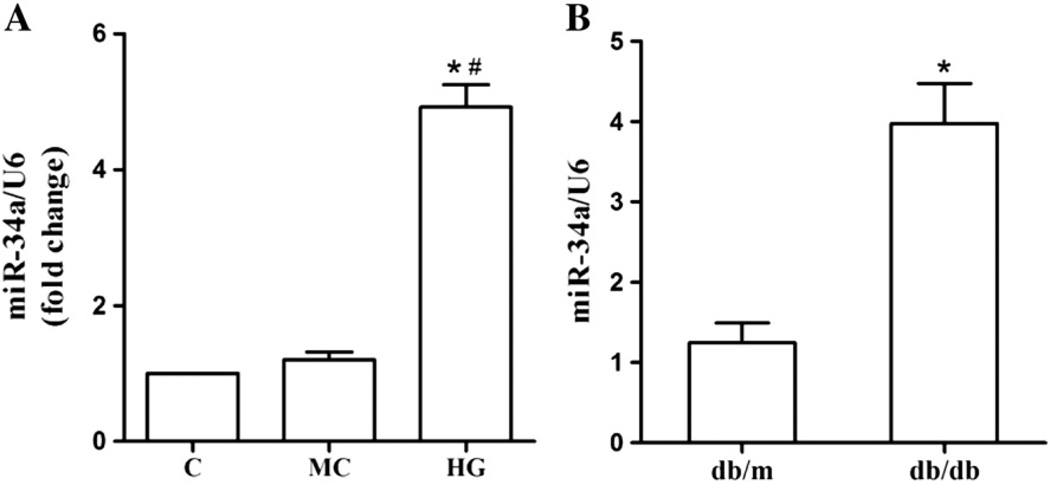
To study the potential role of miR-34a in DN, we first used qRT-PCR to analyze the changes of miR-34a expression. Both MMCs that were exposed to high glucose in vitro and renal glomeruli from db/db DN mice in vivo were tested. As illustrated in Fig. 1A, a significant increase in miR-34a expression was found in MMCs that were treated with 30 mM glucose for 48 h compared with normal control. Also, miR-34a was highly expressed in 12-week-old db/db mice, whose biochemical and histological changes were consistent with that in human disease in this model (Fig. 1B). Taken together, these results suggested that expression level of miR-34a is positively related to DN.
Fig. 1. Expression of miR-34a upon treatment in high glucose condition.
(A) After MMCs were treated with high glucose (30 mM) for 48 h, miR-34a expression was determined by qRT-PCR. Data were expressed as mean ± SEM. *P < 0.05 compared with normal control, #P < 0.05 compared with mannitol control. (B) miR-34a expression in glomeruli from mouse models of type 2 diabetes (db/db mice, 12 weeks of age) was detected by qRT-PCR. Data were shown as mean ± SEM (n = 8 per group). *P < 0.05 compared with the db/m mice. Levels of miR-34a were normalized to U6 small nuclear RNA (miR-34a/U6). C, control; MC, mannitol control; HG, high glucose.
3.2. Down-regulation of miR-34a inhibits MMCs proliferation in vitro and alleviates glomerular hypertrophy in vivo
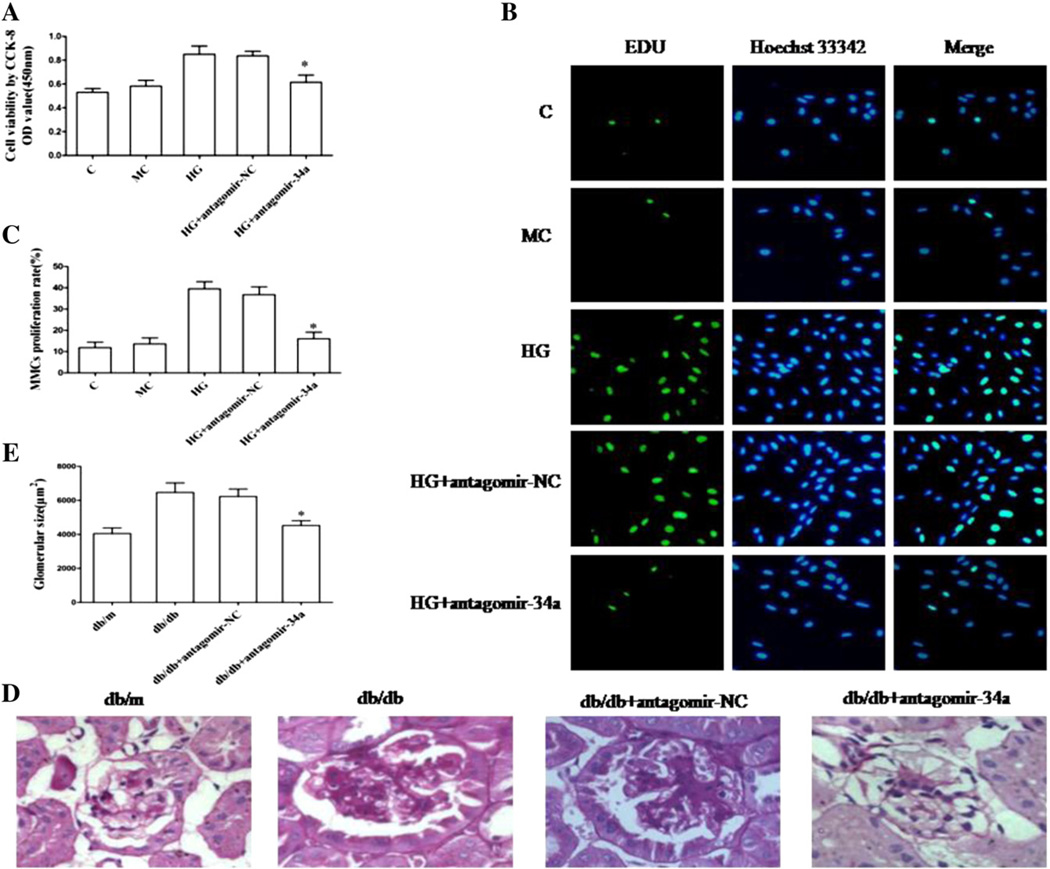
Cell viability was detected with the CCK-8 assay, which can indirectly reflect the proliferative ability of the MMCs. High glucose significantly increased MMCs viability, which could be suppressed by antagomir of miR-34a (Fig. 2A). A similar result was obtained with the EDU incorporation assay. Proliferation rates of MMCs transfected with antagomir-34a was significantly inhibited compared with the antagomir-NC group (Fig. 2B and C). Moreover, we determined the proliferative effect of miR-34a in vivo. The diameter of glomeruli was significantly greater in the db/db group than that in the db/m group. Interestingly, it was smaller in antagomir-34a-treated group than that in the untreated db/db and antagomir-NC-treated groups (Fig. 2D and E). These data convincingly confirmed that down-regulation of miR-34a inhibits the glomerular mesangial cell proliferation in vitro and alleviates glomerular hypertrophy in vivo.
Fig. 2. Effect of miR-34a on cell proliferation in vitro and glomerular hypertrophy in vivo.
(A) Cell counting kit-8 assay. 1 × 104 MMCs of each group were first seeded in a 96-well plate, transfected with antagomir-34a or antagomir NC and then cultured with 5.6 or 30 mM glucose for 48 h. Data were mean ± SEM. *P < 0.05 compared with the group treated with antagomir-NC. (B) EDU incorporation assay. After transfection, MMCs were pre-incubated with 5.6 or 30 mMglucose and were cultured with 10 µM EDU-1640 medium for 2 h. In the image overlay, nuclei of EDU-positive cells were stained cyan, while the blue nuclei were stained by Hoechst 33342. (C) Quantitative measurements of MMCs proliferation rate. Data were mean ± SEM. *P < 0.05 compared with the group treated with antagomir-NC. (D)Morphological observation of glomeruli. Representative photographs of PAS-stained kidney specimens from db/m mice, db/db mice, db/db mice treated with antagomir-NC and db/db mice treated with antagomir-34a. (E) Quantitative measurements of glomerular size. Data were mean ± SEM. *P <0.05 compared with db/db mice treated with antagomir-NC.
3.3. GAS1 is a target for miR-34a
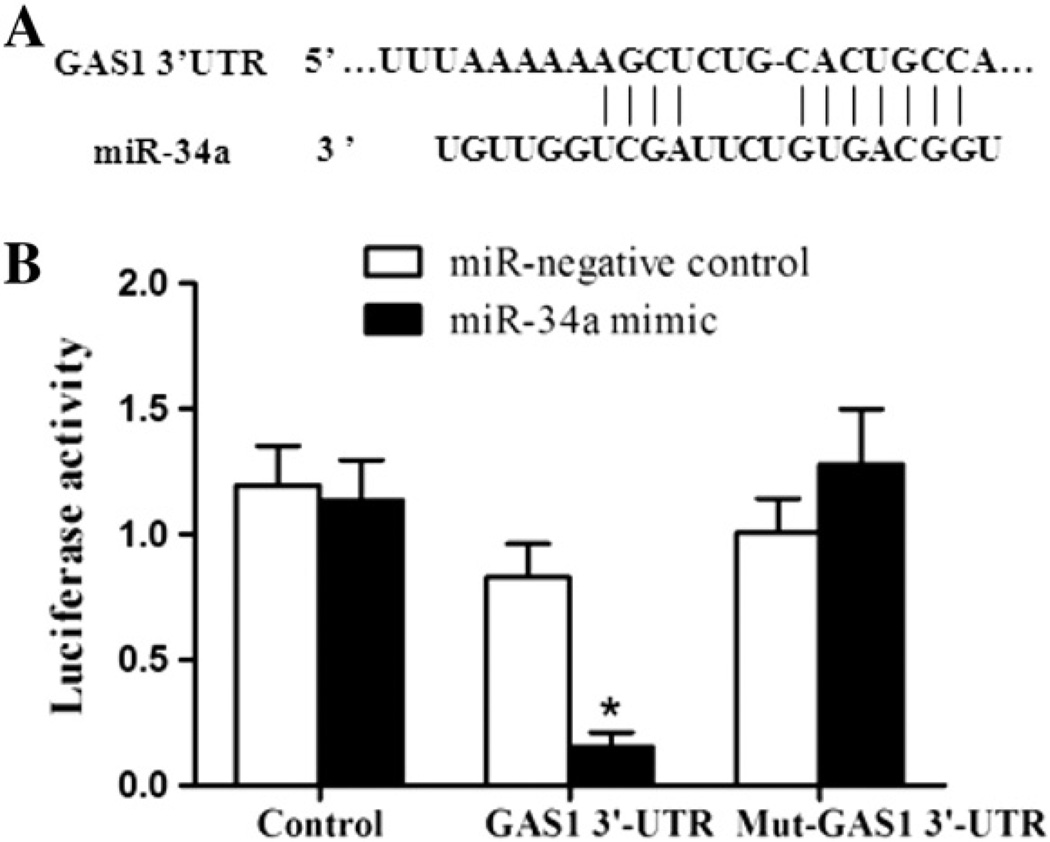
MiRNAs are supposed to inhibit hundreds of mRNA targets, resulting in changes in the cellular phenotype in kidney tissues, such as glomerular hypertrophy and glomerulosclerosis. First, we made an effort to identify potential targets for miR-34a by bioinformatics prediction software. It was found that the gene GAS1 as a putative target gene for miR-34a mediated cell cycle and proliferation. To verify whether miR-34a directly targeted GAS1, luciferase reporter assays were conducted. As shown in Fig. 3A and B, a significant decrease in the luciferase activity was observed when co-transfecting 293T cells with GAS1–3’UTR/plasmid and miR-34a mimics compared with the negative control. This repressive effect was abolished by point mutations in the core binding sites of the GAS1 3’-UTR. All these results indicated that miR-34a has inhibitory effects on GAS1 expression via interaction with the 3’-UTR of GAS1.
Fig. 3. GAS1 3’-UTR is a target of miR-34a.
(A) GAS1 was the target gene of miR-34a by targetscan prediction. (B) The relative luciferase activity in 293T cells was determined after the plasmid with the GAS1 3’-UTR wild-type or mutant was co-transfected with miR-34a mimics or the negative control. Three independent experiments were performed in duplicate. Data are presented as mean ± SEM. *P < 0.05 compared with the cells transfected with the miR-negative control plus GAS1 3’-UTR.
3.4. miR-34a negatively regulates GAS1 expression
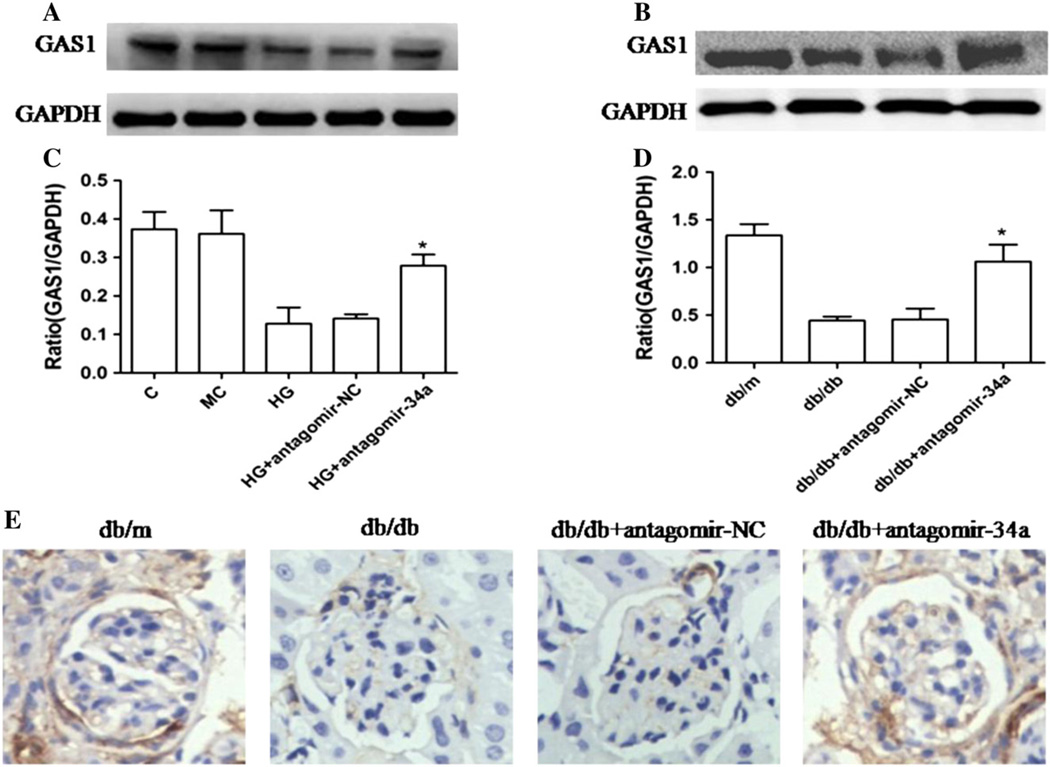
To further confirm that GAS1 was a target gene for miR-34a, western blot analysis was used to detect the expression of GAS1 in MMCs and kidneys, respectively. The expression of GAS1 in both MMCs under high glucose condition and kidneys from db/db mice was significantly up-regulated in the antagomir-34a group compared with that in the untreated group and antagomir-NC group (Fig. 4A–D). In addition, we also detected the protein levels of GAS1 in kidney tissues using immunohistochemical analysis (Fig. 4E). The result coincided with that in western blot assay. Collectively, the above findings suggested that miR-34a negatively regulates GAS1 expression, indicating GAS1 is a direct target of miR-34a.
Fig. 4. Effect of miR-34a on the expression of GAS1 determined by western blot and immunohistochemical analysis in vitro and in vivo.
(A) Representative western blot analysis showed the significant increase of GAS1 protein in antagomir-34a-transfected MMCs compared with antagomir-NC-transfected cells. (B) Representative western blot analysis showed the significant increase of GAS1 proteins in glomeruli of antagomir-34a-treated db/db mice compared with the glomeruli of db/db mice treated with antagomir-NC. (C, D) Quantitative analysis of western blotting. Three independent experiments were performed in duplicate. Data obtained by quantitative densitometry are expressed as mean ± SEM (n=8). *P < 0.05 compared with the db/db mice treated with antagomir-NC. (E) GAS1 protein expression levels were detected in the glomeruli by immunohistochemical staining. Figures are shown at × 400 magnification.
4. Discussion
Emerging evidences show that miR-34a is involved in the pathogenesis of various metabolic diseases such as diabetes mellitus (Nesca et al., 2013; Wei et al., 2013; Yamakuchi, 2012) through regulation of expression of multiple target genes. However, the reports of the abnormal expression and the function of miR-34a in DN are seldom found. In this study, we demonstrated for the first time that miR-34a plays a positive regulatory role in MMCs proliferation, which is associated with DN pathology. And we have provided evidences, both in vitro and in vivo, that down-regulation of miR-34a inhibited mesangial cell proliferation and glomerular hypertrophy. Furthermore, we explored its mechanism via targeting GAS1.
MiR-34a has been reported to be involved in cell proliferation and cell differentiation in many fields (Chiyomaru et al., 2013; Hou et al., 2013). There are some studies indicating the promoting effects of miR-34a in cell proliferation and cell differentiation, which are consistent with our results (Bai et al., 2011; de Antonellis et al., 2011). However, miR-34a has been found previously to be related with cell proliferation mainly as an inhibitor. Our results showed a quite different effect of miR-34a on cell proliferation. The reason for this discrepancy may be caused by the different signaling pathways. As compared with our results, the different role of miR-34a shown in other studies can be illustrated by the different targeting gene of miR-34a. This also shows an interesting and complicated role of miR-34a in different models of cell proliferation.
In this study, we first discovered the changes of cell proliferation after treating with miR-34a antagomir. With the methods of bioinformatics and an evidence showing that GAS1 is involved in glomerular cell proliferation and activation (van Roeyen et al., 2013), GAS1 was thus selected as the target of miR-34a. It has previously been reported that GAS1 is related to a lot of cell activities such as cell proliferation, cell growth, cell activation and cell death (Gobeil, Zhu, Doillon, & Green, 2008; Zarco, Gonzalez-Ramirez, Gonzalez, & Segovia, 2012). Wang et al. (2012) found that GAS1 expression was decreased in gastric cancer and was predictive of a poor prognosis. Restoration of GAS1 expression inhibited cell growth and promoted apoptosis of cancer cells, which was partially associated with the modulation of the Bcl-2/Bax ratio and activating caspase-3. Thus, they suggested that GAS1 might be used as a novel therapeutic candidate for gastric cancer. In another study, van Roeyen et al. (2013) also proved that GAS1 protein inhibited the proliferation of mesangial cells. They discovered that the induction of mesangial cell proliferation by PDGF-BB or -DD led to down-regulation of GAS1 mRNA. However, specific ligands of the PDGF α-receptor, PDGF-AA and -CC, had no effect on GAS1 expression. They conducted transfection of Gas1/Fc plasmid in rat glomerular mesangial cell exhibiting the reduction of cell activation and proliferation. In our study, we identified the relationship between GAS1 and cell proliferation, and proved that GAS1 as a target of miR-34a regulates glomerular cell proliferation, but the detailed mechanism is still not very clear which is worth to be noted in a further study.
MiRNA therapy has already been proved to be effective in treating many diseases through large scale and multicenter clinical trial study. More and more emerging miR-related therapies are under testing and promising for the future novel therapy (Janssen et al., 2013). The results of present studies showed that miR-34a was highly expressed in DN mice as compared with the one in normal kidney tissues, on the other hand, the presence of miR-34a antagomir resulted in the reduction of glomerular hypertrophy under high-glucose condition. Collectively, our results suggested that high expression of miR-34a closely related to the pathological change in glomerular mesangial cells. The level of endogenous miR-34a was positively correlated with glomerular hypertrophy, indicating an association with the progression of DN. Based on all of the evidences above, miR-34a-related therapy might be a novel promising treatment for DN in the future.
In conclusion, our findings suggested that miR-34a plays a critical role in the development of DN, and provided evidences in vitro and in vivo showing that down-regulation of miR-34a inhibited mesangial cell proliferation and glomerular hypertrophy by targeting GAS1, suggesting that miR-34a is a potential new diagnostic and therapeutic target for the treatment of DN.
Acknowledgments
The authors are grateful to Dr. Jinghong Zhao (Department of Nephrology, Xinqiao Hospital, Third Military Medical University) for valuable suggestions concerning study design and preparation of the manuscript. This work was supported by the Clinical Research Foundation of Third Military Medical University (TMMU2011).
Footnotes
Conflicts of Interest: None.
References
- Bai XY, Ma Y, Ding R, Fu B, Shi S, Chen XM. miR-335 and miR-34a Promote renal senescence by suppressing mitochondrial antioxidative enzymes. Journal of the American Society of Nephrology: JASN. 2011;22(7):1252–1261. doi: 10.1681/ASN.2010040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo BC, Gao XM, Winbanks CE, Boey EJ, Tham YK, Kiriazis H, et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(43):17615–17620. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera JR, Sanchez-Pulido L, Rojas AM, Valencia A, Manes S, Naranjo JR, et al. Gas1 is related to the glial cell-derived neurotrophic factor family receptors alpha and regulates Ret signaling. The Journal of biological chemistry. 2006;281(20):14330–14339. doi: 10.1074/jbc.M509572200. [DOI] [PubMed] [Google Scholar]
- Chen H, Sun Y, Dong R, Yang S, Pan C, Xiang D, et al. Mir-34a is upregulated during liver regeneration in rats and is associated with the suppression of hepatocyte proliferation. PloS one. 2011;6(5):e20238. doi: 10.1371/journal.pone.0020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YQ, Wang XX, Yao XM, Zhang DL, Yang XF, Tian SF, et al. Abated microRNA-195 expression protected mesangial cells from apoptosis in early diabetic renal injury in mice. Journal of nephrology. 2012;25(4):566–576. doi: 10.5301/jn.5000034. [DOI] [PubMed] [Google Scholar]
- Chiyomaru T, Yamamura S, Fukuhara S, Yoshino H, Kinoshita T, Majid S, et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PloS one. 2013;8(8):e70372. doi: 10.1371/journal.pone.0070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conserva F, Pontrelli P, Accetturo M, Gesualdo L. The pathogenesis of diabetic nephropathy: Focus on microRNAs and proteomics. Journal of nephrology. 2013;26(5):811–820. doi: 10.5301/jn.5000262. [DOI] [PubMed] [Google Scholar]
- Dang Y, Luo D, Rong M, Chen G. Underexpression of miR-34a in hepatocellular carcinoma and its contribution towards enhancement of proliferating inhibitory effects of agents targeting c-MET. PloS one. 2013;8(4):e61054. doi: 10.1371/journal.pone.0061054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Antonellis P, Medaglia C, Cusanelli E, Andolfo I, Liguori L, De Vita G, et al. MiR-34a targeting of Notch ligand delta-like 1 impairs CD15+/CD133+ tumor-propagating cells and supports neural differentiation in medulloblastoma. PloS one. 2011;6(9):e24584. doi: 10.1371/journal.pone.0024584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid AA, Koubeissi A, Bou-Mjahed R, Khalil NA, Farah M, Maalouf R, et al. Novel carbocyclic nucleoside analogs suppress glomerular mesangial cells proliferation and matrix protein accumulation through ROS-dependent mechanism in the diabetic milieu. Bioorganic & medicinal chemistry letters. 2013;23(1):174–178. doi: 10.1016/j.bmcl.2012.10.122. [DOI] [PubMed] [Google Scholar]
- Evdokiou A, Cowled PA. Growth-regulatory activity of the growth arrest-specific gene, GAS1, in NIH3T3 fibroblasts. Experimental cell research. 1998;240(2):359–367. doi: 10.1006/excr.1998.4011. [DOI] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Ramirez CM, Goedeke L, Suarez Y. MicroRNAs in metabolic disease. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(2):178–185. doi: 10.1161/ATVBAHA.112.300144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil S, Zhu X, Doillon CJ, Green MR. A genome-wide shRNA screen identifies GAS1 as a novel melanoma metastasis suppressor gene. Genes & development. 2008;22(21):2932–2940. doi: 10.1101/gad.1714608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Tang J, Wang Z, Wang C, Chen X, Hou L, et al. Inhibitory effect of microRNA-34a on retinal pigment epithelial cell proliferation and migration. Investigative ophthalmology & visual science. 2013;54(10):6481–6488. doi: 10.1167/iovs.13-11873. [DOI] [PubMed] [Google Scholar]
- Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. The New England journal of medicine. 2013;368(18):1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- Kim YS, Reddy MA, Lanting L, Adler SG, Natarajan R. Differential behavior of mesangial cells derived from 12/15-lipoxygenase knockout mice relative to control mice. Kidney international. 2003;64(5):1702–1714. doi: 10.1046/j.1523-1755.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- Lee KK, Leung AK, Tang MK, Cai DQ, Schneider C, Brancolini C, et al. Functions of the growth arrest specific 1 gene in the development of the mouse embryo. Developmental biology. 2001;234(1):188–203. doi: 10.1006/dbio.2001.0249. [DOI] [PubMed] [Google Scholar]
- Liu L, Hu X, Cai GY, Lv Y, Zhuo L, Gao JJ, et al. High glucose-induced hypertrophy of mesangial cells is reversed by connexin43 overexpression via PTEN/Akt/mTOR signaling. Nephrology, dialysis, transplantation: Official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27(1):90–100. doi: 10.1093/ndt/gfr265. [DOI] [PubMed] [Google Scholar]
- Nesca V, Guay C, Jacovetti C, Menoud V, Peyot ML, Laybutt DR, et al. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia. 2013;56(10):2203–2212. doi: 10.1007/s00125-013-2993-y. [DOI] [PubMed] [Google Scholar]
- Onozaki A, Midorikawa S, Sanada H, Hayashi Y, Baba T, Katoh T, et al. Rapid change of glucose concentration promotes mesangial cell proliferation via VEGF: Inhibitory effects of thiazolidinedione. Biochemical and biophysical research communications. 2004;317(1):24–29. doi: 10.1016/j.bbrc.2004.02.175. [DOI] [PubMed] [Google Scholar]
- Park CW, Kim HW, Ko SH, Lim JH, Ryu GR, Chung HW, et al. Longterm treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. Journal of the American Society of Nephrology: JASN. 2007;18(4):1227–1238. doi: 10.1681/ASN.2006070778. [DOI] [PubMed] [Google Scholar]
- Stebel M, Vatta P, Ruaro ME, Del Sal G, Parton RG, Schneider C. The growth suppressing gas1 product is a GPI-linked protein. FEBS letters. 2000;481(2):152–158. doi: 10.1016/s0014-5793(00)02004-4. [DOI] [PubMed] [Google Scholar]
- Sun YM, Su Y, Li J, Wang LF. Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochemical and biophysical research communications. 2013;433(4):359–361. doi: 10.1016/j.bbrc.2013.02.120. [DOI] [PubMed] [Google Scholar]
- van Roeyen CR, Zok S, Pruessmeyer J, Boor P, Nagayama Y, Fleckenstein S, et al. Growth arrest-specific protein 1 is a novel endogenous inhibitor of glomerular cell activation and proliferation. Kidney international. 2013;83(2):251–263. doi: 10.1038/ki.2012.400. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou X, Zhang Y, Zhu H, Zhao L, Fan L, et al. Growth arrest-specific gene 1 is down-regulated and inhibits tumor growth in gastric cancer. The FEBS journal. 2012;279(19):3652–3664. doi: 10.1111/j.1742-4658.2012.08726.x. [DOI] [PubMed] [Google Scholar]
- Wei R, Yang J, Liu GQ, Gao MJ, Hou WF, Zhang L, et al. Dynamic expression of microRNAs during the differentiation of human embryonic stem cells into insulin-producing cells. Gene. 2013;518(2):246–255. doi: 10.1016/j.gene.2013.01.038. [DOI] [PubMed] [Google Scholar]
- Wolf G, Ziyadeh FN. Molecular mechanisms of diabetic renal hypertrophy. Kidney international. 1999;56(2):393–405. doi: 10.1046/j.1523-1755.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- Yamakuchi M. MicroRNA regulation of SIRT1. Frontiers in physiology. 2012;3:68. doi: 10.3389/fphys.2012.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarco N, Gonzalez-Ramirez R, Gonzalez RO, Segovia J. GAS1 induces cell death through an intrinsic apoptotic pathway. Apoptosis: An international journal on programmed cell death. 2012;17(6):627–635. doi: 10.1007/s10495-011-0696-8. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Luo X, Ding S, Chen J, Chen T, Chen X, et al. MicroRNA-451 regulates p38 MAPK signaling by targeting of Ywhaz and suppresses the mesangial hypertrophy in early diabetic nephropathy. FEBS letters. 2012;586(1):20–26. doi: 10.1016/j.febslet.2011.07.042. [DOI] [PubMed] [Google Scholar]
- Zhang L, Pang S, Deng B, Qian L, Chen J, Zou J, et al. High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-kappaB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. The international journal of biochemistry & cell biology. 2012;44(4):629–638. doi: 10.1016/j.biocel.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Peng H, Chen J, Chen X, Han F, Xu X, et al. MicroRNA-21 protects from mesangial cell proliferation induced by diabetic nephropathy in db/db mice. FEBS letters. 2009;583(12):2009–2014. doi: 10.1016/j.febslet.2009.05.021. [DOI] [PubMed] [Google Scholar]