Abstract
Objective
PTSD is a debilitating stress-related illness associated with trauma exposure. The peripheral and central mechanisms mediating stress response in PTSD are incompletely understood. Recent data suggest that the renin-angiotensin pathway, essential to cardiovascular regulation, is also involved in mediating stress and anxiety. In this study, the authors examined the relationship between active treatment with blood pressure medication, including angiotensin converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARBs), and PTSD symptom severity within a highly traumatized civilian medical population.
Method
Cross-sectional, observational data was analyzed from a larger study, recruiting patients from Grady Memorial Hospital's outpatient population from 2006 to November 2010. Multi-variable linear regression models were fit to statistically evaluate the independent association of being prescribed an ACE-I or ARB with PTSD symptoms, using a sub-set of patients for whom medical information was available (n=505). PTSD diagnosis was assessed using the modified PTSD Symptom Scale (PSS) based on DSM-IV criteria with PTSD symptoms based on PSS and Clinician Administered PTSD Scale (CAPS).
Results
A significant association was determined between presence of ACE-I / ARB medication and decreased PTSD symptoms (mean PSS score 11.4 vs 14.9 for individuals prescribed vs not prescribed ACE-I/ARBs, respectively (p = 0.014)). After adjustment for covariates, ACE-I/ARB treatment remained significantly associated with decreased PTSD symptoms (p = 0.044). Notably, other blood pressure medications, including beta-blockers, calcium channel blockers, and diuretics, were not significantly associated with reduced PTSD symptoms.
Conclusions
These data provide the first clinical evidence supporting a role for the reninangiotensin system in the regulation of stress response in patients diagnosed with PTSD. Further studies should examine whether available medications targeting this pathway should be considered for future treatment and potential protection against PTSD symptoms.
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating, stress-related psychiatric illness associated with trauma exposure. While the lifetime prevalence of PTSD in the general population is estimated to be 5-10%, the prevalence of PTSD in low-income, urban, primary care patients has been estimated to be as high as 45% (1, 2). Higher still is the prevalence of lifetime trauma exposure within this population, approximately 88% (1). Additional research investigating risk and protective factors for PTSD is essential to improving future prevention efforts.
Chronic stress, involving exposure to frequent and early traumatic events, has been implicated in multiple adverse health outcomes, including cardiovascular-associated diseases, such as hypertension (3-5). For example, individuals with PTSD have increased prevalence of blood pressure dysregulation (6-8). Although results from previous studies have been mixed, meta-analyses have demonstrated that PTSD is associated with elevations in resting systolic and diastolic blood pressure (9, 10).
A common approach for the treatment of hypertension involves the pharmacological inhibition of the renin-angiotensin system (RAS) by angiotensin converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARBs). ACE inhibitors prevent the de novo synthesis of angiotensin II, while ARB's block interaction between angiotensin II and its receptor. Many studies have demonstrated that the therapeutic actions of both ACE-I and ARBs, particularly the latter, extend beyond blood pressure reduction (11, 12). The wide-ranging effects of the RAS are due in part to the systemic and local paracrine and autocrine functions of the RAS within individual organs, such as the brain (13) or kidney (14) .
A recent review highlights numerous preclinical studies showing the therapeutic and protective effects of ARBs on the brain, including the reduction of stress, anxiety, brain inflammation and ischemia (12, 15-19). Moreover, recent animal studies have demonstrated that blockade of angiotensin II AT1 receptors or angiotensin II formation can reduce the effects of stress on rodent physiology and behavior (20, 21). These data provide evidence that in animal models, inhibition of brain AT1 receptor activity, by oral or ICV brain injection of ARBs, leads to improvement in stress-related behavior and associated brain pathology (20).
Clinical reports have also described the protective effects of ARBs on cognition (12, 22, 23), quality of life improvements and reductions in depression and anxiety (16, 24). Moreover, a genetic variation in ACE has been identified as altering the risk for major depression, as well as ACE and cortisol plasma levels (25). However, the treatment of psychiatric conditions with ARBs or ACE inhibitors has not been the focus of clinical trials. Further research is clearly needed to elucidate the role of ARBs and ACE inhibitors as therapy in a wide range of stress-related disorders, including PTSD. Using data that has been collected through the Grady Trauma Project from 2006-2010, this analysis examines the cross-sectional, independent association of ACE-I or ARB intake with PTSD symptoms in a highly traumatized population.
Methods
The study was approved by Emory University Institutional Review Board. All procedures of the study were discussed thoroughly with each participant, and all participants provided written informed consent and received monetary compensation for their participation.
Subjects and sample recruitment
This secondary analysis examined data on 505 individuals, who were part of a larger cross-sectional study investigating the genetic and environmental factors that contribute to PTSD. From 2006 to 2010, participants were recruited from the waiting rooms of primary care, obstetric-gynecological clinics or the pharmacy at Grady Memorial Hospital. One of the largest public hospitals in the United States, Grady serves a primarily African American and highly traumatized, low-income, inner-city population. Recruitment took place Monday-Friday during regular clinic hours. Those subjects who agreed to participate completed a number of self-report measures, taking 45-75 minutes to complete.
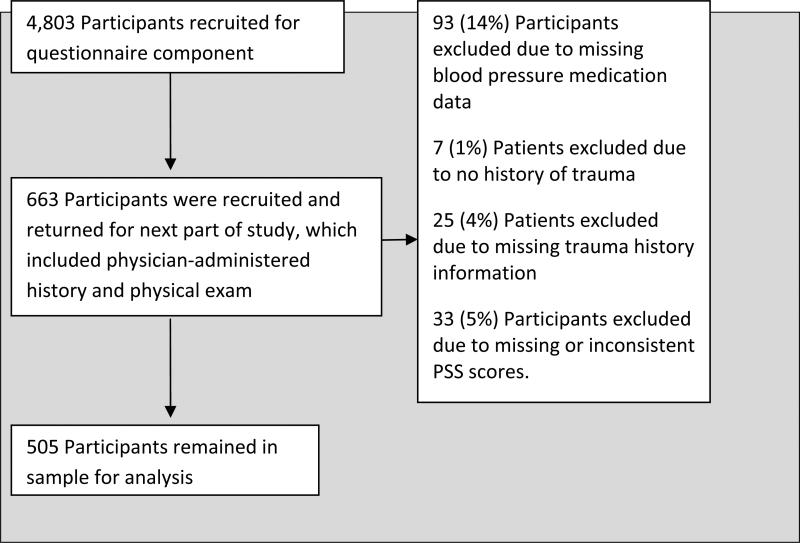
All 4,803 participants who completed the initial interview were asked if they would consent to subsequent study phases. A scheduler, blinded to the participant's information, randomly contacted these participants. 663 returned for subsequent parts of the study, which included a physician-administered medical exam, permission to examine the electronic medical records, and additional self-report measures and structured clinical interviews, including the Clinician Administered PTSD Scale (CAPS).
The primary exposure of interest was taking an ACE-I or ARB; therefore, individuals whose information on blood pressure medications was missing were excluded from analysis (93 subjects (14% of the sample)). Patients who had missing information on the PTSD symptom scale (PSS) were also excluded from the analysis (28 or 4.9% of the remaining sample). To examine the association of ACE-I or ARB with PTSD symptoms among individuals exposed to traumatic events, only individuals who reported one or more traumatic events on the childhood questionnaire (CTQ) or traumatic event inventory (TEI) were included in the analysis, leaving a sample of 505 individuals. A flow chart of the selection process for the sample is provided (Fig. 1).
Figure 1.
Recruitment and Selection of Study Participants
Measurements
Trauma exposure was measured using the TEI, a 14-item screening instrument for lifetime history of traumatic events (26, 27). For each traumatic event, experiencing and witnessing of the event is assessed separately. The TEI also assesses frequency of trauma exposure within each trauma type. Measured as a continuous variable, frequency of exposure to traumatic events was used as a potential covariate. As previous studies have shown associations between chronic stress and blood pressure (4, 5, 9, 28), there may be an indirect association between being on a blood pressure medication and chronic stress, which may be partly measured by frequency of traumatic events.
The primary outcome of interest in this study was PTSD symptom severity; therefore the principal measurement used for analysis was the PSS, a psychometrically valid 17-item self-report scale that measures PTSD symptom severity during the two-week period immediately prior to study assessment (26, 29-31). PSS frequency items (measured as “0: not at all” to “3: 5 or more times a week) were summed to obtain a continuous measure of PTSD symptom severity. We also examined the major subtypes of post-traumatic stress symptoms, including hyperarousal symptoms, avoidance or numbing symptoms and intrusive thoughts, using the symptom-specific subscales of the PSS (30). The categorical diagnosis of PTSD was initially determined based on DSM-IV A-E criterion responses to the PSS questionnaire.
Additionally, information from the CAPS was also used to examine the effect of ACE-I or ARBs on the severity of both current and lifetime PTSD symptoms. An interviewer-administered diagnostic instrument with excellent psychometric properties, the CAPS uses DSM-IV scoring criteria to generate a categorical diagnosis of PTSD, as well as a continuous measure of the extent and severity of lifetime and current post-traumatic stress symptoms (32, 33). While both the PSS and CAPS can generate symptoms to assess PTSD severity, CAPS adds additional information about lifetime PTSD symptoms. For each of the 17 diagnostic criteria, the CAPS rates frequency and intensity scores on a scale of 0 (absent) to 5 (extremely severe). This analysis used the CAPS to obtain both continuous lifetime and current PTSD variables (scores from 0 to 170).
The primary exposure of interest in this study, collected by physicians based on participants’ reports, was whether an individual was prescribed an ACE-I or ARB. The data on ACE-I and ARBs were pooled since there were a small number of individuals on ARBs (17 or 3.16% of the sample) and because of similar mechanisms of action.
Potential covariates assessed in the analysis included: other blood pressure medications, (categorized into beta-blockers, calcium channel blockers (CCBs), diuretics, and other for medications with another mechanism of action), whether an individual was currently on a psychiatric medication, current substance abuse, body mass index (BMI), frequency of adult trauma (as assessed by the TEI), and childhood trauma. Childhood trauma was assessed using the Childhood Trauma Questionnaire (CTQ), a self-report inventory assessing three types of childhood abuse: sexual, physical and emotional. Studies have established internal consistency, stability overtime and criterion validity of both the original 70-item CTQ and the current brief version (34, 35).
Demographic information assessed as potential covariates included: sex, age, current employment, household income level ($0-249, $250-499, $500-999, $1,000-1999, or >$2,000 per month), education (categorized into <12th, high school graduate or GED, some college or technical school, or college graduate and higher education) and race (dichotomized into “African American” and “other,” due to the small number of non-African American subjects in the analysis).
Missing data included information on race (3 or .6% of the sample), income (12 or 2.4% of the sample), employment (3 or .6% of the sample), education (3 or .6% of the sample), current psychiatric medications (247 or 48.9% of the sample), current substance abuse (9 or 1.8% of the sample), adult traumatic experiences (6 or 1.2% of the sample), and childhood traumatic experiences (19 or 3.8% of the sample). Since close to half of the sample had missing data on current psychiatric medication and BMI (247 or 48.9% of the sample and 244 or 48.3% of the sample, respectively), these variables were excluded as potential covariates from modeling analysis.
Analysis
All analysis was performed using SAS 9.2 (Cary, NC) statistical software. To statistically evaluate the independent effect of ACE-I and ARB medication on PTSD symptom severity among patients exposed to trauma, linear regression models were fit with PTSD symptoms, measured by the total PSS score as the continuous outcome variable. Two-way multiplicative interaction between the categorical variable of active treatment with an ACE-I or ARB medication and other covariates were assessed in a full model. Potential confounders were assessed using multiple approaches. First, directed acyclic graphs were constructed using information from previous literature. Second, a two-table approach was used, in which the association of each potential confounder was examined in relation to both PTSD symptoms and active treatment with an ACE-I or ARB. Finally, a backward regression modeling approach was used, in which variables were removed one at a time and assessed for statistical significance and effect on the beta estimate for the main exposure of interest.
Descriptive analysis of the variables was performed, stratified by categorical PTSD diagnosis. Chi-square tests were used to assess the association of PTSD diagnosis with: ACE-I or ARBs, beta-blockers, calcium channel blockers (CCBs), diuretics, sex, race, income, employment, education, current psychiatric medications, and current substance abuse. Two sampled T-tests were used to assess the association of PTSD diagnosis with BMI, age, and adult and childhood trauma (as assessed by the TEI and CTQ, respectively).
To evaluate the effect of different categories of blood pressure medications, including ACE-I and ARBs, on PTSD symptoms, univariate analysis of variance was performed. To statistically evaluate the independent effect of ACE-I and ARBs on PTSD symptoms, multi-variable linear regression models were constructed, using potential confounders, which were previously identified. Co-linearity between the covariates was assessed and linear regression assumptions were checked.
To analyze the effect of treatment with different types of blood pressure medications on the severity of PTSD symptom subtypes, multi-variable linear regression models were created and tested using continuously scaled PTSD symptoms among each subtype as the outcome. A p-value of ≤ 0.05 was considered statistically significant for analysis.
Results
Among the 505 individuals exposed to at least one traumatic event, 180 met criteria for PTSD diagnosis based on PSS score. In the sample, 98 individuals were on ACE-I or ARBs, 63 were on beta-blockers, 53 were on CCBs, 109 on diuretics and 12 were on other blood pressure medications. A significant univariate association was found between PTSD diagnosis and ACE I or ARBs status (Table 1). Of 98 individuals on an ACE-I or ARB, 26 met criteria for PTSD diagnosis using PSS; of 407 individuals not on an ACE-I or ARB, 154 met criteria for PTSD diagnosis (Chi-Square T-value = 4.40, p = 0.036). Covariates demonstrating significant differences based on PTSD diagnosis included: being on a CCB, employment, current psychiatric medication, current substance abuse, total adult trauma experienced and childhood trauma (Table 1). Significantly different potential confounders stratified by ACE-I or ARB included age, education, beta-blockers, CCB and diuretics (Table 2).
Table 1.
| Descriptive Overview of Variables Stratified by PTSD diagnosis (N=505)
| Variables | PTSD (N=180) | No PTSD (N=325) | Analysis | Missing values | |||||
|---|---|---|---|---|---|---|---|---|---|
| Discrete | N | % | N | % | DF | X2 | P | N | % |
| ACE I or ARB | 26 | 14.4 | 72 | 22.2 | 1 | 4.40 | 0.036 | None | 0 |
| Beta Blocker | 17 | 9.4 | 46 | 14.2 | 1 | 2.35 | 0.125 | None | 0 |
| CCB | 12 | 6.7 | 41 | 12.6 | 1 | 4.36 | 0.037 | None | 0 |
| Diuretics | 32 | 17.8 | 77 | 23.7 | 1 | 2.39 | 0.122 | None | 0 |
| Other BP med | 2 | 1.1 | 10 | 3.1 | 1 | *** | *** | None | 0 |
| Sex (Male) | 114 | 63.3 | 189 | 57.2 | 1 | 1.79 | 0.181 | None | 0 |
| Race (Black) | 159 | 89.3 | 297 | 91.7 | 1 | 0.76 | 0.385 | 3 | 0.6 |
| Income / month | 4 | 5.30 | 0.258 | 12 | 2.4 | ||||
| $0-249 | 72 | 41.1 | 105 | 33.0 | |||||
| $250-499 | 16 | 9.1 | 39 | 12.3 | |||||
| $500-999 | 48 | 27.4 | 82 | 25.8 | |||||
| $1,000-1,999 | 28 | 16.0 | 70 | 22.0 | |||||
| ≥$2,000 | 11 | 6.3 | 22 | 6.9 | |||||
| Employed | 30 | 16.9 | 85 | 26.2 | 1 | 6.72 | 0.017 | 3 | 0.6 |
| Education | 3 | 3.98 | 0.264 | 3 | 0.6 | ||||
| <12th grade | 43 | 23.9 | 73 | 22.5 | |||||
| HS Grad/GED | 76 | 42.2 | 144 | 44.3 | |||||
| Some college | 52 | 28.9 | 78 | 24.0 | |||||
| ≥ College | 9 | 5.0 | 30 | 9.23 | |||||
| Psych. Med | 28 | 33.7 | 30 | 17.1 | 1 | 8.89 | 0.003 | 247 | 48.9 |
| Sub. Abuse | 17 | 9.7 | 11 | 3.4 | 1 | 8.25 | 0.004 | 9 | 1.8 |
| Continuous | μ | SD | μ | SD | DF | T | P | N | % |
| BMI μ (SD) | 32.68 | 8.4 | 32.94 | 9.5 | 259 | 0.22 | 0.826 | 244 | 48.3 |
| Age μ (SD) | 42.28 | 11.6 | 41.97 | 13.1 | 497 | −0.26 | 0.796 | 6 | 1.2 |
| TEI* μ (SD) | 4.85 | 2.44 | 2.96 | 2.3 | 487 | −8.53 | <.001 | 6 | 1.2 |
| CTQ**μ (SD) | 51.48 | 20.6 | 38.93 | 14.5 | 263 | −7.10 | <.001 | 19 | 3.76 |
TEI: traumatic events inventory
CTQ: childhood trauma questionnaire
Chi-Square assumptions not met
Table 2.
| Potential Confounders, Stratified by ACE-I or ARB (N= 505)
| Potential Confounders | ACE-I or ARB (N=112) | Not on ACE-I or ARB (N=458) | Analysis | ||||
|---|---|---|---|---|---|---|---|
| Discrete | N | % | N | % | DF | X2 | P |
| Sex (Male)* | 55 | 56.1 | 245 | 60.2 | 1 | 0.54 | 0.461 |
| Race (Black)* | 90 | 92.8 | 366 | 90.4 | 1 | 0.55 | 0.459 |
| Income (per month) | 4 | 4.01 | 0.405 | ||||
| $0-249 | 34 | 35.4 | 143 | 36.0 | |||
| $250-499 | 8 | 8.3 | 47 | 11.8 | |||
| $500-999 | 31 | 32.3 | 99 | 24.9 | |||
| $1,000- 1,999 | 15 | 15.6 | 83 | 20.9 | |||
| ≥$2,000 | 8 | 8.3 | 25 | 6.3 | |||
| Employed* | 16 | 16.5 | 99 | 24.4 | 1 | 2.80 | 0.094 |
| Education* | 3 | 7.94 | 0.047 | ||||
| <12th grade | 19 | 19.4 | 97 | 23.8 | |||
| High School grad/ GED | 36 | 36.7 | 184 | 45.2 | |||
| Some college | 30 | 30.6 | 100 | 24.6 | |||
| College grad. or higher | 13 | 13.3 | 26 | 6.4 | |||
| Psych. Med.* | 17 | 30.9 | 41 | 20.2 | 1 | 2.85 | 0.091 |
| Sub. Abuse* | 5 | 5.2 | 23 | 5.8 | 1 | 0.05 | 0.816 |
| BB | 35 | 35.7 | 28 | 6.9 | 1 | 60.14 | <.0001 |
| CCB | 23 | 23.5 | 30 | 7.4 | 1 | 21.79 | <.0001 |
| Diuretics | 53 | 54.1 | 56 | 13.8 | 1 | 75.87 | <.0001 |
| Continuous | μ | SD | μ | SD | DF | T | P |
| BMI* | 33.73 | 8.5 | 32.61 | 9.28 | 259 | −0.82 | 0.414 |
| Age* | 51.45 | 8.0 | 39.81 | 12.5 | 223 | −11.3 | <.0001 |
| TEI* | 5.49 | 3.6 | 5.42 | 3.6 | 487 | −0.17 | 0.865 |
| CTQ* | 42.43 | 15.8 | 43.56 | 18.4 | 484 | 0.54 | 0.588 |
Variables contain missing values.
Mean PSS scores (total and by subtype) for individuals receiving different blood pressure medications are shown in Table 3. A significant difference in mean total PSS score was only found based on ACE-I or ARB status (11.41 +/- 11.1 (S.D.) for ACE-I/ARB treated and 14.9 +/- 12.9 for non-ACE-I or ARB treated, F = 6.12, p = 0.014). When examined by PTSD subtype, individuals on ACE-I or ARB and/or beta-blockers demonstrated significant differences in mean hyperarousal score (3.90 +/- 4.0 (S.D.) and 5.20 +/- 4.6 on and off ACE-I or ARBs, respectively; 3.88 +/- 3.6 and 5.10 +/- 4.6 on and off beta blockers, respectively). No significant differences in mean avoidance/numbing score were found for any blood pressure medications. Lastly, significant differences in mean intrusive thoughts score were limited to comparisons of individuals on versus not on ACE-I or ARBs (2.48 +/- 3.3 (S.D.) for ACE-I/ARB treated and 3.75 +/- 4.1 for non-ACE-I/ARB treated). Given the comorbidity of depression with PTSD, we also examined the effect of ACE-I / ARB status on depressive symptoms. In the analyzed traumatized sample, individuals on ACE-I/ARBs were found to have lower total BDI scores than individuals not on ACE-I/ARBs, but the results were not statistically significant (13.42 +/- 12.2 (S.D.) compared with 16.19 +/- 12.6, p > 0.05).
Table 3.
| Uni-variable Analysis of Variance of PTSD symptoms by PSS total score and by symptom subtype
| Mean PSS Score | SD | F | Mean Hyperarousal Score | SD | F | Mean Avoid/Numb Score | SD | F | Mean Intrusive Score | SD | F | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACE-I orARB | ||||||||||||
| On (N=98) | 11.41 | 11.1 | 6.12* | 3.90 | 4.0 | 6.69* | 5.03 | 5.4 | 2.51 | 2.48 | 3.3 | 8.16** |
| Off (N=407) | 14.90 | 12.9 | 5.20 | 4.6 | 5.97 | 5.7 | 3.75 | 4.1 | ||||
| Beta Blocker | ||||||||||||
| On (N=63) | 11.42 | 9.4 | 3.60 | 3.88 | 3.6 | 4.11* | 4.84 | 4.7 | 2.05 | 2.70 | 2.8 | 2.97 |
| Off (N=442) | 14.64 | 13.0 | 5.10 | 4.6 | 5.92 | 5.7 | 3.62 | 4.1 | ||||
| CCB | ||||||||||||
| On (N=53) | 12.26 | 11.7 | 1.45 | 4.55 | 4.4 | 0.48 | 4.53 | 5.2 | 2.97 | 3.19 | 3.6 | 0.37 |
| Off (N=452) | 14.47 | 12.8 | 5.00 | 4.5 | 5.94 | 5.7 | 3.54 | 4.0 | ||||
| Diuretic | ||||||||||||
| On (N=109) | 13.18 | 12.0 | 0.97 | 4.65 | 4.4 | 0.63 | 5.40 | 5.5 | 0.65 | 3.13 | 3.9 | 1.21 |
| Off (N=396) | 14.53 | 12.8 | 5.03 | 4.5 | 5.90 | 5.7 | 3.61 | 4.0 |
P<0.05
P<0.01
In multivariate linear regression, there were no statistically significant interactions between treatment status with an ACE-I or ARB and covariates. Covariates that were independently associated with PTSD symptoms were childhood trauma, adult trauma, being male, and unemployed. In backward stepwise regression, beta-blockers and age remained in the model, as these variables confounded the association of ACE-I or ARB with PTSD symptoms. After adjusting for the above covariates, individuals treated with an ACE-I or ARB had significantly decreased risk of current PTSD symptoms compared to individuals not receiving an ACE-I or ARB (β -2.83, S.E 1.4, P=0.044; Table 4).
Table 4 .
| Multi-variable Linear Regression of PSS and CAPS Score
| Outcome: PSS Total Score (N=467) | ACE-I or ARB β Estimate | S.E | T | P |
|---|---|---|---|---|
| Unadjusted effect | −3.51 | 1.4 | −2.47 | 0.014 |
| Adjusted* effect | −2.83 | 1.4 | −2.02 | 0.044 |
| PSS Hyperarousal Score (N=467) | ||||
| Unadjusted effect | −1.30 | 0.5 | −2.59 | 0.010 |
| Adjusted** effect | −1.22 | 0.6 | −2.20 | 0.028 |
| PSS Avoidance Numb Score (N=459) | ||||
| Unadjusted effect | −0.94 | 0.6 | −1.49 | 0.138 |
| Adjusted *** effect | −0.92 | 1.1 | −1.12 | 0.161 |
| PSS Intrusive Score (N=467) | ||||
| Unadjusted effect | −1.27 | 0.4 | −2.86 | 0.005 |
| Adjusted**** effect | −1.01 | 0.5 | −2.20 | 0.029 |
| Lifetime CAPS Score (N=467) | ||||
| Unadjusted effect (ACE-I or ARB) | −4.90 | 3.95 | −1.24 | 0.216 |
| Adjusted¥ effect (ACE-I or ARB) | −1.22 | 0.56 | −2.20 | 0.028 |
| Current CAPS Score (N=417) | ||||
| Unadjusted effect (ACE-I or ARB) | −5.05 | 2.91 | −1.74 | 0.083 |
| Adjusted¥¥ effect (ACE-I or ARB) | −7.16 | 2.78 | −2.57 | 0.010 |
Adjusted for childhood trauma, adult trauma, age, sex, employment, and beta blocker. R2=0.29, F=27.20
2 Adjusted for childhood trauma, adult trauma, age, diuretics, employment and beta blocker. R2=0.22, F=18.20
Adjusted for childhood trauma, adult trauma, age, other BP meds., bet blockers, and income. R2=0.27, F=16.20
Adjusted for childhood trauma, adult trauma, age, sex, and beta blockers. R2=0.22, F=21.17
Adjusted for childhood trauma, adult trauma, age, diuretics, employment and beta blockers. R2=0.22, F=18.20.
Adjusted for childhood trauma, adult trauma, and employment. R2= 0.190, F=22.07
Frequency of childhood and adult trauma were independently associated with all the outcomes of interest, therefore they were included in every model. Unemployment was also independently associated with hyperarousal symptoms, as assessed by the PSS, and current and lifetime PTSD symptoms, as assessed by the CAPS. After adjustment for the above covariates, individuals receiving an ACE-I or ARB had a significantly decreased risk of current PTSD symptoms (β -7.16, S.E 2.78, P=0.010). Age and diuretics also remained in the model examining hyperarousal symptoms as the outcome, as they were found to be confounders. After adjusting for the above covariates, individuals on ACE-I or ARBs had a significantly decreased risk of PTSD hyperarousal symptoms (β -1.22, S.E 0.56, P=0.028; Table 4).
Beta-blockers, age and diuretics were included in the model examining lifetime PTSD symptoms as the outcome, as they were found to be confounders. After adjustment for the above covariates, individuals on an ACE-I or ARB had a significantly decreased risk of lifetime PTSD symptoms (β -1.22, S.E 0.56, P=0.028; Table 4).
Age, beta blockers, other BP medications and income level all remained in the model examining avoidance/numbing symptoms as the outcome, as they were found to be confounders. After adjustment for the above covariates, the effect of being on an ACE-I or ARB remained insignificant.
Lastly, being male was independently associated with intrusive symptoms and remained in the model. Beta-blockers and age were also included, as their presence confounded the effect of ACE-I or ARB on intrusive symptoms. After adjustment for the above covariates, individuals treated with an ACEI or ARB had a significantly decreased risk of PTSD intrusive thoughts symptoms (β -1.01, S.E 0.46, P=0.029; Table 4).
Discussion
Results from this study suggest that ACE-inhibitors and ARB medications have protective effects on PTSD symptoms among individuals exposed to trauma. After adjustment, the effect of ACE-inhibitors and ARBs on the reduction of PTSD symptoms remained significant, both using the PSS and the CAPS measurements (P=0.028 and P=0.010, respectively), the latter of which is thought to be a more thorough measurement of current and lifetime PTSD symptoms.
In addition, this analysis suggests that ACE-I and ARBs may preferentially affect the severity of hyperarousal and intrusive PTSD symptoms. Other medications that have been shown to decrease these symptoms include: Prazosin, Clonidine, Guanfacine, and Propanolol, all of which target the noradrenergic system (36). As the renin-angiotensin system is linked with the noradrenergic system, it may not be surprising that ACE-I and ARBs would affect these specific symptoms (37). Among other effects, angiotensin II activity in the brain has been shown to increase transcription of tyrosine hydroxylase, the rate-limiting enzyme in catecholamine synthesis (19, 36).
Results from this analysis did not demonstrate any interaction in individuals taking both beta-blockers and ACE-I/ARB on PTSD symptoms. In fact, while univariate associations demonstrated a significant effect of beta-blockers on hyperarousal symptoms, these effects did not remain after adjustment for confounders. This was somewhat surprising, given that propanolol has also been used both to ameliorate PTSD symptoms, and, in some studies, to prevent PTSD (36). Nevertheless, the present literature on chemoprophylaxis for individuals exposed to trauma is controversial. Equivocal data exists for the use of alcohol, cortisol, morphine, and propanolol to prevent PTSD in at-risk patients and no census exists about when, for whom, and at what dose prophylaxis would be indicated or cost-effective (38). Additional studies that are better designed to examine whether ACE-I and ARBs can prevent PTSD or reduce symptom severity in at-risk populations are warranted. These include longitudinal cohort or randomized controlled studies that focus on at-risk individuals or those already diagnosed with PTSD and on specific medications.
Limitations of this study include collapsing ARBs and ACE-I categories; this was done for convenience due to the small number of individuals on ARBs, but it may mask important mechanistic subtleties. In addition, the cross-sectional design makes causation difficult to evaluate. Notably, if co-linearity between being on a blood pressure medication and stress-related hypertension were the case in this cohort, we would expect greater PTSD symptom severity to associate with taking blood pressure medications. In contrast, with the ARB/ACE-I class, we find the unexpected decrease in PTSD symptoms associated with medication use. As with many large cross-sectional studies of convenience, we have a number of variables with missing data and the missing values from certain variables such as BMI and psychiatric medication excluded them from analysis. Lastly, chart extraction may not be ideal for determining if individuals are taking medications on a regular basis.
In summary, the present analysis supports the burgeoning preclinical literature describing a role for the renin-angiotensin pathway in stress-related disorders (12, 15-19). While animal models have demonstrated that inhibition of brain AT1 receptor activity reduces of stress-related behaviors (20), this is the first analysis examining the effect of these medications on PTSD symptoms in individuals exposed to trauma. Since ACE-I and ARB medications are safe and widely used to treat hypertension, they may be novel and important targets to consider for treatment and potential protection against PTSD symptoms among certain populations.
Clinical Points.
Certain anti-hypertensive medications may have protective effects on stress-related disorders, such as PTSD. Results from this preliminary study suggest that targeting the renin-angiotensin system through available medications may have protective effects on PTSD symptoms among patients exposed to traumatic events. Further research is needed to examine whether ACE-I and ARBs can prevent PTSD or reduce symptom severity in at-risk populations.
Acknowledgements
This work was primarily supported by National Institutes of Mental Health (MH071537). Support was also received from Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01RR00039), and the Burroughs Wellcome Fund (KJR). Dr. Ressler is a co-founder of Extinction Pharmaceuticals, LLC, for the use of NMDA-acting compounds with psychotherapy. He has received no financial or research support within the last three years from this arrangement, and there he has no competing interests related to this manuscript. Dr. Ressler has also received funding support related to other studies from Burroughs Wellcome Foundation, NARSAD, NIMH and NIDA. Dr. Gillespie has received funding from APIRE/Wyeth, NARSAD, NIDA, and NIMH. Dr. Bradley has received funding from American Foundation for Suicide Prevention. Dr. Wingo has received funding support from NARSAD and an APA Research Fellowship Award.
Footnotes
Disclosure
Nayla M. Khoury, Ann Schwartz, Paul Marvar and Michael Kramer declare no potential competing interests.
References
- 1.Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, et al. Trauma exposure and stress-related disorders in inner city primary care patients. General Hospital Psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler R, Sonnega A, Bromet E. Posttraumatic stress disorder in the National Comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 3.McFarlane AC. The long-term costs of traumatic stress: intertwined physical and psycholgical consequences. World Psychiatry. 2010;9:3–10. doi: 10.1002/j.2051-5545.2010.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hepner PS, Crawford EF, Haji UA, Afari N, Hauger RL, Dashevesky BA, et al. The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Medicine. 2009:7. doi: 10.1186/1741-7015-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehman BJ, Taylor SE, Kiefe CI, Seeman T. Relationship of Early Life Stress and Psychological Functioning to Blood Pressure in the CARDIA Study. Health Psychology. 2009;28(3):338–346. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kibler JL. Posttraumatic stress and cardiovascular disease risk. J Trauma Dissociation. 2009;10(2):135–50. doi: 10.1080/15299730802624577. [DOI] [PubMed] [Google Scholar]
- 7.Tucker P, Jeon-Slaughter H, Pfefferbaum B, Khan Q, Davis NJ. Emotional and biological stress measures in Katrina survivors relocated to Oklahoma. American Journal of Disaster Medicine. 2010;5(2):113–25. doi: 10.5055/ajdm.2010.0013. [DOI] [PubMed] [Google Scholar]
- 8.Ulmer CS, Calhoun PS, Edinger JD, Wagner HR, Beckham JC. Sleep disturbance and baroreceptor sensitivity in women with posttraumatic stress disorder. Journal of Trauma Stress. 2009;22(6):643–7. doi: 10.1002/jts.20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley T, Kaloupek D. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosomatic Medicine. 2001;63:585–594. doi: 10.1097/00006842-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Pole N. Psychophysiology of Posttraumatic Stress Disorder: A Meta-Analysis. Psychological Bulletin. 2007;133(5):725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- 11.Barra S, Vitagliano A, Cuomo V, Vitagliano G, Gaeta G. Vascular and metabolic effects of angiotensin II receptor block-ers. Expert Opinion Pharmacotherapy. 2009;10:173–189. doi: 10.1517/14656560802653180. [DOI] [PubMed] [Google Scholar]
- 12.Anderson C. More direct evidence of potential neuroprotective benefits of Angiotensin receptor blockers. Hypertension. 2010;28:429. doi: 10.1097/HJH.0b013e3283371355. [DOI] [PubMed] [Google Scholar]
- 13.Grobe JL, Grobe CL, Beltz TG, Westphal SG, Morgan DA, Xu D, et al. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metabolism. 2010;12(5):431–42. doi: 10.1016/j.cmet.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Wang Y, Zhou LN, Zheng F. ARB Treatment Prevents the Decrease in Endothelial Progenitor Cells and the Loss of Renal Microvasculature in Remnant Kidney. Am Journal of Nephrology. 2011;33(6):550–7. doi: 10.1159/000328632. [DOI] [PubMed] [Google Scholar]
- 15.Papdemetriou V, Farsang C, Elmfeldt D, Hofman A, Lithell H, Olofsson B, et al. Study on Cognition and Prognosis in the Elderly study group Stroke Prevention with the angiotensin II type 1-receptor blocker candesartan in eldelry patients with isolated systolic hypertension: the Study on Cognition and Prognosis in the Elderly (SCOPE). Journal of American College of Cardiology. 2004;44:1175–1180. doi: 10.1016/j.jacc.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 16.Zanchetti A, Elmfeldt D. Findings and implications of the Study on Cognition and Prognosis in the Elderly (SCOPE)--A review. Blood Pressure. 2006;15:71–79. doi: 10.1080/08037050600771583. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto S, Shimodozono M, Miyata R, Kawahira K. The Angiotensin II type 1 receptor antagonist olmesartan preserves cerebral blood flow and cerebrovascular reserve capacity, and accelerates rehabilitative outcomes in hypertensive patients with a history of stroke. International Journal of Neuroscience. 2010;120:372–380. doi: 10.3109/00207450903389362. [DOI] [PubMed] [Google Scholar]
- 18.Dandona P, Kumar V, Aljada A, Ghanim H, syed T, Hofmayer D, et al. Angiotensin II receptor blocker valsartan suppresses reactive oxygen species generation in leukocytes, nuclear factor-kappa B, in mononuclear cells of normal subjects: evendence of an anti-inflammatory action. J. Clin. Endocrinal. Metab. 2003;88:4496–4501. doi: 10.1210/jc.2002-021836. [DOI] [PubMed] [Google Scholar]
- 19.Saavedra JM, Sanchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benicky J, Sanchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, et al. Angiotensin II AT1 Receptor Blockade Ameliorates Brain Inflammation. Neuropsychopharmacology. 2011;36:857–870. doi: 10.1038/npp.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D, Grecca LL, Head GA, Walther T, Mayorov DN. Blood pressure reactivity to emotional stress is reduced in AT1A- receptor knockout mice on normal, but not high salt intake. Hypertension Research. 2009 doi: 10.1038/hr.2009.59. [DOI] [PubMed] [Google Scholar]
- 22.Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, et al. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010:340. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saxby BK, Harrington F, Wesnes KA, McKeith IG, Ford GA. Candesartan and cognitive decline in older patients with hypertension: a substudy of the SCOPE trial. Neurology. 2008;70:1858–1866. doi: 10.1212/01.wnl.0000311447.85948.78. [DOI] [PubMed] [Google Scholar]
- 24.Weber MA. Angiotensin-II receptor blockers for hypertension and heart failure: quality of life and outcomes. Managed Care and Interface. 2005;18:47–54. [PubMed] [Google Scholar]
- 25.Baghai T, Binder E, C. S, D. S, Eser D, Lucae S, et al. Polymorphisms in the angiotensin-converting enzyme gene are associated with unipolar depression, ACE activity and hypercortisolism. Molecular Psychiatry. 2006;11:1003–1015. doi: 10.1038/sj.mp.4001884. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz AC, Bradley R, Penza KM, Sexton M, Jay D, Haggard P. Pain medication use among patients with posttraumatic stress disorder. Psychosomatics. 2006;47(2):136–142. doi: 10.1176/appi.psy.47.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz AC, Bradley R, Ressler KJ, Sexton M, Sherry A. Treating posttraumatic stress disorder in urban African American mental health patients. Journal of American Psychoanal. Assoc. 2004;53(2):464–465. [PubMed] [Google Scholar]
- 28.Pike JL, Smith TL, Hauger RL, Nicassio PM, Patterson TL, McClintick J, et al. Chronic Life Stress Alters Sympathetic, Neuroendocrine, and Immune Responsivity to an Acute Psychological Stressor in Humans. Psychosomatic Medicine. 1997;59:447–457. doi: 10.1097/00006842-199707000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz AC, R.G. B, Sexton M, Sherry A, Ressler KJ. Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatric Services. 2005;56(2):212–215. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- 30.Foa E, DF T. Comparison of the PTSD Symptom Scale-Interview Version and the Clinician Administered PTSD Scale. Journal of Traumatic Stress. 2000;13:181–191. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 31.Coffey SF, Dansky BS, Falsetti SA, Saladin MF, K.T. B Screening for PTSD in a substance abuse sample: psychometric properties of a modified versionof the PTSD Symptom Scale Self-Report. Posttraumatic Stress Disorder. Journal of trauma stress. 1998;11(2):393–399. doi: 10.1023/A:1024467507565. [DOI] [PubMed] [Google Scholar]
- 32.Weathers FW, Keane TM, Davidson JRT. Clinician-administered PTSD scale: a review of the first ten years of research. Depression and Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 33.Blake DD, Weathers FW, Nagy LM, Kaloupek D, Klauminzer G, Charney DS. A clinician rating scale for assessing current lifetime PTSD: the CAPS-1. Behavior Therapy. 1990;13:187–88. [Google Scholar]
- 34.Bernstein D, Fink I, Handelsman L. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 35.Bernstein D, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of American Academic Child Adolescent Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Strawn JR, Geracioti Jr TD. Noradrenergic Dysfunction and the Psychopharmacology of Posttraumatic Stress Disorder. Depression and Anxiety. 2008;25:260–271. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- 37.Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. American Journal of Physiology. 1992;262(6):763–78. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher S, Creamer M, Forbes D. Preventing post traumatic stress disorder: are drugs the answer? Australian and New Zealand Journal of Psychiatry. 2010;44:1064–1071. doi: 10.3109/00048674.2010.509858. [DOI] [PubMed] [Google Scholar]