Abstract
Introduction
Although social cognitive impairments are key determinants of functional outcome in schizophrenia their neural bases are poorly understood. This study investigated neural activity during imitation and observation of finger movements and facial expressions in schizophrenia, and their correlates with self-reported empathy.
Methods
23 schizophrenia outpatients and 23 healthy controls were studied with functional magnetic resonance imaging (fMRI) while they imitated, executed, or simply observed finger movements and facial emotional expressions. Between-group activation differences, as well as relationships between activation and self-reported empathy, were evaluated.
Results
Both patients and controls similarly activated neural systems previously associated with these tasks. We found no significant between-group differences in task-related activations. There were, however, between-group differences in the correlation between self-reported empathy and right inferior frontal (pars opercularis) activity during observation of facial emotional expressions. As in previous studies, controls demonstrated a positive association between brain activity and empathy scores. In contrast, the pattern in the patient group reflected a negative association between brain activity and empathy.
Conclusions
Although patients with schizophrenia demonstrated largely normal patterns of neural activation across the finger movement and facial expression tasks, they reported decreased self perceived empathy and failed to show the typical relationship between neural activity and self-reported empathy seen in controls. These findings suggest that patients show a disjunction between automatic neural responses to low level social cues and higher level, integrative social cognitive processes involved in self-perceived empathy.
Keywords: Imitation, Observation, Simulation, Schizophrenia, Mirror neuron system, Empathy
Highlights
-
•
Comparable activation patterns were present in both groups for finger and facial stimuli.
-
•
There were no group differences on any of the activation tasks.
-
•
Self-reported empathy differentially related to neural activation in the two groups.
-
•
Empathy related to right inferior frontal activity in controls but not in patients.
-
•
Patients showed a disconnect between low- and high-level social cognitive processes.
1. Introduction
Social dysfunction is among the most debilitating and treatment refractory features of schizophrenia. Rapidly growing evidence indicates that deficits in the domain of social cognition are among the most important determinants of poor functioning. Schizophrenia is characterized by impaired emotion processing, social perception, attributional style, and mentalizing, which account for unique variance in functional outcome above and beyond non-social neurocognitive deficits and clinical symptoms (Green and Horan, 2010). Although these findings demonstrate the unique functional significance of social cognition deficits in schizophrenia, our understanding of their scope (e.g., whether relatively automatic social cognitive processes are also impaired) and neural correlates is limited (Brunet-Gouet et al., 2011).
Social neuroscience research indicates that imitative behavior is a basic prerequisite for the development of social cognition. It has been proposed that a mirror neuron system (MNS) provides the neurophysiological basis for imitation, which facilitates understanding the actions and even emotions of others through a “simulation” mechanism. First described in the ventral premotor and inferior parietal cortices of monkeys (Rizzolatti and Craighero, 2004), neurons with mirroring properties fire both when performing and merely observing actions performed by another agent. More recent electrophysiological studies in monkeys provide evidence of neurons with mirroring properties in the lateral intraparietal area (Shepherd et al., 2009) and ventral intraparietal area (Ishida et al., 2010) in the intraparietal sulcus, the dorsal premotor and primary motor cortices (Cisek and Kalaska, 2004, Dushanova and Donoghue, 2010, Tkach et al., 2007), the supplementary motor area, and the medial temporal cortex (Mukamel et al., 2010). These findings demonstrate that mirroring is a neuronal property present in many neural systems in the primate brain. Brain imaging studies in humans have also shown that multiple areas in the frontal and parietal cortices are active during action observation, execution and imitation (Caspers et al., 2010, Iacoboni, 2005, Iacoboni, 2009, Iacoboni et al., 1999). This common coding of motor perception and motor action is believed to enable us to represent and understand the actions of others in terms of our own actions.
The MNS also appears to be involved in higher-level socio-emotional processes, such as decoding and empathizing with the emotional states of others. Several fMRI studies have examined MNS activity during observation and imitation of facial emotional expressions (Carr et al., 2003, Dapretto et al., 2006, Leslie et al., 2004, Schulte-Ruther et al., 2007). Both imitation and observation of facial expressions activate a neural network that includes mirroring areas, the insula and the limbic system (i.e., the amygdala). Consequently, it has been proposed that one way of empathizing is through the embodiment of the facial emotional expressions displayed by others, enabling the translation of an observed expression into its internally felt emotional significance. Consistent with this notion, MNS activation has been linked to individual differences in self-reported empathy (e.g., Gazzola et al., 2006, Kaplan and Iacoboni, 2006, Schulte-Ruther et al., 2007). Furthermore, diminished MNS activation has been documented in autism spectrum disorders, which are characterized by imitative and empathic disturbances in response to simple hand movements and facial expressions (Dapretto et al., 2006, Iacoboni and Dapretto, 2006, Williams et al., 2006).
Although disturbances in the MNS and the “social brain” have been theoretically linked to schizophrenia (e.g., Burns, 2006, Iacoboni, 2009), very little work has directly evaluated this area. While behavioral studies show impaired imitation of complex hand movements and facial emotional expressions in schizophrenia (e.g., Kohler et al., 2008, Lee et al., 2014, Matthews et al., 2013, Park et al., 2008, Varcin et al., 2010), the few studies of MNS activity have been inconsistent. Three electrophysiological studies examined Mu suppression, a hypothesized biomarker of MNS activity. The first reported normal Mu suppression in schizophrenia during observation of hand movements and social interaction stimuli, but diminished Mu suppression during observation of basic biological motion (point light animation) (Singh et al., 2011). The second focused on hand movement stimuli and found normal or, in one task condition, enhanced Mu suppression in schizophrenia, which correlated with higher levels of psychotic symptoms (McCormick et al., 2012). The third reported that a small sample of drug-free patients showed diminished Mu suppression during a video depicting a handshake (only hands shown), and that this disturbance did not improve with four weeks of treatment with antipsychotic medications (Mitra et al., 2014). Thus, EEG studies appear to be intact under at least some experimental conditions.
A few studies have used transcranial magnetic stimulation (TMS) paradigms to study MNS activity in schizophrenia. One found that patients demonstrated reduced motor evoked potential facilitation during hand action observation (Enticott et al., 2008). A series of studies by Mehta et al. found that unmedicated, though not medicated, patients had reduced motor evoked potentials during action observation, and that individual differences among patients correlated with scores on performance measures of facial affect perception and theory of mind (Mehta et al., 2012, Mehta et al., 2013). Thus, TMS studies provide more consistent evidence of impaired MNS activation in schizophrenia.
Although fMRI paradigms have been extensively used to examine MNS activity in healthy and clinical samples, to our knowledge such paradigms have only been applied in one prior study of schizophrenia. Thakkar et al. (2014) used a finger movement observation–imitation task and found that individuals with schizophrenia (n = 16) showed lower activation than healthy controls (n = 16) in one MNS region, the right inferior parietal lobe, during observation of finger movements, but higher activation than controls in this region during imitation of finger movements. Neither neural responses to faces nor self-perceived empathy was examined. The current study applied an observation–imitation–execution paradigm to individuals with schizophrenia that included both finger movement and facial emotional expression conditions. The goals of the study were to (1) compare MNS activity in patients and controls on these two types of stimuli, and (2) examine whether individual differences in self-reported empathy differentially relate to neural activation in patients and controls.
2. Methods
2.1. Participants
Twenty-three outpatients with schizophrenia and 23 healthy controls participated in the study. Schizophrenia patients were 18–60 years of age and recruited from outpatient clinics at the VA Greater Los Angeles Healthcare System and through local board and care facilities. Patients were clinically stable and received the Structural Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Axis I Disorders (SCID; First et al., 1996) to confirm diagnosis of schizophrenia. Patients were medicated at clinically determined dosages with 17 receiving atypical antipsychotics, one receiving typical antipsychotics, and five receiving both types of antipsychotic medication. The mean dose of antipsychotic medication was equivalent to 282.51 mg/day of chlorpromazine (SD = 162.49) (Andreasen et al., 2010). Exclusion criteria for patients included (1) substance abuse or dependence in the last 6 months, (2) IQ < 70, (3) history of loss of consciousness > 1 h, (4) identifiable neurological disorder, and (5) not sufficiently fluent in English.
Healthy control participants were recruited through flyers posted in the local community, newspaper advertisements, and website postings. Exclusion criteria for control participants included (1) history of schizophrenia or other psychotic disorder, bipolar disorder (no history of a manic or hypomanic episode), recurrent depression (no subjects were experiencing a depressive episode at the time of testing), dysthymia, history of substance dependence, or any substance abuse in the last 6 months based on the SCID, (2) avoidant, paranoid, schizoid, and schizotypal disorders based on the SCID for Axis II (First et al., 1994), (3) history of loss of consciousness > 1 h, (4) schizophrenia or other psychotic disorder in a first-degree relative, (5) significant neurological disorder, and (5) not sufficiently fluent in English. All participants were evaluated for their capacity to give informed consent and provided written informed consent after all procedures were fully explained, according to procedures approved by the institutional review boards at the University of California, Los Angeles (UCLA) and the Greater Los Angeles VA Health Care System.
2.2. Clinical and empathy measures
For patients, we assessed clinical symptoms using the expanded Brief Psychiatric Rating Scale (BPRS; Kopelowicz et al., 2008, Lukoff et al., 1986, Overall and Gorham, 1962) and examined the BPRS total score as well as the BPRS mean subscales for positive symptoms, negative symptoms, and depression/anxiety. All interviewers were trained through the Treatment Unit of the VA Desert Pacific Mental Illness Research, Education, and Clinical Center. SCID interviewers were trained to a minimum kappa of 0.75 for key psychotic and mood items, and BPRS raters were trained to a minimum intraclass correlation of .80 (Ventura et al., 1993). In addition, all participants filled out the Interpersonal Reactivity Inventory (IRI; Davis, 1983), indicating to what extent 28 short phrases described them on a 5-point scale (from “does not describe me at all” to “describes me very well”). This measure was chosen because it taps a variety of aspects of empathy, although it does not directly address motoric aspects like mimicry.
2.3. Activation paradigm
2.3.1. Stimuli
Finger and face stimuli were presented to subjects through magnet-compatible goggles. For hands, five stimulus sets were assembled using two color videos that displayed either an index finger movement or a middle finger movement, as in previous studies (Iacoboni et al., 1999). Each 2.5 s video began with a left hand resting on a surface in a relaxed position with the palm down and fingers facing the subject. After 500 ms, the video depicted upward movement of either the index or middle finger from the resting position, which then remained stationary for the remainder of the video. Each stimulus set consisted of eight videos that displayed index or middle finger movements (each shown four times) in random order. For faces, five stimulus picture sets were assembled from the NimStim set of facial expressions (Tottenham et al., 2002) each containing randomly ordered depictions of prototypical expressions of four emotions (happy, sad, angry, and afraid), as in previous studies (Carr et al., 2003, Dapretto et al., 2006, Pfeifer et al., 2008). This stimulus set has undergone extensive validation and been widely used in social neuroscience (Tottenham et al., 2009). Each stimulus set consisted of eight pictures presented for 2.5 s each in which different individuals displayed the four emotions (each emotion shown twice), with males and females in equal proportion.
2.3.2. Tasks
Subjects were presented with five runs of stimuli. The task structure was identical across all six experimental conditions, regardless of whether the condition involved video, picture, or word stimuli. Each run consisted of six blocks of 20 s each, including one block for each of the three finger tasks and for each of the three face tasks. Within each block, eight sequential 2.5 s stimuli were presented with no time gap between each of them.
Finger tasks: (1) Observe: subjects were asked to “just look at the movement in each finger” shown in a set of eight videos, (2) Imitate: subjects were asked to “imitate the movement in each finger” with their own right hand shown in a set of eight videos, and (3) Execute: subjects were asked to “make the movement described by each word” with their right hand and, instead of videos, the words “Lift Index” and “Lift Middle” were each presented four times for 2.5 s in a random order.
Face tasks: (1) Observe: subjects were asked to “just look at the expression on each face” in a set of eight pictures, (2) Imitate: subjects were asked to “imitate the expression on each face” on their own face in a set of eight pictures, and (3) Execute: subjects were asked to “make the expression described by each word” in their face and, instead of pictures, the words “Happy”, “Sad”, “Angry”, and “Afraid” were each presented two times for 2.5 s in a random order. The observe/imitate/execute conditions for the finger and face tasks were counterbalanced across runs.
A 2.5 s instruction screen was presented before each of the six trial blocks; all instruction screens were modeled. Each run began and ended with an 8 s rest period and the task blocks were separated by five rest periods of 16 s (blank screen), which were not modeled and served as an implicit baseline. Subjects were given a short break between each run. Each run was preceded by a reminder of the instructions.
Prior to the scanning session, subjects were familiarized with the task during a standardized 30-minute training session. The session included detailed instruction about the tasks and extensive practice on the finger and face tasks. At the end of the practice session subjects completed one run of the paradigm that was videotaped and possible between-group differences in behavior during the imitate tasks were examined (Dapretto et al., 2006). Three raters who were blind to group membership were asked to rate how well each subject performed the imitate fingers and faces tasks on a 5-point Likert scale (ranging from 1 = very poorly to 5 = very well). Inter-rater agreement was good for both fingers and faces (Kappas = .84 and .85, respectively). For fingers, the ratings were high and comparable across groups (Schizophrenia: M = 4.4, SD = .5; Control: M = 4.7, SD = .4; t(44) = − 1.42, p > .05). For faces, ratings were lower in the Schizophrenia (M = 3.5; SD = .7) than the Control (4.3, SD = .4) group, t(44) = − 5.34, p < .01. Thus, although the mean ratings suggest that both groups sufficiently understood and performed the tasks, the patients showed lower accuracy when imitating face expressions.
2.4. MRI data acquisition & processing
Images were acquired on a Siemens 3 T (Erlangen, Germany) Trio MRI scanner. For functional runs we acquired 116 T2*-weighted echoplanar images (EPIs) [repetition time (TR) 2000 ms; echo time (TE) 30 ms; flip angle = 75°; 33 slices; slice thickness 4 mm; matrix 64 × 64; FOV 220 mm]. To allow for T1 equilibrium the first two volumes of each functional scan are automatically discarded before data collection begins. Two sets of structural images were also acquired for registration of functional data: a T2-weighted matched-bandwidth high-resolution scan with the same slice prescription as the EPI [repetition time (TR) 6540 ms; echo time (TE) 13 ms; flip angle = 120°; 33 slices; slice thickness 4 mm; matrix 128 × 128; FOV 220 mm]; and a T1 weighted magnetization prepared rapid-acquisition gradient echo image (MPRAGE) [TR, 1900 ms; TE 3.43 ms; flip angle = 9°; 160 sagittal slices; slice thickness 1 mm; matrix 256 × 256; FOV 256 mm]. Visual stimuli were timed and presented with Presentation software (Neurobehavioral Systems, Albany, CA) through magnet-compatible LCD goggles.
Image preprocessing and data analysis were performed with FSL version 4.1.4 (Centre for Functional Magnetic Resonance Imaging of the Brain software library, www.fmrib.ox.ac.uk/fsl) (Smith et al., 2004). Images were realigned to the middle volume to compensate for any head motion using MCFLIRT (Jenkinson et al., 2002). The data were temporally filtered with a high-pass filter cutoff of 100 s and spatially smoothed with a 6 mm full width at half maximum Gaussian kernel in three dimensions. Two patients and one control were excluded from the analyses due to excessive movement artifact, defined as global movement ≥ .40 mm, across three or more runs. Additionally, for five patients, one (n = 3) or two (n = 2) runs were removed due to excessive movement artifact. The final sample sizes were 23 patients and 23 controls.
Statistical analyses were performed at the single subject level using a general linear model (GLM) with the fMRI Expert Analysis Tool (FEAT). After convolution with a canonical double-gamma hemodynamic response function, each block type (observe, imitate, execute for finger and face tasks) was included as a regressor in the GLM. In addition, the instruction screens (orthogonalized with respect to regressors of interest) were included in the model as a nuisance regressor. Six parameters for motion correction were included as regressors of non-interest to control for motion artifact. Three types of contrasts were examined: (1) Each of the six main experimental tasks was compared to implicit baseline (i.e., unmodeled rest periods); (2) Within the finger and the face tasks, contrasts were estimated between the imitate vs. observe and the imitate vs. execute tasks; (3) Between the finger and face tasks, contrasts were estimated between the three corresponding tasks ([observe finger–implicit baseline vs. observe face–implicit baseline], [imitate finger–implicit baseline vs. imitate face–implicit baseline], and [execute finger–implicit baseline vs. execute face–implicit baseline]). Mirror neuron regions typically activate more during execution than observation of an action, and highest during imitation of an action, which involves both execution and observation (Iacoboni, 2009).
First level contrast estimates were computed for each run and then registered to standard space (Montreal Neurological Institute, MNI) in three stages. The middle volume of each run of individual EPI data was registered first to the co-planar matched-bandwidth high-resolution T2-weighted image and subsequently, the co-planar volume was registered to the T1-weighted MPRAGE. Both of these steps were carried out using FLIRT (affine transformations: EPI to co-planar, 3° of freedom; co-planar to MPRAGE, 6° of freedom) (Jenkinson et al., 2002). Finally registration of the MPRAGE to MNI space (FSL's MNI Avg152, T1 2 × 2 × 2 mm) was carried out with FLIRT (affine transformation, 12° of freedom). Contrast estimates for each subject were then computed treating each run as a fixed effect.
Prior to group-level analyses, a mask was created that comprised of activity across all six experimental conditions in the combined sample (n = 46) using a threshold of z > 2.3 and corrected for multiple comparisons using cluster-based Gaussian random field theory controlling family-wise error across the whole brain at p < 0.05. The purpose of the mask was to conservatively limit our analyses to regions that activated for any condition in the whole sample and avoid spurious effects that might be associated with activation in one condition. Within this masked area we applied cluster thresholding as described below for all voxels included in the mask to correct for multiple comparisons. Then, a group level analysis was performed to calculate a group mean for each contrast treating each subject as a random effect. All group images were thresholded at the same level as the mask (z > 2.3, p < 0.05). Within the GLM as implemented by FSL, all between group comparisons used one-way ANOVAs for each contrast of interest.
Finally, to explore whether brain activity was differentially related to self-reported empathy across groups, separate whole brain analyses were conducted within the GLM as implemented by FSL using two-group ANCOVA comparisons with a continuous covariate (IRI summary score) for the six main experimental conditions. Although concerns have been raised about whole brain analyses using a two step-inferential process (aka “double dipping”; Lieberman et al., 2009, Vul et al., 2009), the current approach involved a one step approach to identify brain regions that were associated with self-reported empathy and did not include any secondary inferential statistical analyses. These analyses were performed in every voxel included in the mask, which was identified independently, and significance levels were set at z > 2.3, with correction for multiple comparisons at the cluster level (p < 0.05).
3. Results
3.1. Descriptive information
As shown in Table 1, the groups did not significantly differ in sex, age, or ethnicity. The patients had lower personal education levels than controls but the groups did not differ in parental education. The schizophrenia group had a typical age of onset, was chronically ill, and showed mild to moderate levels of clinical symptoms at the time of testing.
Table 1.
Demographic and descriptive data.
| Schizophrenia | Controls | Statistic | |
|---|---|---|---|
| Sex (% male) | 73.9% | 69.6% | χ2(1,46) = .11 |
| Age (SD) | 46.5 (11.1) | 46.7 (6.9) | t(44) = .08 |
| Ethnicity | |||
| White | 60.9% | 73.9% | χ2(4,58) = 1.58 |
| African American | 34.8% | 26.1% | |
| Hispanic | 0% | 4.3% | |
| Marital status | |||
| Never married | 65.2% | 34.8% | χ2(2,58) = 4.73 |
| Currently married | 8.7% | 26.1% | |
| Ever married | 26.1% | 39.1% | |
| Education (SD) | 12.9 (1.6) | 14.9 (1.6) | t(56) = 4.25⁎ |
| Parental education (SD) | 13.6 (3.3) | 14.9 (2.9) | t(56) = 1.35 |
| Age of onset (SD) | 20.6 (5.7) | ||
| Duration of illness (SD) | 26.9 (11.8) | ||
| BPRS | |||
| Positive symptoms (SD) | 1.5 (0.5) | ||
| Negative symptoms (SD) | 1.7 (0.8) | ||
| Total (SD) | 33.7 (6.4) |
Notes:
p < .001.
3.2. Group comparisons for the finger tasks
3.2.1. Finger tasks vs. rest
Signal changes during the imitate and execute finger tasks were seen in a large set of cortical and subcortical areas in both groups, a finding consistent with previous studies in healthy subjects (see Fig. 1 and Supplemental Table 1). No group differences were seen for any of the three finger tasks.
Fig. 1.
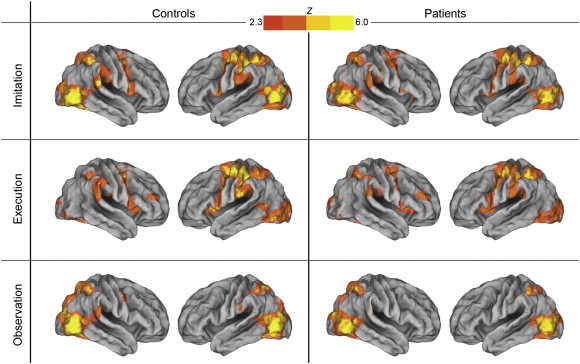
Imitation, execution, and observation of finger movements in controls and patients. Both groups showed activation across several cortical regions, including prefrontal, premotor, inferior and superior parietal, and occipital cortices. There were no group differences on any of the finger tasks.
3.2.2. Imitate finger versus other finger tasks
Comparisons between the imitate and execute finger tasks showed greater activity in lateral visual areas for the imitate task in both groups and greater activity in medial visual areas for the execute task in both groups (Supplemental Table 2). There were no group differences for these tasks. In the imitate versus observe finger task comparisons, the imitate task showed greater activity in the frontal and parietal areas, and basal ganglia, all areas of well known motor significance, compared to the observe task, in both groups. There were a few regions in the controls in the posterior occipital area in which observe was greater than imitate, but no group differences.
3.3. Group comparisons for face tasks
3.3.1. Face tasks versus rest
As shown in Fig. 2 and Supplemental Table 3, for both groups, imitate and execute face tasks demonstrated widespread signal changes in a large set of cortical and subcortical areas, a finding that is again consistent with previous studies. The observe task produced signal changes in occipital areas in both groups. Further, this task showed signal changes in premotor areas that were bilateral in the control group and only in the right hemisphere the patients. There were, however, no significant between group differences for any of the face movement tasks.
Fig. 2.
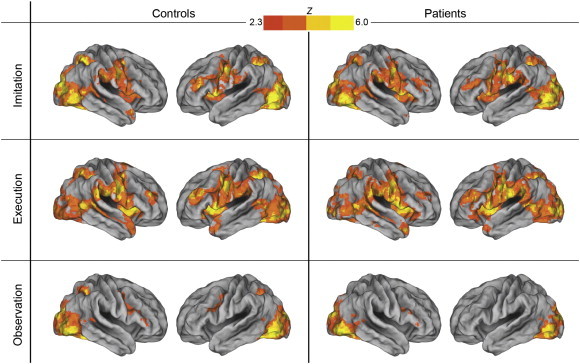
Imitation, execution, and observation of facial expressions in controls and patients. Both groups showed activation in the imitation and execution conditions across several regions including prefrontal, premotor, inferior and superior parietal, and occipital cortices. For the observe task, there were signal changes in occipital areas and premotor cortex. There were no group differences for any of the face tasks.
3.3.2. Imitate face versus other face tasks
The imitate face task showed greater activity in visual areas than the execute task (Supplemental Table 4) in both groups. The execute task showed more activity than imitate in the basal ganglia, anterior cingulate cortex, and middle temporal gyrus, in both groups. The imitate task also showed greater activity than the observe task in a large number of cortical and subcortical areas, but no regions showed activity with the reverse contrast. There were no significant between group differences in any of these comparisons.
3.4. Group comparisons for contrasts between the finger and face tasks
Separate comparisons between the finger and face stimuli for each of the imitate, execute, and observe tasks were carried out (see Fig. 3 and Supplemental Table 5). The contrast of the imitate finger versus imitate face tasks demonstrated clear differences in the primary motor cortex that correspond to its rough somatotopic organization. Finger movements produced greater activity in the knob of the left central sulcus and neighboring areas in the precentral and postcentral sulci. In contrast, face movements produced greater activity in more ventral areas bilaterally along the central sulcus. The imitate face task also resulted in greater activity in a number of other cortical and subcortical areas.
Fig. 3.
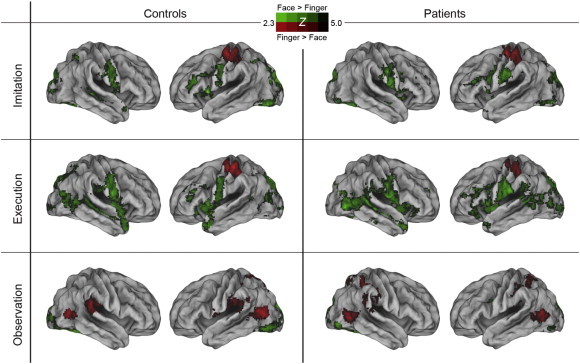
Contrasts of corresponding finger versus face tasks in controls and patients. The finger versus face contrasts for imitation and execution tasks showed activation differences in primary motor cortex that correspond to its rough somatotopic organization in both groups. Finger movements produced greater activity in the knob of the left central sulcus and neighboring areas in the precentral and postcentral sulcus (shown in red) whereas face movements produced greater activity in more ventral areas bilaterally along the central sulcus (shown in green). For observe tasks, finger movements produced greater activation in lateral occipital cortex and middle temporal gyrus, while facial expressions produced higher activity in a large section of visual cortex including medial and lateral occipital areas. There were no group differences for any of the contrasts.
The contrasts of execute finger versus execute face produced results similar to the imitate contrasts, also with no group differences. In the observe tasks, for finger minus face, there was activation in the lateral occipital cortex and middle temporal gyrus in both groups. For the contrast of face minus finger, both groups showed higher activity in a large section of the visual cortex including the medial and lateral occipital areas. There were no significant group differences for the observe tasks.
3.5. Correlations with self-reported empathy
Previous studies have reported correlations in healthy subjects between self-reported empathy and brain activity in tasks similar to the ones used here. Thus, we investigated whether correlations with a summary index from the widely used IRI differ between the schizophrenia and control groups for each of the six main experimental tasks. We chose to use a summary index to limit the number of correlational analyses and reduce the chance of false positive results. Between group comparisons on the four IRI subscales are presented in Table 2. The schizophrenia group reported significantly lower scores than controls on the Perspective taking and Empathic concern subscales, and numerically lower Fantasy subscale scores. However, patients reported numerically higher Personal Distress scores than controls, raising concerns that higher scores on this subscale may not necessarily reflect more adaptive levels of empathy. We therefore created a summary empathy index from the Perspective taking, Empathic concern, and Fantasy subscales rather than the more commonly used index based on all four subtests. This decision was supported by the relatively high correlations between the three subscales and the composite score within each group (correlations for schizophrenia group: .87 for Perspective tasking; .68 for Fantasy; and .91 for Empathic concern. Correlations for controls: .76 for Perspective tasking; .78 for Fantasy; and .86 for Empathic concern). Patients (M = 48.45, SD = 10.55) had significantly lower scores than controls (M = 56.97, SD = 10.55), t(44) = − 2.74, p < .01, d = − .81, on this summary index.
Table 2.
Group comparisons on the Interpersonal Reactivity Inventory (IRI).
| Schizophrenia | Controls | t value | Effect size | |
|---|---|---|---|---|
| Perspective taking | 15.75 | 20.51 | − 3.68⁎⁎ | − 1.09 |
| (4.69) | (4.06) | |||
| Empathic concern | 18.20 | 21.34 | − 3.14⁎ | − .83 |
| (4.06) | (3.48) | |||
| Fantasy | 14.50 | 15.12 | − .42 | − .12 |
| (4.10) | (5.72) | |||
| Personal distress | 11.00 | 9.08 | 1.38 | .41 |
| (5.84) | (3.23) | |||
| IRI summary indexa | 48.45 | 56.97 | − 2.74⁎ | − .81 |
| (10.55) | (10.55) |
Notes: Degrees of freedom for t-tests = 44. Effect sizes presented as Cohen's d.
IRI summary index comprised of scores form the Perspective taking, Empathic concern, and Fantasy subscales.
p < .01.
p < .001.
Within the GLM we used two-group ANCOVAs with a continuous covariate (IRI index) to evaluate relations between self-reported empathy and activation during each of the six experimental conditions. For the three finger tasks, there were no significant within group correlations with the IRI above threshold and there were also no significant between group differences in correlation. For the face tasks, there were no significant within group or between group differences in correlations with the IRI for the imitate or execute tasks. However, for the observe face task, there was a significant group difference for the correlation between IRI scores and activity in a single cluster in the lateral inferior prefrontal cortex (pars opercularis) in the right hemisphere (see Supplemental Table 6). As shown in Fig. 4, to further characterize this interaction effect we examined scatter plots of the relationship between IRI scores and activation levels (beta values) in the IFG cluster in the two groups. The groups showed opposing patterns of association. Higher IRI scores were associated with higher IFG activation levels in controls whereas higher IRI scores were associated with lower IFG activation levels in patients1.
Fig. 4.
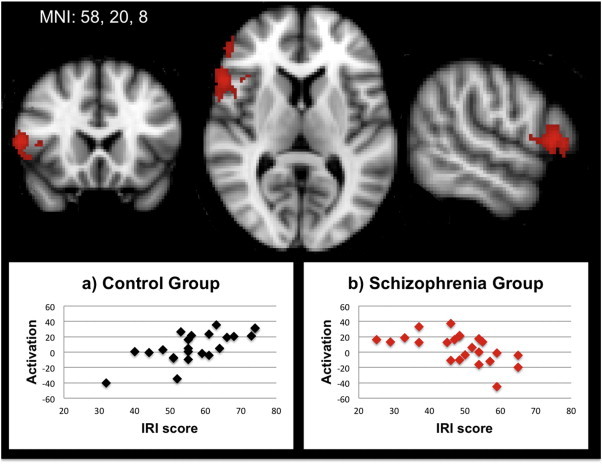
Contrast of correlation between IRI summary scores and activation during the observe face task in controls minus patients. To illustrate the nature of the interaction, scatterplots are presented separately by group for controls (panel a) and patients (panel b).
4. Discussion
This study examined whether clinically stable outpatients with schizophrenia demonstrate neural activation differences from healthy controls during an action observation–execution–imitation paradigm involving finger movements and facial expressions. As a group, patients showed an activation pattern that was similar to controls across the finger and face tasks. There were no significant between-group differences for any of the contrasts examined. Self-reported empathy, however, differentially related to right inferior frontal activity (pars opercularis) during the observe face task between groups, as controls showed a positive relationship between these variables whereas patients tended to show a negative relationship. Similar relations between self-reported empathy and inferior frontal activity during action observation tasks have been previously reported in a number of studies on healthy adults (e.g., Chakrabarti et al., 2006, Cheng et al., 2009, Gazzola et al., 2006, Hooker et al., 2008, Hooker et al., 2010, Jabbi et al., 2007, Kaplan and Iacoboni, 2006, Schulte-Ruther et al., 2007), as well as children (Pfeifer et al., 2008). Furthermore, decreased activation in this region has been found to correlate with higher levels of behavioral problems in children with autism spectrum disorders (Dapretto et al., 2006). The schizophrenia patients in the current study failed to show the typical pattern of correlation between inferior frontal activity and self-reported empathy.
All subjects received extensive training prior to scanning and although there were no group differences on independent ratings of finger imitation performance, patients' received lower (yet reasonably good) accuracy ratings than controls for imitating facial expressions. The patients' poorer imitation for faces is consistent with prior studies showing impaired voluntary imitation of face and complex hand gesture movements in schizophrenia (e.g., Kohler et al., 2008, Lee et al., 2014, Matthews et al., 2013, Park et al., 2008). Despite these differences, the patient group showed comparable activation to controls during the finger and face imitation and execution tasks. Indeed, when comparing task-related activation in tasks involving different body parts (hands, faces) we observed differential activity in motor areas in both groups that is consistent with the known rough somatotopic organization of these areas.
Consistent with a number of prior studies (see Achim et al., 2011, Smith et al., 2012) patients showed lower self-reported empathy than controls on the IRI (particularly Perspective taking and Empathic concern), whereas their pattern of task-related brain activation in this study was comparable to control subjects. These between-group differences, in conjunction with the correlational results for IRI relations with regional activation levels, raise the question of why patients show a relative lack of correspondence between brain activity during basic tasks and self-reported empathy. The sensory–motor tasks we adopted are generally conceptualized as tasks engaging stimulus-driven, low level forms of social cognition. Self-reports on empathic predispositions, in contrast, involve an explicit understanding of one's own experience. Healthy controls showed a significant positive relation between a low-level, automatic social cognitive process (activation in the IFG region of the mirror neuron system in response to viewing faces) and a high-level, effortful social cognitive process (self-reported empathic abilities). In patients, we saw a mismatch between these low-level and high-level processes, with patients actually tending to show a negative relationship between them. Since self-reports about one's own empathic abilities involves the integration of several higher-level processes, such as insight and emotional awareness, the disjunction seen in patients could stem from disturbances in insight. Indeed, insight is often impaired in schizophrenia and has been proposed as a factor that contributes to the disturbances in self-perceived empathy seen in this disorder (Lysaker et al., 2013, Smith et al., 2013). An alternative possibility is that the capacity for automatic empathic responding at the neural level is intact in schizophrenia, but their diminished self-reported empathy indicates that this intact neural capacity is less frequently utilized in the course of their daily lives due to a relatively impoverished social environment.
Although it has been hypothesized that impaired neural mirroring is associated with schizophrenia (Burns, 2006) our findings are not consistent with this notion. Our findings, however, are consistent with two prior EEG studies reporting normal Mu suppression in central electrodes in recent-onset and chronically ill schizophrenia outpatients during the processing of social stimuli (McCormick et al., 2012, Singh et al., 2011). Mu suppression is considered an EEG index of neural mirroring. Furthermore, in a companion EEG study that included subjects in the current study, we found that schizophrenia patients and healthy controls demonstrated comparable patterns of Mu suppression across a range of experimental conditions involving observation/execution of hand movements and observation of videos depicting different levels of social interaction (Horan et al., in revision). These converging findings suggest that some aspects of the neural response during imitation and observation of social stimuli may be intact in schizophrenia.
It should be noted, however, that several studies suggest that mirroring-related processes are not fully intact in schizophrenia. For example, we failed to replicate, in a larger sample, a recent fMRI study by Thakkar et al. (2014) that found that individuals with schizophrenia showed diminished inferior parietal cortex activation during a finger movement observation task. Differences across studies could reflect their use of a considerably more complex finger sequencing task that included animated, symbolic, and spatial conditions, as well as some differences in data analytic approaches (e.g., cluster thresholding). In behavioral paradigms, patients have shown impaired voluntary imitation of facial expressions and complex gestures, and also diminished spontaneous mimicking of others' behaviors (e.g. yawning, face expressions) (e.g., Kohler et al., 2008, Lee et al., 2014, Matthews et al., 2013, Park et al., 2008, Varcin et al., 2010). Overtly mirroring the emotions displayed by others, and hence communicating that we understand them, play a critical role in interpersonal exchanges — i.e., the nonconscious behavioral mimicry also known as the ‘chameleon effect’ (Chartrand and Bargh, 1999). Such overt mirroring disturbances, which are particularly apparent in patients with high levels of the negative symptom, “affective flattening”, can be expected to contribute to strained social communication. It is unclear why covert mirroring, which has been found to be intact in schizophrenia (Kring et al., 1999), would not be effectively translated into overt mirroring in schizophrenia. It is possible that some markers of mirroring are more effective in capturing reduced mirroring in schizophrenia. Indeed, a few studies using transcranial magnetic stimulation and magnetoencephalography paradigms have reported impaired MNS functioning in schizophrenia (Enticott et al., 2008, Kato et al., 2011, Mehta et al., 2012). Further research is needed to clarify the experimental conditions under which MNS functioning appears intact vs. impaired in schizophrenia.
Some limitations of the current study should be considered. First, the number of subjects was relatively small and may have impacted the power to detect group differences. Although the current sample sizes are larger than the majority of fMRI studies reporting disturbances in social cognitive processes in schizophrenia (e.g., Harvey et al., 2013, Sugranyes et al., 2011, Thakkar et al., 2014), the lack of significant between group differences must be interpreted with appropriate caution. Second, as described in the Methods section, we used a mask that restricted our analyses to regions that were activated above threshold in the entire sample, and this decision has pros and cons. Although this relatively conservative approach constrains the analyses to mirroring related regions, it may bias against finding significant between group differences. Third, it is unclear whether our findings in medicated, chronically ill patients are generalizable to other types of samples (e.g., unmedicated patients). Finally, given difficulties with self-reflection and other relevant processes that could influence self-reported empathy in schizophrenia (e.g., alexithymia; Kubota et al., 2012), incorporating alternative assessment methods (e.g., based on clinician ratings) could be informative in future neuroimaging studies.
In summary, schizophrenia patients showed essentially normal BOLD responses during observation, execution, and imitation of finger movements and facial emotional expressions. However, the patients exhibited a disconnection between their self-reported empathy and their cortical activity during the facial emotion task. These findings suggest that the patients may be unable to benefit from the smooth connection between automatic, low-level and integrative, high-level social cognitive processes that is believed to underlie adaptive social functioning. The understanding of social cognitive deficits and their neural bases under this perspective deserves to be explored more carefully by future studies of schizophrenia.
Financial disclosure
Dr. Green reports having received consulting fees from Abbott Laboratories, Amgen, Cypress, Lundbeck, and Teva. He has received speaking fees from Otsuka and Sunovion. The rest of the authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgments
Support for this study came from a VA Career Development Award (William P. Horan, Ph.D.), NIMH Grants MH065707 and MH43292 (Michael F. Green, PhD), and the Attias Family Foundation (Marco Iacoboni, MD PhD). The authors wish to thank Amanda Bender, Michelle Dolinsky, Crystal Gibson, Cory Tripp, and Katherine Weiner for their assistance in data collection.
Footnotes
The same significant interaction effect was found when these analyses were re-done with the more commonly used IRI total score based on all four IRI subscales.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2014.06.006.
Appendix A. Supplementary data
Supplementary tables.
References
- Achim A.M., Ouellet R., Roy M.A., Jackson P.L. Assessment of empathy in first-episode psychosis and meta-analytic comparison with previous studies in schizophrenia. Psychiatry Res. 2011;190:3–8. doi: 10.1016/j.psychres.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet-Gouet E., Achim A.M., Vistoli D., Passerieux C., Hardy-Bayle M.C., Jackson P.L. The study of social cognition with neuroimaging methods as a means to explore future directions of deficit evaluation in schizophrenia? Psychiatry Res. 2011;190:23–31. doi: 10.1016/j.psychres.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Burns J. The social brain hypothesis of schizophrenia. World Psychiatry. 2006;5:77–81. [PMC free article] [PubMed] [Google Scholar]
- Carr L., Iacoboni M., Dubeau M.C., Mazziotta J.C., Lenzi G.L. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S., Zilles K., Laird A.R., Eickhoff S.B. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti B., Bullmore E., Baron-Cohen S. Empathizing with basic emotions: common and discrete neural substrates. Soc. Neurosci. 2006;1:364–384. doi: 10.1080/17470910601041317. [DOI] [PubMed] [Google Scholar]
- Chartrand T.L., Bargh J.A. The chameleon effect: the perception–behavior link and social interaction. J. Pers. Soc. Psychol. 1999;76:893–910. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chou K.H., Decety J., Chen I.Y., Hung D., Tzeng O.J., Lin C.P. Sex differences in the neuroanatomy of human mirror-neuron system: a voxel-based morphometric investigation. Neuroscience. 2009;158:713–720. doi: 10.1016/j.neuroscience.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Cisek P., Kalaska J.F. Neural correlates of mental rehearsal in dorsal premotor cortex. Nature. 2004;431:993–996. doi: 10.1038/nature03005. [DOI] [PubMed] [Google Scholar]
- Dapretto M., Davies M.S., Pfeifer J.H., Scott A.A., Sigman M., Bookheimer S.Y., Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983;44:113–126. [Google Scholar]
- Dushanova J., Donoghue J. Neurons in primary motor cortex engaged during action observation. Eur. J. Neurosci. 2010;31:386–398. doi: 10.1111/j.1460-9568.2009.07067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott P.G., Hoy K.E., Herring S.E., Johnston P.J., Daskalakis Z.J., Fitzgerald P.B. Reduced motor facilitation during action observation in schizophrenia: a mirror neuron deficit? Schizophr. Res. 2008;102:116–121. doi: 10.1016/j.schres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W., Benjamin L. New York State Psychiatric Institute; New York, NY: 1994. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (Version 2.0) [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L., Williams J.B.W. Patient Edition. Biometrics Research; New York: 1996. Structured Clinical Interview for DSM-IV Axis I Disorders. [Google Scholar]
- Gazzola V., Aziz-Zadeh L., Keysers C. Empathy and the somatotopic auditory mirror system in humans. Curr. Biol. 2006;16:1824–1829. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- Green M.F., Horan W.P. Social cognition in schizophrenia. Curr. Dir. Psychol. Sci. 2010;19:243–248. [Google Scholar]
- Harvey P.O., Zaki J., Lee J., Ochsner K., Green M.F. Neural substrates of empathic accuracy in people with schizophrenia. Schizophr. Bull. 2013;39:617–628. doi: 10.1093/schbul/sbs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C.I., Verosky S.C., Germine L.T., Knight R.T., D'Esposito M. Mentalizing about emotion and its relationship to empathy. Soc. Cogn. Affect. Neurosci. 2008;3:204–217. doi: 10.1093/scan/nsn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C.I., Verosky S.C., Germine L.T., Knight R.T., D'Esposito M. Neural activity during social signal perception correlates with self-reported empathy. Brain Res. 2010;1308:100–113. doi: 10.1016/j.brainres.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W.P., Pineda J.A., Wynn J.K., Iacoboni M., Green M.F. Some Markers of Mirroring Appear Intact in Schizophrenia: Evidence from Mu Suppression. Soc. Cogn. Affect. Neurosci. 2014 doi: 10.3758/s13415-013-0245-8. in revision. (in press) [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Neural mechanisms of imitation. Curr. Opin. Neurobiol. 2005;15:632–637. doi: 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Imitation, empathy, and mirror neurons. Annu. Rev. Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat. Rev. Neurosci. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Woods R.P., Brass M., Bekkering H., Mazziotta J.C., Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Ishida H., Nakajima K., Inase M., Murata A. Shared mapping of own and others' bodies in visuotactile bimodal area of monkey parietal cortex. J. Cogn. Neurosci. 2010;22:83–96. doi: 10.1162/jocn.2009.21185. [DOI] [PubMed] [Google Scholar]
- Jabbi M., Swart M., Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kaplan J.T., Iacoboni M. Getting a grip on other minds: mirror neurons, intention understanding, and cognitive empathy. Soc. Neurosci. 2006;1:175–183. doi: 10.1080/17470910600985605. [DOI] [PubMed] [Google Scholar]
- Kato Y., Muramatsu T., Kato M., Shibukawa Y., Shintani M., Mimura M. Magnetoencephalography study of right parietal lobe dysfunction of the evoked mirror neuron system in antipsychotic-free schizophrenia. PLoS One. 2011;6:e28087. doi: 10.1371/journal.pone.0028087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C.G., Martin E.A., Stolar N., Barrett F.S., Verma R., Brensinger C., Bilker W., Gur R.E., Gur R.C. Static posed and evoked facial expressions of emotions in schizophrenia. Schizophr. Res. 2008;105:49–60. doi: 10.1016/j.schres.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelowicz A., Ventura J., Liberman R.P., Mintz J. Consistency of Brief Psychiatric Rating Scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology. 2008;41:77–84. doi: 10.1159/000111551. [DOI] [PubMed] [Google Scholar]
- Kring A.M., Kerr S.L., Earnst K.S. Schizophrenic patients show facial reactions to emotional facial expressions. Psychophysiology. 1999;36:186–192. [PubMed] [Google Scholar]
- Kubota M., Miyata J., Sasamoto A., Kawada R., Fujimoto S., Tanaka Y., Sawamoto N., Fukuyama H., Takahashi H., Murai T. Alexithymia and reduced white matter integrity in schizophrenia: a diffusion tensor imaging study on impaired emotional self-awareness. Schizophr. Res. 2012;141:137–143. doi: 10.1016/j.schres.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Chun J.W., Yoon S.Y., Park H.J., Kim J.J. Involvement of the mirror neuron system in blunted affect in schizophrenia. Schizophr. Res. 2014;152:268–274. doi: 10.1016/j.schres.2013.10.043. [DOI] [PubMed] [Google Scholar]
- Leslie K.R., Johnson-Frey S.H., Grafton S.T. Functional imaging of face and hand imitation: towards a motor theory of empathy. Neuroimage. 2004;21:601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Berkman E.T., Wager T.D. Correlations in social neuroscience aren't voodoo: commentary on Vul et al. (2009) Perspect. Psychol. Sci. 2009;4:299–307. doi: 10.1111/j.1745-6924.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoff D., Nuechterlein K.H., Ventura J. Manual for the expanded Brief Psychiatric Rating Scale. Schizophr. Bull. 1986;12:578–602. doi: 10.1093/schbul/12.4.578. [DOI] [PubMed] [Google Scholar]
- Lysaker P.H., Hasson-Ohayon I., Kravetz S., Kent J.S., Roe D. Self perception of empathy in schizophrenia: emotion recognition, insight, and symptoms predict degree of self and interviewer agreement. Psychiatry Res. 2013;206:146–150. doi: 10.1016/j.psychres.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Matthews N., Gold B.J., Sekuler R., Park S. Gesture imitation in schizophrenia. Schizophr. Bull. 2013;39:94–101. doi: 10.1093/schbul/sbr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick L.M., Brumm M.C., Beadle J.N., Paradiso S., Yamada T., Andreasen N. Mirror neuron function, psychosis, and empathy in schizophrenia. Psychiatry Res. 2012;201:233–239. doi: 10.1016/j.pscychresns.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta U.M., Basavaraju R., Thirthalli J., Gangadhar B.N. Mirror neuron dysfunction — a neuro-marker for social cognition deficits in drug naive schizophrenia. Schizophr. Res. 2012;141:281–283. doi: 10.1016/j.schres.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Mehta U.M., Thirthalli J., Basavaraju R., Gangadhar B.N., Pascual-Leone A. Reduced mirror neuron activity in schizophrenia and its association with theory of mind deficits: evidence from a transcranial magnetic stimulation study. Schizophr. Bull. 2013 doi: 10.1093/schbul/sbt155. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S., Nizamie S.H., Goyal N., Tikka S.K. Unchanging mirror neuron activity in schizophrenia patients over 4 weeks of treatment: evidence from a 192 channel quantitative electroencephalography study. Biol. Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.12.012. (in press) [DOI] [PubMed] [Google Scholar]
- Mukamel R., Ekstrom A.D., Kaplan J., Iacoboni M., Fried I. Single-neuron responses in humans during execution and observation of actions. Curr. Biol. 2010;20:750–756. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall J.E., Gorham D.R. The Brief Psychiatric Rating Scale. Psychol. Rep. 1962;10:799–812. [Google Scholar]
- Park S., Matthews N., Gibson C. Imitation, simulation, and schizophrenia. Schizophr. Bull. 2008;34:698–707. doi: 10.1093/schbul/sbn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Iacoboni M., Mazziotta J.C., Dapretto M. Mirroring others' emotions relates to empathy and interpersonal competence in children. Neuroimage. 2008;39:2076–2085. doi: 10.1016/j.neuroimage.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M., Markowitsch H.J., Fink G.R., Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J. Cogn. Neurosci. 2007;19:1354–1372. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- Shepherd S.V., Klein J.T., Deaner R.O., Platt M.L. Mirroring of attention by neurons in macaque parietal cortex. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9489–9494. doi: 10.1073/pnas.0900419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh F., Pineda J., Cadenhead K.S. Association of impaired EEG mu wave suppression, negative symptoms and social functioning in biological motion processing in first episode of psychosis. Schizophr. Res. 2011;130:182–186. doi: 10.1016/j.schres.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Beckmann C.F., Woolrich M.W., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Niazy R.K., Flitney D.E., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith M.J., Horan W.P., Karpouzian T.M., Abram S.V., Cobia D.J., Csernansky J.G. Self-reported empathy deficits are uniquely associated with poor functioning in schizophrenia. Schizophr. Res. 2012;137:196–202. doi: 10.1016/j.schres.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Smith M.J., Horan W.P., Cobia D.J., Karpouzian T.M., Fox J.M., Reilly J.L., Breiter H.C. Performance-based empathy mediates the influence of working memory on social competence in schizophrenia. Schizophr. Bull. 2013 doi: 10.1093/schbul/sbt084. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugranyes G., Kyriakopoulos M., Corrigall R., Taylor E., Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLoS One. 2011;6:e25322. doi: 10.1371/journal.pone.0025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar K.N., Peterman J.S., Park S. Altered brain activation during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am. J. Psychiatry. 2014;171:539–548. doi: 10.1176/appi.ajp.2013.13040498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach D., Reimer J., Hatsopoulos N.G. Congruent activity during action and action observation in motor cortex. J. Neurosci. 2007;27:13241–13250. doi: 10.1523/JNEUROSCI.2895-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Borscheid A., Ellertsen K., Marcus D.J., Nelson C.A. Categorization of facial expressions in children and adults: establishing a larger stimulus set. J. Cogn. Neurosci. 2002;S74 ( http://www.macbrain.org/faces/index.htm) [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Marcus D.J., Westerlund A., Casey B.J., Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varcin K.J., Bailey P.E., Henry J.D. Empathic deficits in schizophrenia: the potential role of rapid facial mimicry. J. Int. Neuropsychol. Soc. 2010;16:621–629. doi: 10.1017/S1355617710000329. [DOI] [PubMed] [Google Scholar]
- Ventura J., Green M.F., Shaner A., Liberman R.P. Training and quality assurance with the brief psychiatric rating scale: ‘the drift busters’. Int. J. Methods Psychiatr. Res. 1993;3:221–224. [Google Scholar]
- Vul E., Harris C., Winkielman P., Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect. Psychol. Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Williams J.H.G., Waiter G.D., Gilchrist A., Perrett D.I., Murray A.D., Whiten A. Neural mechanisms of imitation and ‘mirror neuron’ functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.


