Beyond PMTCT
Prevention of mother-to-child transmission of HIV (PMTCT) is one of public health’s greatest successes. Chemoprophylaxis for HIV-infected pregnant women averted more than 100,000 infections between 2003 and 2010 and 600,000 since 1995 (1). The success of these programs in low and middle income countries (LMICs) has prompted leaders to aim for an “AIDS-free generation” and the elimination of mother-to-child transmission of HIV by 2015 (2).
The prevention-first agenda is both sensible and laudable: preventing pediatric infections is indisputably better than having to treat them. However, in the rush to scale up PMTCT programs and with the overly optimistic expectations that such programs alone would eliminate pediatric HIV, pediatric HIV treatment has lagged behind. PMTCT programs have raced ahead while pediatric HIV care suffers from weak and fragmented systems for case finding, antiretroviral treatment, and clinical follow-up. As a result, countless children become sick or die from HIV, often undiagnosed.
Pediatric HIV treatment will remain essential: even if the ambitious e-MTCT goal of reducing the number of new pediatric infections by 90% is reached, roughly 40,000 infants will continue to be infected each year (2). And while the numbers of infected children will diminish, children will continue to be born exposed to HIV (See HIV-exposed Infants paper in this series). Although preventing pediatric infections is the ideal, caring for these exposed and infected children is a practical and ethical necessity that has not been fully addressed (3).
In this paper, we review the history and development of today’s largely prevention-focused approach to pediatric HIV and consider changes which will bring a more assertive agenda to addressing the needs of infected children. Pediatric HIV diagnosis, care and treatment—a critical component of the global agenda - must receive the same attention and resources that PMTCT and adult care and treatment have received from researchers, donors and policymakers.
Background
The evolution of PMTCT interventions resulting in vertical transmission rates of 1% or less in the developed world demonstrates that MTCT elimination is possible (4). Moreover, such success has validated strategies that use antenatal care for identifying women with HIV; initiate appropriate antiretroviral (ARV) prophylaxis during pregnancy, labor and delivery; provide postpartum ARV prophylaxis for mother and/or child; and support safe infant feeding practices to prevent transmission through breastfeeding.
In LMICs, the evolution of PMTCT programming has been dynamic, with World Health Organization (WHO) guidelines changing 4 times in the last decade. PMTCT has evolved from an extremely time-limited intervention to one that is more proactive and effective, addressing the lifelong care and treatment needs of both the infant and the mother. WHO guidelines for prophylactic ARV regimens have progressed from single-dose nevirapine (NVP) to short course zidovudine (AZT); AZT-based “Option A”; to “Option B,” which initiates maternal triple-drug ART during pregnancy through breastfeeding; and now the novel “Option B+,” pioneered in Malawi which initiates all HIV-infected pregnant and breastfeeding women on lifelong ART irrespective of CD4 count or clinical stage (5). With each change, LMICs attempt, with the best intentions, to harmonize their guidelines with global recommendations. Over time, this has resulted in confusion, with success hampered by implementation challenges.
Option B+ offers numerous operational advantages over earlier approaches, essentially providing treatment to all pregnant and breastfeeding women, as the first large-scale example of “test-and-treat” (6). Yet even as prevention has evolved, most LMICs continue to experience significant levels of mother-to-child transmission because the success of PMTCT programming is predicated on women’s access to the PMTCT gateway via antenatal care and smooth, consistent implementation of programs. Even where encouraging increases to 50–60% antenatal care coverage for women are seen, these data often reflect only initial enrollment and initiation into PMTCT programs and certainly not completion of a multi-faceted PMTCT cascade. Indeed, a recent meta-analysis of the magnitude of lost to follow up in sub-Saharan Africa PMTCT programs was larger than previously thought. An estimated 49% of HIV-positive pregnant women are lost between ANC registration and delivery, while about 34% of HIV-exposed infants are lost to follow-up by 3 months and 45% of infants are lost after HIV testing (7).
For those women enrolled and retained in the PMTCT cascade, supply chain problems, stigma, limited quality and availability of medicines, test kits and other commodities undermine PMTCT effectiveness. Still other women—those who become infected during pregnancy and breastfeeding—will continue to be missed if we rely solely on the current prevention-first strategy and not expand strategies to identify such women (8).
History of PMTCT
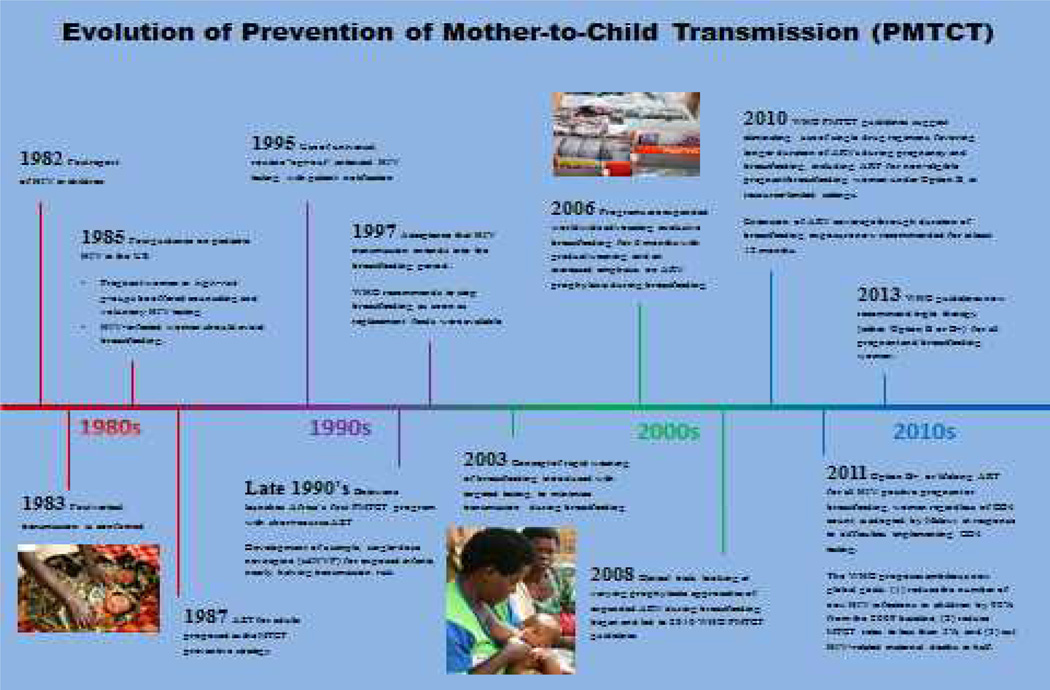
Recognition that we now have the tools to achieve elimination of vertical transmission of pediatric HIV merits a closer examination of where we started, where we are now, and where we are headed (Figure 1).
Figure 1.
Timeline of PMTCT Milestones
1980’s
The first case of pediatric HIV in the United States was reported in 1982 (9), 18 months after the first report of HIV in adults (10). By 1983, parental risk for HIV transmission to child was identified, confirming that most pediatric HIV infections occurred via transmission from mother-to-child and that one in four HIV-infected mothers transmitted HIV to their infants (11). No specific prevention interventions existed at that time other than identification of HIV status and, if infected, to avoid pregnancy. By 1985, the first guidance on pediatric HIV in the US recommended that pregnant women in high-risk groups be offered counseling and voluntary HIV testing, and that HIV-infected women should avoid breastfeeding (12). In 1987, the approval of AZT for adults was subsequently proposed as a MTCT preventive strategy. The 67% reduction in MTCT in the “076 AZT trial” was the first demonstration of “treatment as prevention”. Unfortunately, these interventions were too complex to administer (e.g., the protocol required both oral and intravenous AZT, the need for a sustainable infrastructure, and sustained attendance of women to ANC, which was not the norm) and therefore not feasible for delivery in LMICs at that time. Subsequent research focused on simpler options to achieve similar results (13).
1990’s
In February 1995, recommendations expanded from selective testing of high-risk, pregnant women to HIV education and voluntary routine testing for all pregnant women in the US, leading to the universal, routine “opt-out” antenatal HIV testing with patient notification. By the late 1990’s, enhanced affordability of AZT enabled Botswana to launch Africa’s first PMTCT program with short-course AZT while new research added a simple, single-dose nevirapine (sdNVP) for enhanced efficiency, nearly halving transmission risk (14). In 1997, the recommendation to stop breastfeeding as soon as replacement feeds were available was the first programmatic acknowledgement that HIV transmission extends into the breastfeeding period. This recommendation became problematic in LMICs, however, as adequate supplies of safe infant formula could not be assured. The realization that the risk of transmission must be balanced against optimal feeding practices became important in PMTCT programs, and growing consideration was given to infant survival beyond the risk of transmission (15,16).
2000’s
In 2000, a five-year NVP donation to developing countries expanded the availability of PMTCT for most LMICs. Additional research indicated that combining AZT and sdNVP was highly effective and capable of reducing MTCT to rates seen with triple ARV in resource-rich countries, which became the global standard for PMTCT. However, delays in program implementation, in part due to supply chain management problems of AZT and sdNVP, resulted in the majority of HIV-infected pregnant women in LMICs never receiving prophylaxis. Further, emerging concerns about rapidly developing resistance from sdNVP were becoming clear.
Recognition of breastfeeding as the cornerstone of infant survival in LMICs spurred research on safe breastfeeding interventions. (See Infant and Young Child Feeding Paper in this series). In 2003, the concept of rapid weaning of breastfeeding with targeted testing to minimize transmission risks during breastfeeding was introduced. In 2006, calls for exclusive breastfeeding for 6 months with gradual weaning and an increased emphasis on ARV prophylaxis during breastfeeding were expanded. While this represented a clear progression in thinking, correct programmatic implementation and messaging around this strategy was mired in confusion. Only when normative bodies began incorporating not only HIV, but child survival into consideration of reducing risks did this issue progress. By 2008, clinical trials examined varying prophylactic approaches of expanded ARV during breastfeeding and led to implementation of such programs by 2010 (5,17–23).
2010 and beyond
The 2010 WHO PMTCT guidelines recommended eliminating the use of single drug regimens, favoring longer duration of ARVs during pregnancy and breastfeeding, including ART for non-eligible pregnant/breastfeeding women under Option B, in resource-limited settings. The 2010 guidelines also recommended extending ARV coverage through duration of breastfeeding exposure, now recommended for at least 12 months. In 2011 “Option B+, or lifelong ART for all HIV positive pregnant/breastfeeding women regardless of CD4 count, was adopted by Malawi in response to difficulties implementing CD4 testing. Preliminary data from Malawi’s growing B+ programs reported a rapid increase in the number of pregnant and breastfeeding women on ART, with a 77% over 12 months retention rate (24).
In 2011, UNAIDS and PEPFAR proposed ambitious new global goals: (1) to reduce the number of new HIV infections in children by 90% from the 2009 baseline; and (2) to cut HIV-related maternal deaths in half. The 2013 WHO guidelines now recommend triple therapy (either Option B or B+) for all pregnant and breastfeeding women (25).
Challenges for the prevention-first strategy
Limitations in PMTCT
While the global response to care for infected and exposed children has matured, the existing pediatric HIV strategy remains focused on PMTCT at the expense of a comprehensive approach to identifying and treating pediatric infections (26). The evolution of PMTCT has been impressive but preventing vertical transmission still presents challenges under even the best of circumstances. The current PMTCT gateway is simply linked to presenting at an ANC clinic and getting tested as part of prenatal care, however, many women never access ANC and are therefore never initiated. Even when successfully initiated, the challenge of retaining women in care (27) and adherence to ART for the duration of pregnancy and breastfeeding remains a significant barrier (28–30).
Furthermore, some women will decline ART (31), and since no one should ever be pressured to initiate treatment, other options are needed. While guidelines are vague, having a response is essential. For this small proportion of women, it may be appropriate and ethical to continue, for instance Option A, although it may tax the existing health systems capacity in many LMICs. In addition, even if the current PMTCT cascade is implemented seamlessly, late incident infections, or women becoming infected following the initial HIV test are of concern, and new testing paradigms are needed to identify women who acquire HIV during pregnancy or breastfeeding (see EID and Treatment 2.0 papers in this series).
The struggle for pediatric care and treatment
Pediatric HIV care and treatment is not always available for HIV-exposed or infected children, nor is there always a clear segue for these infants into child-specific care systems. Child-oriented HIV care and treatment is neither routinely provided by maternal and child health (MCH) clinics or by adult HIV clinics, leaving children with HIV betwixt and between. Worse, in most countries only physicians can administer ART in children, stretching already-thin human resources for pediatric treatment. Finally, the prevention first approach fails to address the growing cohort of children born exposed but uninfected. Nor does it address the health consequences of ARV exposure in utero and drug resistance among those children born infected and exposed to maternal ARVs (See HIV-exposed infants paper in this series).
Adding to the burden for healthcare workers, case finding for children missed by PMTCT is problematic. DNA-PCR testing for infants often requires a long turnaround time, during which patients may be lost to follow-up or delay ART initiation (See EID paper in this series). For infected children, eligibility criteria have historically been more complicated than for adults, though the 2013 guidelines move us closer to a more streamlined, holistic response calling for universal treatment for all infected children under-5 (25). Still, with significant numbers of children missed by current PMTCT efforts, new approaches to case-finding must be introduced or those children will be lost (see Case finding paper in this series).
Even if a child is diagnosed and deemed ART eligible, other challenges remain. The 2010 guidelines first called for universal treatment of all HIV-infected children <2 (32), and the 2013 guidelines expanded this to all children under 5 (25) but in truth, neither is currently the norm. Currently recommended pediatric ARV regimens can be more complex to administer than those for adults. For example, the current preferred first line regimen for HIV-infected children <3 years of age requires at least two separate drug formulations, one of which requires a cold chain and a foul tasting syrup, presenting challenges from a feasibility and acceptability standpoint. And while the availability of a once daily fixed dose combination that can be used for both first line ART in adults and pregnant women is a major step forward, no such formulation exists for children; and when a regimen change is indicated, there are far fewer second line options for children compared with adults.
Quality clinical monitoring of infants and children on ART is also more challenging than for adults as health care workers are often less comfortable evaluating and treating infants and children. The success of PMTCT has resulted in an increasingly smaller population of HIV-infected children, so some providers, particularly those in more remote and isolated areas, may manage one or two HIV-infected children in a given year. It is no wonder then that management of pediatric HIV care and treatment are skills that few clinicians, physicians and nurses alike feel confident to deliver. Further, treatment occurs in the context of health systems which are particularly weak around pediatric HIV, leading to stockouts of pediatric ARV formulations, as well as other critical supplies such as HIV rapid test kits, DNA-PCR sample materials and reagents. (See the EID and Treatment 2.0 papers in this series). Finally, the shifting role of the utility of CD4 and a decreasing emphasis on immunologic monitoring requires consideration of how to implement viral load monitoring for children, when even PCR testing for infant diagnosis is still so problematic.
A public health approach to pediatric HIV
A “public health approach” providing pediatric care necessitates adapting health programs to the capacities of the health systems, akin to Treatment 2.0 for children (See Treatment 2.0 paper in this series), maximizing health impact using the resources available. Options B and B+ benefit from harmonization with adult treatment guidelines, allowing health systems to use the same regimen for PMTCT and first-line adult care. This harmonization should facilitate more effective supply chain management and continued decreases in ART costs.
By simplifying enrollment and diagnostic requirements (i.e., negating the need for an initial CD4 count for initiation), Option B+ succeeds in treating the mother while offering protection for the infant. Since Malawi rolled out B+, many countries have followed suit (Figure 2). Particularly with WHO guidelines now recommending adult treatment initiation at 500 cells/mm3—which should include a majority of women tested—many LMIC will likely find that CD4 tests required by Option B is not a worthwhile allocation of scarce resources. The evolution of PMTCT requires bringing the same public health approach to pediatric care and treatment. It should, at a minimum, address:
Figure 2.
Expansion of B+ throughout Africa(53)
1. Case finding
DNA-PCR testing is the gold standard for determining HIV status in infants but its limited availability and long turnaround time leads to missed opportunities and loss to follow-up (33). Expanded case finding efforts would help identify both infants and older children missed by PMTCT and should include broader opt-out testing strategies, including provider-initiated testing in under-5 clinics, testing of children of adults already in ART care, nutrition clinics, outpatient departments, community case treatment, and other environments (See Case Finding paper in this series). Data is clear that mortality is reduced significantly the earlier that ART is initiated in infants (around 7 weeks of age) as every 10-fold increase in viral load over time, raises the risk of illness and death 8.5 times among infected children(34). If we wait until 6–8 weeks to do the first PCR on these children, we risk losing these infected infants before their HIV results are even available (35,36).
Point-of-care (POC) DNA-PCR testing may be advantageous in some environments although will likely be subject to the complexities of training, human resources, supply chain and cost. Most importantly however, a qualitative option for POC DNA testing, in addition to a quantitative option for measuring HIV viral load, is a long-term goal so that the same POC test could be used for monitoring disease in those infected (quantitative) and confirming infection in infants (qualitative).
Regardless, diagnosing and enrolling children into care must become simpler and keeping them in care a more frequent occurrence, so new ideas are needed (see Case Finding and Linkage and Retention paper in this series). For instance, initiating HIV-exposed children on ART prior to a definitive laboratory diagnosis may seem disquieting, but the knowledge that many children are slipping through untreated to uncertain fates perhaps tips the risk-benefit in favor of presumptive treatment. Data from Tanzania indicates better mortality outcomes when WHO guidelines for presumptive HIV diagnosis (91% positive predictive value) are employed to evaluate symptomatic HIV-exposed infants with initiation of ART while awaiting definitive diagnosis by HIV DNA PCR (37).
2. Eligibility
Expanding eligibility by increasing the eligibility age for ART initiation based on antibody testing, prior to confirmation by DNA-PCR is worthy of consideration. The current WHO threshold for treatment initiation based on antibody testing is 18 months (38), although this cutoff varies by country and should be reconsidered based on the evidence (39). An earlier cutoff would facilitate immediate ART for more children. In Malawi, for example, antibody testing is sufficient for patients 12 months and older. A 9-month threshold for serologic testing, with confirmatory testing by DNA-PCR at that time might facilitate earlier treatment for previously unknown infected children and corresponds to a routine immunization visit, offering a more realistic opportunity for a test tied to an existing health visit, to document HIV-free survival in these children (See Laboratory paper in this series).
3. Regimen selection
For adult treatment and PMTCT, the shift to a single first-line regimen provides operational advantages for the supply chain and for providers, whose training can be simplified. A similar shift in pediatric treatment would also yield advantages but current pediatric ART regimens are fragmented across age groups in terms of eligibility and regimen choice, are expensive, require cold storage throughout the supply chain and are difficult to administer to children (See Costing and Treatment 2.0 papers in this series).
In the short term, country programs should make decisions to narrow preferred pediatric regimens. Fixed-dose combinations simplify the supply chain and are easier to administer. For instance, bypassing LPV/r for ART unexposed infants in favor of a triple-nucleoside plus NVP regimen, and omitting the lead-in dose which has complicated the regimen, is worthy of consideration. Breakthrough infections in infants previously exposed to NNRTIs, as well as with extended NVP during breastfeeding in settings not yet able to implement B/B+ still occur so while this would not negate the need for LPV/r based regimens altogether, it could provide a simpler option for a significant proportion of young children on treatment (40,41). Increasing evidence of the safety of tenofovir and efavirenz use during pregnancy has paved the way for Option B+ regimens to be harmonized with other adult first-line therapy. Development of newer pediatric fixed-dose formulations (e.g., TDF/3TC/EFV) will also facilitate greater harmonization, as would better tolerated and more potent NNRTI-sparing regimens that could mitigate adherence issues secondary to LPV/r intolerance. In addition, raltegravir and ritonavir-boosted darunavir have been used successfully among children in LMICs that have developed LPV/r-resistance (42) and the recent approval of dalutegravir for children >12 years is another promising development (43). These advances are collectively expanding the availability of newer approaches to ART therapy in children, and will certainly influence future guidelines for pediatric treatment.
4. Service delivery paradigm
In most countries, PMTCT is in the purview of antenatal/MCH clinics, while treatment of pediatric infection usually falls under care and treatment programs that are adult-focused, with very few specialized pediatric ART clinics. Integrating B+ into the existing infrastructure of ANC or MCH clinics or adult HIV clinics should be driven not just by necessity or opportunism, but through a clear philosophical shift towards an integrated approach to service delivery. The siloed, vertical approach for PMTCT is unsteady in the best of circumstances and does not work when prevention fails and children become infected. Indeed, pediatric HIV has been left vastly under-resourced compared to the suite of MCH initiatives (maternal health, child survival and immunization) and adult HIV treatment.
Wherever PMTCT and pediatric HIV are most effectively integrated should be a topic of discussion and operational research to identify models which provide an adequate environment for children. Each will offer distinct advantages and drawbacks. Where pediatric care is integrated with PMTCT, it creates an opportunity to further strengthen retention for all HIV-exposed children but its full integration remains elusive. Improved retention will facilitate definitive diagnoses in children and better monitoring for child survival and maternal health (See Retention paper in this series). In short, pediatric care, including PMTCT should be available at multiple points throughout health systems of LMIC and not limited solely to ANC.
Time to rethink the PMTCT paradigm?
Innovations in service delivery could potentially increase the number of women who successfully complete the PMTCT cascade, boosting the impact of PMTCT programs, and strengthen connections to care for HIV-exposed and -infected children. The 2013 WHO guidelines have accelerated ART and provision of care and treatment for adults and children into a new era. The recommendation to initiate all adult patients at CD4s below 500cells/mm3, pushes eligibility closer to universal ART, and it is likely that future guidelines will bring us closer to this aspirational goal. This convergence of prophylactic and therapeutic guidelines offers an opportunity to reconsider the PMTCT model in which women present for antenatal care and a cascade is initiated.
As this model, and PMTCT regimens in general, correctly evolved over time, the possibility exists that these functions may no longer be necessary in many settings. In most LMIC, PMTCT is a vertical and siloed system of care originally designed to determine eligibility for mono, then dual- or triple therapy. With the current treatment-centered models, the paradigm of PMTCT is shifting and the previous focus on determining eligibility is far less important. With the increasing availability of Option B+, the cascade is simplified, but to be seamless, it must simplify further and a primary emphasis on retaining women and their children in care must predominate. Initiation of the B/B+ approaches may not require the infrastructure inherent in previous iterations of PMTCT programming, and the time may be ripe for a more integrated approach.
Regardless of the specific approach, we must do a better job integrating PMTCT with other programs; some success has been achieved in this area but more needs to be done. But before such changes are made, resources directed at defining operational consequences and successes are needed. If such inquiry proves fruitful, the presumed cost savings resulting from increased efficiencies achieved through true integration can be directed towards treatment for more patients. Simplified guidelines may create greater flexibility in delivery systems, allowing for new approaches to integrated care for PMTCT, treatment for women, and treatment for children. The critical next question is whether the resources previously and correctly spent on determining eligibility should continue, or whether those resources should be reconfigured with a primary emphasis on retention along the treatment cascade in care for both infected mothers and their children.
The questions now are whether the care of HIV-infected pregnant women be shifted towards adult ART treatment centers within health centers where their HIV care is assured; or is it more feasible to have ART clinics with comprehensive care for mothers and babies, to ensure proper treatment for both; or, should HIV-infected pregnant women receive their care and treatment in ANC (and MCH clinics) with children’s care relegated to existing pediatric care. Alternatively, it may be time to expand family clinics, where all family members affected by HIV can receive follow-up care, prophylaxis and treatment. Although presently, most family clinics are adult clinics where children can also receive treatment but it may be appropriate to re-envision family clinics as places that have dedicated time and space in which the needs of the whole family are addressed. Primary care-oriented approaches to HIV care and treatment present ideal opportunities to integrate HIV care with existing services including SRH, MCH, IMCI, U5 clinics (44–48). In addition to providing incentives to seek care as a family, integrated family-centered approaches have strong impacts on pediatric case-finding and enrolment, as well as pediatric clinical outcomes, including cotrimoxazole coverage, ART adherence and retention in care (48). More research is urgently needed to determine the optimal service models for delivery of care.
Monitoring and evaluation are pivotal to assessing our current progress, shortfalls and comparing alternative approaches, but current M&E efforts often are not able to document the impact that programmers and policy makers need to advocate for dwindling resources. For PMTCT, this means generation of estimates of HIV-free survival for infants, and good health and survival for their mothers. Current systems don’t offer such data and largely document programmatic progress (i.e., number of women accessing ANC, the number of children receiving DNA-PCR testing), but only for the duration of the PMTCT program. Strategies, such as a recently completed PTMCT evaluation performed in Malawi that provided population-based estimates of vertical transmission rates through immunization clinic sero-surveillance are urgently needed(49).
Moving pediatric HIV care forward
While a thorough evaluation of Option B+ is ongoing, the global community is optimistic of its effectiveness. Expanding the “test and treat” paradigm requires continued advocacy to keep the focus on children as well as their mothers; those protected as a result of PMTCT efforts, and those who are currently slipping through the cracks. As a global community we can’t rest when 60% of HIV-infected pregnant women initiate PMTCT while not having a clear understanding of how many of those complete and when many other women and their children never realize the benefit of PMTCT.
We need to set higher national targets, advocate for expanded funding for PMTCT and pediatric care and treatment and expanded clinical guidelines and legislation allowing nurses and non-clinicians to prescribe ART. Family clinics should be defined by the child’s needs and not as another mechanism for adults to get treatment quickly without the lines. We need to determine the best way to deliver PMTCT services—to determine if the best way is beyond ANC clinics. In the global HIV response, integration is the new mantra. We need to ensure that our PMTCT efforts are integrated as well.
Modernizing pediatric HIV should occupy a greater share of attention and resources. While the ambitious Global MTCT Elimination Plan is inspiring and well-founded, it is unacceptable to ignore the children who will need HIV care (2). We argue for a dual strategy: an all-out push to prevent vertical transmission, complemented by a public health approach to pediatric care and treatment for those children for whom PMTCT foreseeably fails. We are optimistic that PMTCT efforts will someday render pediatric HIV a rarity; but until that day arrives, children living with HIV deserve the benefits of a health system designed to deliver efficient and effective care.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the US Agency for International Development, the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry, nor those of the US government.
References
- 1.Abrams EJ, Simonds RJ, Modi S, Rivadeneira E, Vaz P, Kankasa C, et al. PEPFAR scale-up of pediatric HIV services: innovations, achievements, and challenges. J Acquir Immune Defic Syndr. 2012 Aug 15;60(Suppl 3):S105–S112. doi: 10.1097/QAI.0b013e31825cf4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Countdown to Zero: Global Plan Towards The Elimination of New HIV Infections Amoung Children by 2015 and Keeping Their Mothers Alive. [Google Scholar]
- 3.Kellerman SE, Sugandhi N. Pediatric AIDS in the Elimination Agenda. PLoS Med. 2013 Aug 27;10(8):e1001503. doi: 10.1371/journal.pmed.1001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Linstow ML, Rosenfeldt V, Lebech AM, Storgaard M, Hornstrup T, Katzenstein TL, et al. Prevention of mother-to-child transmission of HIV in Denmark, 1994–2008. HIV Med. 2010 Aug;11(7):448–456. doi: 10.1111/j.1468-1293.2009.00811.x. [DOI] [PubMed] [Google Scholar]
- 5.Schouten EJ, Jahn A, Midiani D, Makombe SD, Mnthambala A, Chirwa Z, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. The Lancet. 2011 Jul;378(9787):282–284. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- 6.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. The Lancet. 3. 373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 7.Sibanda E, Weller I, Hakim J, Cowan F. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS. 2013 Sep 19; doi: 10.1097/QAD.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rollins N, Mahy M, Becquet R, Kuhn L, Creek T, Mofenson L. Estimates of peripartum and postnatal mother-to-child transmission probabilities of HIV for use in Spectrum and other population-based models. Sex Transm Infect. 2012 Dec 1;88(Suppl 2):i44–i51. doi: 10.1136/sextrans-2012-050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.jasonbardi. [cited 2013 Sep 20];First Pediatric Cases of HIV: Recalling 1981 [Internet]. UCSF AIDS 2012 Blog. Available from: http://aids2012.ucsf.edu/2012/07/22/first-pediatric-cases-of-hiv-recalling-1981/
- 10.Pneumocystis Pneumonia --- Los Angeles.htm. [Google Scholar]
- 11.Ammann AJ. Is there an acquired immune deficiency syndrome in infants and children? Pediatrics. 1983 Sep;72(3):430–432. [PubMed] [Google Scholar]
- 12.Current Trends Recommendations for Assisting in the Prevention of Perinatal Transmission of Human T-Lymphotropic Virus Type III Lymphadenopathy-Associated Virus and Acquired Immunodeficiency Syndrom.htm. [PubMed] [Google Scholar]
- 13.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. The Lancet. 1999 Sep 4;354(9181):795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 14.Eshleman SH, Becker-Pergola G, Deseyve M, Guay LA, Mracna M, Fleming T, et al. Impact of Human Immunodeficiency Virus Type 1 (HIV-1) Subtype on Women Receiving Single-Dose Nevirapine Prophylaxis to Prevent HIV-1 Vertical Transmission (HIV Network for Prevention Trials 012 Study) . J Infect Dis. 2001 Oct 1;184(7):914–917. doi: 10.1086/323153. [DOI] [PubMed] [Google Scholar]
- 15.Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet. 2000 Feb 5;355(9202):451–455. [PubMed] [Google Scholar]
- 16.Nicoll A, Killewo JZ, Mgone C. HIV and infant feeding practices: epidemiological implications for sub-Saharan African countries. AIDS. 1990 Jul;4(7):661–665. [PubMed] [Google Scholar]
- 17.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, et al. Maternal or Infant Antiretroviral Drugs to Reduce HIV-1 Transmission. New England Journal of Medicine. 2010;362(24):2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kesho Bora Study Group. de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011 Mar;11(3):171–180. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 19.Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, et al. Prevention of Mother-to-Child Transmission of HIV-1 Through Breastfeeding by Treating Mothers With Triple Antiretroviral Therapy in Dar es Salaam, Tanzania: The Mitra Plus Study. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2009 Nov;52(3):406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 20.Kumwenda NI, Hoover DR, Mofenson LM, Thigpen MC, Kafulafula G, Li Q, et al. Extended Antiretroviral Prophylaxis to Reduce Breast-Milk HIV-1 Transmission. New England Journal of Medicine. 2008;359(2):119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 21.Mofenson LM. Prevention in Neglected Subpopulations: Prevention of Mother-to-Child Transmission of HIV Infection. Clinical Infectious Diseases. 2010 May 15;50(s3):S130–S148. doi: 10.1086/651484. [DOI] [PubMed] [Google Scholar]
- 22.Taha TE, Kumwenda J, Cole SR, Hoover DR, Kafulafula G, Fowler MG, et al. Postnatal HIV-1 Transmission after Cessation of Infant Extended Antiretroviral Prophylaxis and Effect of Maternal Highly Active Antiretroviral Therapy. J Infect Dis. 2009 Nov 15;200(10):1490–1497. doi: 10.1086/644598. [DOI] [PubMed] [Google Scholar]
- 23.Thomas TK, Masaba R, Borkowf CB, Ndivo R, Zeh C, Misore A, et al. Triple-Antiretroviral Prophylaxis to Prevent Mother-To-Child HIV Transmission through Breastfeeding—The Kisumu Breastfeeding Study, Kenya: A Clinical Trial. PLoS Med. 2011 Mar 29;8(3):e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chimbwandira F, Mhango E, Midiani D, Makombe SD, Mwsansambo C, Njala J, et al. Impact of an Innovative Approach to Prevent Mother-to-Child Transmission of HIV-Malawi, July 2011-September 2012. MMWR. 2013;8(62):148–151. [PMC free article] [PubMed] [Google Scholar]
- 25.WHO | Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection [Internet] WHO; [cited 2013 Sep 20]. Available from: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html. [PubMed] [Google Scholar]
- 26.Barker PM, Mphatswe W, Rollins N. Antiretroviral Drugs in the Cupboard are Not Enough: The Impact of Health Systems’ Performance on Mother-to-Child Transmission of HIV. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011 Feb;56(2):e45–e48. doi: 10.1097/QAI.0b013e3181fdbf20. [DOI] [PubMed] [Google Scholar]
- 27.Braun M, Kabue MM, McCollum ED, Ahmed S, Kim M, Aertker L, et al. Inadequate Coordination of Maternal and Infant HIV Services Detrimentally Affects Early Infant Diagnosis Outcomes in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2011 Apr 15;56(5):e122–e128. doi: 10.1097/QAI.0b013e31820a7f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feinstein L, Dimomfu BL, Mupenda B, Duvall S, Chalachala JL, Edmonds A, et al. Antenatal and delivery services in Kinshasa, Democratic Republic of Congo: care-seeking and experiences reported by women in a household-based survey. Tropical Medicine & International Health. 2013;18(10):1211–1221. doi: 10.1111/tmi.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guliani H, Sepehri A, Serieux J. Determinants of prenatal care use: Evidence from 32 low-income countries across Asia, Sub-Saharan Africa and Latin America. Health Policy Plan. 2013 Jul 26;:czt045. doi: 10.1093/heapol/czt045. [DOI] [PubMed] [Google Scholar]
- 30.Finlayson K, Downe S. Why Do Women Not Use Antenatal Services in Low- and Middle-Income Countries? A Meta-Synthesis of Qualitative Studies. PLoS Med. 2013 Jan 22;10(1):e1001373. doi: 10.1371/journal.pmed.1001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Option B+: Understanding perspectives/experiences of women living with HIV in. [Google Scholar]
- 32.Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access: Recommendations for a Public Health Approach: 2010 Revision [Internet] Geneva: World Health Organization; 2010. [cited 2013 Oct 4]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK138576/ [PubMed] [Google Scholar]
- 33.Dube Q, Dow A, Chirambo C, Lebov J, Tenthani L, Moore M, et al. Implementing early infant diagnosis of HIV infection at the primary care level: experiences and challenges in Malawi. Bulletin of the World Health Organization. 2012 Sep;90(9):699–704. doi: 10.2471/BLT.11.100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorvaldsson O, Paintsil E, Northrup V, Andiman W. Cumulative Viremia-Copy Years Predicts Morbidity and Mortality in Perinatally HIV-Infected Children (IDWeek 2013) San Francisco, CA: 2013. [cited 2013 Oct 6]. Available from: https://idsa.confex.com/idsa/2013/webprogram/Paper41666.html. [Google Scholar]
- 35.Sherman G. Recognizing risk in HIV-exposed and HIV-infected infants and children. Diagnosing HIV infection in infants: are we there yet? Seattle. 2012 [Google Scholar]
- 36.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early Antiretroviral Therapy and Mortality among HIV-Infected Infants. New England Journal of Medicine. 2008;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shea1 S, Bradford1 J, Mgawe1 M, Chacky2 P, Kayabu1 A, Minde1 M, Sanders1 J, Bisimba3 J, Mwita1 L, Tolle1 M. Presumptive diagnosis of HIV infection in HIV-exposed infants and children under 18 months of age at a paediatric HIV centre in Tanzania´s lake zone [Internet] Kuala Lampur, Malaysia: 2013. [cited 2013 Sep 19]. Available from: http://pag.ias2013.org/Abstracts.aspx?AID=3019. [Google Scholar]
- 38.Read JS. Diagnosis of HIV-1 Infection in Children Younger Than 18 Months in the United States. Pediatrics. 2007 Dec 1;120(6):e1547–e1562. doi: 10.1542/peds.2007-2951. [DOI] [PubMed] [Google Scholar]
- 39.Rouzioux C, Costagliola D, Burgard M, Blanche S, Mayaux MJ, Griscelli C, et al. Estimated Timing of Mother-to-Child Human Immunodeficiency Virus Type 1 (HIV-1) Transmission by Use of a Markov Model. Am J Epidemiol. 1995 Dec 15;142(12):1330–1337. doi: 10.1093/oxfordjournals.aje.a117601. [DOI] [PubMed] [Google Scholar]
- 40.Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral Treatment for Children with Peripartum Nevirapine Exposure. New England Journal of Medicine. 2010 Oct 14;363(16):1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus Ritonavir-Boosted Lopinavir for HIV-Infected Children. New England Journal of Medicine. 2012;366(25):2380–2389. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirk BL, Gomila A, Matshaba M, Marape M, Joel DR, Anabwani G, et al. Early Outcomes of Darunavir- and/or Raltegravir-Based Antiretroviral Therapy in Children with Multidrug-Resistant HIV at a Pediatric Center in Botswana. J Int Assoc Provid AIDS Care. 2013 Mar 1;12(2):90–94. doi: 10.1177/1545109712463073. [DOI] [PubMed] [Google Scholar]
- 43. [cited 2013 Sep 20];FDA Approval: Dolutegravir for HIV-1 Infection [Internet] Available from: http://www.medscape.org/viewarticle/810717.
- 44.Myer L, Manuelli V, Abrams E, McIntyre J, Bekker L. Optimization of ART Initiation in Pregnancy through Linkage of Services vs Integration of ART into Antenatal Care. Atlanta, Georgia: 2013. [Google Scholar]
- 45.Tolle MA. A package of primary health care services for comprehensive family-centred HIV/AIDS care and treatment programs in low-income settings. Tropical Medicine & International Health. 2009;14(6):663–672. doi: 10.1111/j.1365-3156.2009.02282.x. [DOI] [PubMed] [Google Scholar]
- 46.Leeper SC, Montague BT, Friedman JF, Flanigan TP. Lessons learned from family-centred models of treatment for children living with HIV: current approaches and future directions. Journal of the International AIDS Society. 2010;13(Suppl 2):S3. doi: 10.1186/1758-2652-13-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeGennaro V, Zeitz P. Embracing a family-centred response to the HIV/AIDS epidemic for the elimination of pediatric AIDS. Global Public Health. 2009;4(4):386–401. doi: 10.1080/17441690802638725. [DOI] [PubMed] [Google Scholar]
- 48.Luyirika E, Towle MS, Achan J, Muhangi J, Senyimba C, Lule F, et al. Scaling Up Paediatric HIV Care with an Integrated, Family-Centred Approach: An Observational Case Study from Uganda. PLoS ONE. 2013 Aug 6;8(8):e69548. doi: 10.1371/journal.pone.0069548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinunu M, Schouten E, Wadonda N, Kajawo E, Eliya M, Moyo K, et al. Evaluating the Impact of Prevention of Mother-to-Child Transmission of HIV (PMTCT) in Malawi through Immunization Clinic-based Surveillance. Washington DC, USA: 2012. [cited 2013 Oct 6]. Available from: http://www.iasociety.org/Abstracts/A200746136.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pires R. Uganda, MSH Photo Fellowship 2013. 2013. [Google Scholar]
- 51.Pires R. Uganda, MSH Photo Fellowship 2013. [Google Scholar]
- 52.Zelman W. DRC and Ethiopia, MSH Photo Fellowship 2013. [Google Scholar]
- 53.Gieselman A, Phelps BR, Bachman G. PMTCT and Community: updates & PEPFAR perspectives: HIV/AIDS TWG. 2013. [Google Scholar]