Abstract
Although a number of recent studies have examined functional connectivity at rest, few have assessed differences between connectivity both during rest and across active task paradigms. Therefore, the question of whether cortical connectivity patterns remain stable or change with task engagement continues to be unaddressed. We collected multi-scan fMRI data on healthy controls (N = 53) and schizophrenia patients (N = 42) during rest and across paradigms arranged hierarchically by sensory load. We measured functional network connectivity among 45 non-artifactual distinct brain networks. Then, we applied a novel analysis to assess cross paradigm connectivity patterns applied to healthy controls and patients with schizophrenia. To detect these patterns, we fit a group by task full factorial ANOVA model to the group average functional network connectivity values. Our approach identified both stable (static effects) and state-based differences (dynamic effects) in brain connectivity providing a better understanding of how individuals’ reactions to simple sensory stimuli are conditioned by the context within which they are presented. Our findings suggest that not all group differences observed during rest are detectable in other cognitive states. In addition, the stable differences of heightened connectivity between multiple brain areas with thalamus across tasks underscore the importance of the thalamus as a gateway to sensory input and provide new insight into schizophrenia.
Keywords: connectivity, fMRI, schizophrenia, static & dynamic connectivity, thalamus, posterior temporal areas
1. Introduction
Functional connectivity is an approach that helps to assess the integrity of neural circuits by examining the covariance in activity across brain regions and can be assessed using a seed-based analysis approach or independent component analysis (ICA)(Calhoun and Adali, 2012; Erhardt et al., 2011a). Seed-based approaches assess the temporal correlation between a seed region and individual brain voxels (Cordes et al., 2002; Fox et al., 2005) whereas ICA is a data-driven approach which identifies spatially distinct but temporally related brain networks (Calhoun et al., 2001a).
To date, most studies have focused only on the analysis of functional connectivity during performance of a single task. Such an approach does not take advantage of the within-subject pattern of response which likely occurs across tasks, and which can be of benefit in a number of applications (Calhoun and Adali, 2009; Calhoun et al., 2006, 2008). However, one of the challenges associated with studying the resting state is that connectivity changes could reflect differences dependent on the cognitive states of the individual's brain, rather than consistent structural or functional-based differences in brain connectivity (Repovš and Barch, 2012). The results reported by previous studies limit our ability to understand whether observed cortical connectivity deficits in schizophrenia represent consistent characteristics rather than differences in cognitive state or task response.
Functional MRI (fMRI) results have been used to better understand the pathophysiology of schizophrenia, in particular to assess the disconnection hypothesis of schizophrenia (Friston and Frith, 1995; Woodward, 2012). These differences include a link between prefrontal cortex activation and vulnerability to psychosis (Fusar-Poli, 2007), reduced network activation during executive task performance (Minzenberg, 2009), and abnormal activation patterns in working memory tasks (Glahn et al., 2005). There has been growing interest in investigating the integrity of the neural circuits in schizophrenia that work together to support sensory, cognitive, and emotional processes (Calhoun et al., 2009;Liu et al., 2012; Yu et al., 2013).
Previous seed-based and ICA studies (Allen et al., 2011; Bassett et al., 2011; Cole et al., 2011; Garrity et al., 2007; Woodward et al., 2011) that examined functional connectivity in schizophrenia during rest found reduced connectivity for schizophrenia patients(SPs) within the default mode network, frontal network, cingulo-opercular network and cerebellar network. Several other studies (Anticevic et al., 2011; Diaconescu et al., 2011; Fornito et al., 2011) examined task-related functional connectivity in schizophrenia that largely focused on specific brain networks. These studies have also provided evidence for alterations in functional connectivity across a range of tasks where each task was studied separately. ICA provides measures of functional connectivity (within component coherence) as well as functional network connectivity (FNC) which measures changes in connectivity across networks (Jafri et al., 2008).
To gain a broader understanding of brain function and dysfunction as a dynamic process, we must examine how cognition changes under an established progression of task manipulations. Dynamic changes across tasks have been investigated with FNC and functional connectivity. Arbabshirani et al. (Arbabshirani et al., 2012) compared dynamic FNC changes across two tasks including resting state and an auditory oddball task in 28 healthy controls(HCs). Results of this study showed decreased FNC during task relative to rest among numerous network pairs. Also, Repovš et al. (Repovš and Barch, 2012) examined differences in functional connectivity during rest and a working memory task with increasing memory loads; SPs and their siblings had reduced connectivity between the frontal network and cingulo-opercular network with cerebellar network relative to HCs and their healthy siblings demonstrating network differences related to genetic risk. These group differences did not change as a function of task state or memory load. Although Arbabshirani et al. (Arbabshirani et al., 2012) compared FNC across two task in HC and Repovš et al.(Repovš and Barch, 2012) identified group differences in functional network across tasks, none of these studies evaluated changes in FNC across a hierarchy of tasks between the HC and SP groups.
The goal of this study is to determine whether cortical connectivity patterns remain stable or change across a hierarchy of sensory tasks. To the best of our knowledge there has been no study to investigate this issue in a variety of different FNC networks in a multi-task hierarchy with a relatively large number of subjects. This present study examined FNC across a hierarchy of sensory tasks with varying levels of sensory load. Data for each participant were gathered across multiple fMRI scanning sessions over the course of up to two months (1~2 months) with prospective randomization of task presentation and close monitoring of SPs to ensure clinical stability. Our goal was to track connectivity changes in SPs and HCs as sensory load increased. Using multiple tasks in addition to multiple conditions within a single task allows us to recognize that individuals’ reactions to sensory stimuli are conditioned by the circumstances in which such stimuli are presented and measurements at separate time points allows us to better assess state versus trait group differences. We sought to determine whether SPs and HCs showed significant FNC differences among brain regions across the task hierarchy by modeling the temporal dependency between functional networks derived from fMRI data. The tasks defined a natural hierarchy related to sensory load and included a rest task, two levels of auditory sensory gating, and two levels of multisensory perception with auditory and audio-visual stimuli. We remained skeptical of the notion that rest differences necessarily equate to characteristic differences in cognition between SPs relative to HCs. We hypothesized that data collected using a sensory load task hierarchy including rest will provide evidence of both stable (static effects) and state-based differences (dynamic effects).
2. Methods and Materials
2.1. Participants
This study combined existing data from 95 subjects. Informed consent was obtained from all subjects according to institutional guidelines at the University of New Mexico Human Research Protections Office, and all data were anonymized prior to group analysis. Inclusion criteria for patient selection included diagnosis of schizophrenia or schizoaffective disorder between 18 to 65 years of age. Each SP completed the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 2002a) for diagnostic confirmation and evaluation for co-morbidities. The imaging sessions (three cumulative hours) were completed in 1-2 sessions within up to two months (1~2 months) to reduce subject fatigue. SP had to demonstrate retrospective and prospective clinical stability to be included in this investigation. The Clinical Core (COBRE Stability Clinic) affiliated with this project determined retrospective stability from relevant psychiatric records documenting no change in symptomatology or type/dose of psychotropic medications occurred during the three months prior to the referral. The Clinical Core assessed prospective stability during three consecutive weekly visits and during each imaging assessment. Prospective stability was defined as no change in clinical symptoms > 2 points from the positive symptom items on the Positive and Negative Syndrome Scale (Kay SR et al., 1987). No score of “worse” or “much worse” on the Clinical Global Impression (Guy W, 1976) no suicidal or violent ideation, and no psychiatric or medical hospitalizations. The doses of antipsychotic medications were converted to olanzapine equivalents (Gardner et al., 2010). SPs with a history of neurological disorders including head trauma (loss of consciousness > 5 minutes), mental retardation, or history of active substance dependence or abuse (except for nicotine) within the past year were excluded. All SPs had a negative toxicology screen for drugs of abuse at the start of the study. HCs were recruited from the same geographic location and completed the Structured Clinical Interview for DSM-IV Axis I Disorders – Non-Patient Edition to rule out Axis I conditions (First et al., 2002a). SPs and HCs were matched on parental educational level (p<0.05), a less biased estimate of pre-morbid educational attainment potential (Saykin et al., 1991).We assessed symptom variability among each item of the PANSS positive symptom scores. Consistent with our inclusion criteria, the included subjects had minimal variance (< 2) associated with each symptom measure. Table-1 provides demographic characteristics of the participants and Table-2 lists the medications of the patient group.
Table 1.
Demographic and clinical variables for SPs and HCs.
| SP (SD) (n=42) | HC(SD) (n=53) | t or x2 (p-value) | |
|---|---|---|---|
| Demographics | |||
| Age | 37.38 ( 13.44) | 35.92 (11.97) | −0.56 (0.57) |
| Gender (M/F) | 33/9 | 39/14 | 0.32 (0.57) |
| Age on onset | 20.04 (8.03) | ||
| Illness duration | 16.22 (12.91) | ||
| Calgary | 3.25 (1.01) | ||
| Depression CGI | |||
| PCEL | |||
| CODEM-6 | 4.4 (2.11) | 4.75 (1.89) | 0.082(0.41) |
| CODEM-7 | 4.93 (2.24) | 4.97 (2.27) | 0.082(0.93) |
| PANSS | |||
| Positive | 14.35 (5.27) | ||
| Negative | 13.95 (5.28) | ||
| General | 28.71 (9.63) | ||
| Medications | |||
| OE(mg/day) | 16.78 (12.95) |
Abbreviations: PANSS= Positive and Negative Syndrome Scale. CGI = Clinical Global Impression. PCEL: Primary caregiver education level. CODEM-6: Highest Level of Education for Primary Caretaker until 18 years old. CODEM-7: Highest Level of Education for Secondary Caretaker until subject was 18 years old. Educational levels as follows 1: grade 6 or less, 2: grade 7-12, 3: graduated high school, 4: part college, 5: graduated 2 year college, 6: graduated 4 year college, 7: graduate or professional school, 8: completed graduate or professional school
Table-2.
Medication list for the patient group.
| Antipsychotics* | Dosage Range** |
|---|---|
| Aripiprazole (n = 3) | 10 - 15 mg |
| Clozapine (n = 4) | 50 - 400 mg |
| Fluphenazine (oral, n = 1) | 10 mg |
| Haloperidol (oral, n = 1) | 5 mg |
| Haloperidol decanoate (n = 2) | 50 mg |
| Olanzapine (n = 1) | 20 mg |
| Perphenazine (n = 1) | 8 mg |
| Quetiapine (n = 1) | 200 - 800 mg |
| Risperidone (oral, n =6) | 1 - 4 mg |
| Risperidoneconsta (n =6) | 12.5 - 50 mg |
| Thiothixene (n = 1) | 60 mg |
| Ziprasidone (n = 1) | 160 mg |
Eight of the 28 patients were treated with multiple antipsychotics. This table lists either the long acting injection or the antipsychotic with the higher olanzapine equivalents.
mg/day or dose of long acting injection
2.2. Task Hierarchy
The tasks represented cognitive paradigms that were analyzed separately (Mayer AR et al., 2012; Stone DB et al., 2011). Each task represented a different cognitive demand: resting state, pre-attentional sensory processing and multisensory processing. These studies were embedded in a larger study that explicitly proposed that SP would have deficits at multiple levels including pre-attentive sensory processing, integration of sensory information across modalities and impaired working memory performance. For the present investigation, we arranged the sensory tasks into five levels according to the amount of sensori-motor processing required by participants during the task. A subset of the subjects (SPs:22/42, HCs:23/53) were reported previously in Mayer et al. (Mayer et al., 2012) based on the original hypotheses of the sensory gating response. The Stone et al. (Stone et al., 2011) paper describes the paradigm but reports on event related potential results and does not include the fMRI results presented here.
Resting state fMRI: On the first level, participants performed a simple rest task. Subjects were instructed to keep their eyes open during the scan and stare passively at a central fixation cross, as this is suggested to facilitate network delineation compared to eyes-closed conditions (Mayer et al., 2012).
Sensory gating: Tasks on the second and third levels were variants of the paired click paradigm for testing sensory gating (Van et al., 2010). On the second level, participants were presented with a 5ms tone at either 2 kHz or 3 kHz. On the third level, participants were presented with paired tones - either identical 2 kHz tones or a 2 kHz tone followed by a 3 kHz tone. Both of these tasks were passive in that a response was not required.
Multi-modal sensory integration: Tasks on the fourth and fifth levels were designed to test multisensory integration and employed a simple forced choice behavioral task (Stone et al., 2011). A perspective digital drawing was used as a visual background with a fixation point, and participants were instructed to maintain fixation throughout the task. On the fourth level, an auditory stimulus (500 Hz tone) was presented to participants at two different volumes, 80dB to simulate a sound near the participant (NEAR) and 64dB to simulate it being farther away (FAR). On the fifth level, the above auditory stimulus was presented synchronously with a visual stimulus - an image of a soccer ball appeared in one of two possible positions (NEAR or FAR) in the participant's lower visual field with size and position consistent with the perspective drawing. The NEAR visual stimulus was presented in the participant's peripheral visual field and the FAR stimulus was presented closer to fixation in central visual field. Participants underwent fMRI scanning while deciding whether the stimuli presented were NEAR or FAR with a button press. Participants all performed the task at a high level of performance more than 88% percent correct in both groups across task conditions.
Since data acquisition across the tasks takes 3 hours, fMRI data collection occurred across 1-2 scanning sessions completed within up to two months. During this time participants underwent clinical, functional, and neuropsychological assessment as well as magnetoencephalography data acquisition across 1-3 visits. As mentioned above, a brief clinical stability questionnaire was obtained at each study visit to ensure stability across the multiple study visits. Task order for the MRI scan sessions was randomized and there is no significant group differences in the task randomization at p<0.05 level.
2.3. Data Acquisition
All images were collected on a single 3-Tesla Siemens Trio scanner with a 12-channel radio frequency coil. High resolution T1-weighted structural images were acquired with a five-echo MPRAGE sequence with TE = 1.64, 3.5, 5.36, 7.22, 9.08 ms, TR = 2.53 s, TI = 1.2 s, flip angle = 7°, number of excitations = 1, slice thickness = 1 mm, field of view = 256 mm, resolution = 256×256. T2*-weighted functional images were acquired using a gradient-echo EPI sequence with TE = 29 ms, TR = 2 s, flip angle = 75°, slice thickness = 3.5 mm, slice gap = 1.05 mm, field of view 240 mm, matrix size = 64×64, voxel size = 3.75 mm×3.75 mm×4.55 mm. Resting state scans consisted of 149 volumes. Tasks on the second (19 trials/condition) and third levels (20 trials/condition) acquired 111 volumes and tasks on the fourth (17trials/condition) and fifth levels (17 trials/condition) acquired 181 volumes per run.
2.4. Data Preprocessing
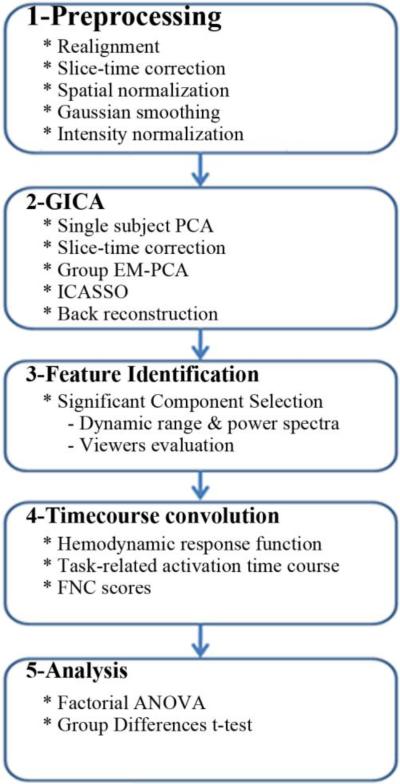
For preprocessing the MATLAB-based (www.mathworks.com) SPM-5 toolbox (www.fil.ion.ucl.ac.uk/spm/software/spm5) was used. To remove T1 equilibration effects, the first four volumes were discarded. Next, the INRIalign algorithm (Freire L et al., 2002) was used to realign the images, and slice-timing correction was applied using the middle slice as the reference frame in the functional data pipeline. The data was then spatially normalized to the standard Montreal Neurological Institute space (Friston et al., 1995) using a nonlinear (affine + low frequency direct cosine transform basis functions) registration, resampled to 3 mm × 3 mm × 3 mm voxels, and smoothed using a Gaussian kernel (FWHM=5mm) with a full-width at half-maximum of 10 mm. The preprocessed time series data was scaled to a mean of 100. This intensity normalization improves the test-retest reliability of the Group Independent Component Analysis (GICA)(Allen et al., 2011). See Fig. 1, step 1.
Fig.1.
Schematic of the analysis pipeline
2.5. Group Independent Component Analysis (GICA)
We used the GIFT Toolbox (http://mialab.mrn.org/software/gift/) and infomax algorithm (Bell and Sejnowski, 1995) for GICA. We performed a subject-specific data reduction principle component analysis retaining 100 principal components (PC) using a standard economy-size decomposition (Allen et al., 2011). The relatively large number of subject-specific PCs has been shown to stabilize subsequent back-reconstruction (Erhardt et al., 2011b). Then reduced data from all subjects and all sessions were concatenated together and put through another reduction step. To use memory more efficiently, further group data reduction was performed using an expectation maximization (EM) principle component analysis algorithm (Roweis, 1998) and 75 PCs were retained.
We used a relatively high model order ICA (number of components, C = 75), since such models yield refined components that correspond to known anatomical and functional segmentation (Abou-Elseoud et al., 2010; Kiviniemi et al., 2009). In order to estimate the reliability of the decomposition (Himberg et al., 2004), the Infomax ICA algorithm was applied repeatedly in Icasso (http://research.ics.aalto.fi/ica/icasso/) and resulting components were clustered.
The ICA results, once estimated and fixed, can be considered as a set of weighted seed maps (Joel SE et al., 2011). The ICA algorithm is trying to identify maximally independent sets of maps (which can overlap) each of which are represented by a strongly coherent (correlated) time-course. In the case of a distributed set of regions, there are multiple locations which are highly correlated to one another, and thus can be considered a node in this sense. Indeed, this is a major strength of multivariate approaches like ICA, as one knows that all the voxels with strong weights in a given component are highly correlated, and thus it makes sense to consider them a node and use FNC to evaluate the inter-relationship among these nodes. In contrast, for a seed-based approach, one knows the correlation to the seed region, but the correlation between any two voxels which are correlated to the seed may not be correlated with one another (Erhardt EB et al., 2011a). Thus, biologically, we would argue it is more interpretable to work with ICA-defined regions than it does seed-derived regions. Another benefit to the ICA approach is the artifact components have some overlap, and this provides spatial filtering or ‘cleaning’ of the remaining variance which can improve our ability to, e.g. classify groups based on the results (Erhardt EB et al., 2011a).
All rest and task data were analyzed in one group ICA instead of separate ICAs so that a tighter comparison between rest and tasks could be performed without introducing additional variability from the required matching of components if separate ICA analyses were performed. It has been shown in multiple previous papers that group ICA characterizes individual variation such as might occur across sessions quite well (Allen EA et al., 2011; Calhoun VD and Adali T, 2012; Erhardt EB et al., 2011b). Since the ICA model constrains the fluctuations of each voxel in a given component to have the same time course, each ICA component can be considered a temporally coherent network (Erhardt EB et al., 2011a). Next we calculate the within session cross-correlation among ICA timecourses (called FNC) and subsequently model the cross-session effects as described in the paper. Therefore, comparing the time-courses of different components in the resting state and during the tasks provides insights into the change in dynamics across the task hierarchy between the independent component networks. See Fig. 1, step 2.
2.6. Feature Identification
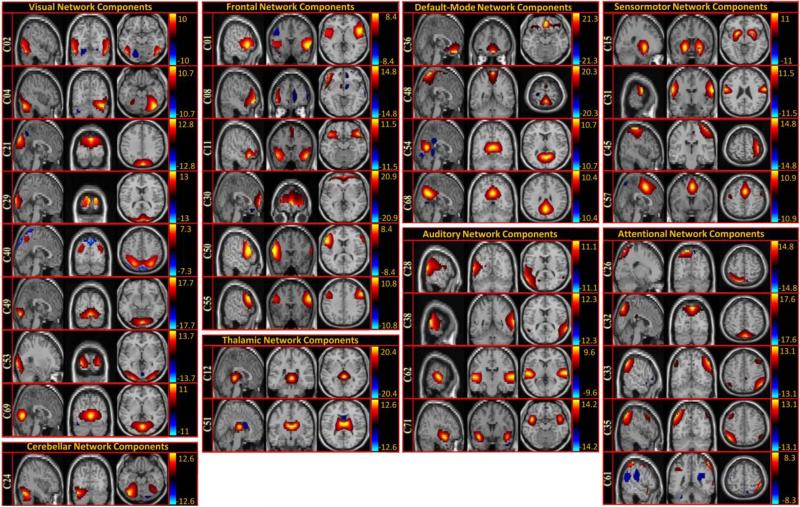
We used a two-step process to identify non-artifactual components that contain features associated with resting state networks and task performance on a sensory task (Robinson et al., 2009). See Fig. 1, step 3. In the first step we examined the power spectra with two criteria in mind: dynamic range and low frequency/high frequency ratio. Dynamic range refers to the difference between the peak power and minimum power at frequencies to the right of the peak in the power spectra. Low frequency to high frequency power ratio is the ratio of the integral of spectral power below 0.10 Hz to the integral of power between 0.15 and 0.25 Hz (Allen et al., 2011). For the second step, three expert reviewers evaluated the components for functional relevance. In this evaluation, if a component exhibited peak activation in gray matter, low spatial overlap with known vascular, ventricular, motion, and susceptibility artifacts, and time courses dominated by low frequency fluctuations (Cordes et al., 2000), it was classified as a non-artifactual component. At the end of the evaluation, the components were separated into two broad classes: artifactual and non-artifactual components. Of the 75 components returned by the GICA, 45 were identified as non-artifactual components. See Fig-2.
Fig-2.
Maps of the components identified as non-artifactual in static FNC or dynamic FNC analysis: Of the 75 components returned by the GICA, 45 were identified as non-artifactual components. Only 34 of these non-artifactual components showed static FNC or dynamic FNC effects. 34 non-artifactual components are divided into groups based on their anatomical and functional properties and include visual network, thalamic network, cerebellar network, frontal network, attentional network, default mode network, sensory motor network, and Auditory networks.
2.7. Timecourse convolution
To get FNC scores during the resting state scan, time courses (TC) of separate components in the resting state were correlated with one another by using a cosine similarity measure that can be computed as follows:
Where TCCx and TCCy are time courses of two separate components and n is length of time courses. For other tasks in the analysis, we isolated activations related to particular tasks within an fMRI scanning session. The design matrix denoting stimulus presentation (when the stimuli occur for each task) during fMRI scanning sessions were convolved with a hemodynamic response function. The resulting function was normalized on a zero-to-one scale. These functions were termed hemodynamic predictor functions. A hemodynamic predictor function models the expected pattern of activation associated with a task and can be thought of as a weight expressing the degree to which component activation at a particular time would associate with a given task. Each task's hemodynamic predictor function was then multiplied with the component time courses from the GICA to yield a task-related component time course. A task-related component time course indicates the activation of a particular GICA component solely as it pertains to a given task performed in the fMRI scanner, and is zero where the task does not influence activity. Task-related component time courses for separate components within a task were then correlated with one another exclusively over non-zero areas of the hemodynamic predictor function using a cosine similarity measure to yield task-related FNC scores for pairs of components. See Fig. 1, step 4. The statistical tests described below were performed on these FNC scores.
2.8. Data Structure
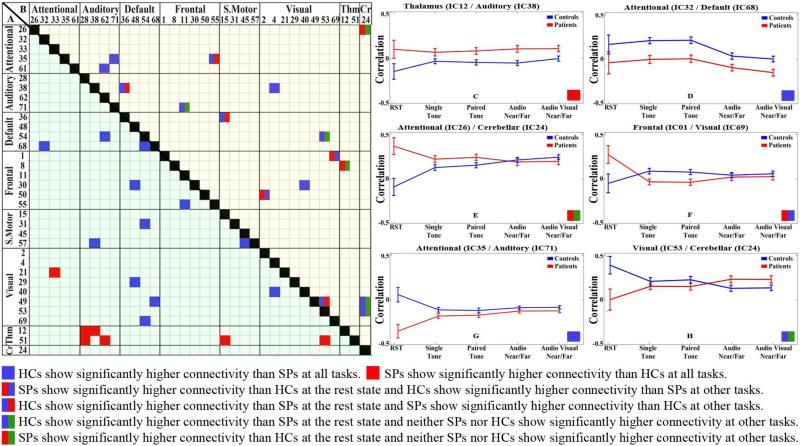
For each pair of components identified by the GICA, a vector of FNC results was created with values for every task performed by every subject. This allowed us to address questions about FNC effects between SPs and HCs at distinct levels of the hierarchy. We evaluated effects in two FNC categories. First, static FNC component pairs (see Fig. 3A) showed consistency between SP and HC groups across levels of the task hierarchy (see Fig. 3C). Second, dynamic FNC showed differences in connectivity between SP and HC groups at different levels of the task hierarchy (see Fig. 3D). By using these two categories, we were able to identify static and dynamic group differences for SPs and HCs across task.
Fig-3.
A) static FNC matrix(lower part). Pairwise correlations of component pairs showed static FNC effects at the α> 0.001 level. B) dynamic FNC matrix(upper part). Pairwise correlations of component pairs showed dynamic FNC effects at the α≤ 0.001 level. C-D) Samples for static FNC; thalamus (IC12) / auditory networks (IC38), attentional network (IC32) / default mode network (IC68).E-F-G-H) Sample for dynamic FNC; attentional network (IC26) / cerebellar network (IC24), frontal network (IC01) / visual network (IC69), attentional network (IC35) / auditory network (IC71), visual network (IC35) /cerebellar network(IC24)
2.9. Data Analysis
We maintained an interest in where we observed static and dynamic connectivity effects, and how this analysis approach may provide insights about current findings on connectivity in schizophrenia. To detect differential (state-dependent) connectivity effects, we fit a 2x5 (Group x Task) full factorial ANOVA model to the group average FNC values. To assess medication effects, we repeated the analysis for significant component pairs from the static FNC and dynamic FNC effects with a median split of the olanzapine equivalents. See Fig. 1, step 5. With 45 non-artifactual components in our data set, 990 pairwise comparisons were performed.
We examined component pairs that showed static FNC offset between groups throughout the hierarchy of tasks by using a factorial ANOVA model at α > 0.001 level. The retained pairs demonstrated a main effect of group but did not show signs of a diagnosis-by-task interaction. We then averaged FNC values across tasks to control for individual subject effects and performed two-sample t-tests to identify those component pairs that showed significant static FNC effects (p<0.001) (see Fig-3C).
Decisions about whether a particular component pair showed significant dynamic FNC effects was based on an F-test of the model including the task-by-diagnosis interaction term (factorial ANOVA model at α ≤ 0.001 level)(see Figure-3D). We defined the results as a significant diagnosis-by-task interaction. According to our hierarchy of tasks these component pairs have FNC differences between SPs and HCs as a function of task level.
To determine the proportion of false discoveries in the set of declared discoveries, we used a method as described in Soric et al. (Soric, 1989). For n number of experiments with r discoveries, α is the probability of wrong null-hypothesis rejections where r/n> α and for r rejections of null hypotheses the proportion of fallacies Q has least upper bound: Qmax= (n/r-1)α /(1-α)<1. In our study, Qmax (dynamic FNC) = 0.06 and Qmax(static FNC) = 0.05. Based on this analysis, at least 94% of dynamic FNC and 95% of static FNC discoveries are true.
3. Results
3.1. Static Functional Network Connectivity Effects
We obtained 18 significant pairs (see Fig-3A lower-left portion of the connectivity image) that showed static FNC effects. The significant static effects with a main effect of group and no interaction between group and task are shown in Fig-3C, D denoted by the solid blue or red squares. We present our component pair results by first specifying the anatomic representation followed by the independent component(IC) number.
Our study found higher static FNC in SP relative to HC (solid red squares) between the following thalamic network pairs (see solid red blocks in Fig. 3A): thalamus (IC12) / auditory networks (IC28), thalamus (IC51) / auditory networks (IC28), thalamus (IC12) / auditory networks (IC38), and the thalamus (IC51) / auditory networks (IC62), thalamus (IC51) / sensory motor network (putamen)(IC15) and the thalamus(IC51) / visual network (extrastriate cortex)(IC53). The thalamic FNC are the only network pairs that show increased static FNC in SP relative to HC with only one exception, attentional network (IC33) / visual network (IC21).
The remaining static FNC results describe higher connectivity in HC relative to SP (solid blue squares). These static FNC effects were observed in the default mode network, in particular, connectivity relating to the posterior cingulate cortex (IC54) / cingulate cortex (IC68). While we did not find overall static FNC patterns governing the interaction of the default mode network with other component networks, we found a number of individual brain regions showing stable differences between SPs and HCs. The posterior cingulate cortex showed reduced connectivity for SPs in the following pairs: default mode network(IC54) / auditory networks (IC62), dorsal precuneus, default mode network(IC68) /attentional network (IC32), post-central gyrus; default mode network (IC54) / sensory motor network (IC31), default mode network (IC54) / visual network (IC69) and lingual gyrus; default mode network(IC68) / visual network (IC49). We also observed static FNC effects in other parts of the default mode network (IC48) / frontal network (IC30) and default mode network (IC48) / visual network (IC29).
Other component pairs showing static FNC effects at the α> 0.001 level are summarized in Fig. 3A.
3.2. Dynamic Functional Network Connectivity Effects
We obtained 16 significant pairs (see Fig-3B) that showed dynamic FNC effects. Component pairs that yielded a significant dynamic FNC effect showed different patterns of interaction (and often negligible FNC differences) across the active tasks (See Fig. 3D, F, G, H) and resting state measures also showed considerable differences.
We found significant dynamic FNC in the thalamus, posterior cingulate cortex, and posterior temporal area for the following pairs: thalamus (IC12) / frontal network (IC08); default mode network (cingulate cortex) (IC54) / visual network (extrastriate cortex) (IC53); auditory networks (posterior temporal area) (IC38) /default mode network (orbito frontal cortex) (IC36); and auditory networks (posterior temporal area) (IC38) /visual network (extrastriate cortex)(IC04). SPs showed significantly higher FNC than HCs in resting state on the thalamus (IC12) and frontal network (IC08) pair, while HCs FNC was higher with the task-related paradigms.
Unlike the observed static FNC effects, SPs had higher FNC than HCs in the resting state relative to task activated conditions in the following pairs of networks: thalamus (IC12) / frontal network (IC08), attentional network (IC26) / cerebellar network (IC24), frontal network (IC01) / visual network (IC69) and frontal network (Broca's area) (IC50) / visual network (IC02).
Some visual network components had the opposite pattern. For example, two visual network components: visual networks (extrastriate cortex) (IC53) / visual networks (IC49) which is located close to the calcarine fissure. Also, they showed significant dynamic FNC effects with the cerebellar network (IC24)/ visual networks (IC49) and cerebellar network (IC24)/ visual networks (IC53). Both showed positive FNC among SPs and HCs throughout the hierarchy, although HCs showed more FNC than SPs at resting state. Other component pairs showing dynamic FNC effects at the α≤ 0.001 level are summarized in Fig. 3B.
We, also, used a paired t-test to check the effect of antipsychotic dose (olanzapine equivalents), PANSS score, age and gender to the FNC values at p<0.05 and did not detect any significant effect on the significant static or dynamic effects reported in the study.
4. Discussion
The method used in this study identifies static and dynamic differences in cortical networks by determining whether SPs and HCs showed significant FNC differences among brain regions across the task hierarchy. To detect these differences, we fit a group by task full factorial ANOVA model to the group average FNC values. This method provides a better approach of recognizing the reactions of SPs and HCs to simple sensory stimuli that are conditioned by the circumstances. The present study highlights a number of FNC differences between SPs and HCs, indicative of both stable abnormalities (static FNC) and task-dependent effects (dynamic FNC). Static FNC differences in SPs impacted multiple networks involving the thalamus, posterior cingulate, and posterior temporal cortices. Dynamic FNC differences in SPs affected similar anatomic locations, but the affected number of components was less.
Among the regions where we observed significant static FNC effects, the thalamus and posterior temporal area (centered on posterior superior temporal gyrus but extending into middle temporal gyrus) deserve particular mention (see cluster of red boxes denoting Thalamus/Auditory FNC in Fig. 3A). The temporal lobe has long been implicated in schizophrenia including multiple studies demonstrating auditory-related deficits (Hamm et al., 2011; Meda et al., 2012) in addition to structural changes in posterior temporal regions (Cullen et al., 2012; Mathiak et al., 2011). This is consistent with differences identified in the posterior temporal lobe region in response to unisensory auditory stimuli during the multisensory integration paradigm; only the response to multisensory stimuli showed enhanced responses in SP compared to HC as reported in Stone et al. (Stone DB et al., 2011). These group differences provide motivation for understanding how connectivity patterns differ in response to these different stimulus conditions. Interestingly, no significant group differences were reported in Mayer et al. (Mayer et al., 2012) for sensory gating, indicating that the current analysis approach may provide enhanced sensitivity to identify group differences through the comparison across task thereby identifying both stable abnormalities (static FNC) and task-dependent effects (dynamic FNC).
Furthermore, thalamic abnormalities in schizophrenia are well-documented (Goff and Coyle, 2001; Shenton et al., 2001; Woodward, 2012). Recent research suggests that these regions play a role related to production of auditory verbal hallucinations (Hoffman and Hampson, 2012) along with the putamen, which also showed a significant static thalamic connectivity effect.
The present study is consistent with previous results suggesting that in addition to cortical deficits, the thalamus plays an important role in the cortical abnormalities of schizophrenia. We found static FNC effects between the thalamus and posterior temporal area, the putamen, and extrastriate cortex. In all cases, SPs had significantly higher connectivity than HCs, across all levels of the sensory load hierarchy.
In this study, we used a high noise model of schizophrenia instead of a direct measure of brain signal variability. The use of the high noise model (Daniel PK et al., 2006; Rolls ET et al., 2008; Tononi G and Edelman GM, 2000;Susan WG et al., 2009) here is referring to the dampened stimulus evoked responses, similar to the previous descriptions that indicate default mode activity is not sufficiently dampened during stimulus presentation in SZ relative to HC. The group differences in static FNC may represent an inability for brain connectivity to be modulated by external stimuli consistent with the high noise model of schizophrenia (see Fig. 3D). Interestingly, these areas are not only a part of the default mode network they may also represent broader group differences signifying a lack of flexibility in SP. Our data supports the conclusion that these effects, along with other differences involving the posterior cingulate and the default mode network, represent stable characteristics underlying schizophrenia.
We also found significant static FNC differences involving elements of the default mode network, particularly the posterior cingulate. This provides support for the hypothesis (Dosenbach et al., 2007; Garrity et al., 2007; Jafri et al., 2008) that aberrant default mode network connectivity in resting state reflects the existence of a stable static FNC characteristic that does not depend on cognitive state or task response in a particular default mode network sub-region. Future studies can also focus on changes in the dynamics of functional connectivity, a new area of investigation which provides interesting results in the healthy brain (Allen et al., 2012).
Our study identified dynamic FNC group differences across the active tasks between both the Fronto-Parietal networks (frontal network (IC08)) / cingulo-opercular network (thalamus (IC12) and Fronto-Parietal networks (attentional network (IC26)) / cerebellar network (IC24). These results show differences in connectivity in a similar network as the previous study by Repovš et al. (Repovš G and Barch DM, 2012) showing group differences in connectivity with increasing working memory load between the Fronto-Parietal networks. Furthermore, connectivity patterns varied across components including in some cases differences in connectivity with increasing sensory load. While this study did not employ a working memory paradigm, the similar pattern of dynamic connectivity changes suggests that the dynamic connectivity patterns form a more general pattern across sensory and cognitive paradigms. In addition, we also found other dynamic FNC effects which are summarized in Fig. 3B. Although, 990 comparisons limit our ability to fully describe the results, we feel this approach provides a novel method for assessing trait-based and state-based differences between clinical groups.
The majority of the previous studies that examined functional connectivity in schizophrenia during rest, found reduced connectivity for SPs while some other studies found stronger connectivity during the performance of the task (Harrison et al., 2008; Shirer et al., 2012). Most of these studies were limited to a few preselected brain regions using a seed-based approach. In this study, we used an ICA approach (FNC) to extract all measurable non-artifactual brain networks (Jafri et al., 2008). The results of this study support the previous studies described above by identifying group differences in commonly reported networks including: the attentional network, auditory networks, default mode network, frontal network, sensory motor network, visual network, thalamus and cerebellar network. Also, our results provide additional information about how each of these networks responds to different sensory loads through testing both static FNC and dynamic FNC changes. Our study extends the previous results by identifying group differences in 18 static FNC and 16 dynamic FNC in 8 different networks in a multi task hierarchy with a large number of subjects.
There are likely some spatial differences in the networks across tasks. For ICA analyses, we have two options. The first option is using separate ICA analyses for each task which then requires component matching to make any statements regarding task hierarchy. This is a time consuming and error prone process (especially for large numbers of subjects) which is less robust to noise (Du, Y. et al., 2014). The second option is incorporating all rest and task data in one group ICA which eliminates the need to match components across ICA analyses due to a common analytic model and thereby allows for tighter comparisons between rest and tasks. It has been shown in multiple previous papers that group ICA characterizes individual variation such as might occur across sessions quite well (Allen EA et al., 2011; Calhoun VD and Adali T, 2012; Erhardt EB et al., 2011b). Next we calculate the within subject/within session (task) cross-correlation among ICA timecourses (called FNC) and subsequently model the cross-session effects as described in the paper. Such an approach thus allows for each component to have a distinct timecourse for each task/subject.
4.1. Limitations and Future Work
We consider the current results a first step in developing more sophisticated models to map hierarchical patterns across different brain regions to better understand how information content influences brain function at all levels of cortical functioning. Furthermore, the task hierarchy defined in the current study was based on a study designed to assess function across the sensory to cognitive hierarchy by sampling differences at pre-attentional sensory levels, multisensory integration with motor response, and working memory performance. In the current study we limited the analysis to the sensory tasks based in part on the recent results linking sensory deficits to cognitive impairments in schizophrenia. Furthermore, the current design attempts to systematically assess the perceptual/arousal state of participants which is included as a sub-domain of the Research Domain Criteria established to recognize the variability within diagnostic categories and the similarities across diagnostic categories. Additional task hierarchies are likely to yield different results within the dynamic FNC framework. In this section we discuss limitations of the current study and future work.
One challenge with performing a multi-task analysis is to determine the expected hierarchal relationship between these tasks. Here, we chose the two simplest assumptions: static versus dynamic along the preselected hierarchical arrangement. There are a number of possible reasons that this assumption may fail with the current dataset, including the following examples: (a) levels 4 and 5 require a response, whereas levels 2 and 3 do not. So from the broader cognitive perspective, levels 4 and 5 clearly require higher cognitive load than levels 2 and 3 suggesting that higher order brain areas may follow a relationship across the established hierarchical framework. (b) Similarly, the change from level 4 to 5 represents an addition of a visual stimulus with presentation of the exact same auditory stimulus in both levels which, again, one might argue, would add complexity at the cognitive level, but the auditory cortex response might be expected to remain constant from level 4 to 5. Despite these considerations, we identified a natural hierarchy(linearly increased) in some of the IC pairs, such as, static FNC effects: attentional network (IC33) / visual network (IC21), thalamus (IC51) / auditory networks (IC62), default mode network (IC48) / visual network (IC29) and dynamic FNC effects: visual network (IC49) / visual network (IC53), auditory networks (IC38) / visual network (IC04), auditory networks (IC62) / attentional network(IC61). 990 comparisons limit our ability to fully describe the results, but we feel this approach provides a novel method for assessing trait-based and state-based differences between clinical groups.
Only subjects able to complete all tasks were retained in the final analysis. Such a rigorous research protocol inevitably excluded more symptomatic subjects who were unable to complete the entire protocol, as confirmed by comparing our final sample with the entire sample that began this study. Thus, our results may have limited generalizability to the entire spectrum of symptom severity across schizophrenia. Second, all of our SPs were taking antipsychotic medications (as noted in our inclusion criteria) whereas HCs were not. Antipsychotic dose and type did not change for the duration of the research protocol (up to two months). As mentioned above we found no relationship between medication dose and FNC measures, however, a main effect of medication cannot be eliminated in this study.
Another challenge is performing a timecourse convolution with a multi-task analysis. While the entire resting-state task time course was analyzed, for other tasks, a hemodynamic predictor function was multiplied with the component time courses from the GICA to yield a task-related component time course. Although, this causes some loss of data in the time course, it helps to reduce noise and improve the correctness of the correlation results between the component pairs (Arbabshirani et al., 2012; Mayer et al., 2012). Based on the different questions that are being asked in rest versus task-based activations, it is unclear how to better address these differences.
In future studies, we intend to explore aspects that may improve the current methodology. An important extension to the current study is to include cognitive tasks with established pathophysiology in schizophrenia, such as working memory, delayed match-to-sample, reinforcement learning, or Go/No-Go tasks.
5. Conclusion
In conclusion, we used a novel analysis to investigate cross paradigm connectivity patterns applied to a large dataset of patients with schizophrenia contrasted with healthy controls. The results provided evidence of both static FNC differences between SPs and HCs and differences modulated by the rest and task hierarchy. This suggests that FNC differences observed only at rest or during performance of particular tasks are not necessarily indicative of the fundamental characteristics of cognition in schizophrenia.
Research Highlights.
We questioned whether cortical connectivity patterns remain stable or task-dependent
A novel analysis was applied to assess cross paradigm connectivity patterns
Stable abnormalities and task-dependent effects exist
Not all differences observed during rest are detectable in other cognitive states
ACKNOWLEDGMENTS
The authors thank MIALAB group members and COBRE group members.
The authors declare no conflict of interest. This research was supported by NIH 1R01-EB006841, NIH 1R01-EB005846, NIH 2R01-EB000840, NIH 1 P20 RR021938-01 and DOE DEFG02-08ER64581 to V.D.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Elseoud A, Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V. The effect of model order selection in group PICA. Hum Brain Mapp. 2010;31:1207–1216. doi: 10.1002/hbm.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Wei Y, Eichele T, Calhoun VD. Capturing inter-subject variability with group independent component analysis of fMRI data: a simulation study. NeuroImage. 2011;59:4141–4159. doi: 10.1016/j.neuroimage.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Calhoun VD. Tracking whole-brain connectivity dynamics in the resting state. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Barch DM. Emotion effects on attention, amygdale activation, and functional connectivity in schizophrenia. Schizophr. Bull. 2011 doi: 10.1093/schbul/sbq168. doi:10.1093/schbul/sbq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbabshirani MR, Havlicek M, Kiehl KA, Pearlson GD, Calhoun VD. Functional Network Connectivity During Rest and Task Conditions: A Comparative Study. Human Brain Mapping. 2012 doi: 10.1002/hbm.22118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Nelson BG, Mueller BA, Camchong J, Lim KO. Altered resting state complexity in schizophrenia. Neuroimage. 2011;59:2196–2207. doi: 10.1016/j.neuroimage.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T. Feature-based Fusion of Medical Imaging Data. IEEE Transact on Info Tech in Biomed. 2009;13:1–10. doi: 10.1109/TITB.2008.923773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T. Multi-subject Independent Component Analysis of fMRI: A Decade of Intrinsic Networks, Default Mode, and Neurodiagnostic Discovery. IEEE Reviews in Biomedical Engineering. 2012;5:60–73. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Hum Brain Mapp. 2001a;13:43–53. doi: 10.1002/hbm.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Kiehl KA, Astur R, Pekar JJ, Pearlson GD. A method for multitask fMRI data fusion applied to schizophrenia. Hum Brain Mapp. 2006;27:598–610. doi: 10.1002/hbm.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Eichele T, Pearlson G. Functional brain networks in schizophrenia: a review. Front. Hum. Neurosci. 2009;3:17. doi: 10.3389/neuro.09.017.2009. doi:10.3389/neuro.09.017.2009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol. Psychiatry. 2011;70:43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton V, Carew JD, Arfanakis K, Maravilla K. Hierarchical clustering to measure connectivity in fMRI resting-state data. MagnReson Imaging. 2002;4:305–317. doi: 10.1016/s0730-725x(02)00503-9. [DOI] [PubMed] [Google Scholar]
- Cullen AE, De Brito SA, Gregory SL, Murray RM, Williams SC, Hodgins S. Temporal Lobe Volume Abnormalities Precede the Prodrome: A Study of Children Presenting Antecedents of Schizophrenia. Schizophr Bull. 2012 doi: 10.1093/schbul/sbs128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel PK, Elizabeth R, Eric C. Failing to deactivate: resting functional abnormalities in autism. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaconescu AO, Jensen J, Wang H, Willeit M, Menon M, Kapur S, McIntosh AR. Aberrant effective connectivity in schizophrenia patients during appetitive conditioning. Front. Hum. Neurosci. 2011;4:239. doi: 10.3389/fnhum.2010.00239. Doi: 10.3389/fnhum.2010.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Allen EA, He H, Sui J, Calhoun VD. Comparison of ICA based fMRI artifact removal: single subject and group approaches. Proceeding. (Hamburg, Germany) 2014 doi: 10.1109/EMBC.2014.6943768. [DOI] [PubMed] [Google Scholar]
- Erhardt EB, Allen EA, Damaraju E, Calhoun VD. On network derivation, classification, and visualization: a response to Habeck and Moeller. Brain Connectivity. 2011a;1:1–19. [PMC free article] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011b;32:2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. New York State Psychiatric Institute, Biomedical Research.; 2002a. [Google Scholar]
- Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol. Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anti correlated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clinical Neuroscience. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Fusar-Poli P. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neuro Sci Behav. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. The American Journal of Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant default mode functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept of Health, Education and Welfare ADM; 1976. pp. 76–338. [Google Scholar]
- Hamm JP, Gilmore CS, Picchetti NA, Sponheim SR, Clementz BA. Abnormalities of neuronal oscillations and temporal integration to low- and high-frequency auditory stimulation in schizophrenia. Biol Psychiatry. 2011;69:989–996. doi: 10.1016/j.biopsych.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Lopez-Sola M, Hernandez-Ribas R, Deus J, Ortiz H, Soriano-Mas C, Yucel M, Pantelis C, Cardoner N. Consistency and functional specialization in the default mode brain network. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA. 2008;105:9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Hampson M. Functional connectivity studies of patients with auditory verbal hallucinations. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel SE, Caffo BS, Van Zijl PC, Pekar JJ. On the relationship between seed-based and ICA-based measures of functional connectivity. MagnReson Med. 2011;66:644–657. doi: 10.1002/mrm.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Starck T, Remes J, Long X, Nikkinen J, Haapea M, et al. Functional segmentation of the brain cortex using high model order group PICA. Hum Brain Mapp. 2009;30:3865–3886. doi: 10.1002/hbm.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kaneko Y, Ouyang X, Li L, Hao Y, Chen EYH. Schizophrenic Patients and Their Unaffected Siblings Share Increased Resting-State Connectivity in the Task-Negative Network but Not Its Anticorrelated Task-Positive Network. Schizophrenia Bulletin. 2012;38:285–294. doi: 10.1093/schbul/sbq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiak K, Ackermann H, Rapp A, Mathiak KA, Shergill S, Riecker A, et al. Neuromagnetic oscillations and hemodynamic correlates of P50 suppression in schizophrenia. Psychiatry Res. 2011;194:95–104. doi: 10.1016/j.pscychresns.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Ruhl D, Merideth F, Ling J, Hanlon FM, Bustillo J, Cañive J. Functional imaging of the hemodynamic sensory gating response in schizophrenia. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Gill A, Stevens MC, Lorenzoni RP, Glahn DC, Calhoun VD, et al. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71 doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovš G, Barch DM. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Basso G, Soldati N, Sailer U, Jovicich J, Bruzzone L, et al. A resting state network in the motor control circuit of the basal ganglia. BMC Neurosci. 2009;10:137. doi: 10.1186/1471-2202-10-137. doi:10.1186/1471-2202-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9:696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- Roweis S. EM algorithms for PCA and SPCA. Adv. Neural Inf. Process Syst. 1998:626–632. [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48:618–24. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding Subject-Driven Cognitive States with Whole-Brain Connectivity Patterns. Cereb. Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soric B. Statistical “Discoveries” and Effect-Size Estimation. Journal of the American Statistical Association. 1989;84:608–610. [Google Scholar]
- Stone DB, Urrea LJ, Aine CJ, Bustillo JR, Clark VP, Stephen JM. Unisensory processing and multisensory integration in schizophrenia: a high-density electrical mapping study. Neuropsychologia. 2011;49:3178–3187. doi: 10.1016/j.neuropsychologia.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Edelman GM. Schizophrenia and the mechanisms of conscious integration. Brain Res Rev. 2000;31:391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- Van DKR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND. Thalamocortical dysconnectivity in schizophrenia. The American Journal of Psychiatry. 2012;1:1092–9. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Rogers B, Heckers S. Functional resting state networks are differentially affected in schizophrenia. Schizophr. Res. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Shen H, Zhang H, Zeng L, Xue Z, Hu D. Functional connectivity-based signatures of schizophrenia revealed by multiclass pattern analysis of resting-state fMRI from schizophrenic patients and their healthy siblings. BioMedical Engineering Online. 2013;12:10. doi: 10.1186/1475-925X-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]