Abstract
BACKGROUND & AIMS
Many colon cancers produce the hormone progastrin, which signals via autocrine and paracrine pathways to promote tumor growth. Transgenic mice that produce high circulating levels of progastrin (hGAS) have increased proliferation of colonic epithelial cells and are more susceptible to colon carcinogenesis than control mice. We investigated whether progastrin affects signaling between colonic epithelial and myofibroblast compartments to regulate tissue homeostasis and cancer susceptibility.
METHODS
Colonic myofibroblast numbers were assessed in hGAS and C57BL/6 mice by immunohistochemistry. Human CCD18Co myofibroblasts were incubated with recombinant human progastrin (rhPG)(1–80) for 18 hours, and proliferation was assessed in the presence of pharmacologic inhibitors. The proliferation of human HT29 colonic epithelial cells was assessed after addition of conditioned media from CCD18Co cells incubated with progastrin. The effects of the insulin-like growth factor (IGF)-I receptor antagonist AG1024 were investigated in cultured HT29 cells and on the colonic epithelium of hGAS mice compared with mice that did not express transgenic progastrin (controls).
RESULTS
The colonic mucosa of hGAS mice contained greater numbers of myofibroblasts that expressed α–smooth muscle actin and vimentin than controls. Incubation of CCD18Co myofibroblasts with 0.1 nmol/L rhPG(1–80) increased their proliferation, which required activation of protein kinase C and phosphatidylinositol-3 kinase. CCD18Co cells secreted IGF-II in response to rhPG(1–80), and conditioned media from CCD18Co cells that had been incubated with rhPG(1–80) increased the proliferation of HT29 cells. The colonic epithelial phenotype of hGAS mice (crypt hyperplasia, increased proliferation, and altered proportions of goblet and enteroendocrine cells) was inhibited by AG1024.
CONCLUSIONS
Progastrin stimulates colonic myofibroblasts to release IGF-II, which increases proliferation of colonic epithelial cells. Progastrin might therefore alter colonic epithelial cells via indirect mechanisms to promote neoplasia.
Keywords: Mouse Model, Colon Cancer, PKC, PI3K
The colonic epithelium is organized into crypts that rapidly self-renew via stem cells, which are believed to be located at or near the crypt base.1 Tissue self-renewal is precisely regulated to maintain normal homeostasis and avoid malignant transformation. Under normal conditions, colonic epithelial stem cells self-renew asymmetrically and daughter cells divide further in the transit-amplifying region in the bottom two-thirds of the crypt. Cells then differentiate into secretory (goblet, enter-oendocrine) or absorptive (colonocyte) lineages as they migrate up the crypt axis until they are shed into the lumen. Surrounding the colonic crypts are sheaths of mesenchymal cells, including myofibroblasts, which participate in regulating homeostasis through reciprocal signaling with the epithelial compartment. Myofibroblasts produce Wnt signals2 and several growth factors, such as hepatocyte growth factor and insulin-like growth factors (IGFs),3 which increase colonic epithelial cell proliferation. Factors that disrupt homeostasis in this niche may therefore render the colon more susceptible to development of cancer.
Progastrin is a precursor of the fully processed hormone gastrin and is produced by cotranslational cleavage of the precursor molecule preprogastrin (reviewed in Dockray et al4). Amidated forms of gastrin are produced mainly by G cells in the gastric antrum, where they function as gastric acid secretagogues. Gastrin precursors are also biologically active; in particular, they disrupt colonic mucosal homeostasis and are involved in development of colon cancer. Preprogastrin is expressed by many colon cancers,5 but because these tumors lack the enzymes required to process progastrin to amidated forms,6–8 immature gastrins such as progastrin and glycine-extended gastrin (G-Gly) are secreted, leading to high local and circulating concentrations of these hormones.9 Progastrin is mitogenic to colonic cell lines10,11 and increases colonic epithelial proliferation in progastrin-overexpressing (hGAS) mice.12–14 hGAS mice have colonic crypt hyperplasia, increased numbers of colonic goblet cells, and increased susceptibility to colonic tumor development after treatment with the carcinogen azoxymethane,15,16 possibly as a result of persistent epithelial mitosis following DNA-damaging stimuli.13
We therefore hypothesized that progastrin regulates colonic homeostasis and carcinogenesis susceptibility by exerting effects on the myofibroblast compartment of the colonic mucosa in addition to its well-documented effects on epithelial cells. We have assessed colonic myofibroblast numbers in hGAS mice and have used a human colonic myofibroblast cell line (CCD18Co) to investigate the effect of exogenous recombinant human progastrin (rhPG) (1–80) on myofibroblasts in vitro. Various pharmacologic agents were used to investigate the signaling pathways involved.
Materials and Methods
Animals
Ten- to 12-week-old C57BL/6 mice (Charles River Laboratories, Margate, Kent, England) and hGAS mice12 on the C57BL/6 genetic background were maintained at the University of Liverpool in England. Animals were fed a commercially prepared diet, given water ad libitum, and maintained on a 12:12-hour light/dark cycle. All experiments were performed with UK Home Office (Animals Scientific Procedures Act 1986) and local ethical committee approval.
Treatment With AG1024
Groups of 5 sex-matched hGAS and C57BL/6 mice aged 10 weeks were treated with 3 injections of 30 μg AG1024 (Merck Chemicals Ltd, Nottingham, England) in 15% dimethyl sulfoxide (DMSO) or vehicle control intraperitoneally at 0, 24, and 48 hours and were killed 52 hours after the first injection.
Tissue Preparation and Scoring
Colons were fixed in 4% formalin and embedded in paraffin. Four-micrometer sections of distal colon were stained with H&E for morphological assessment or Alcian blue/periodic acid–Schiff for goblet cell identification or underwent immunohistochemistry for chromogranin A (CgA), Ki67, or DCAMKL-1. Fifty hemi-crypts per mouse were assessed for the number of mitotic, goblet, Ki67-positive, CgA-positive, and DCAMKL-1–positive cells. Data are presented as positive cells per hemi-crypt or on a cell positional basis.13
Immunohistochemistry
CgA and DCAMKL-1 immunohistochemistry was performed using an EnVision Plus Kit (Dako UK Ltd, Cambridge-shire, England). Sections were subjected to heat-mediated antigen retrieval (10 mmol/L citric acid buffer), incubated for 1 hour at room temperature with rabbit anti-CgA (1/1000; Abcam, Cambridge, England) or rabbit anti–DCAMKL-1 (1/50; Cambridge Bioscience Ltd, Cambridge, England), washed and incubated with biotin-labeled anti-rabbit polymer and 3,3′-dia-minobenzidine chromogen, and counterstained with hematoxylin. Ki67 immunohistochemistry was also performed following heat-mediated antigen retrieval, but after overnight incubation with rat anti-Ki67 primary antibody (1/20; Dako) at 4°C, the signal was detected with a biotin-conjugated rabbit anti-rat secondary antibody (1/200; Dako), Vectastain ABC (Vector Laboratories, Peterborough, England), and 3,3′-diaminobenzidine chromogen (Sigma-Aldrich Company Ltd, Gillingham, England).
For evaluation of myofibroblast cell numbers, sections were incubated with rabbit anti—α–smooth muscle actin (SMA) antibody (1/100; Abcam) in 10% normal goat serum and/or guinea pig anti-vimentin antibody (1/50; Fitzgerald Industries International, North Acton, MA) in 1% bovine serum albumin overnight at 4°C. Sections were then incubated with Texas Red–conjugated anti-rabbit and/or fluorescein isothiocyanate–conjugated anti–guinea pig immunoglobulins (Jackson ImmunoResearch Laboratories, Cambridgeshire, England) (1/200 or 1/600 dilutions, respectively) and mounted with Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstain (Vector Laboratories Ltd). Three fields of view (40× objective lens) per mouse were scored for total number of mucosal cells (by DAPI-stained nuclei) and for positively stained mucosal cells using a fluorescence microscope (BX51; Olympus Microscopy, Essex, England). Data are presented as the mean percentage of α-SMA–or vimentin-positive cells per total number of mucosal cells.
CCD18Co Cell Culture
The human colonic myofibroblast cell line CCD18Co (American Type Culture Collection, Manassas, VA) was cultured at 37°C with 5% CO2 in Eagle’s modified essential media (LGC Standards, Teddington, England) containing 10% fetal calf serum (FCS) (Gibco Invitrogen Ltd, Paisley, Scotland), 0.5% 2 mmol/L L-glutamine (Sigma-Aldrich), and penicillin/streptomycin (Sigma-Aldrich). Cells were detached using trypsin/EDTA (Sigma-Aldrich) and seeded at 1000 cells/well in 96-well plates. Media was changed to serum-free media at 24 and 48 hours to synchronize the cell cycle. At 72 hours, cells were treated with rhPG(1–80),17 gastrin-17 (G-17), G-Gly, or 50 mmol/L phorbol myristate acetate (PMA), a known inducer of protein kinase C (PKC), for 18 hours. Cells were also pretreated for 20 minutes before addition of 0.1 nmol/L rhPG(1–80) with the cholecystokinin-2 receptor (CCK-2R) inhibitor YM022 (10 nmol/L; Tocris Bioscience, Bristol, England), PKC inhibitor RO-32-0432 (1 μmol/L; Calbiochem, Nottingham, England), mitogen-activated protein kinase (MAPK) inhibitors PD98059 (20 μmol/L; Calbiochem) and U0126 (10 μmol/L; Cell Signaling Technology, Beverly, MA), or the phosphoinositide 3-kinase (PI3K) inhibitor LY294002 (20 μmol/L; Calbiochem). Serum-free media and media containing 10% FCS were used as negative and positive controls.
Generation of Conditioned Media From CCD18Co Cells
CCD18Co cells were grown for 24 hours and media was replaced with serum-free media. This process was repeated 24 hours later and 72 hours after initial plating (48 hours after being in serum-free media). Cells were then treated with 0.1 nmol/L rhPG(1–80) in fresh serum-free media or serum-free media alone (negative control). Conditioned media was collected 18 hours posttreatment and centrifuged at 1000 rpm for 5 minutes to remove cells.
Protein Extraction and Western Blotting
Conditioned media was harvested from CCD18Co cells on 3 separate occasions and proteins were concentrated and desalted using Amicon Ultra-15 centrifugal filter units, 3kDa (Millipore UK Ltd, Watford, England). Samples were electrophoresed, transferred onto nitrocellulose membranes, and blocked with 5% nonfat milk. Blots were probed with goat anti-human IGF-II (1/1250; R&D Systems, Abingdon, England), followed by horseradish peroxidase–conjugated rabbit anti-goat secondary antibody (1/2000; Dako), and the signal was detected using Supersignal (Thermo Scientific, Runcorn, England) and a ChemiDoc imaging system (Bio-Rad, Hertford-shire, England).
HT29 Cell Culture
The human colonic epithelial cell line HT29 (American Type Culture Collection) was cultured using the same conditions as for CCD18Co cells. Seventy-two hours after plating (the last 48 hours in serum-free media), cells were treated with 0.1 nmol/L rhPG(1–80) or various types of conditioned media, either in the presence or absence of the IGF-1R inhibitor AG1024 (Merck) at 3.2 μmol/L. Serum-free media and media containing 10% FCS were used as negative and positive controls.
Primary Culture of Murine Colonic Myofibroblasts
Colonic myofibroblasts were isolated and cultured from 2 C57BL/6 and hGAS mice aged 6 weeks.18 Briefly, colons were rinsed, cut into small pieces, and placed in Dulbecco’s modified Eagle medium with 10% fetal bovine serum, EDTA, and dithiothreitol. After shaking and filtering through a 100-μm filter, tissue fragments were digested using type I collagenase (Gibco Life Technologies, Grand Island, NY) and deoxyribonuclease I (Roche, Basel, Switzerland), washed, filtered again, and cultured until almost confluent. One thousand viable cells per well were plated in 96-well plates, media was changed to serum-free media on day 2, and proliferation was assessed using the ViaLight Cell Proliferation BioAssay Kit (Lonza Biologics, Slough, England). Differences in proliferation between wild-type and hGAS colonic myofibroblasts were determined on 2 separate occasions. Secretion of IGF-I into the media from C57BL/6 myofibroblasts with and without stimulation by 0.1 nmol/L rhPG(1–80) for 18 hours was quantified by enzyme-linked immunosorbent assay (Abcam; Ab100695).
Assessment of Cell Proliferation In Vitro
Changes in cell proliferation were assessed using the ViaLight Plus Cell Proliferation/Cytotoxicity Kit (Lonza Biologics), which produces luminescence proportional to adenosine triphosphate levels. Each treatment was tested in 8 wells of a 96-well plate, and each experiment was repeated on at least 3 separate occasions. Data are presented as the mean increase in relative luminescence units (RLUs) compared with serum-free media ± the standard error of the mean.
Nested Reverse-Transcriptase Polymerase Chain Reaction
RNA was extracted from CCD18Co, HT29, and AGSGR19 (a human gastric cancer cell line stably transfected with the gastrin/CCK-2R) cells using TRI Reagent (Sigma-Aldrich), purified using the RNeasy Mini Kit (Qiagen, Crawley, England) and reverse transcribed using the Transcriptor First-Strand cDNA Synthesis Kit (Roche). Nested polymerase chain reaction (PCR) was performed using primers for the gastrin/CCK-2R as previously described: external forward (sense), 5′-TTGGAGCTGGCCATTAG-3′; external reverse (anti-sense), 5′-CACTGTCGCCGTCAAAG-3′; internal forward (sense), 5′-ATGCTCATCATCGTGGTC-3′; internal reverse (antisense), 5′-AGAGATAAGCCCGTAGGC-3′.20 PCR product was visualized on an agarose gel using a ChemiDoc imager (Bio-Rad, Hertfordshire, England).
Quantitative Real-Time PCR
A modified Weiser technique21 was used to detach colonic epithelial cells from a minimum of 6 sex-matched 10-to 12-week-old C57BL/6 and hGAS mice. Cells were washed with phosphate-buffered saline and RNA was extracted using a High Pure Tissue RNA Kit (Roche) followed by reverse transcription using a QuantiTect Reverse Transcription Kit (Qiagen). Quantitative reverse-transcription (RT)-PCR was performed using a Light Cycler 480 (Roche) and the QuantiTect SYBR Green primer assay for Lgr5 (NM_010195) and GAPDH (NM_008084). Reactions were performed at 95°C for 15 minutes, followed by 40 cycles of 94°C for 15 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. Relative quantification was performed using the comparative Ct method.
Statistical Analyses
A 2-tailed, 2-sample Student t test assuming unequal variance, or a 2-way analysis of variance with either Bonferroni or Tukey correction where appropriate, was used to determine significant differences between mouse groups and cell line treatments. The Mann–Whitney U test was used to assess primary myofibroblast cultures. The modified median test was used to determine significant differences at individual cell positions.22 Significance was defined as P < .05 by t test, analysis of variance, or Mann–Whitney U test and by differences at ≥3 consecutive cell positions by modified median test.
Results
Numbers of Colonic Pericryptal Myofibroblasts Are Increased in hGAS Mice
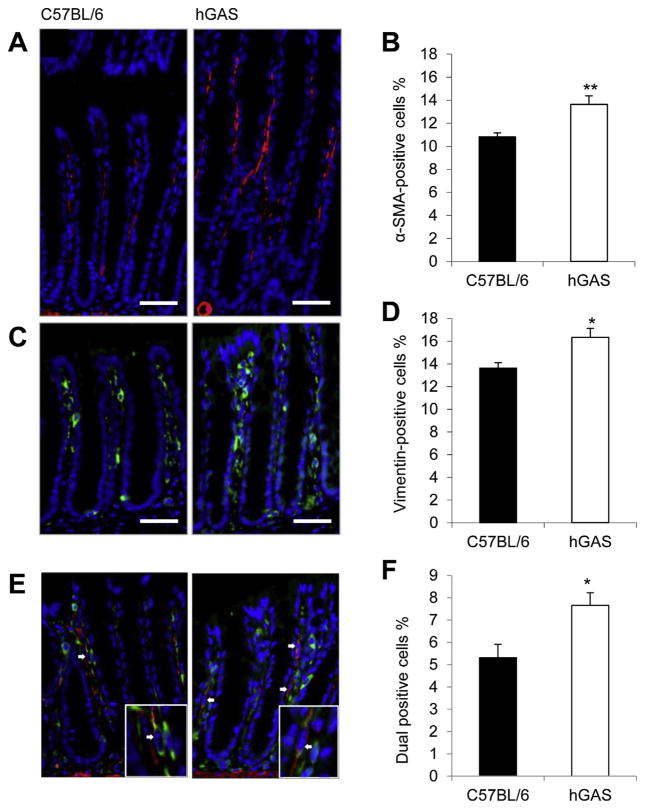
To investigate whether progastrin has any effect on the myofibroblast compartment of the colonic mucosa, we assessed the numbers of colonic mucosal myofibroblasts in hGAS mice. Because hGAS mice display colonic crypt hyperplasia,12 we assessed the total number of positively stained cells as a percentage of the total number of mucosal cells (counted by DAPI-stained nuclei) to account for changes in crypt length. hGAS mice showed a mean of 13.7% ± 0.7% α-SMA–positive cells compared with 10.8% ± 0.3% in C57BL/6 mice (Figure 1A and B). Similarly, hGAS mice showed 16.3% ± 0.8% vimentin-positive cells compared with 13.6% ± 0.5% in C57BL/6 mice (Figure 1C and D). To define a difference in the myofibroblast compartment, we further assessed the colocalization of α-SMA with vimentin and found a significant increase in dual-labeled cells in hGAS (7.7% ± 0.6%) compared with C57BL/6 mice (5.3% ± 0.6%; Figure 1E and F). Less that 1% of α-SMA– or vimentin-positive cells in both hGAS and C57BL/6 mice expressed the proliferation marker Ki67 (data not shown). This phenotype persisted in animals housed in a different animal facility at Columbia University in New York, suggesting that it is not influenced by bacterial flora or diet (data not shown).
Figure 1.
Immunofluorescence images of (A) α-SMA, (C) vimentin, and (E) both (indicated by arrows) α-SMA (red) and vimentin (green) in the colonic mucosa of C57BL/6 (left panel) and hGAS (right panel) mice. Mean percentage of (B) α-SMA–positive, (D) vimentin-positive, and (F) dual-labeled cells per total mucosal cells. n = 6 mice/ group. **P < .01, *P < .05 by Student t test.
CCD18Co Cells Proliferate in Response to Exogenous rhPG(1–80) But Not G-17 or G-Gly
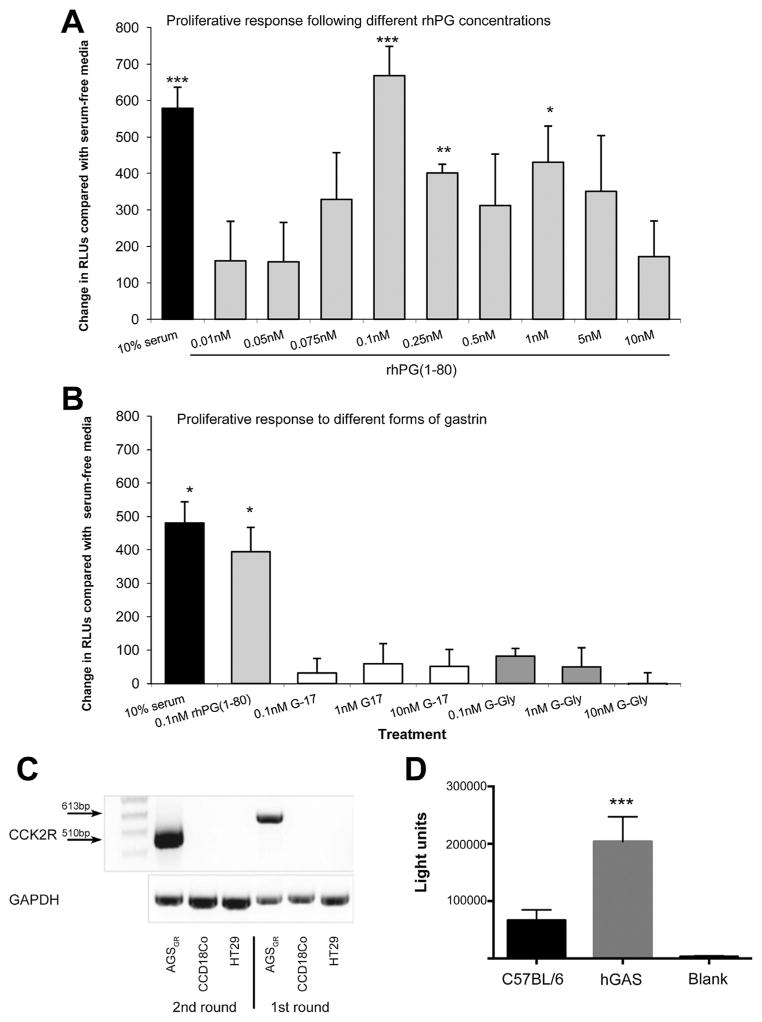
Because an increased circulating concentration of progastrin was associated with increased numbers of colonic mucosal myofibroblasts in vivo, we next used an in vitro approach to investigate whether progastrin caused any direct effects on colonic myofibroblast cells. CCD18Co cells showed a significant increase in proliferation after incubation with 10% FCS compared with serum-free media alone (73% increase, P < .01). Administration of rhPG(1–80) resulted in a “bell-shaped” dose-response curve with increased cell proliferation observed after treatment with 0.1 nmol/L (86%, P < .01), 0.25 nmol/L (40%, P < .01), and 1 nmol/L (53%, P < .01) rhPG(1–80) (Figure 2A). However, G-17 or G-Gly at 0.1 nmol/L, 1 nmol/L, or 10 nmol/L did not affect CCD18Co cell proliferation (Figure 2B). We also assessed the proliferation of colonic myofibroblasts in primary culture from C57BL/6 and hGAS mice and observed a significant increase in proliferation of hGAS compared with C57BL/6 myofibroblasts (Figure 2D). We confirmed that the majority (~70%) of primary stromal cells coexpressed α-SMA and vimentin, suggesting that myofibroblasts were the most abundant cell population (Supplementary Figure 1).
Figure 2.
Increase in RLUs (indicative of proliferation) compared with serum-free media in CCD18Co cells 18 hours after treatment with (A) 0.01 to 10 nmol/L rhPG(1–80) or (B) 0.1 to 10 nmol/L G-17 or G-Gly. (C) Nested RT-PCR showing first-and second-round PCR for the gastrin/CCK-2 receptor in AGSGR, HT29, and CCD18Co cells. (D) Luminescence units of primary myofibroblast cultures of C57BL/6 and hGAS colon. *P < .05, **P < .01, and ***P < .001 compared with serum-free media by Student t test for cell lines and ***P < .001 by Mann–Whitney U test for primary culture.
Previous studies involving hGAS mice have suggested that their colonic phenotype may involve a signaling pathway that includes the gastrin/CCK-2 receptor.23 We therefore assessed whether CCD18Co myofibroblasts (or HT29 cells) expressed this receptor and determined by nested RT-PCR that they did not (Figure 2C).
rhPG(1–80) Induces Proliferation of CCD18Co Cells via the PKC and PI3K Pathways
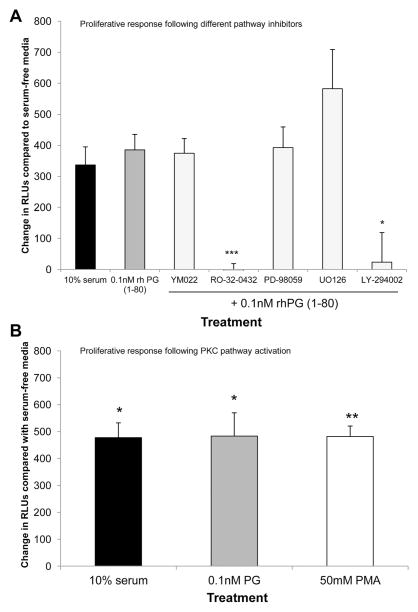
CCD18Co cells pretreated with the PKC (RO-32-0432) or PI3K (LY-294002) inhibitors before treatment with 0.1 nmol/L rhPG(1–80) showed significant reductions in cell proliferation compared with cells treated with 0.1 nmol/L rhPG(1–80) alone. CCD18Co cell proliferation was not altered significantly by pretreatment with the CCK-2R inhibitor YM022 or the ERK inhibitors PD-98059 or UO126 before addition of 0.1 nmol/L rhPG(1–80) (Figure 3A). To confirm the role of PKC in progastrin-mediated signaling, CCD18Co cells were treated with the PKC activator PMA. Cell proliferation was significantly increased after treatment with 50 mmol/L PMA, suggesting that activation of PKC increases proliferation in these cells (Figure 3B).
Figure 3.
(A) Increase in RLUs (indicative of proliferation) compared with serum-free media in CCD18Co cells 18 hours after treatment with 10% serum, 0.1 nmol/L rhPG(1–80) alone, or 0.1 nmol/L rhPG(1–80) after pre-treatment with YM022, RO-32-0432, PD-98059, LY-294002, or U0126. (B) Increase in RLUs compared with serum-free media in CCD18Co cells 18 hours after treatment with 10% serum, 0.1 nmol/L rhPG(1–80), or 50 mmol/L PMA. *P < .05, **P < .01, ***P < .01 by Student t test compared with serum-free media.
rhPG(1–80) and CCD18Co-Conditioned Media Induce Proliferation of HT29 Cells
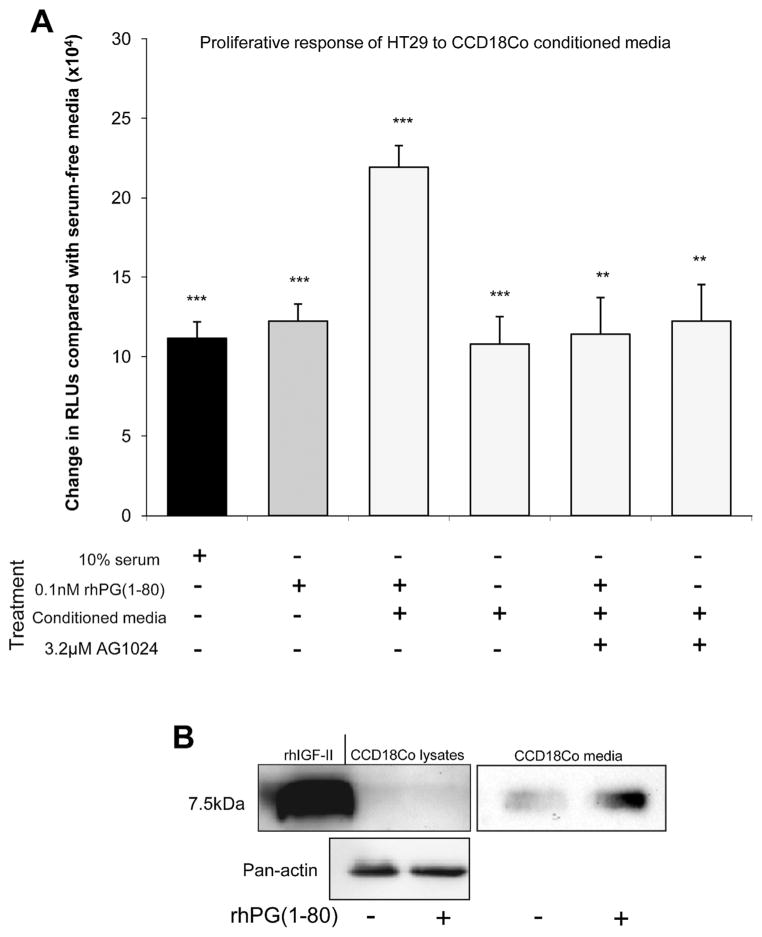
Like CCD18Co cells, human colonic epithelial HT29 cells showed a significant increase in proliferation after treatment with 10% FCS compared with serum-free media. A similar increase was observed after treatment with 0.1 nmol/L rhPG(1–80). Although 0.1 nmol/L rhPG(1–80) alone and conditioned media from CCD18Co cells both significantly increased HT29 proliferation, a greater increase in proliferation was observed when CCD18Co cells were treated for 18 hours with 0.1 nmol/L rhPG(1–80) and the resulting conditioned media was used to stimulate HT29 cells (Figure 4A). This effect was probably due to rhPG(1–80)–induced IGF signaling, because addition of the IGF-1R inhibitor AG1024 to rhPG(1–80)–treated, CCD18Co-conditioned media reduced HT29 proliferation to approximately the same levels as those seen after treatment with 10% FCS, 0.1 nmol/L rhPG(1–80), or non—rhPG(1–80)–treated CCD18Co-conditioned media (Figure 4A). Because CCD18Co cells have previously been shown not to secrete IGF-I24 and because we determined that primary myofibroblast cultures from C57BL/6 mouse colon treated with or without 0.1 nmol/L rhPG(1–80) did not secrete IGF-I (data not shown), we hypothesized that IGF-II may have been responsible for these enhanced proliferative effects. We therefore analyzed conditioned media from CCD18Co cells by Western blot. Greater amounts of IGF-II were found in the media from rhPG(1–80)–treated CCD18Co cells (2.44-fold) compared with the media from myofibroblasts that had been incubated in serum-free media. Mature IGF-II was found at very low abundance in untreated and rhPG(1–80)–treated CCD18Co cells and no difference in expression was observed (Figure 4B).
Figure 4.
(A) Increase in RLUs (indicative of proliferation) compared with serum-free media in HT29 cells 18 hours after treatment with 10% serum, 0.1 nmol/ L rhPG(1–80), CCD18Co-conditioned media 18 hours after rhPG(1–80) treatment, CCD 18Co-conditioned media alone, and CCD18Co-con-ditioned media 18 hours after treatment with rhPG(1–80) in the presence of AG1024 or CCD18Co-conditioned media in the presence of AG1024. (B) Western blot showing mature IGF-II expression in CCD18Co cells and secretion by CCD18Co cells into the media with or without rhPG(1–80).**P < .01 and ***P < .001 compared with serum-free media by Student t test.
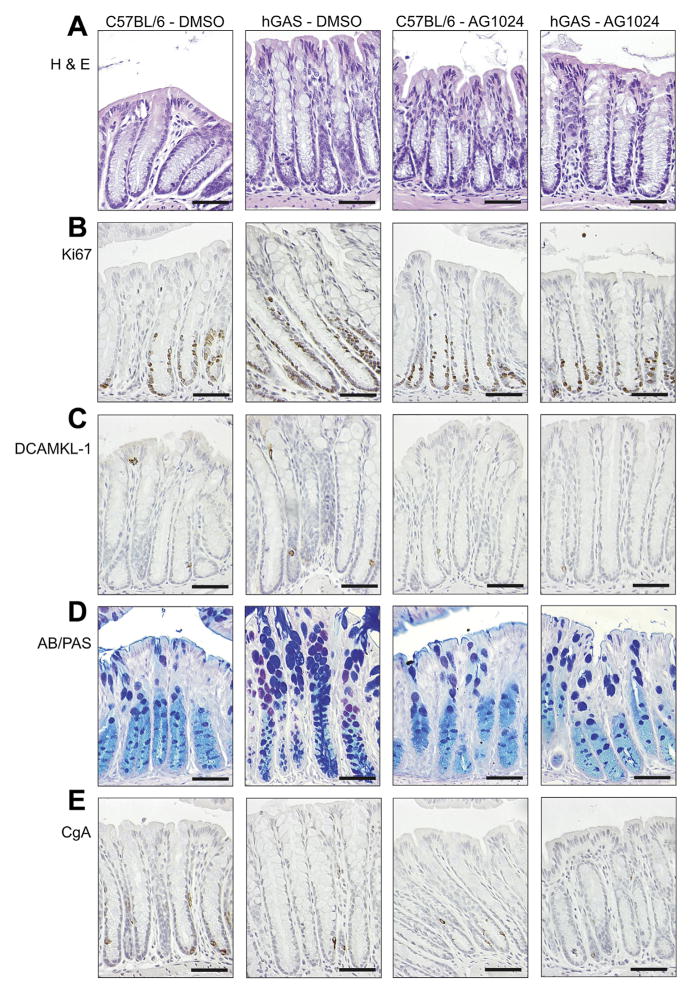
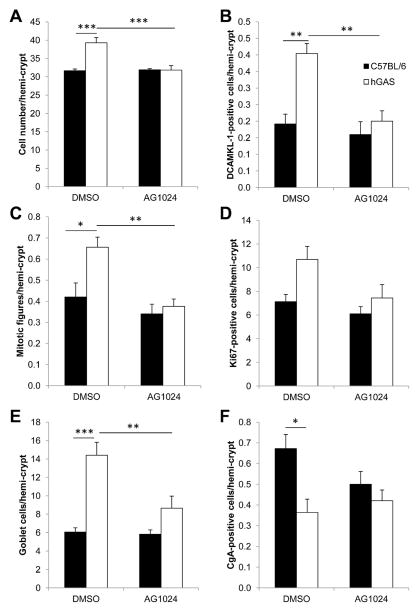
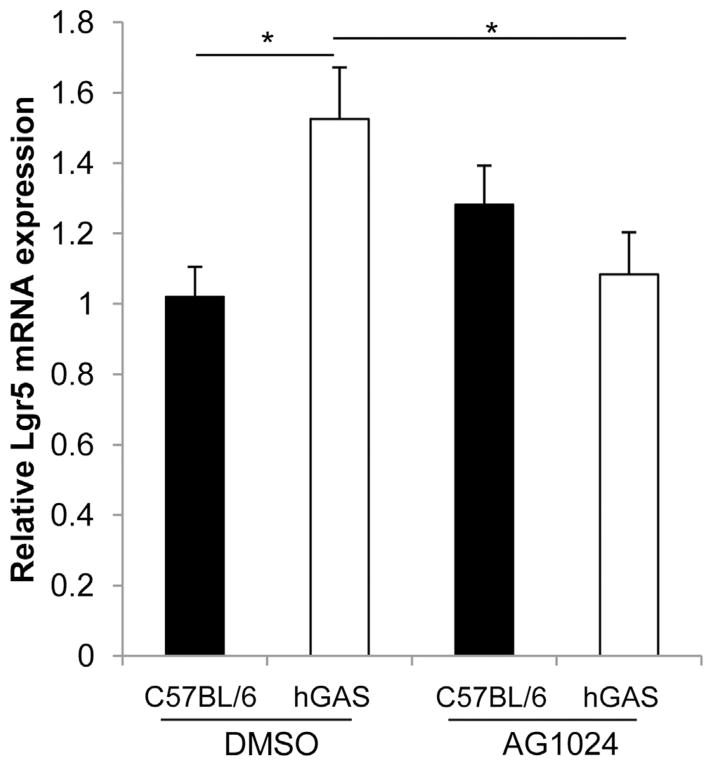
AG1024 Reduces Colonic Hyperplasia, Epithelial Cell Proliferation, DCAMKL-1 Protein Expression, and Lgr5 Gene Expression in hGAS Mice
Because IGF-II has previously been shown to enhance the proliferation of HT29 cells25,26 and was secreted in greater abundance into the media from CCD18Co cells treated with rhPG(1–80) (Figure 4B), we hypothesized that the observed increase in colonic epithelial mitosis in hGAS mice (Figures 5A and 6C) was also mediated by signaling via IGF-1R. We therefore administered the IGF-1R antagonist AG1024 to hGAS and C57BL/6 mice and assessed distal colonic crypt length (Figure 6A), mitotic figures per hemi-crypt (Figure 6C), Ki67-positive cells per hemi-crypt (Figure 6D), DCAMKL-1–positive cells per hemi-crypt (Figure 6B), and relative expression of Lgr5 transcripts (Figure 7) 52 hours after drug administration.27 As previously described,13,15,23 cell number per hemi-crypt, mitotic figures per hemi-crypt, number of DCAMKL-1–positive cells per hemi-crypt, and Lgr5 expression were significantly increased in the colons of DMSO-treated hGAS mice compared with C57BL/6 mice (Figures 5–7 and Supplementary Figure 2). Administration of AG1024 to hGAS mice, however, resulted in significant reductions in the number of total cells, mitotic cells, DCAMKL-1–positive cells, and Lgr5 messenger RNA (mRNA) transcripts in the colon compared with DMSO-treated hGAS mice. All of these parameters were reduced to levels similar to those observed in C57BL/6 mice treated with either DMSO or AG1024 (Figures 5A and C, 6A–C, and 7 and Supplementary Figure 3). Quantification of colonic epithelial cell proliferation by Ki67 immunohistochemistry also showed a similar trend to that seen for mitotic index, but this did not reach statistical significance (Figures 5B and 6D). Thus, AG1024 reversed the increased colonic epithelial proliferation observed in hGAS animals, possibly as a result of inhibiting the effects of IGF-II secreted from colonic myofibroblasts as a result of progastrin stimulation.
Figure 5.
Photomicrographs showing representative (A) H&E, (B) Ki67, (C) DCAMKL-1, (D) Alcian blue/periodic acid–Schiff (AB/PAS), and (E) CgA staining in C57BL/6 and hGAS distal colon after treatment with DMSO or AG1024 as indicated. Scale bars = 50 μm.
Figure 6.
(A) Cell number, (B) DCAMKL-1-positive cells, (C) mitotic figures, (D) Ki67-positive cells, (E) goblet cells, and (F) CgA-positive cells per hemi-crypt in DMSO- or AG1024-treated C57BL/6 (black) or hGAS (white) distal colon. n = 5 mice/group. *P < .05, **P < .01, ***P < .001 by 2-way analysis of variance with Bonferroni post hoc test.
Figure 7.
Quantitative real-time PCR analysis of relative Lgr5 mRNA expression in epithelial cell–enriched preparations from C57BL/6 (black) and hGAS (white) mice treated with DMSO or AG1024. n = 6 sex-matched mice/group. *P < .05 by 2-way analysis of variance and Tukey post hoc test.
Administration of AG1024 Results in Reversal of Colonic Goblet Cell Hyperplasia and Endocrine Cell Hypoplasia in hGAS Mice
As previously observed in hGAS mice on the FVB/N genetic background,12,28 hGAS mice on the C57BL/6 genetic background treated with DMSO (Figures 5D and 6E and Supplementary Figure 2A) showed a significant increase in colonic epithelial goblet cell numbers compared with C57BL/6 mice. Fewer colonic enteroendocrine cells were also observed in hGAS mice, suggesting that there is an alteration in differentiation toward all cell types in the secretory cell lineage (Figures 5E and 6F and Supplementary Figure 2B). Treatment of hGAS mice with AG1024 for 52 hours resulted in a significant reduction in colonic goblet cell numbers compared with hGAS mice treated with DMSO. The numbers of colonic goblet cells were similar to those observed in C57BL/6 mice treated with either DMSO or AG1024 (Figures 5D and 6E and Supplementary Figure 3A). Although hGAS mice treated with DMSO showed reduced enteroendocrine cell numbers compared with C57BL/6 mice as assessed by CgA immunohistochemistry, no significant changes in enteroendocrine cell numbers were detected in either strain after administration of AG1024 (Figures 5E and 6F and Supplementary Figure 3B). These results therefore suggest that altered epithelial cell differentiation in hGAS colonic crypts may also be partially mediated by progastrin-induced IGF signaling.
Discussion
We assessed how transgenic progastrin over-expression affects the myofibroblast compartment of murine colonic mucosa, and we confirmed that hGAS mice that have been backcrossed onto the C57BL/6 genetic background retained the procarcinogenic colonic phenotype of crypt hyperplasia and increased colonic epithelial mitosis (Figures 5 and 6).12–15,23 We showed increased numbers of colonic goblet cells as previously described12,28 and also found decreased numbers of colonic enteroendocrine cells (Figures 5E and 6F). The differentiation of intestinal secretory cell lineages is predominantly regulated by the expression of transcription factors including Math1, Klf4, and neurogenin3.1 Progastrin has previously been shown to alter colonic Klf4 expression, and this may be responsible for the altered numbers of colonic goblet cells observed.28 Factors responsible for reduced numbers of enteroendocrine cells in the hGAS colon are not yet known, but the altered expression of Lgr-5 and DCAMKL-1 in these animals15,23 suggests that an altered colonic stem cell niche may contribute.
Because pericryptal myofibroblasts signal in a paracrine manner with colonic epithelial cells via secretion of a wide variety of cytokines, growth factors, chemokines, and inflammatory mediators,3 we hypothesized that progastrin may affect colonic homeostasis and thus susceptibility to colon cancer at least partly via effects on colonic myofibroblasts. We found a significant increase in the proportion of colonic pericryptal myofibroblasts in hGAS mice (Figure 1) and hypothesized that progastrin acts as a growth factor for these cells, causing them to secrete other growth factors that in turn increase the proliferation of adjacent epithelial cells.
We developed our in vivo observations by investigating the direct effects of progastrin on a human colonic myofibroblast cell line in vitro and showed that CCD18Co proliferation was significantly increased after treatment with 0.1 nmol/L rhPG(1–80) (Figure 2A). Human patients with colorectal cancer have previously been shown to have circulating progastrin concentrations in the range of 0.015 to 0.132 nmol/L.9 To elucidate the molecular pathway(s) involved in rhPG(1–80)–induced proliferation, we pretreated CCD18Co cells with pharmacologic inhibitors of CCK-2R, PKC, MAPK, or PI3K. Significant decreases in proliferation were observed with RO-32-0432 and LY294002 (Figure 3A), suggesting that progastrin increases CCD18Co cell proliferation by signaling via activation of PI3K and PKC. Progastrin(6–80) has been shown to exert antiapoptotic effects in epithelial cell lines in vitro, and inhibition of MAPK and PI3K pathways attenuated this antiapoptotic effect.29,30 However, inhibition of the MAPK pathway in our studies did not attenuate progastrin-mediated myofibroblast proliferation. Because pharmacologic inhibition of CCK-2R did not result in decreased proliferation (Figure 3A) and because we did not detect the presence of this receptor in CCD18Co cells by nested RT-PCR (Figure 2B), it is unlikely that signaling via the CCK-2 receptor is responsible for the effects of progastrin on myofibroblasts in vitro. In addition, CCK-2R antagonists have not previously been shown to inhibit progastrin-induced proliferation in epithelial cell lines.10,31 Recently, we have shown that progastrin required the presence of the CCK-2 receptor in vivo to exert pro-proliferative and antiapoptotic effects and cause progenitor cell expansion in the colon; however, the nature of the interaction between progastrin and this receptor remains to be determined.23 Although biotinylated progastrin shows strong binding to several colonic cell lines, this was not CCK-2R dependent.17 Indeed, the identity of a progastrin receptor remains an issue of intense investigation. Recent studies have suggested that Annexin A2,32,33 ferric ions,34 and charged glycosaminoglycans17 are involved.
To assess interactions between myofibroblasts and epithelial cells in progastrin-related signaling, we treated HT29 cells with rhPG(1–80) and observed increased proliferation in response to 0.1 nmol/L (Figure 4A). rhPG(1–80) 0.1 to 1.0 nmol/L has previously been shown to promote proliferation of pluripotent rat intestinal cell lines.35 Proliferation was also increased after treatment of HT29 cells with media conditioned for 18 hours by CCD18Co cells, suggesting that colonic myofibroblasts secrete factors that are mitogenic to colonic epithelial cells. Because treatment with AG1024 did not further reduce HT29 cell proliferation when progastrin was absent, additional factors may be secreted from colonic myofibroblasts that control progastrin-independent proliferation (Figure 4A). Similar signaling pathways have also been observed in the gastric mucosa, where myofibroblasts reciprocally signal with the epithelium via the IGF pathway.36 The IGF family consists of IGF-I, IGF-II, the IGF-1R receptor, and 6 IGF-binding proteins (IGFBP1–6), which sequester IGFs and therefore block activation of IGF-1R.37 The pathway has well-described roles in stimulating colonic cell proliferation and inhibiting apoptosis and has been implicated in the development of colorectal38 as well as several other cancers. IGF family members have also been shown to promote intestinal cell differentiation in vitro.39
In view of the role of the IGF family in epithelial-myofibroblast signaling in the stomach, we treated HT29 cells with conditioned media from rhPG(1–80)–treated CCD18Co cells in the presence of the IGF-1R inhibitor AG1024 and showed that HT29 proliferation was attenuated to the level observed after treatment with CCD18Co-conditioned media alone (Figure 4A). Blockade of IGF-1R in HT29 cells using a monoclonal antibody has also been shown to inhibit proliferation.40 Additionally, we have shown increased IGF-II secretion after rhPG(1–80) treatment of CCD18Co myofibroblasts (Figure 4B). Further to these observations in vitro, we showed in hGAS mice in vivo that AG1024 reduced epithelial cell proliferation, Lgr5 gene expression, DCAMKL-1 labeling, and goblet cell numbers to levels similar to those observed in the distal colon of DMSO- or AG1024-treated C57BL/6 mice (Figures 5–7). We propose that this is likely to be due at least in part to AG1024 inhibiting the progastrin-induced release of IGF-II from pericryptal myofibroblasts (or its liberation from IGFBPs), thus suppressing the proliferation of colonic epithelial cells. In view of the altered numbers of DCAMKL-1 and differentiated cell types and altered gene expression of Lgr5 observed in AG1024-treated hGAS mice, it is distinctly possible that these effects arise as a result of alterations in cells that occupy the colonic stem cell niche.
In conclusion, elevated serum concentrations of progastrin resulted in colonic crypt hyperplasia, increased colonic mitosis, goblet cell hyperplasia, enteroendocrine cell hypoplasia, and increased numbers of pericryptal myofibroblasts in hGAS mice. rhPG(1–80) 0.1 nmol/L stimulated the proliferation of CCD18Co myofibroblast cells via PKC and PI3K pathways and resulted in IGF-II secretion. Inhibition of IGF-1R signaling decreased progastrin-stimulated colonic epithelial proliferation in vitro and in vivo. These data therefore suggest important roles for progastrin in the regulation of colonic homeo-stasis and carcinogenesis via regulation of mesenchymal-epithelial signaling.
Supplementary Material
Acknowledgments
The authors thank E. Bennett, D. Berry, K. Gittins, L. McLaughlin, and K. Andrews for experimental assistance.
Funding
Supported by a grant from the North West Cancer Research Fund (to D.M.P. and A.V.).
Abbreviations used in this paper
- CCK-2R
cholecystokinin-2 receptor
- CgA
chromogranin A
- DAPI
4′,6-diamidino-2-phenylindole
- DMSO
dimethyl sulfoxide
- FCS
fetal calf serum
- G-Gly
glycine-extended gastrin
- G-17
gastrin-17
- hGAS
high circulating levels of progastrin
- IGF
insulin-like growth factor
- MAPK
mitogen-activated protein kinase
- mRNA
messenger RNA
- PI3K
phosphoinositide 3-kinase
- PKC
protein kinase C
- PMA
phorbol myristate acetate
- rhPG
recombinant human progastrin
- RLU
relative luminescence unit
- RT-PCR
reverse-transcription polymerase chain reaction
- SMA
smooth muscle actin
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2013.03.012
Conflicts of interest
The authors disclose no conflicts.
References
- 1.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 2.Gregorieff A, Pinto D, Begthel H, et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Powell DW, Adegboyega PA, Di Mari JF, et al. Epithelial cells and their neighbors I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol. 2005;289:G2–G7. doi: 10.1152/ajpgi.00075.2005. [DOI] [PubMed] [Google Scholar]
- 4.Dockray GJ, Varro A, Dimaline R, et al. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119–139. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 5.Finley GG, Koski RA, Melhem MF, et al. Expression of the gastrin gene in the normal human colon and colorectal adenocarcinoma. Cancer Res. 1993;53:2919–2926. [PubMed] [Google Scholar]
- 6.Ciccotosto GD, McLeish A, Hardy KJ, et al. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology. 1995;109:1142–1153. doi: 10.1016/0016-5085(95)90572-3. [DOI] [PubMed] [Google Scholar]
- 7.Nemeth J, Taylor B, Pauwels S, et al. Identification of progastrin derived peptides in colorectal carcinoma extracts. Gut. 1993;34:90–95. doi: 10.1136/gut.34.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Solinge WW, Nielsen FC, Friis-Hansen L, et al. Expression but incomplete maturation of progastrin in colorectal carcinomas. Gastroenterology. 1993;104:1099–1107. doi: 10.1016/0016-5085(93)90279-l. [DOI] [PubMed] [Google Scholar]
- 9.Siddheshwar RK, Gray JC, Kelly SB. Plasma levels of progastrin but not amidated gastrin or glycine extended gastrin are elevated in patients with colorectal carcinoma. Gut. 2001;48:47–52. doi: 10.1136/gut.48.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollande F, Imdahl A, Mantamadiotis T, et al. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology. 1997;113:1576–1588. doi: 10.1053/gast.1997.v113.pm9352860. [DOI] [PubMed] [Google Scholar]
- 11.Singh P, Xu Z, Dai B, et al. Incomplete processing of progastrin expressed by human colon cancer cells: role of noncarboxyamidated gastrins. Am J Physiol. 1994;266:G459–G468. doi: 10.1152/ajpgi.1994.266.3.G459. [DOI] [PubMed] [Google Scholar]
- 12.Wang TC, Koh TJ, Varro A, et al. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996;98:1918–1929. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ottewell PD, Watson AJ, Wang TC, et al. Progastrin stimulates murine colonic epithelial mitosis after DNA damage. Gastroenterology. 2003;124:1348–1357. doi: 10.1016/s0016-5085(03)00288-9. [DOI] [PubMed] [Google Scholar]
- 14.Ferrand A, Bertrand C, Portolan G, et al. Signaling pathways associated with colonic mucosa hyperproliferation in mice overexpressing gastrin precursors. Cancer Res. 2005;65:2770–2777. doi: 10.1158/0008-5472.CAN-04-0978. [DOI] [PubMed] [Google Scholar]
- 15.Ramanathan V, Jin G, Westphalen CB, et al. P53 gene mutation increases progastrin dependent colonic proliferation and colon cancer formation in mice. Cancer Invest. 2012;30:275–286. doi: 10.3109/07357907.2012.657814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh P, Velasco M, Given R, et al. Progastrin expression predisposes mice to colon carcinomas and adenomas in response to a chemical carcinogen. Gastroenterology. 2000;119:162–171. doi: 10.1053/gast.2000.8527. [DOI] [PubMed] [Google Scholar]
- 17.Dubeykovskiy A, Nguyen T, Dubeykovskaya Z, et al. Flow cytometric detection of progastrin interaction with gastrointestinal cells. Regul Pept. 2008;151:106–114. doi: 10.1016/j.regpep.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manieri NA, Drylewicz MR, Miyoshi H, et al. Igf2bp1 is required for full induction of Ptgs2 mRNA in colonic mesenchymal stem cells in mice. Gastroenterology. 2012;143:110–121. doi: 10.1053/j.gastro.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varro A, Noble PJ, Wroblewski LE, et al. Gastrin-cholecystokinin(B) receptor expression in AGS cells is associated with direct inhibition and indirect stimulation of cell proliferation via paracrine activation of the epidermal growth factor receptor. Gut. 2002;50:827–833. doi: 10.1136/gut.50.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hori H, Nakata H, Iguchi G, et al. Oncogenic ras induces gastrin/ CCKB receptor gene expression in human colon cancer cell lines LoVo and Colo320HSR. J Lab Clin Med. 2003;141:335–341. doi: 10.1016/S0022-2143(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 21.Flint N, Cove FL, Evans GS. A low-temperature method for the isolation of small-intestinal epithelium along the crypt-villus axis. Biochem J. 1991;280:331–334. doi: 10.1042/bj2800331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potten CS, Owen G, Roberts SA. The temporal and spatial changes in cell proliferation within the irradiated crypts of the murine small intestine. Int J Radiat Biol. 1990;57:185–199. doi: 10.1080/09553009014550431. [DOI] [PubMed] [Google Scholar]
- 23.Jin G, Ramanathan V, Quante M, et al. Inactivating cholecystokinin-2 receptor inhibits progastrin-dependent colonic crypt fission, proliferation, and colorectal cancer in mice. J Clin Invest. 2009;119:2691–2701. doi: 10.1172/JCI38918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemers E, Duval C, McCaig C, et al. Insulin-like growth factor binding protein-5 is a target of matrix metalloproteinase-7: implications for epithelial-mesenchymal signaling. Cancer Res. 2005;65:7363–7369. doi: 10.1158/0008-5472.CAN-05-0157. [DOI] [PubMed] [Google Scholar]
- 25.Lahm H, Suardet L, Laurent PL, et al. Growth regulation and co-stimulation of human colorectal cancer cell lines by insulin-like growth factor I, II and transforming growth factor alpha. Br J Cancer. 1992;65:341–346. doi: 10.1038/bjc.1992.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szepeshazi K, Schally AV, Groot K, et al. Antagonists of growth hormone-releasing hormone (GH-RH) inhibit IGF-II production and growth of HT-29 human colon cancers. Br J Cancer. 2000;82:1724–1731. doi: 10.1054/bjoc.2000.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deutsch E, Maggiorella L, Wen B, et al. Tyrosine kinase inhibitor AG1024 exerts antileukaemic effects on STI571-resistant Bcr-Abl expressing cells and decreases AKT phosphorylation. Br J Cancer. 2004;91:1735–1741. doi: 10.1038/sj.bjc.6602190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng H, Pritchard DM, Yang X, et al. KLF4 gene expression is inhibited by the notch signaling pathway that controls goblet cell differentiation in mouse gastrointestinal tract. Am J Physiol Gastro-intest Liver Physiol. 2009;296:G490–G498. doi: 10.1152/ajpgi.90393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel O, Marshall KM, Bramante G, et al. The C-terminal flanking peptide (CTFP) of progastrin inhibits apoptosis via a PI3-kinase-dependent pathway. Regul Pept. 2010;165:224–231. doi: 10.1016/j.regpep.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Rengifo-Cam W, Umar S, Sarkar S, et al. Antiapoptotic effects of progastrin on pancreatic cancer cells are mediated by sustained activation of nuclear factor-{kappa}B. Cancer Res. 2007;67:7266–7274. doi: 10.1158/0008-5472.CAN-07-1206. [DOI] [PubMed] [Google Scholar]
- 31.Baldwin GS, Hollande F, Yang Z, et al. Biologically active recombinant human progastrin(6-80) contains a tightly bound calcium ion. J Biol Chem. 2001;276:7791–7796. doi: 10.1074/jbc.M009985200. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar S, Swiercz R, Kantara C, et al. Annexin A2 mediates up-regulation of NF-kappaB, beta-catenin, and stem cell in response to progastrin in mice and HEK-293 cells. Gastroenterology. 2011;140:583–595. doi: 10.1053/j.gastro.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkar S, Kantara C, Singh P. Clathrin mediates endocytosis of progastrin and activates MAPKs: role of cell surface annexin A2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G712–G722. doi: 10.1152/ajpgi.00406.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrand A, Lachal S, Bramante G, et al. Stimulation of proliferation in the colorectal mucosa by gastrin precursors is blocked by des-ferrioxamine. Am J Physiol Gastrointest Liver Physiol. 2010;299:G220–G227. doi: 10.1152/ajpgi.00046.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh P, Lu X, Cobb S, et al. Progastrin1-80 stimulates growth of intestinal epithelial cells in vitro via high-affinity binding sites. Am J Physiol Gastrointest Liver Physiol. 2003;284:G328–G339. doi: 10.1152/ajpgi.00351.2002. [DOI] [PubMed] [Google Scholar]
- 36.McCaig C, Duval C, Hemers E, et al. The role of matrix metalloproteinase-7 in redefining the gastric microenvironment in response to Helicobacter pylori. Gastroenterology. 2006;130:1754–1763. doi: 10.1053/j.gastro.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 37.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 38.Nosho K, Yamamoto H, Taniguchi H, et al. Interplay of insulin-like growth factor-II, insulin-like growth factor-I, insulin-like growth factor-I receptor, COX-2, and matrix metalloproteinase-7, play key roles in the early stage of colorectal carcinogenesis. Clin Cancer Res. 2004;10:7950–7957. doi: 10.1158/1078-0432.CCR-04-0875. [DOI] [PubMed] [Google Scholar]
- 39.Ewton DZ, Kansra S, Lim S, et al. Insulin-like growth factor-I has a biphasic effect on colon carcinoma cells through transient inactivation of forkhead1, initially mitogenic, then mediating growth arrest and differentiation. Int J Cancer. 2002;98:665–673. doi: 10.1002/ijc.10229. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Zhang Y. Growth inhibition of insulin-like growth factor I receptor monoclonal antibody to human colorectal cancer cells. Cancer Invest. 2008;26:230–236. doi: 10.1080/07357900701508975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.