Abstract
The use of intravenous bisphosphonates (pamidronate or zoledronic acid) is the cornerstone for the management of multiple myeloma-(MM-) related bone disease. However, osteonecrosis of the jaw (ONJ) is a rare, but sometimes difficult to manage, adverse effect of bisphosphonates therapy. A retrospective review of all MM patients who were treated with bisphosphonates in our department, from 2003 to 2013, and developed ONJ was performed. According to inclusion criteria, 38 patients were studied. All these patients were treated as conservatively as possible according to the American Association of Oral and Maxillofacial Surgeons criteria. Patients were managed with observation, oral antibacterial mouth rinse with chlorhexidine, oral antibiotics, pain control with analgesics, nonsurgical sequestrectomy with or without simultaneous administration of antibiotics, or major surgery with or without antibiotics. Healing of the lesions was achieved in 23 (60%) patients who were treated with conservative measures; the median time to healing was 12 months (95% CI: 4–21). The number of bisphosphonates infusions influenced the time to healing: the median time to healing for patients who received <16 infusions was 7 months and for those with >16 infusions was it 14 months (P = 0.017). We conclude that a primarily nonsurgical approach appears to be a successful management strategy for bisphosphonate-related ONJ.
1. Introduction
Bisphosphonate-related osteonecrosis of the jaws (BRONJ) is an avascular osteonecrosis of the jaws, associated mainly with intravenous administrated bisphosphonates but also with oral bisphosphonates. Intravenous bisphosphonates are used for the management of bone disease and bone metastases, caused by multiple myeloma and other solid tumors, for example, breast cancer, prostate cancer, and lung cancer [1, 2]. BPs main action is to inhibit osteoclast function and subsequent bone resorption, resulting in the prevention of loss of bone mass and skeletal related events, such as pathologic fractures and pain, caused by the underlying disease [3, 4]. A great number of patients with cancer benefit from the therapeutic results of BPs. Nevertheless bisphosphonate-related osteonecrosis of the jaw (BRONJ) has been described as an adverse effect of these drugs in various malignancies [5–8], with negative effect on the quality of life of the patients [9].
The diagnosis of osteonecrosis is clinical and according to suggested criteria [10] requires the presence of exposed bone in the jaw area for more than eight weeks, in a patient under current or previous treatment with a bisphosphonate, with no history of radiation therapy to the head and/or neck area.
The incidence of BRONJ ranges considerably due to various factors, such as type of bisphosphonate, type of cancer, way of administration, time of exposure, and number of infusions [11–14]. The risk of developing BRONJ in multiple myeloma patients receiving intravenous zoledronic acid or pamidronate is relatively high. Previous studies from our team as well as from other groups have identified tooth extraction or chronic trauma of the oral mucosa caused by poorly fitting dentures, poor oral hygiene, and number and duration of zoledronic acid administration as the main triggering factors for the development of ONJ [12, 15–18]. However, spontaneous development of BRONJ is also possible and has been reported [12, 17].
Several approaches have been evaluated for the treatment of patients who developed BRONJ and many management strategies have been proposed. Nevertheless, it seems that taking preventative measures is the most effective way to face BRONJ. In our current study we report on the outcome of our series of MM patients who developed ONJ and discuss management issues.
2. Materials and Methods
A retrospective review of multiple myeloma patients who were diagnosed with BRONJ from July 2003 until September 2013 and were treated in the Department of Clinical Therapeutics (Athens, Greece) was conducted. All the patients reporting symptoms and/or signs compatible with the probability of development of osteonecrosis were prospectively evaluated. BRONJ was diagnosed by a specialized maxillofacial surgeon (IM) according to the following criteria: patients, with no history of head and/or neck radiotherapy, currently or previously treated with bisphosphonates and presence of exposed bone in the maxilla and/or the mandible for more than eight weeks. All cases with denosumab associated necrosis were excluded, as well as cases in which the whole treatment was not performed by the same group, to avoid data that was not confirmed.
From 105 patients with osteonecrosis of the jaws under treatment with antiresorptive agents for any reason (solid tumor metastasis, multiple myeloma, etc.), thirty eight patients were selected according to the aforementioned criteria, that is, multiple myeloma patients with osteonecrosis of the jaw, caused by IV bisphosphonate therapy, who were treated in our clinic from the time of diagnosis of their disease. Biopsy was performed, if exclusion of myelomatous involvement was necessary. All species removed surgically (sequestra debridement) were also histologically evaluated.
The determination of the stage of osteonecrosis was made according to the definition and staging system published by the American Association of Oral and Maxillofacial Surgeons (AAOMS) updated position paper as follows: stage 0, no clinical evidence of necrotic bone, but nonspecific clinical findings and symptoms; stage 1, exposed and necrotic bone in patients who are asymptomatic and have no evidence of infection; stage 2, exposed and necrotic bone associated with infection as evidenced by pain and erythema in the region of the exposed bone with or without purulent drainage; and stage 3, exposed and necrotic bone in patients with pain, infection, and one or more of the following: exposed and necrotic bone extending beyond the region of alveolar bone (i.e., inferior border and ramus in the mandible, maxillary sinus, and zygoma in the maxilla) resulting in pathologic fracture, extra-oral fistula, oral-antral/oral-nasal communication, or osteolysis extending to the inferior border of the mandible or sinus floor.
A standardized and comprehensive history was obtained from each patient at the initial consultation. Data was abstracted, using a standardized template that collected patient information, medical history, and dental history, including recent dental extractions. Information concerning myeloma treatment, for example, number of infusions, duration of BP exposure, time for healing, and time of death, was also evaluated. All patients underwent comprehensive clinical evaluation and panoramic and/or intraoral periapical radiographs, when a com beam CT scan was performed in some cases. Management was provided according to general guidelines designed to minimize symptoms and/or achieve resolution of lesions.
The protocol we have followed since 2003 for all patients diagnosed with BRONJ was established based on the data of bibliography and the observation and personal experience of the attendant maxillofacial surgeon (IM). According to our protocol bisphosphonate therapy was interrupted in patients who developed BRONJ at the time of diagnosis according to guidelines [14]. Initial management in all cases was as conservative as possible. Regardless of stage, chlorhexidine rinses were prescribed for the majority of patients and mobile fragments of bone were managed with non-surgical sequestrectomy (simple removal of mobile bone fragments), typically without the need for local anesthesia. In patients with BRONJ and no signs of inflammation, avoidance of surgical dental treatment (extractions, implant therapy, and oral surgery procedures), amelioration of oral hygiene, and use of oral antiseptic mouth rinses (chlorhexidine 0.12% for 3 weeks per month, other antiseptic for 1 week per month) were recommended. Patients with artificial dentures were advised to remove them, in order to reduce the contact of the denture with the exposed bone and avoid further trauma of the mucosa. When inflammation was present, antimicrobial chemotherapy was given, usually metronidazole 500 mg twice a day for 2 weeks or aminopenicillins in combination with metronidazole for 15 days in more severe cases. Alternative choice for patients allergic to aminopenicillin was moxifloxacin for 10 days, as post antibiotic effect makes this treatment equal to a 15-day therapy. According to literature, the use of clindamycin in patients with BRONJ is not indicated after 2005 [12]. When bone spindles were present, only minor debridement procedures were attempted, in order to reduce trauma of the adjacent soft tissues. Observation and/or minor debridement procedures were also attempted, in case of spontaneous apoptosis of sequestra. When radiographic appearance of a sequestrum was observed, minor surgical sequestrectomy under local anesthesia and antibiotic treatment was attempted. Patients at stage 3 or patients who showed recurrence were treated with major surgical intervention, that is, peripheral ostectomy under general anaesthesia and antibiotic therapy.
Absence of exposed necrotic bone, absence of any signs of inflammation of the soft tissues, complete healing of the mucosa, and absence of subjective complains about pain and/or numbness for more than 3 months were considered as complete healing criteria.
3. Results
A total of thirty eight multiple myeloma patients were diagnosed with BRONJ, 25 males (66%) and 13 females (34%). The patients' age at time of BRONJ diagnosis ranged from 29 to 83 years, with mean age of 66 years. Twenty-six patients developed BRONJ in the mandible, 11 in the maxilla, and one patient in both mandible and maxilla. Thirty-three patients (87%) were treated with zoledronic acid (Zometa; Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA) of 4 mg infused over 15 minutes every 4 weeks, 1 patient with pamidronate (Aredia; Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA), of 90 mg infused every 4 weeks, and 4 patients (11%) were treated with both zoledronic acid and pamidronate. Mean number of BP infusions was 25.5 (6–83). The triggering factor of BRONJ development was oral surgery, such as tooth extraction in 22 cases, chronic mucosa trauma from artificial dentures in 5 cases, periodontal and/or periapical inflammation in 4 cases. Seven cases developed spontaneously, six of them at the mylohyoid ridge (Table 1).
Table 1.
Patients' clinical characteristics.
| Patient | Gender | Age at diagnosis | BP therapy | Number of infusions | Stage of BRONJ | Triggering factor |
|---|---|---|---|---|---|---|
| A.G. | Male | 81 | ZA | 12 | 1 | Spontaneous |
| A.K. | Male | 61 | ZA and Pam | 19 | 3 | Extraction |
| A.E. | Female | 70 | ZA | 25 | 1 | Spontaneous |
| B.I. | Male | 50 | ZA | 20 | 2 | Extraction |
| B.A. | Male | 76 | ZA | 11 | 1 | Spontaneous |
| B.Ir. | Female | 53 | ZA | 25 | 2 | Extraction |
| B.D. | Male | 65 | Z.A and Pam | 80 | 2 | Extraction |
| B.P. | Female | 43 | ZA | 26 | 3 | Extraction |
| B.E. | Female | 79 | ZA | 42 | 3 | Extraction |
| B.S. | Male | 81 | ZA | 32 | 2 | Trauma from dentures |
| D.A. | Male | 63 | ZA | 28 | 2 | Extraction |
| D.Z. | Male | 59 | ZA | 6 | 1 | Extraction |
| D.E. | Female | 72 | ZA | 13 | 0 | Trauma from dentures |
| G.M. | Male | 82 | ZA | 12 | 2 | Extraction |
| K.K. | Male | 74 | ZA | 17 | 3 | Spontaneous |
| K.M. | Female | 72 | ZA | 39 | 1 | Spontaneous |
| K.E. | Female | 68 | ZA | 22 | 3 | Trauma from dentures |
| K.N. | Male | 78 | ZA | 58 | 2 | Extraction |
| K.P. | Male | 66 | ZA | 17 | 2 | Extraction |
| K.V. | Male | 73 | ZA | 30 | 0 | Periapical abscess |
| K.I. | Male | 69 | Pam | 25 | 2 | Extraction |
| M.T. | Female | 59 | ZA | 31 | 1 | Trauma from dentures |
| P.O. | Female | 61 | ZA | 48 | 2 | Extraction |
| P.G. | Male | 81 | ZA | 59 | 1 | Trauma from dentures |
| P.V. | Female | 57 | ZA | 83 | 2 | Periapical abscess |
| P.T. | Male | 61 | ZA | 15 | 1 | Spontaneous |
| P.M. | Female | 69 | ZA | 21 | 1 | Trauma from dentures |
| P.Ma. | Female | 71 | ZA | 36 | 1 | Periodontal Inflammation |
| P.K. | Male | 59 | ZA | 8 | 3 | Extraction |
| P.D. | Male | 61 | ZA and Pam | 34 | 2 | Extraction |
| S.E. | Male | 65 | Z.A | 13 | 3 | Extraction |
| S.D. | Male | 61 | Z.A | 26 | 3 | Extraction |
| S.K. | Male | 55 | Z.A | 65 | 2 | Periodontal Inflammation |
| S.G. | Male | 80 | Z.A | 45 | 2 | Spontaneous |
| T.P. | Male | 29 | ZA and Pam | 38 | 2 | Extraction |
| V.C. | Male | 50 | ZA | 25 | 3 | Extraction |
| X.E. | Female | 67 | ZA | 26 | 0 | Extraction |
| Z.L. | Male | 72 | ZA | 17 | 3 | Extraction |
|
| ||||||
| Total | Male: 25 female: 13 |
66 Years (29–83) |
ZA: 33 Pam: 1 ZA + Pam: 4 |
25.5 (6–83) |
St 0: 3 St 1: 8 St 2: 17 St 3: 10 |
Extraction: 22 Trauma Dentures: 5 Periodontal/periapical inflammation: 4 Spontaneous: 7 |
ZA: zoledronic Acid; Pam: pamidronate.
Biopsy and histological assessment of the sequestra were performed in 29 cases, which confirmed the complication. Three patients (8%) were diagnosed with stage 0, eight patients (21%) with stage 1, seventeen cases (45%) with stage 2, and ten (26%) with stage 3 ONJ (Table 2).
Table 2.
Management of ONJ by stage.
| Stage | N | CHL rinses and observation plus removal of bony edges | Antibiotics plus removal of bony edges | Spontaneous apoptosis of sequestra | Minor surg. intervention-Sequstrectomy | Major surgical intervention |
|---|---|---|---|---|---|---|
| 0 | 3 | 0 | 2 (67%) | 0 | 1 (33%) | 0 |
| 1 | 8 | 1 (12.5%) | 1 (12.5%) | 5 (62.5%) | 1 (12.5%) | 0 |
| 2 | 17 | 1 (5.9%) | 4 (23.5%) | 1 (5.9%) | 11 (64.7%) | 0 |
| 3 | 10 | 1 (10%) | 3 (30%) | 1 (10%) | 3 (30%) | 2 (20%) |
|
| ||||||
| Total | 38 | 3 (7.9%) | 10 (26.3%) | 7 (18.4%) | 16 (42.1%) | 2 (5.3%) |
Three patients were treated only with observation, mouth rinses with chlorhexidine 0.12% for 3 weeks per month, other antiseptic for 1 week per month, in order to avoid disturbance of the oral flora, and removal of the bony edges of the lesion. One showed complete healing, one remained stable, without any signs of inflammation or pain until death, and one patient developed higher stage of ONJ (stage 2) and was treated with antibiotics. Ten patients were treated with chlorhexidine 0.12% for 3 weeks per month, other antiseptic for 1 week per month, and antibiotics, whenever inflammation appeared. Eight of these patients remained stable for a mean follow-up of 24 months (3–48), one was completely healed after 8 months, with a 5 months follow-up after healing and one patient developed a higher stage of ONJ and is scheduled for surgery, whenever his health status permits. Seven patients had spontaneous apoptosis of sequestra and they all showed complete healing. Mean follow-up was 27 months (8–40) after the confirmation of healing. No recurrence was observed in any of these patients, until the last-follow up or until death. Conservative sequestrectomy was attempted after a meantime of 12 months under antibiotic therapy in 16 cases. Eleven of these cases showed complete healing; one case was not yet completely healed at the time of the last follow-up, one patient died during the follow-up after healing period, and three cases underwent a second minor surgery before achieving complete healing. Major surgical intervention was attempted in 2 patients with stage 3 BRONJ. Complete healing was observed in both cases, although one patient underwent a second surgery after a period of 5 months, in order to reverse the failure of the first surgery. The other patient underwent two surgeries in different locations each time—one in the maxilla and one in the mandible, since he had developed ONJ bilateral in the maxilla and the mandible. Mean follow-up after healing in both cases was more than 6 months (Table 3).
Table 3.
Results of ONJ treatment.
| Treatment | N | Stable | Complete healing | Regression |
|---|---|---|---|---|
| CHL rinses and observation plus removal of bony edges | 3 | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) |
| Antibiotics plus removal of bony edges | 10 | 8 (80%) | 1 (10%) | 1 (10%) |
| Spontaneous apoptosis of sequestra | 7 | 0 | 7 (100%) | 0 |
| Minor surg. intervention-Sequestrectomy | 16 | 1 (6.25%) | 15 (93.75%) | 0 |
| Major surgical intervention | 2 | 0 | 2 (100%) | 0 |
|
| ||||
| Total | 38 | 10 (26.3%) | 26 (68.4%) | 2 (5.3%) |
In patients where healing was stated (N = 24, 63%), by removal of bony edges, spontaneous apoptosis of the sequestra, or sequestrectomy, the median time to healing was 12 months (95% CI 4–21). A statistically significant difference (P = 0.017) was found between groups with more and less than 16 infusions of bisphosphonates, when median time to healing for those with <16 infusions was 7 months and median time to healing for those with >16 infusions was 14 months (P = 0.017; Figure 1).
Figure 1.
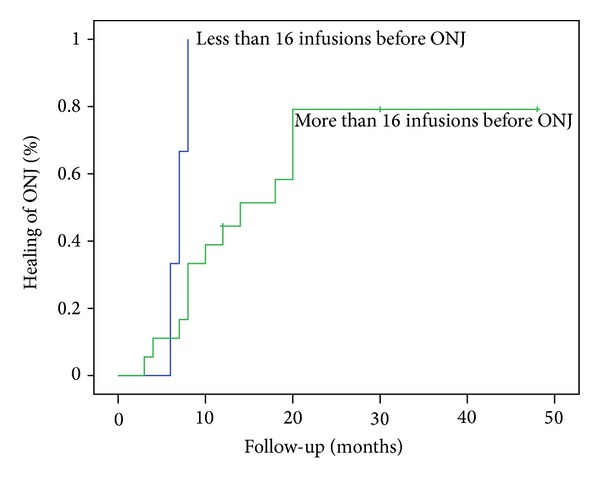
Median time to healing in association with the number of infusions of bisphosphonates.
4. Discussion
The incidence of BRONJ is yet undetermined. According to many studies of patients with multiple myeloma, breast, or prostate cancer, who received intravenous amino-BP therapy, the occurrence of osteonecrosis is estimated to be approximately 4–11% [7, 13, 18]. In our study which included only multiple myeloma patients, the incidence of ONJ was almost 6%. The probability of developing BRONJ ranges due to various risk factors. The number and frequency of infusions, but mainly the cumulative dose of BP, are strongly associated with the risk of BRONJ [12, 19]. Invasive dental procedures, that is, tooth extractions, implant therapy, oral surgery, as well as mucosa trauma by poor fitting dentures have been reported as the most important triggering factors of developing this complication. However, spontaneous development of BRONJ occurs in approximately 20% of the patients who develop BRONJ [20, 21]. Indeed, in our study 57.9% of the patients who developed osteonecrosis underwent dental extraction, 13.2% had chronic mucosa trauma by artificial dentures, 10.5% of ONJ patients developed ONJ due to periodontal and/or periapical inflammation, and in 18,4% patients it occurred spontaneously, which comes in agreement with the latest reviews. The mean number of infusions was 25.5 and the mean time of BP exposure was 36.5 months. In the present study, lesions occurred more frequently in the mandible than in the maxilla (67% versus 33%). This ratio is also confirmed by several studies [22–24].
The management of BRONJ is a difficult goal to achieve and still remains controversial, since consensus standard protocol has not yet been established. According to the guidelines of the AAOMS, treatment strategies of BRONJ emphasize mainly the elimination of pain and inflammation and the reduction of the exposure of the necrotic bone and secondarily they emphasize the complete healing of the lesion. Several methods have been proposed, which can be categorized as nonsurgical or conservative [25–27] and surgical approaches [28, 29].
Nonsurgical treatment includes a combination of antiseptic mouth rinses, antimicrobial chemotherapy, when inflammation occurs, and nonsurgical sequestrectomy and/or debridement. The outcomes of most studies [24–27] seem to be satisfactory. According to one of the largest—in terms of patients—retrospective study by Lerman et al., 71–80% of the cases, treated conservatively improved or remained asymptomatic and stable [25]. In our study 63% of the patients who were treated with conservative measures (removal of bony edges, spontaneous apoptosis of sequestra, or minor surgical intervention) achieved complete healing and another 23.7% remained asymptomatic and stable, while in 5.2% of the patients major surgical interpretation was performed, because of failure of the conservative treatment. Van den Wyngaert et al. suggest that there are several factors, such as stage of ONJ, patient's health condition, time of exposure to BP, type of BP therapy, use of chemotherapy before ONJ, which should be considered in order to proceed to a specific treatment of ONJ, although it seems that strictly conservative treatment at low stages of the complication can lead to healing in about half of the cases [26]. In agreement with the above results a study by Moretti et al. confirms management of pain with minimally invasive treatment in more than 60% of the cases, while all of the patients who underwent sequestrectomy—spontaneously or gently induced by the surgeon—achieved complete healing [27]. In the present study 87.5% of stage 1 patients, 59% of stage 2, and 50% of stage 3 patients were healed.
On the other hand, radical surgical treatment of ONJ, including extensive sequestrectomy and limited or extensive bone resection, has showed healing of BRONJ in several studies [29–33]. The results of the study by Wilde et al. showed that 88% of the patients, treated surgically, achieved complete healing of ONJ. Nevertheless, a statistically high failure rate in stage 3 ONJ, approximately 36%, may initiate doubts about the efficiency of the surgery, while adequate surgical planning and high degree of experience on the determination of the resection margins are clearly pointed out by the author. Stockmann et al., at a study with 80 patients, report a success rate of about 89%, which declined to 84% within 14 months postoperatively [31]. The outcomes of a review by Kühl et al. showed that, when comparing the results of conservative and surgical treatment of BRONJ, it seems that there is no difference regarding the success of treatment (e.g., 60.5% versus 60.4%), although it appeared that complete healing of BRONJ after conservative treatment is only successful in low stages of the complication [32]. We also conclude (P = 0.017) that the number of BP infusions is associated with the median time to healing. Patients who received less than 16 infusions achieved healing in the half time, compared with patients who received more than 16 infusions (7 versus 14 months).
Other therapeutic approaches, such as medical ozone [34] and ND:YAG laser stimulation [35, 36] have given encouraging results in the management of patients with ONJ but the experience with these methods is limited.
In the present study, major surgical intervention was decided only at high levels of ONJ or in case of failure of conservative measures. Both patients who underwent major surgery achieved complete healing. Due to bisphosphonates discontinuation, many cases (7) of spontaneous apoptosis of the sequestra have been observed. The mean time of sequestra formation was 10.2 months where the mean time for minor surgery intervention (15 patients) was 15.6 months. It could be a reasonable thought that in that period of time the bone turnover in the necrotic area starts to work. When treatment with IV bisphosphonates could be stopped, it is reasonable to treat patients conservatively until the time where sequestra formation seems to start. Therefore, in agreement with the AAOMS guidelines, we believe that the cost-benefit for patients who are already debilitated by their malignancy leans to more conservative treatment strategies of ONJ with satisfactory results and surgical intervention should be performed only in cases of failure of the above strategies.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. New England Journal of Medicine. 1996;334(8):488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Yee GC, Somerfield MR, et al. American society of clinical oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. Journal of Clinical Oncology. 2007;25(17):2464–2472. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 3.Fleisch H. Bisphosphonates: mechanisms of action. Endocrine Reviews. 1998;19(1):80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 4.Rogers MJ, Gordon S, Benford HL, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88(12):2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. Journal of Oral and Maxillofacial Surgery. 2003;61(9):1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 6.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. Journal of Oral and Maxillofacial Surgery. 2004;62(5):527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. Journal of Clinical Oncology. 2005;23(34):8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 8.Migliorati CA, Schubert MM, Peterson DE, Seneda LM. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer. 2005;104(1):83–93. doi: 10.1002/cncr.21130. [DOI] [PubMed] [Google Scholar]
- 9.Miksad RA, Lai K, Dodson TB, et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist. 2011;16(1):121–132. doi: 10.1634/theoncologist.2010-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws. American Association of oral and maxillofacial surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. Journal of Oral and Maxillofacial Surgery. 2007;65(3):369–376. doi: 10.1016/j.joms.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos MA, Kastritis E, Moulopoulos LA, et al. The incidence of osteonecrosis of the jaw in patients with multiple myeloma who receive bisphosphonates depends on the type of bisphosphonate. Blood. 2005;106(11):p. 637. [Google Scholar]
- 12.Marx RE, Cillo JE, Jr., Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. Journal of Oral and Maxillofacial Surgery. 2007;65(12):2397–2410. doi: 10.1016/j.joms.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Durie BG, Katz M, Crowley J, et al. Osteonecrosis of the Jaw and bisphosphonates. New England Journal of Medicine. 2005;353(1):99–102. doi: 10.1056/NEJM200507073530120. [DOI] [PubMed] [Google Scholar]
- 14.Terpos E, Sezer O, Croucher PI, et al. The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network. Annals of Oncology. 2009;20(8):1303–1317. doi: 10.1093/annonc/mdn796. [DOI] [PubMed] [Google Scholar]
- 15.Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. Journal of Oral and Maxillofacial Surgery. 2007;65(3):415–423. doi: 10.1016/j.joms.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 16.Vahtsevanos K, Kyrgidis A, Verrou E, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. Journal of Clinical Oncology. 2009;27(32):5356–5362. doi: 10.1200/JCO.2009.21.9584. [DOI] [PubMed] [Google Scholar]
- 17.Van den Wyngaert T, Huizing MT, Vermorken JB. Osteonecrosis of the jaw related to the use of bisphosphonates. Current Opinion in Oncology. 2007;19(4):315–322. doi: 10.1097/CCO.0b013e32819f820b. [DOI] [PubMed] [Google Scholar]
- 18.Campisi G, Fedele S, Fusco V, Pizzo G, Di Fede O, Bedogni A. Epidemiology, clinical manifestations, risk reduction and treatment strategies of jaw osteonecrosis in cancer patients exposed to antiresorptive agents. Future Oncology. 2014;10(2):257–275. doi: 10.2217/fon.13.211. [DOI] [PubMed] [Google Scholar]
- 19.Kastritis E, Terpos E, Melakopoulos I, et al. The cumulative dose but not the frequency of infusions is a risk factor for the development of osteonecrosis of the jaw (ONJ) in myeloma patients who receive zoledronic acid (ZA). American Society of Hematology Annual Meeting; 2013; [Google Scholar]
- 20.Wilkinson GS, Kuo Y, Freeman JL, Goodwin JS. Intravenous bisphosphonate therapy and inflammatory conditions or surgery of the jaw: a population-based analysis. Journal of the National Cancer Institute. 2007;99(13):1016–1024. doi: 10.1093/jnci/djm025. [DOI] [PubMed] [Google Scholar]
- 21.Migliorati CA, Siegel MA, Elting LS. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncology. 2006;7(6):508–514. doi: 10.1016/S1470-2045(06)70726-4. [DOI] [PubMed] [Google Scholar]
- 22.Stanton DC, Balasanian E. Outcome of surgical management of bisphosphonate-related osteonecrosis of the jaws: review of 33 surgical cases. Journal of Oral and Maxillofacial Surgery. 2009;67(5):943–950. doi: 10.1016/j.joms.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 23.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. Journal of Bone and Mineral Research. 2007;22(10):1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 24.Scoletta M, Arduino PG, Dalmasso P, Broccoletti R, Mozzati M. Treatment outcomes in patients with bisphosphonate-related osteonecrosis of the jaws: a prospective study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2010;110(1):46–53. doi: 10.1016/j.tripleo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Lerman MA, Xie W, Treister NS, Richardson PG, Weller EA, Woo S. Conservative management of bisphosphonate-related osteonecrosis of the jaws: staging and treatment outcomes. Oral Oncology. 2013;49(9):977–983. doi: 10.1016/j.oraloncology.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Van den Wyngaert T, Claeys T, Huizing MT, Vermorken JB, Fossion E. Initial experience with conservative treatment in cancer patients with osteonecrosis of the jaw (ONJ) and predictors of outcome. Annals of Oncology. 2009;20(2):331–336. doi: 10.1093/annonc/mdn630. [DOI] [PubMed] [Google Scholar]
- 27.Moretti F, Pelliccioni GA, Montebugnoli L, Marchetti C. A prospective clinical trial for assessing the efficacy of a minimally invasive protocol in patients with bisphosphonate-associated osteonecrosis of the jaws. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2011;112(6):777–782. doi: 10.1016/j.tripleo.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Carlson ER, Basile JD. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. Journal of Oral and Maxillofacial Surgery. 2009;67(5):85–95. doi: 10.1016/j.joms.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Wilde F, Heufelder M, Winter K, et al. The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2011;111(2):153–163. doi: 10.1016/j.tripleo.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Bedogni A, Saia G, Bettini G, et al. Long-term outcomes of surgical resection of the jaws in cancer patients with bisphosphonate-related osteonecrosis. Oral Oncology. 2011;47(5):420–424. doi: 10.1016/j.oraloncology.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Stockmann P, Burger M, von Wilmowsky C, et al. The outcome after surgical therapy of bisphosphonate-associated osteonecrosis of the jaw-results of a clinical case series with an average follow-up of 20 months. Clinical Oral Investigations. 2013;18(4):1299–1304. doi: 10.1007/s00784-013-1092-2. [DOI] [PubMed] [Google Scholar]
- 32.Kühl S, Walter C, Acham S, Pfeffer R, Lambrecht JT. Bisphosphonate-related osteonecrosis of the jaws—a review. Oral Oncology. 2012;48(10):938–947. doi: 10.1016/j.oraloncology.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 33.Patel V, McLeod NMH, Rogers SN, Brennan PA. Bisphosphonate osteonecrosis of the jaw-a literature review of UK policies versus international policies on bisphosphonates, risk factors and prevention. British Journal of Oral and Maxillofacial Surgery. 2011;49(4):251–257. doi: 10.1016/j.bjoms.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Ripamonti CI, Cislaghi E, Mariani L, Maniezzo M. Efficacy and safety of medical ozone (O3) delivered in oil suspension applications for the treatment of osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates: preliminary results of a phase I-II study. Oral Oncology. 2011;47(3):185–190. doi: 10.1016/j.oraloncology.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Vescovi P, Merigo E, Manfredi M, et al. Nd:YAG laser biostimulation in the treatment of bisphosphonate-associated osteonecrosis of the jaw: clinical experience in 28 cases. Photomedicine and Laser Surgery. 2008;26(1):37–46. doi: 10.1089/pho.2007.2181. [DOI] [PubMed] [Google Scholar]
- 36.Luomanen M, Alaluusua S. Treatment of bisphosphonate-induced osteonecrosis of the jaws with Nd:YAG laser biostimulation. Lasers in Medical Science. 2012;27(1):251–255. doi: 10.1007/s10103-011-0929-7. [DOI] [PubMed] [Google Scholar]


