Abstract
The objective of this study was to find out the impact of environmental conditions on the survival of intestinal parasites on environmental surfaces commonly implicated in the transmission of these parasites. The study was performed by incubating Cryptosporidium and Giardia (oo)cysts on environmentally relevant surfaces such as brushed stainless steel, formica, ceramic, fabric, and skin. Parallel experiments were conducted using clean and soiled coupons incubated under three temperatures. The die-off coefficient rates (K) were calculated using first-order exponential formula. For both parasites, the fastest die-off was recorded on fabric, followed by ceramic, formica, skin, and steel. Die-off rates were directly correlated to the incubation temperatures and surface porosity. The presence of organic matter enhanced the survivability of the resting stages of test parasites. The decay rates calculated in this study can be used in models for public health decision-making process and highlights the mitigation role of hand hygiene agents in their prevention and control.
1. Introduction
Populations in different parts of the world face diverse parasitic challenges. The public health managers around the world face different challenges because the diversity of parasites endemic in an area is influenced by a variety of factors [1–4]. For example, Enterobius vermicularis is more prevalent in temperate areas [5], and incidence of Ascaris lumbricoides is more common in tropical regions [6].
To reduce the infectious disease load in communities, public health managers have a variety of intervention tools; however, the effectiveness of these tools is impacted by environmental, cultural, and socioeconomic conditions. For example, hand washing can be effective under a broad variety of conditions [7–9]. However, the effectiveness of these interventions is impacted by personal and cultural norms [10]. The role of hand washing as an intervention for improving public health safety is well established. However, it is practiced significantly differently across societies and cultures. For example, in Peru, only 11% of people wash their hands after defecation and the use of soap is still more rare [11]. In a recent survey, we found that 14% of children and adults in India and 7% in Bangladesh carry the neglected enteric parasites on their hands [12]. Furthermore, these parasites have been implicated in impacting both the physical and the cognitive development of children in the affected regions of the world highlighting the mitigational role of hygiene [13]. In addition to personal hygiene practices, environmental factors also influence the richness of parasite species and intensity of infection in the host species [14] by impacting the survival of the environmental stages of these intestinal parasites. Among environmental conditions, temperature and relative humidity are known to have significant effect on the survival of (oo)cysts. Temperature changes have been associated with the parasite development rates [15, 16], while moisture and relative humidity have been reported for maintaining parasite pressure [17, 18]. Optimum moisture and temperature conditions are critical for maintaining the vitality of food stored in (oo)cysts until they are ready for the next stage in their life cycle [19]. Given the importance of temperature and relative humidity in parasite life cycle, it is imperative to study the impact of these factors on intestinal parasites that have been neglected in the scientific studies during the recent years [7].
Environmental, cultural, and socioeconomic conditions factors along with nature of public health threat such as pathogen survival are important inputs in public health decision-making processes. Reliability of any model is tied to the accuracy of data used in the model. Information on the survival of intestinal parasites and public health relevant conditions is sparse and scattered in the literature. There is a need for comprehensive study to generate scientific data to facilitate decision-making process for addressing the public health threat from intestinal parasites.
In this study, survival of Cryptosporidium and Giardia (oo)cysts on animate and inanimate surfaces was investigated under environmental conditions of public health relevance.
2. Methods
2.1. Parasites
C. parvum oocysts (Iowa isolate) were obtained from the Sterling Parasitology Laboratory, the University of Arizona, Tucson, AZ, USA. The Giardia muris cysts were obtained from Dr. Shivaji Ramalingam (Oregon Health Sciences University, Portland, OR, USA). Upon receipt, all the stocks of (oo)cysts were stored at 4°C until being used for assays. Concentrations of all the stocks and working solutions were determined by direct count using a hemocytometer.
2.2. Coupons for Survival Studies
Five types of materials representing animate and inanimate prototype surfaces of different porosity and surface roughness were selected for the study. The materials included brushed stainless steel 18 Ga (MetalsDepot, Winchester, KY, USA), formica (Home Depot, Gilbert, AZ, USA), ceramic plates (Home Depot, Gilbert, AZ, USA), fabric (100% cotton) (Testfabrics, West Pittson, PA, USA), and synthetic skin (Bioscience, Castro Valley, CA, USA). For each material, coupons measuring 1 square inch (2.5 cm × 2.5 cm) were used in this study. Porosity of the selected material was determined using water saturation method [20].
2.3. Experimental Conditions
Survival of Cryptosporidium and Giardia (oo)cysts was studied on brushed stainless steel, formica, ceramic, fabric, and synthetic skin. For each test parasite, (oo)cysts were inoculated on each type of material with and without organic matter—10 mg/0.2 mL bovine serum albumen (Mallinckrodt, Paris, KY, USA). The inoculated coupons were incubated at 15°C/50% RH, 25°C/50% RH, and 37°C/70% RH for a specified time period. All the experiments were performed in triplicate. After the specified incubation period, inoculated coupons were processed to study the viability/infectivity of each parasite.
2.4. Environmental Chamber
Inoculated coupons were placed in glass jars that were set up according to ASTM Standard E96. The relative humidity inside these jars was maintained by using anhydrous calcium chloride as a desiccant. These coupon holding jars were placed in trays inside environmental chambers that were built according to the ASTM method D3273 using polypropylene tanks. Digital thermometer/hygrometers were installed in environmental chambers to constantly monitor the relative humidity and temperature.
2.5. Viability and Infectivity Studies
The viability of Cryptosporidium oocysts and Giardia cysts was determined using methods described previously [21, 22]. Sample processing and PCR conditions were based on the procedure previously described [22].
2.6. Calculation of Die-Off Rates
The first-order exponential formula was used to simulate (oo)cysts die-off on five surfaces at different environmental conditions. The equation is as follows:
| (1) |
where K is the die-off rate coefficient and y 0 and y t are the numbers of (oo)cysts at time zero, under initial conditions and at time t, respectively. If normalized by the initial numbers of (oo)cysts, (1) can be rewritten as follows:
| (2) |
where
| (3) |
In (2), K is independent of the initial numbers of parasitic (oo)cysts and represents a constant die-off rate over the entire incubation period.
3. Results
The survival/infectivity of Cryptosporidium oocysts and Giardia cysts was studied under different environmental conditions, and inactivation kinetics are presented here. In general, parasites survival was inversely correlated with the storage temperature and porosity of the surface. The temporal decay of Cryptosporidium oocysts and Giardia cysts under various environmental conditions (temperature, relative humidity, and organic material) and on different surfaces (microlevel variation in porosity and roughness) is presented in Figures 1 and 2, and the results are summarized in Table 1. On clean (nonsoiled) surfaces stored at 25°C, the die-off rate of Giardia cysts ranged from −0.25754 to −0.7764, whereas, for similar samples stored at 12°C and 37°C, the cysts die-off rate ranged from −0.14704 to −0.34612 and −0.36001 to −0.89851, respectively. Similarly, under various test conditions, the overall trends of the Cryptosporidium oocysts die-off were similar to the one of Giardia cysts; however, the level of die-off for both parasites was different under comparable conditions. The Cryptosporidium oocysts die-off on clean surfaces incubated at 37°C ranged from −0.16308 to −1.08403, whereas incubation at 12°C and 25°C resulted in oocysts die-off rates ranging from −0.04837 to −0.46996 and −0.09552 to −0.58958, respectively. Presence of organic matter on the surface had a protective effect on the survival of both parasites; however, this protective effect was more consistent for Giardia cysts than Cryptosporidium oocysts (Table 1). The results of this study concur with previous studies reporting the importance of environmental conditions such as temperature on the survival and transmission of intestinal parasites [23–25].
Figure 1.
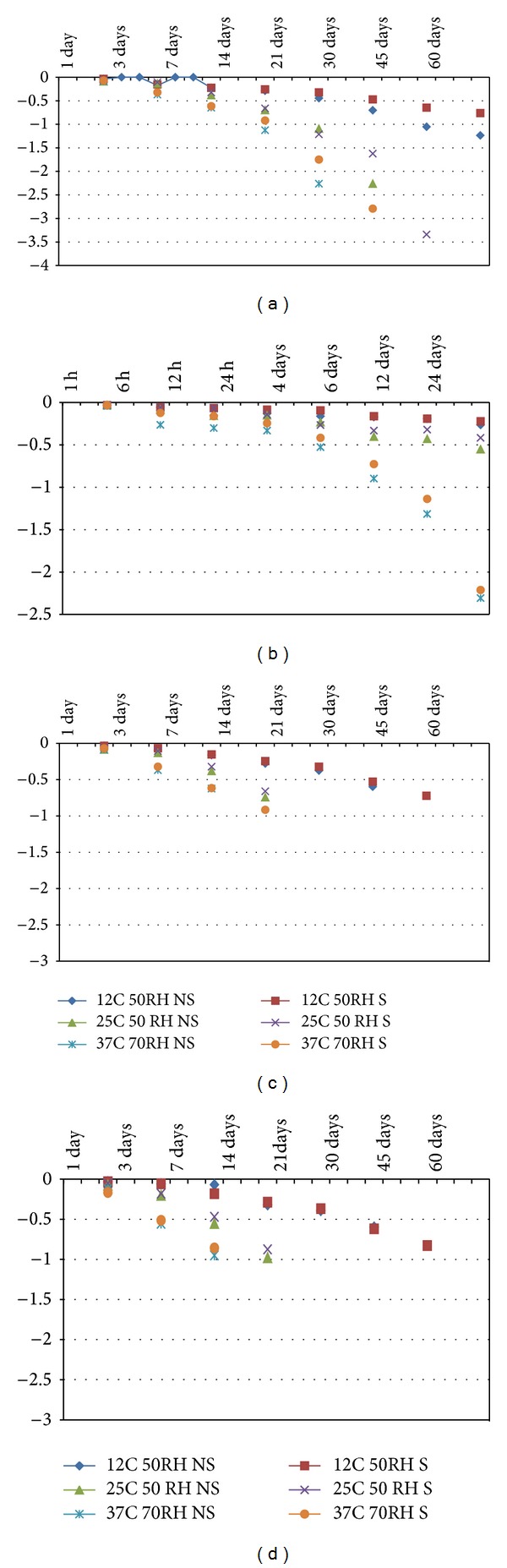
K values for the first-order die-off rate of Giardia on (a) stainless steel, (b) skin, (c) formica, and (d) fabric.
Figure 2.
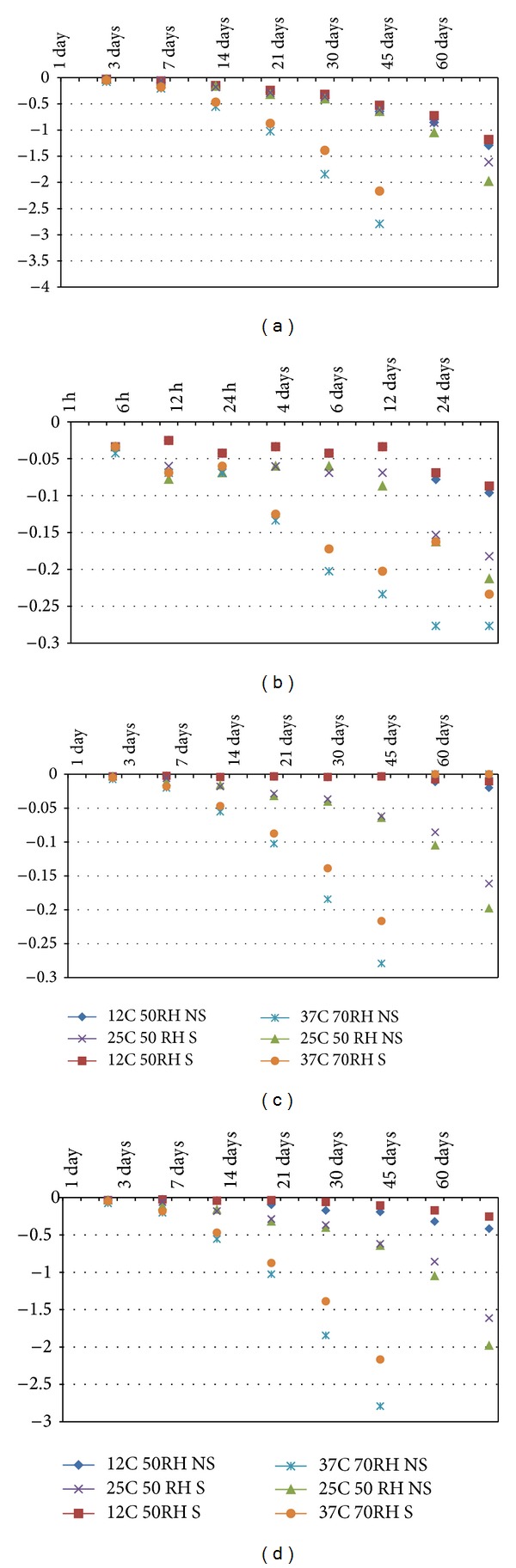
K values for the first-order die-off rate of Cryptosporidium on (a) stainless steel, (b) skin, (c) formica, and (d) fabric.
Table 1.
Summary of die-off rates (the first-order K values) of Giardia cysts and Cryptosporidium oocysts on a variety of surfaces incubated in different environmental conditions.
| Parasite | Test material | Test conditions | |||||
|---|---|---|---|---|---|---|---|
| 12°C 50% RH | 25°C 50% RH | 37°C 70% RH | |||||
| NS | S | NS | S | NS | S | ||
| Giardia | Fabric | −0.24609 | −0.3409 | −0.45487 | −0.39509 | −0.54514 | −0.5132 |
| Ceramic | −0.2572 | −0.29622 | −0.35363 | −0.28729 | −0.36001 | −0.47733 | |
| Formica | −0.2572 | −0.29622 | −0.33656 | −0.28729 | −0.36001 | −0.48086 | |
| Skin | −0.14704 | −0.11569 | −0.25754 | −0.20598 | −0.74951 | −0.6331 | |
| Stainless steel | −0.34612 | −0.35842 | −0.7764 | −1.04699 | −0.89851 | −1.07793 | |
| Average K | −0.25073 | −0.28149 | −0.4358 | −0.44453 | −0.58264 | −0.63648 | |
|
| |||||||
| Cryptosporidium | Fabric | −0.16294 | −0.09118 | −0.58958 | −0.5037 | −1.08403 | −0.85624 |
| Ceramic | −0.46996 | −0.40785 | −0.58958 | −0.5037 | −1.08403 | −0.85624 | |
| Formica | −0.0663 | −0.04954 | −0.58958 | −0.5037 | −0.81303 | −0.64218 | |
| Skin | −0.04837 | −0.04609 | −0.09552 | −0.08723 | −0.16308 | −0.13251 | |
| Stainless steel | −0.46996 | −0.40785 | −0.58958 | −0.5037 | −1.08403 | −0.85624 | |
| Average K | −0.24351 | −0.2005 | −0.49077 | −0.42041 | −0.84564 | −0.66868 | |
S: surface soiled with 5% organic matter; NS: surface not soiled (clean).
4. Discussion
In surface water, oocysts and cysts can survive for months [26–29]. Under natural conditions, the die-off rate of Cryptosporidium oocysts in water is 0.005–0.037 log10-units per day. For Giardia, the die-off rate is higher and more temperature-dependent, varying between 0.015 log10-units per day at 1°C and 0.28 log10-units per day at 23°C [27]. However, when present on surfaces or in solids (soil or sludge), different parasites may respond differently to variations in environmental conditions such as temperature, relative humidity (RH), porosity, and organic matter. For example, under conditions of severe desiccation, Eimeria remains viable for a significantly longer period of time than the Cryptosporidium oocysts [30]. After reviewing the available data on the relation of die-off of different parasites to moisture and temperature, the USEPA concluded that no single microorganism is really adequate to represent all other parasites [31].
4.1. Effect of Temperature
Over the course of the study, higher temperature resulted in increased die-off of Cryptosporidium and Giardia. Temperature has been regarded as one of the most critical factors in the (oo)cysts survival in the environment [29, 31–33]. In saturated soil at 37°C, 1-log reduction in the viability of Cryptosporidium oocysts was recorded in 10 days, whereas storage of oocysts for months under similar conditions at 15°C did not result in the loss of viability [34, 35]. In a study, 106 oocysts of Cryptosporidium oocysts were stored for 10 days in dry soils at 32°C, and no loss of viable oocysts was recorded by PCR-based assay [35]. Similar survival trends have been reported by others, reporting complete die-off in 24 weeks, 8 weeks, and 72 hours for 15°C, 25°C, and 37°C, respectively [35, 36]. The higher inactivation reported by these researchers may be due to diurnal changes in temperature which can result in the rapid breakdown of energy reserves, which are vital for viability and infectivity of resting stages of parasites [35, 36]. In addition, in the presence of bacterial extracellular enzymes, in just 7 hours, 1-log reduction in the number of viable/infectious Cryptosporidium oocysts has been reported [34]. These findings are critical when comparing the results of studies conducted in clean environment and under field conditions.
Various estimates have been made for rate of inactivation of parasite (oo)cysts. These resting forms of enteric parasites are more resistant to environmental conditions than the nonencysted forms. The survival of (oo)cysts depends on the type of parasites and the conditions to which they are exposed. Defining a quantified relationship between the die-off rate and environmental stresses for enteric parasite-contaminated environmental surfaces has received little attention in the literature. The USEPA has formulated default die-off values applicable to all kinds of (oo)cysts for different temperatures and incubation conditions [37]. The die-off rates of different parasites reported in this study for 15°C, 25°C, and 37°C are supported by results of previous studies. O'Donnell et al. reported die-off rates of 10(−0.2) and 10(−0.3) per month for Ascaris ova in aerobically treated sludge and anaerobic treated sludge, respectively [38]. For Giardia, die-off rates of 0.029 log10 day−1 and 0.37 log10 day−1 have been reported for water and sediment, respectively [39]. For Cryptosporidium oocysts, kept in water, soils, and feces? Needs to clarify K values of −0.041 and −0.130 per day have been reported for 15°C and 25°C, respectively [40]. In the present study, similar die-off K values were estimated for Cryptosporidium oocysts deposited on formica for one week.
4.2. Surface Porosity and Roughness
Survival of some common parasite (oo)cysts in solids (sand, loam, and sludge) of different porosity has been studied [34, 37]; however, no information is available on the survival of the (oo)cysts of the neglected intestinal parasites such as Cryptosporidium and Giardia lamblia on animate and inanimate surfaces. In general, greater viability loss was noted for all test enteric parasitic (oo)cysts incubated on fabric and ceramic compared to steel and formica. This difference in viability of various types of surfaces appears to be inversely related to the surface porosity. It can be argued that parasites (oo)cysts on higher porosity surfaces may be exposed to increased osmotic stress, which can be a factor for higher die-off rate on such surfaces. It is known that desiccation can cause lethal impact on oocysts and they can lose viability after being subjected to stress driven by the interaction of water contents and texture of storage material [38, 41]. Nasser et al. found that incubation for 10 days in dry loamy soil at 32°C resulted in a 2.5-log reduction in oocysts by cell culture assay but no such decrease was recorded by PCR assay [34]. However, only 1-log reduction was recorded in saturated soil at similar temperature. They also demonstrated that the viability of oocysts in saturated loamy soil at 15°C remained unchanged for months. Jenkins et al. reported that estimated K values for soil at 25°C were increased from 0.014 to 0.416 day−1 when the soil water potential was decreased from −0.10 to −3.2 MPa [42]. Therefore, the direct correlation between higher die-off rates of parasites (oo)cysts and higher porosity of surfaces noted in this study is supported by previous reports.
4.3. Organic Load
Presence of organic matter and soil load is known to impact the survival of parasites. In the present study, presence of organic matter generally enhanced the survival of parasites (oo)cysts. Based on the extensive amount of parasite survival data, USEPA has calculated generic die-off values of 0.000533/hour, 0.00041/hour, and 0.7845/hour for Ascaris ova in moist soil, on soil surface, and on plant surface, respectively [37]. It has been shown that soil particle or organic matter provides protection to parasites against environmental factors affecting their viability. This supports the finding of the present study reporting better survival of all parasites (oo)cysts when studied under soiled conditions.
Due to experimental and logistic limitations, most of the studies investigating parasite survival under different environmental conditions do not use factorial experimental design. Therefore, data from such studies cannot be used to isolate the impact of temperature from other variables on the survival of parasite (oo)cysts. This is the first study that used a factorial experimental design to investigate the impact of temperature, relative humidity, organic load, and surface type on the survival of parasites' resting stages [(oo)cysts] of parasites. Although with the present knowledge it is not possible to precisely define the role of environmental variables (temperature, RH, porosity, organic matter, the nutrient concentration, and the parasitic/microbial communities) in the survival of parasites (oo)cysts, recovery of neglected enteric parasitic (oo)cysts/ova from naturally contaminated hands of children reported previously [11] coupled with the results of the present study provide valuable information for predicting their survival under variable conditions of public health significance and help in designing hygienic measures for interruption of their dissemination to both animal and human populations.
Acknowledgments
Dr. Absar Alum's research is supported by RB, and Joseph R. Rubino and M. Khalid Ijaz are engaged in R&D work at RB, Montvale, NJ, USA.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Fan PC. Review of enterobiasis in Taiwan and offshore islands. Chinese Journal of Microbiology and Immunology. 1998;31(4):203–210. [PubMed] [Google Scholar]
- 2.Gleason NN, Horwitz MS, Newton LH, Moore GT. A stool survey for enteric organisms in Aspen, Colorado. American Journal of Tropical Medicine and Hygiene. 1970;19(3):480–484. doi: 10.4269/ajtmh.1970.19.480. [DOI] [PubMed] [Google Scholar]
- 3.Parekh UC, Naik PA, Udani PM, Shah PM. Parasitic infestations in pre-school children of urban and rural communities. Indian Pediatrics. 1972;9(6):332–336. [PubMed] [Google Scholar]
- 4.Sengbusch HG. Studies on enterobiasis. III—the incidence of pinworm infection in a mysore school. The Indian Journal of Pediatrics. 1970;37(6):229–238. doi: 10.1007/BF02807265. [DOI] [PubMed] [Google Scholar]
- 5.Vermund SH, Wilson CM. Pinworm (Enterobius vermicularis) Seminars in Pediatric Infectious Diseases. 2000;11(4):252–256. [Google Scholar]
- 6.Stepek G, Buttle DJ, Duce IR, Behnke JM. Human gastrointestinal nematode infections: are new control methods required? International Journal of Experimental Pathology. 2006;87(5):325–341. doi: 10.1111/j.1365-2613.2006.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alum A, Rubino JR, Ijaz MK. The global war against intestinal parasites-should we use a holistic approach? International Journal of Infectious Diseases. 2010;14(9):e732–e738. doi: 10.1016/j.ijid.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 8.Han AM, Hlaing T. Prevention of diarrhoea and dysentery by hand washing. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1989;83(1):128–131. doi: 10.1016/0035-9203(89)90737-2. [DOI] [PubMed] [Google Scholar]
- 9.Wilson JM, Chandler GN. Hand-washing reduces diarrhoea episodes: a study in Lombok, Indonesia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1991;85(6):819–821. doi: 10.1016/0035-9203(91)90468-e. [DOI] [PubMed] [Google Scholar]
- 10.Han AM, Oo KN, Aye TA, Hlaing T. Personal toilet after defaecation and the degree of hand contamination according to different methods used. The American Journal of Tropical Medicine and Hygiene. 1986;89:237–241. [PubMed] [Google Scholar]
- 11.Ijaz MK, Talukder KA, Aslam M, et al. Natural contamination of human hands with enteric parasites in Indian Subcontinent. World Journal of Clinical Infectious Diseases. 2013;3(2):13–19. [Google Scholar]
- 12.Ijaz MK, Rubino JR. Impact of infectious diseases on cognitive development in childhood and beyond: potential mitigational role of hygiene. Open Infectious Diseases Journal. 2012;6(1):65–70. [Google Scholar]
- 13.Huttly SR, Lanata CF, Gonzales H, et al. Observations on handwashing and defecation practices in a shanty town of Lima, Peru. Journal of Diarrhoeal Diseases Research. 1994;12(1):14–18. [PubMed] [Google Scholar]
- 14.Mas-Coma S, Valero MA, Bargues MD. Effects of climate change on animal and zoonotic helminthiases. OIE Revue Scientifique et Technique. 2008;27(2):443–457. [PubMed] [Google Scholar]
- 15.Hudson PJ, Cattadori IM, Boag B, Dobson AP. Climate disruption and parasite-host dynamics: patterns and processes associated with warming and the frequency of extreme climatic events. Journal of Helminthology. 2006;80(2):175–182. doi: 10.1079/joh2006357. [DOI] [PubMed] [Google Scholar]
- 16.Kutz SJ, Hoberg EP, Polley L, Jenkins EJ. Global warming is changing the dynamics of Arctic host-parasite systems. Proceedings of the Royal Society B: Biological Sciences. 2005;272(1581):2571–2576. doi: 10.1098/rspb.2005.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appleton CC, Gouws E. The distribution of common intestinal nematodes along an altitudinal transect in KwaZulu-Natal, south africa. Annals of Tropical Medicine and Parasitology. 1996;90(2):181–188. doi: 10.1080/00034983.1996.11813042. [DOI] [PubMed] [Google Scholar]
- 18.Moyer BR, Drown DM, Clayton DH. Low humidity reduces ectoparasite pressure: implications for host life history evolution. Oikos. 2002;97(2):223–228. [Google Scholar]
- 19.Grice RL, Prociv P. In vitro embryonation of Syphacia obvelata eggs. International Journal for Parasitology. 1993;23(2):257–260. doi: 10.1016/0020-7519(93)90148-r. [DOI] [PubMed] [Google Scholar]
- 20.RILEM. RILEM recommendations—absorption of water by immersion under Vacuum, Materials and Structures. RILEM CPC-11.3. 1984;101:393–394. [Google Scholar]
- 21.Alum A, Rubino JR, K. Ijaz M. Comparison of molecular markers for determining the viability and infectivity of Cryptosporidium oocysts and validation of molecular methods against animal infectivity assay. International Journal of Infectious Diseases. 2011;15(3):e197–e200. doi: 10.1016/j.ijid.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Alum A, Sbai B, Asaad H, Rubino JR, K. Ijaz M. ECC-RT-PCR: a new method to determine the viability and infectivity of Giardia cysts. International Journal of Infectious Diseases. 2012;16(5):e350–e353. doi: 10.1016/j.ijid.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Mangal TD, Paterson S, Fenton A. Predicting the impact of long-term temperature changes on the epidemiology and control of schistosomiasis: a mechanistic model. PLoS ONE. 2008;3(1) doi: 10.1371/journal.pone.0001438.e1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moyer BR, Drown DM, Clayton DH. Low humidity reduces ectoparasite pressure: implications for host life history evolution. Oikos. 2002;97(2):223–228. [Google Scholar]
- 25.Nwosu CO, Madu PP, Richards WS. Prevalence and seasonal changes in the population of gastrointestinal nematodes of small ruminants in the semi-arid zone of north-eastern Nigeria. Veterinary Parasitology. 2007;144(1-2):118–124. doi: 10.1016/j.vetpar.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Chauret C, Chen P, Springthrope S, Sattar S. Effect of environmental stressors on the survival of Cryptosporidium oocysts. Proceedings of the American Water Works Association Water Quality Technology Conference; November 1995; New Orleans, Louisiana. American Water Works Association Denver; [Google Scholar]
- 27.DeRegnier DP, Cole L, Schupp DG, Erlandsen SL. Viability of Giardia cysts suspended in lake, river, and tap water. Applied and Environmental Microbiology. 1989;55(5):1223–1229. doi: 10.1128/aem.55.5.1223-1229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medema GJ, Schijven JF. Modelling the sewage discharge and dispersion of cryptosporidium and giardia in surface water. Water Research. 2001;35(18):4307–4316. doi: 10.1016/s0043-1354(01)00161-0. [DOI] [PubMed] [Google Scholar]
- 29.Robertson LJ, Campbell AT, Smith HV. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Applied and Environmental Microbiology. 1992;58(11):3494–3500. doi: 10.1128/aem.58.11.3494-3500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas DM, Stanton NL, Seville RS. A stable Eimerian assemblage in Wyoming ground squirrels (Spermophilus elegans elegans): maintaining viability over winter. Journal of the Helminthological Society of Washington. 1995;62(1):1–5. [Google Scholar]
- 31.Jenkins MB, Walker MJ, Bowman DD, Anthony LC, Ghiorse WC. Use of a sentinel system for field measurements of Cryptosporidium parvum oocyst inactivation in soil and animal waste. Applied and Environmental Microbiology. 1999;65(5):1998–2005. doi: 10.1128/aem.65.5.1998-2005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins MB, Anguish LJ, Bowman DD, Walker MJ, Ghiorse WC. Assessment of a dye permeability assay for determination of inactivation rates of Cryptosporidium parvum oocysts. Applied and Environmental Microbiology. 1997;63(10):3844–3850. doi: 10.1128/aem.63.10.3844-3850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen A. The proportion of helminth infections in a community in western Kenya which would be treated by mass chemotherapy of schoolchildren. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998;92(2):144–148. doi: 10.1016/s0035-9203(98)90721-0. [DOI] [PubMed] [Google Scholar]
- 34.Nasser AM, Tweto E, Nitzan Y. Die-off of Cryptosporidium parvum in soil and wastewater effluents. Journal of Applied Microbiology. 2007;102(1):169–176. doi: 10.1111/j.1365-2672.2006.03048.x. [DOI] [PubMed] [Google Scholar]
- 35.Fayer R, Trout JM, Jenkins MC. Infectivity of Cryptosporidium parvum oocysts stored in water at environmental temperatures. Journal of Parasitology. 1998;84(6):1165–1169. [PubMed] [Google Scholar]
- 36.King BJ, Keegan AR, Monis PT, Saint CP. Environmental temperature controls Cryptosporidium oocyst metabolic rate and associated retention of infectivity. Applied and Environmental Microbiology. 2005;71(7):3848–3857. doi: 10.1128/AEM.71.7.3848-3857.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.EPA. Sewage Sludge Pathogen Transport Model.Health Effects Research Laboratory, Office of Research and Development. Cincinnati, Ohio, USA: 1980. (OH. EPA 600/1-81-049a NTIS PB82-109000). [Google Scholar]
- 38.O’Donnell CJ, Meyer KB, Jones JV, et al. Survival of parasite eggs upon storage in sludge. Applied and Environmental Microbiology. 1984;48(3):618–625. doi: 10.1128/aem.48.3.618-625.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karim MR, Manshadi FD, Karpiscak MM, Gerba CP. The persistence and removal of enteric pathogens in constructed wetlands. Water Research. 2004;38(7):1831–1837. doi: 10.1016/j.watres.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 40.Peng X, Murphy T, Holden NM. Evaluation of the effect of temperature on the die-off rate for Cryptosporidium parvum oocysts in water, soils, and feces. Applied and Environmental Microbiology. 2008;74(23):7101–7107. doi: 10.1128/AEM.01442-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King BJ, Monis PT. Critical processes affecting Cryptosporidium oocyst survival in the environment. Parasitology. 2007;134(3):309–323. doi: 10.1017/S0031182006001491. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins MB, Bowman DD, Ghiorse WC. Inactivation of Cryptosporidium parvum oocysts by ammonia. Applied and Environmental Microbiology. 1998;64(2):784–788. doi: 10.1128/aem.64.2.784-788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


