Summary
Peptidoglycan surrounds the bacterial cytoplasmic membrane to protect the cell against osmolysis. The biosynthesis of peptidoglycan, made of glycan strands crosslinked by short peptides, is the target of antibiotics like β-lactams and glycopeptides. Nascent peptidoglycan contains pentapeptides that are trimmed by carboxypeptidases to tetra- and tripeptides. The well-characterized DD-carboxypeptidases hydrolyze the terminal D-alanine from the stem pentapeptide to produce a tetrapeptide. However, few LD-carboxypeptidases that produce tripeptides have been identified, and nothing is known about substrate specificity in these enzymes. We report biochemical properties and crystal structures of the LD-carboxypeptidases LdcB from Streptococcus pneumoniae, Bacillus anthracis, and Bacillus subtilis. The enzymes are active against bacterial cell wall tetrapeptides and adopt a zinc-carboxypeptidase fold characteristic of the LAS superfamily. We have also solved the structure of S. pneumoniae LdcB with a product mimic, elucidating the residues essential for peptidoglycan recognition and the conformational changes that occur on ligand binding.
Highlights
-
•
A peptidoglycan, peptide stem-trimming carboxypeptidase, LdcB, has been characterized
-
•
The crystal structure of LdcB has been solved with a peptidoglycan mimic bound
-
•
The LdcB structure undergoes significant conformational change on binding ligand
-
•
The exquisite substrate specificity of LdcB has also been demonstrated in vitro
Peptidoglyan is an essential layer surrounding the bacterial cytoplasmic membrane that is matured and trimmed by carboxypeptidases. Hoyland et al. describe the structure of one such carboxypeptidase in the presence of a product mimic, explaining the molecular specificity of the enzyme family.
Introduction
Bacteria surround their cytoplasmic membrane with a large, semipermeable barrier, the peptidoglycan (PG) sacculus, which not only protects the cell from lysis from turgor but also helps to maintain cell shape. PG comprises repeating units of the N-acetylglucosamine-N-acetylmuramic acid (GlcNAc—MurNAc) disaccharide that polymerizes into long glycan chains. Pentapeptides, usually comprising L-Ala—D-γ-Glu[Gln]—L-Lys—D-Ala—D-Ala, extend from the MurNAc moiety. In Gram-negative bacteria and some Gram-positive Firmicutes, the L-Lys at position 3 is replaced by meso-diaminopimelic acid (A2pm), which is amidated in Bacillus subtilis (Vollmer et al., 2008a). Peptides protruding from adjacent glycan chains are crosslinked by transpeptidases to form the net-like PG sacculus, which the cell has to enlarge in order to grow and to divide (Sauvage et al., 2008). Newly inserted PG matures and is turned over by PG hydrolases, and the balance between synthesis and degradation of PG is key to normal cell division (Vollmer et al., 2008b). The importance of PG to the healthy physiology of a bacterium is highlighted by the fact that its biosynthesis is the target of some of our most successful antibiotics, β-lactams such as penicillin and glycopeptides such as vancomycin, resistance to which in pathogens is an urgent and currently unmet human health challenge.
In most species, the terminal D-alanine of the nascent pentapeptide stem is hydrolysed by DD-carboxypeptidases from the class C penicillin binding protein (PBP) family (Lovering et al., 2012). LD-carboxypeptidases subsequently hydrolyze the L-Lys[A2pm]—D-Ala bond to release the tripeptide. Though tripeptides are found in many bacteria, very few of the corresponding LD-carboxypeptidases have been identified. Membrane preparations of B. subtilis have shown strong LD-carboxypeptidase activity against cell-wall-derived tetrapeptides (Arminjon et al., 1977), though the specific enzyme involved has not been isolated. The best characterized LD-carboxypeptidase is LD-carboxypeptidase A (LdcA), a cytoplasmic, penicillin-insensitive member of the S66 family of serine peptidases that is involved in PG recycling and is essential for cells entering the stationary phase (Metz et al., 1986; Templin et al., 1999). The cytoplasmic localization of LdcA precludes its involvement in the production of cell wall tripeptides. Other LD-carboxypeptidases either must be active within the periplasm of Gram-negative bacteria or are anchored to the exterior of the cell membrane in Gram-positives.
Several LD-carboxypeptidase genes have been identified recently, including dacB from Lactococcus lactis and Streptococcus pneumoniae (Barendt et al., 2011; Courtin et al., 2006), csd6 from Helicobacter pylori (Sycuro et al., 2012) and pgp2 from Campylobacter jejuni (Frirdich et al., 2014), though it should be noted that csd6 and pgp2 share no sequence similarity with dacB. The corresponding null mutants exhibited increased amounts of tetrapeptides in the cell wall and a concomitant decrease in tripeptide content (Sycuro et al., 2012; Frirdich et al., 2014). The S. pneumoniae dacB mutant also displayed severe morphological defects, developing into small, spherical cells or elongating into long, thick cells, as a result of division asymmetry produced by random septum localization (Barendt et al., 2011). Both the H. pylori csd6 and the C. jejuni pgp2 mutants have a morphological defect, displaying a straight shape instead of the normal helical morphology, indicating that csd6 and pgp2 also contribute to cell shape maintenance (Frirdich et al., 2014; Sycuro et al., 2012). In C. jejuni (Frirdich et al., 2012) and H. pylori (Sycuro et al., 2010), cell shape is also linked to pathogenicity, and PG fragments released by growing cells are important signaling molecules for immune recognition and modulation in animals ranging from insects to man (Royet et al., 2011). Therefore, the PG plays an important role in bacterial shape maintenance, viability, virulence and immune signaling.
In this study, we have investigated the biochemical activity of S. pneumoniae and B. subtilis DacB against cell wall sacculi and synthetic tetrapeptides. Both proteins are shown to be active against cell-wall-derived tetrapeptides and synthetic tetrapeptides lacking the sugar moiety but are inactive against tetrapeptides terminating in L-alanine, confirming their classification as LD-carboxypeptidases. We therefore propose to rename these enzymes LD-carboxypeptidase B (LdcB). We present the crystal structures of both LdcB enzymes and the ortholog from Bacillus anthracis, all of which belong to the LAS (lysostaphin, D-Ala-D-Ala metallopeptidases, sonic hedgehog) family of zinc-dependent peptidases. The structure of S. pneumoniae LdcB with MurNAc—L-Ala—D-γ-Gln—L-Lys—(D-Asn) bound reveals the molecular basis of PG recognition and the key roles played in catalysis by active site elements. It is surprising that the active site is reminiscent, yet reversed, of that found in pancreatic carboxypeptidase A, suggesting that the enzymes have converged on a common catalytic mechanism.
Results
Role for LdcB In Vivo
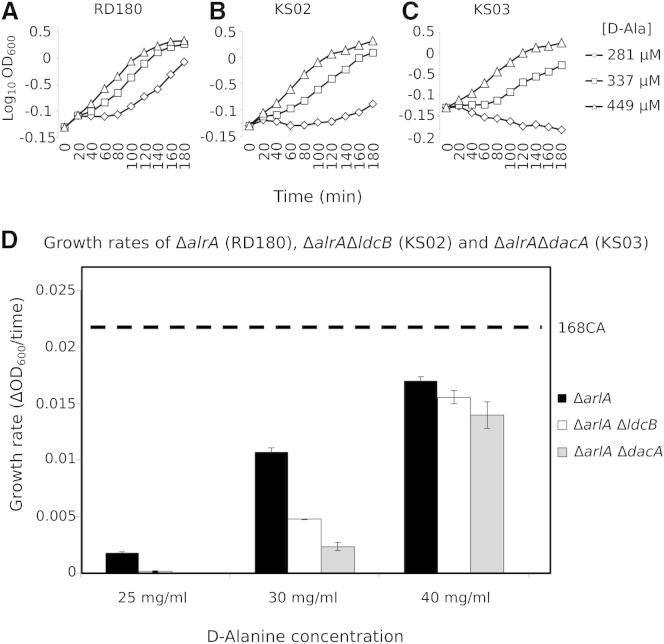
The mature PG of S. pneumoniae and B. subtilis contains mostly tripeptides, indicating the presence of PG-active peptidases in these organisms (Atrih et al., 1999; Bui et al., 2012). Both species contain DacA orthologs, DD-carboxypeptidases that cleave pentapeptides to tetrapeptides; however, the LD-carboxypeptidases that produce tripeptides from the tetrapeptides have remained elusive until recently. The PG of a S. pneumoniae dacB mutant contained mainly tetrapeptides, suggesting strongly that SpDacB is an LD-carboxypeptidase (hereinafter renamed as SpLdcB) (Barendt et al., 2011). Single mutants of the dacA and dacB orthologs in B. subtilis, dacA, and yodJ (hereinafter renamed as ldcB), had no obvious phenotype during vegetative growth, indicating that the trimming of peptides or the released D-Ala is not required for growth under laboratory conditions (Atrih et al., 1999). To test whether the released D-Ala becomes important under D-Ala limiting conditions, we constructed dacA and ldcB null mutants in a B. subtilis strain auxotrophic for D-Ala (an alanine racemase [alrA] null mutant) and assayed growth in the presence of D-Ala. The D-Ala-dependent strain, RD180, required at least 450 μM D-Ala for normal growth, and it grew more slowly at lower D-Ala concentrations (Figure 1). However, strains also bearing either the dacA (KS03) or the ldcB (KS02) alleles exhibited even more severe growth rate dependencies on D-Ala, with the dacA mutant the most severely affected. These data suggest strongly that LdcB is an LD-carboxypeptidase that releases D-Ala from the cell wall, which can be used to support growth under D-Ala-limiting conditions.
Figure 1.
Effect of dacA and ldcB Alleles in a D-alanine Auxotroph
(A–C) The growth of strains (A) RD180 (ΔalrA), (B) KS02 (ΔldcBΔalrA), and (C) KS03 (ΔdacAΔalrA) incubated at 37°C in LB medium supplemented with different concentrations of D-alanine was determined by monitoring the culture at OD600 and plotting it against time of incubation.
(D) The calculated growth rate of each strain at three different concentrations of exogenous D-alanine. The values shown represent the average of three independent experiments. The dashed line shows the growth rate of wild-type B. subtilis strain 168CA under the same conditions. The error bars represent the SD of growth rate means at a confidence level of 95%.
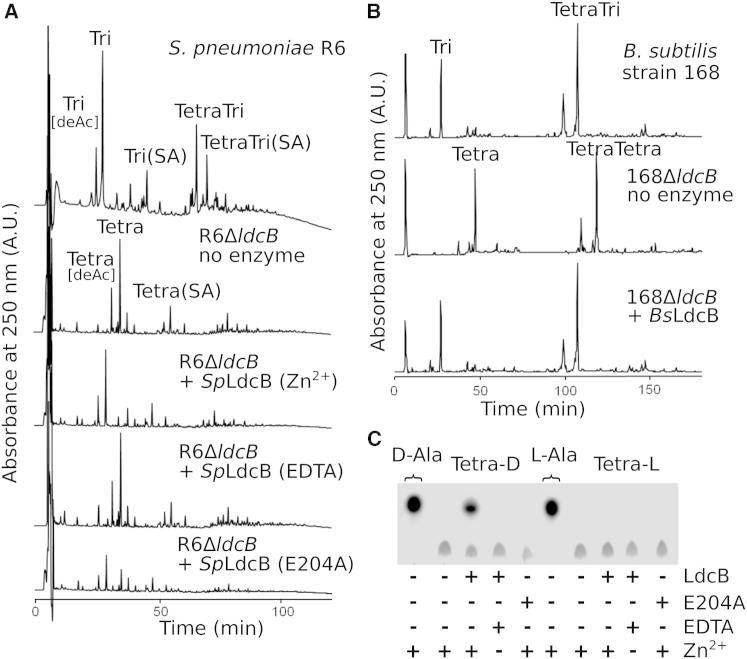
To test directly whether LdcB functions as an LD-carboxypeptidase, we generated S. pneumoniae and B. subtilis ldcB mutants and isolated their cell wall PG. Muropeptides were released with cellosyl and analyzed by HPLC (Figures 2A and 2B). The ldcB mutant strains lacked tripeptides and contained mainly tetrapeptides in their PGs, consistent with previous work indicating that LdcB is the main enzyme trimming cell wall tetrapeptides (Barendt et al., 2011). To obtain more direct evidence for an enzymatic activity, purified, recombinant LdcB enzymes from S. pneumoniae and B. subtilis (termed SpLdcB and BsLdcB) were incubated with PG isolated from the respective ldcB mutant strains before digesting with cellosyl and analyzing the resulting muropeptides by HPLC (Figures 2A and 2B). Both SpLdcB and BsLdcB almost quantitatively digested PG tetrapeptides with a carboxy-terminal D-Ala producing the tripeptides, proving their LD-carboxypeptidase activity. SpLdcB was active in the presence of Zn2+ ions and inactive in the presence of EDTA, suggesting a metal-ion-dependent peptidase mechanism.
Figure 2.
Activity of SpLdcB and BsLdcB against Peptidoglycan and Peptides
(A) HPLC chromatograms of muropeptides from S. pneumoniae strains, obtained after incubating PG with or without SpLdcB or SpLdcB(E204A) in the presence of Zn2+ or EDTA, followed by digestion with cellosyl and reduction with sodium borohydride. The muropeptides are: Tri, GlcNAc—MurNAc(r)—L-Ala—D-γ-Gln—L-Lys; Tetra, GlcNAc—MurNAc(r)—L-Ala—D-γ-Gln—L-Lys-D-Ala; TetraTri, GlcNAc—MurNAc—L-Ala—D-γ-Gln—L-Lys—D-Ala—L-Lys—D-γ-Gln—L-Ala—MurNAc(r)—GlcNAc; TetraTetra, GlcNAc—MurNAc—L-Ala—D-γ-Gln—L-Lys—(D-Ala)—D-Ala—L-Lys—D-γ-Gln—L-Ala—MurNAc(r)—GlcNAc; deAc, deacetylation at GlcNAc; SA, L-Ser—L-Ala branch (at L-Lys). GlcNAc, N-acetylglusosamine; MurNAc(r), N-acetylmuramitol.
(B) HPLC chromatograms of muropeptides from B. subtilis strains, obtained after incubating PG with or without BsLdcB, followed by digestion with cellosyl and reduction with sodium borohydride. Muropeptides: Tri, GlcNAc—MurNAc(r)—L-Ala—D-γ-Glu—meso-Dap(NH2); Tetra, GlcNAc—MurNAc(r)—L-Ala—D-γ-Glu—meso-Dap(NH2)—D-Ala; TetraTri, GlcNAc—MurNAc—L-Ala—D-γ-Glu—meso-Dap(NH2)—D-Ala-meso-Dap(NH2)— D-γ-Glu—L-Ala—MurNAc(r)—GlcNAc; TetraTetra, GlcNAc—MurNAc—L-Ala—D-γ-Glu—meso-Dap(NH2)—(D-Ala)—D-Ala—meso-Dap(NH2)— D-γ-Glu—L-Ala—MurNAc(r)—GlcNAc; meso-Dap(NH2), meso-diaminopimelic acid (amidated).
(C) TLC of SpLdcB incubated with various substrates.
SpLdcB and BsLdcB Are Catalytically Active against Synthetic Cell Wall Tetrapeptides
In addition to removing the terminal D-Ala from S. pneumoniae cell wall PG tetrapeptides (Figure 2A), SpLdcB was also tested against synthetic tetrapeptides (L-Ala—D-Gln—L-Lys—D-Ala [tetra-D]) and its L-isomer, L-Ala—D-Gln—L-Lys—L-Ala [tetra-L]). Though these substrates lack the glycan component and differ further from the natural substrate by the replacement of D-γ-glutamine with D-glutamine, they have the potential to discriminate between LD- and LL-carboxypeptidase activities. SpLdcB was active solely against the tetra-D ligand (Figure 2C), an activity that was dependent on the presence of zinc and abrogated totally in the presence of EDTA (Figure 2C).
Overall Structure of LdcB
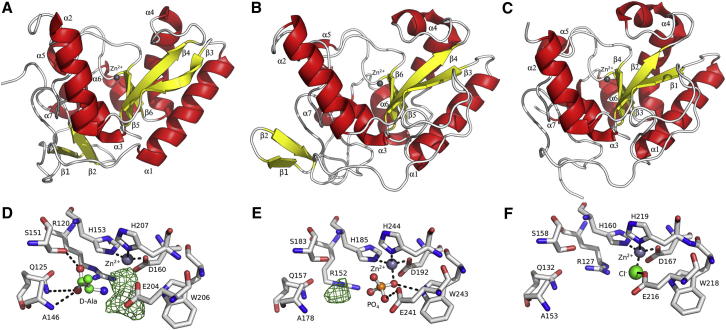
The crystal structures of SpLdcB and Bacillus anthracis LdcB (BaLdcB) were solved by single wavelength anomalous dispersion (SAD); BsLdcB was solved by molecular replacement. The LdcB enzymes are single domain proteins comprising two subdomains separated by a V-shaped cleft (Figures 3A–3C). The first subdomain comprises a four-stranded, antiparallel β sheet (β4–β7) that opposes the cleft, and the outside face of the β sheet is flanked by three α helices (α1, α4, and α6). The second subdomain comprises an N-terminal, three-stranded, antiparallel β sheet (β1–β3), somewhat discrete from the four helical bundle (α2, α3, α5, and α7) that makes up the rest of the subdomain. Though the BaLdcB structure contains the same subdomain, it is less well structured in this region than BsLdcB and SpLdcB, and, as a consequence, the β1–β3 sheet is not formed. Neither subdomain is formed by a contiguous, linear part of the primary sequence; six loops (β3–α1, β4–α2, α3–β5, β6–α5, α5–β7, and α6–α7) connect the two subdomains.
Figure 3.
Structure of LdcB
(A–C) Cartoon representations of (A) SpLdcB, (B) BsLdcB, and (C) BaLdcB; α helices (red) and β strands (yellow) are numbered. The loops between secondary structure elements are colored silver. The bound zinc ion is shown as a gray sphere.
(D–F) The active sites of (D) SpLdcB, (E) BsLdcB, and (F) BaLdcB. A zinc ion is coordinated in a tetrahedral manner in the structures by two invariant histidines and a well-conserved aspartate. Residual electron density in SpLdcB, shown in (D), corresponds to D-alanine in the S1′ subsite, and an unknown compound in S1, adjacent to the zinc ion. Electron density corresponding to a phosphate ion, a component of the crystallization medium, in the S1 subsite of BsLdcB shown in (E) is a mimic of the tetrahedral transition state. A second feature in the S1′ subsite is unmodeled. The residual difference density features are shown contoured at a level of 3σ and hydrogen bonds are drawn as dashed black lines.
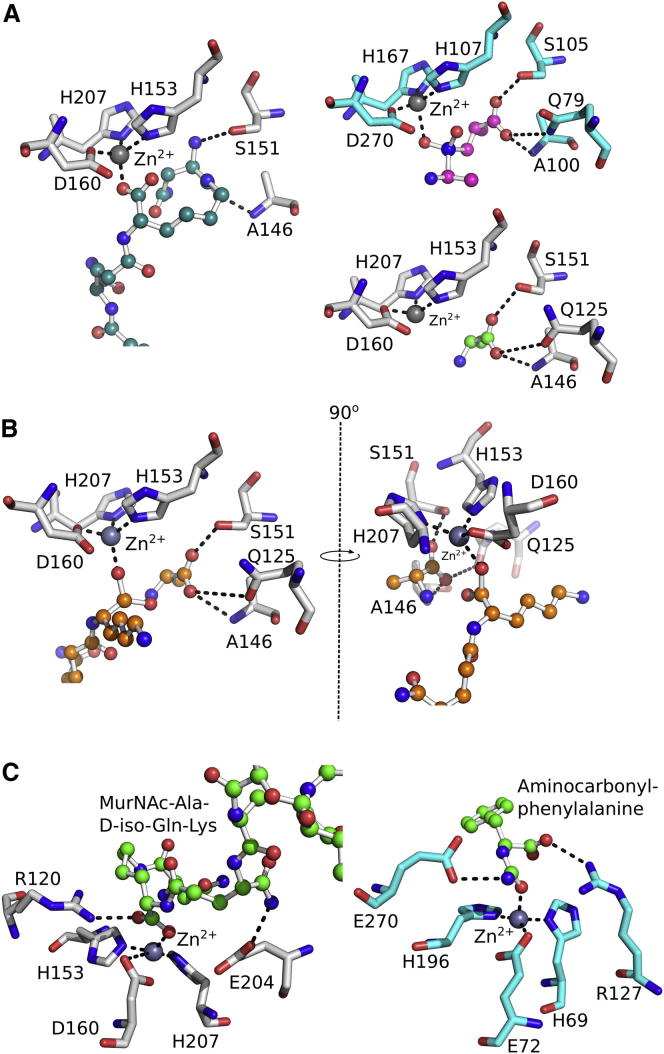
The cleft between subdomains is 15–18 Å wide at the top and 15 Å deep and narrows down to the base where a Zn2+ ion, added to the enzyme during purification, is located (Figures 3A–3C), which is coordinated in a tetrahedral manner by three amino acids. In SpLdcB, the liganding residues are His153, Asp160, and His207 (Figure 3D); the equivalents in BsLdcB/BaLdcB are His185/His160, Asp192/Asp167, and His244/His219 (Figures 3E and 3F). These zinc ligands are universally conserved in LdcB sequences, consistent with an important functional role in these enzymes. The active site of SpLdcB contains unattributed residual electron density that occupies the position of the fourth ligand in the tetrahedral coordination sphere of the zinc (Figure 3D). To use the nomenclature common to proteases, the unattributed electron density extends as far as the S1′ subsite, the location ordinarily occupied by the scissile amino acid. The electron density in the S1′ subsite is of a size, shape, and location corresponding to that of alanine. The ligand is stabilized in this position by van der Waals’ contacts between the alanyl methyl group and the phenolic side chains of Tyr191 and Tyr201 and by hydrogen bonds to the side chains of Glu204 and the main chain amide nitrogen of Tyr144. Despite the high resolution of the SpLdcB diffraction data, it was not possible to discriminate between either alanyl stereoisomer, both of which are present in the crystallization conditions. We have modeled D-Ala because it is the enzymatic product of these enzymes. However, residual electron density adjacent to the zinc ion could not be modeled satisfactorily with any ligand arising from the enzymatic activity, the crystallization media, or purification media; consequently, the remaining electron density has been left unmodeled. Mass differences between native and denatured protein samples also failed to identify candidate molecules corresponding to the residual electron density. Residual electron density in the vicinity of the fourth zinc ligand in BsLdcB was fulfilled by a phosphate anion, a component of the crystallization conditions (Figure 3E), which refined satisfactorily. The phosphate is located so as to mimic the tetrahedral transition state of amide bond hydrolysis. There is a further unmodeled electron density feature (Figure 3E), this time in the S1′ subsite, that we have not been able to identify unambiguously.
Comparison of LdcB Structures
SpLdcB, BaLdcB, and BsLdcB share pairwise sequence identities of ∼30%, and they superimpose on ∼155 common Cα atoms with a root-mean-square deviation (rmsd) of 1.5 Å–1.8 Å (Figures 3A–3C). The major differences between the LdcB structures are limited to the rather flexible and unconstrained N- and C-termini and the occasional longer loop in one structure in comparison to the other, as a direct consequence of sequence insertions. The active sites of SpLdcB, BaLdcB, and BsLdcB are, to all extents and purposes, indistinguishable (Figures 3D–3F). A further BaLdcB structure has been solved that contains no bound zinc ion, but there are negligible differences between it and its counterpart with zinc bound (Figure S1 available online). The active site of the LdcB enzymes is, therefore, sculpted in a robust fashion, competent for peptidolysis once the catalytic machinery is completed by the incorporation of zinc.
Structural Comparison with Other Peptidases
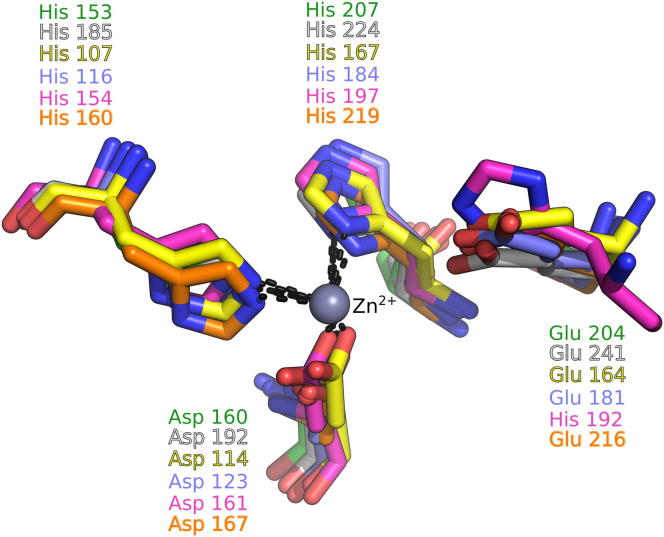
Using SpLdcB as a search model in DALI (Holm and Rosenström, 2010), over 100 significantly similar structures were identified in the Protein Data Bank (PDB), including the structure of VanXYG, a dipeptidase/DD-carboxypeptidase (PDB ID 4f78 [Meziane-Cherif et al., 2014]; DALI Z score = 17.0); an endolysin from the Listeria bacteriophage A500 (PDB ID 2vo9 [Korndörfer et al., 2008]; Z = 9.5); VanX, a DD-dipeptidase (PDB ID 1r44 [Bussiere et al., 1998]; Z = 6.6); and the N-terminal domain of sonic hedgehog, a protease involved in embryogenesis (PDB ID 2wg4 [Bishop et al., 2009]; Z = 5.3). Equivalent results were obtained using the structures of BaLdcB and BsLdcB as queries in DALI. The matched structures are all members of the wider LAS superfamily of proteases (Bochtler et al., 2004), which, although they share very little sequence identity, possess markedly similar active sites (Figure 4). By comparison with the VanXYG structure (Meziane-Cherif et al., 2014), the active site of the LdcB proteins is open and more solvent accessible. The open nature of the active site is a direct consequence of the absence of the β4-α5/bisubstrate selectivity loop in LdcB, which enables the recognition of PG, a molecule far greater in size than the dipeptides utilized by VanXYG (Meziane-Cherif et al., 2014).
Figure 4.
A Comparison of the Active Sites of LdcB and Other LAS Family Members
LdcB shares an active site closely superimposable on those of other LAS family enzymes. The zinc is coordinated by a H-X(3–6)-D-X(not conserved)-H motif. A glutamate that plays a key role in catalysis is also shown; note that, in D-Ala-D-Ala carboxypeptidase from Streptomyces albus, the equivalent amino acid is histidine, the first amino acid in the H-X-H motif common to most LAS carboxypeptidases. The structures shown, distinguished by the coloring of carbon atoms, are SpLdcB (white), BsLdcB (green), BaLdcB (orange), Enterococcus faecalis D,D-dipeptidase/D,D-carboxypeptidase VanXYG (yellow; PDB ID 4f78), Enterococcus faecium aminodipeptidase VanX (blue; PDB ID 1r44) and S. albus D-Ala-D-Ala carboxypeptidase (pink; PDB ID 1lbu).
As with other LAS superfamily members, LdcB contains the characteristic zinc binding motif (Figure 4), with a consensus sequence of H-X(3–6)-D-X(not conserved)-H. The fourth zinc ligand in the structures of other LAS family members tends to be a solute from the crystallization milieu or a water that is likely to act as the nucleophile in the reaction (Bussiere et al., 1998). A second conserved motif in the LAS superfamily, H-X-H, of which the first histidine is the catalytic residue (Bochtler et al., 2004), is missing from LdcB enzymes; the catalytic histidine is replaced spatially by glutamate (i.e., Glu204 in SpLdcB).
SpLdcB Cocrystallized with a Reaction Product
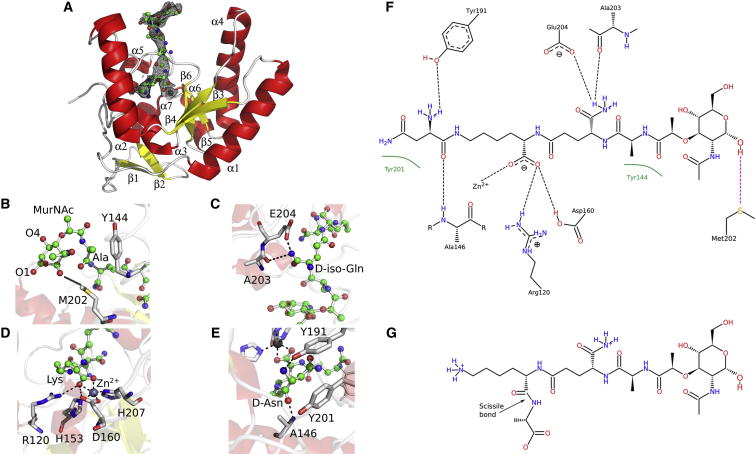
Despite attempting to crystallise LdcB enzymes with a range of synthetic ligands, one crystal structure was obtained, that of SpLdcB in complex with MurNAc—L-Ala—D-γ-Gln—L-Lys—(D-Asn) (Figure 5A). This ligand is a close chemical analog of the true reaction product, as it contains half of the repeating unit of the glycan component of PG and comprises a tripeptide stem peptide that is branched at the L-lysine, the location of the crosslink position in natural PG (Figures 5F and 5G). There are two chemical differences between this synthetic mimic and the true reaction product. First, the ligand lacks GlcNAc, the second sugar moiety of the glycan repeating unit in PG. Second, the terminal D-Asn group is covalently attached by a peptide bond between the main chain carbonyl of the terminal D-Asn and the side chain Nζ of the penultimate lysine. In crosslinks between peptide stems, the side chain Nζ of the penultimate lysine is linked by a peptide bond to the main chain carbonyl of a D-Ala. Hence, the L-Lys—(D-Asn) linkage is a faithful mimic of the natural L-Lys—(D-Ala), albeit the chemistry of the side chains of the terminal D-amino acids clearly differ.
Figure 5.
SpLdcB with Bound MurNAc—L-Ala—D-γ-Gln—L-Lys—(D-Asn)
(A) Cartoon representation of SpLdcB, drawn using the same color scheme as in Figure 3. The MurNAc—Ala—D-γ-Gln—L-Lys—(D-Asn) is drawn as a ball-and-stick model with Refmac-weighted 2Fobs-Fcalc electron density displayed at a contour level of 1 σ.
(B–E) The interactions made between the ligand and the protein at each subsite are shown successive panels: (B) S3; (C) S2; (D) S1, and (E) S1′. Key van der Waals’ interactions are shown as a transparent silver line.
(F) A schematic of the interactions of SpLdcB with the bound peptidoglycan mimic. Hydrogen-bond interactions are shown with a black dotted line, and van der Waals’ forces are shown with a green arc.
(G) A schematic of the tetrapeptide substrate of SpLdcB, drawn in the same manner as the peptidoglycan mimic in (F). The scissile bond is highlighted by an arrow. Crosslinks to other stem peptides would take place via the lysine’s terminal amino group on the left.
There are six molecules of SpLdcB in this crystallographic asymmetric unit, though none of the interfaces represent a stable multimeric form. Five of six protein chains could be built and refined satisfactorily; the electron density for the sixth copy could not be improved by any procedure to permit its reliable modeling, and its absence from the model contributes directly to the slightly inflated final Rwork and Rfree values (Table 1). The five built molecules are highly superimposable, with each other and with the apo SpLdcB structure (rmsds between 0.5 Å and 1.1 Å; Table 2). In chains B, C, and E, the zinc is coordinated by a likely nucleophilic water to form tetrahedral coordination geometry. In chain D, the fourth zinc ligand is not evident, perhaps due to local disorder. Residual electron density was retained throughout refinement in only one of the five molecules, chain A, into which MurNAc—L-Ala—D-γ-Gln—L-Lys—(D-Asn) could be built and refined unambiguously (Figure 5A).
Table 1.
Summary of X-Ray Data Collection and Refinement Statistics
| Parameters | SpLdcB | BsLdcB | SpLdcB with Ligand | BaLdcB | BaLdcB with Zinc |
|---|---|---|---|---|---|
| PDB ID | 4OX5 | 4OX3 | 4OXD | 4JID | 4MPH |
| Data collection | |||||
| Space group | I222 | P212121 | C2 | I222 | I222 |
| Unit Cell Dimensions | |||||
| a, b, c (Å) | 48.8, 60.4, 138.2 | 42.7, 53.7, 102.4 | 346.0, 42.5, 79.3 | 76.6, 113.0, 124.6 | 76.8, 112.3, 125.7 |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 | 90, 93.1, 90 | 90, 90, 90 | 90, 90, 90 |
| Wavelength (Å) | 0.98 | 0.97 | 0.92 | 0.97856 | 1.27696 |
| Resolution (Å) | 20.0–1.80 (1.84–1.80) | 47.6–1.70 (1.73–1.70) | 47.8–2.80 (2.95–2.80) | 29.7–2.30 (2.36–2.30) | 28.3–2.03 (2.06–2.03) |
| Multiplicity | 24.3 (14.2) | 3.2 (1.7) | 3.4 (3.4) | 4.2 (3.6) | 11.8 (9.9) |
| Rmergea | 0.077 (0.108) | 0.072 (0.235) | 0.134 (0.635) | 0.093 (0.542) | 0.101 (0.511) |
| I/σI | 34.4 (2.4) | 11.9 (2.4) | 7.8 (1.9) | 13.5 (2.4) | 32.1 (4.2) |
| Completeness (%) | 98.6 (86.9) | 95.7 (89.3) | 98.3 (98.3) | 98.3 (94.3) | 99.8 (96.9) |
| Refinement | |||||
| Resolution (Å) | 20.0–1.80 | 47.6–2.0 | 47.8–2.80 | 29.7–2.3 | 28.3–2.03 |
| No. of reflections | 25,656 (1,157) | 35,526 (1,057) | 47,327 (1,979) | 22,730 (1,226) | 67,494 (3,374) |
| Rwork/Rfreeb | 0.191/0.253 | 0.176/0.247 | 0.273/0.335 | 0.194/0.259 | 0.151/0.189 |
| No. of Atoms | |||||
| Protein | 1,476 | 1,753 | 7,014 | 3,040 | 2,970 |
| No. of proteins/AU | 1 | 1 | 6 | 2 | 2 |
| Ion/ligand | 3/22 | 1/5 | 21/51 | -/4 | 2/12 |
| Water | 197 | 230 | 111 | 424 | 466 |
| B Factors (Å2) | |||||
| Protein | 41.8 | 16.0 | 44.3 | 45.3 | 35.8 |
| Ion/ligand | 36.9/50.7 | 10.7/23.8 | 38.7/69.7 | −/49.8 | 17.9/60.0 |
| Water | 48.2 | 31.6 | 28.7 | 43.6 | 46.2 |
| Rmsd | |||||
| Bond lengths (Å) | 0.018 | 0.015 | 0.005 | 0.013 | 0.0012 |
| Bond angles (°) | 1.96 | 1.76 | 0.84 | 1.36 | 0.991 |
| Ramachandran | |||||
| Favored (%) | 96.1 | 96.7 | 95.8 | 97.6 | 98.0 |
| Allowed (%) | 100 | 100 | 99.6 | 99.7 | 100 |
Highest resolution shell shown in parentheses. AU, asymmetric units.
Rmerge = ΣhΣi|Ii(h) − 〈I(h)〉/ΣhΣiIi(h), where Ii(h) and 〈I(h)〉 are the ith and mean measurement of the intensity of reflection h.
Rwork/Rfree = Σ|Fpobs − Fpcalc|/ΣFpobs, where Fpobs and Fpcalc are the observed and calculated structure factor amplitudes, respectively.
Table 2.
Rmsd Values between Ligand-Bound SpLdcB Chains (4OXD) and apo SpLdcB (4OX5)
| 4OX5 and 4OXD Chain | 4OX5 | 4OXD Chain A | 4OXD Chain B | 4OXD Chain C | 4OXD Chain D | 4OXD Chain E |
|---|---|---|---|---|---|---|
| 4OX5 | – | 0.73 | 0.57 | 0.66 | 0.69 | 0.80 |
| 4OXD chain A | 0.73 | – | 0.63 | 0.64 | 0.76 | 1.03 |
| 4OXD chain B | 0.57 | 0.63 | – | 0.56 | 0.65 | 0.86 |
| 4OXD chain C | 0.66 | 0.64 | 0.56 | – | 0.56 | 0.64 |
| 4OXD chain D | 0.69 | 0.76 | 0.65 | 0.56 | – | 0.61 |
| 4OXD chain E | 0.80 | 1.03 | 0.86 | 0.64 | 0.61 | – |
The structure of the ligand-bound form of SpLdcB is entirely consistent with its LD-carboxypeptidase function. First, the MurNAc moiety is situated at the very top of the intersubdomain cavity and makes little contact with the protein. This part of the ligand has the highest B factors (a mean of 93 Å3, versus a mean of 83 Å3 for the intact ligand and a mean of 45 Å3 for all atoms modeled) and, thus, the weakest electron density. The MurNAc O1 and O4 atoms, which define the location of the flanking β1- to β4-linked GlcNAc sugars in PG, project away from the protein surface so that the glycan chain would be accommodated by the protein without steric clashes. The N-acetyl group at C1 is stabilized against the side chain of Met202 by van der Waals’ forces (Figure 5B).
The Cα and Cβ atoms of L-alanine at position 1 of the peptide pack against the face of Tyr144, a conserved amino acid in many LdcB sequences, to discriminate against the D-stereoisomer in this subsite, S3. L-alanine is favored by contacts with Tyr144 and Met202, which discriminate against all L-amino acids bulkier than alanine (Figure 5B). The NH2 group of the D-γ-Gln of position 2 is located in subsite S2, 2.3 Å away from and in hydrogen bonding distance of Glu204 (Figure 5C), an invariant amino acid in LdcB sequences. Stereoselectivity at this position is maintained because L-γ-glutamine would clash with Tyr144. The γ-glutamine is selected for by specific interactions with the main chain carbonyl of Ala203 and the side chain of Glu204 while ensuring that the scissile peptide bond is in the catalytic position. These key aspects of molecular recognition could not occur with commonly occurring amino acids. Canonical glutamine can be accommodated at this position in the synthetic peptide, tetra-D, because the absence of contacts with one face of the peptide provides scope for the conformational changes needed to accommodate glutamine in the absence of the glycan strands of the natural substrate.
Position 3 of the peptide is occupied by L-lysine. D-lysine could not be accommodated at this position because of the presence nearby of Glu107. As expected of a reaction product complex, the lysine carboxylate is located adjacent to the active site zinc in subsite S1 (Figure 5D). One of the carboxylic oxygens completes the zinc tetrahedral geometry with His153/Asp160/His207, and the second oxygen forms a salt bridge with Arg120, which is either arginine or lysine in LdcB orthologs. The L-lysine carboxylate thus represents a posthydrolysis state, placed appropriately for the peptide bond of the substrate to be hydrolyzed by the water molecule that fulfils the fourth zinc ligand in the absence of substrate.
The side chain of the L-lysine twists back against the rest of the peptide and projects into the protein core to place the terminal D-Asn branch in subsite S1′, against the main chain atoms of Tyr144 and the side chains of Tyr191, Tyr201, and His207 (Figure 5E). The carbonyl of the L-Lys—(D-Asn) peptide bond is contacted by the side chain of Ser151. However, it should be noted that subsite S1′ would be occupied by the scissile D-alanine in the presence of the reaction substrate, and support for this hypothesis comes from the structure of VanXYG solved in complex with a phosphinate transition state mimic (Figure 6A) (PDB ID 4muq) (Meziane-Cherif et al., 2014). The phosphinate moiety is a transition state mimic of peptide bond hydrolysis because the reaction proceeds via a tetrahedral intermediate that phosphinate mimics. VanXYG superimposes on SpLdcB with an rmsd of 1.6 Å on 144 matched Cαs, and the alanyl carboxylate moiety of the phosphinate is situated in an analogous position to that of the L-Lys—(D-Asn) peptide linkage observed in SpLdcB (Figure 6A). The phosphate atoms of the phosphinate transition state mimic superimpose almost identically with the phosphate anion modeled adjacent to the zinc ion in the structure of BsLdcB (Figure 3D). Other than Ser151, a role in determining specificity for the terminal D-alanine can be invoked for Gln125, and both these amino acids are strictly conserved. The amide nitrogen of Ala146 also participates in stereoselectivity, but as this is a main chain atom, it is perhaps no surprise that sequence conservation is not maintained at this position.
Figure 6.
A Comparison of the Ligand-Bound Active Sites Carboxypeptidases
(A) The active site of SpLdcB (white) with bound MurNAc—L-Ala-D-γ-Gln—L-Lys—(D-Asn) (turquoise carbons) is compared to that of VanXYg (blue) containing a phosphinate transition state analog (pink carbons) of D-Ala—D-Ala (PDB ID 4muq). The phosphinate in VanXYg coordinates the active site zinc in a similar manner to that of the lysyl carboxylate in the MurNAc—L-Ala-D-γ-Gln—L-Lys—(D-Asn) bound to SpLdcB. The D-alanyl moiety of the phosphinate ligand in VanXYg, which occupies subsite S1′, is matched by the D-alanine (green carbons) in SpLdcB (white) and the D-Asn of the MurNAc—L-Ala-D-γ-Gln—L-Lys—(D-Asn) ligand. The zinc ions are shown as gray spheres.
(B) The proposed positions of the two posthydrolysis ligands (orange carbons) in the active site of SpLdcB (white). The D-alanine from the SpLdcB structure occupies the S1′ site of the protein, while the lysine side chain has been rotated and modeled in an extended conformation. The altered conformation of the lysine presents the Nζ to the surface of the protein, in an ideal position to accommodate peptide crosslinks. The zinc ions are shown as gray spheres.
(C) The active sites of SpLdcB and pancreatic carboxypeptidase A (PDB ID 1hdu [Cho et al., 2002]) are compared. In each structure, the Zn2+ ion is coordinated by two histidines (His69 and His196 in carboxypeptidase A; His153 and His207 in SpLdcB) and an acidic amino acid (Glu72 in carboxypeptidase A; Asp160 in SpLdcB). The reversed locations of Arg127 and Glu270 in carboxypeptidase A relative to Arg120 and Glu204 in SpLdcB is matched by a reversal of the path of the respective substrates through the active sites and the likely retention of a common catalytic mechanism.
However, crosslinks between peptide stems cannot be accommodated in the observed conformation because of the burial of the branched amino acid in subsite S1′ (Figure 6B). Mere rotation of the side chain torsion angles of the L-lysine would result in a more thermodynamically favored extended conformation for this side chain to result in it passing beyond Arg120, Leu128, and Tyr132 to project beyond the surface of the protein (Figure 6B; Figure S2). In this conformation, crosslinked peptide stems attached to the terminal Nζ would not be sterically occluded by the body of the protein, consistent with the observed cleavage of crosslinked peptides (Figure 2A). In some bacteria, the lysine is replaced with diaminopimelic acid, a carboxy derivative of lysine that is amidated in bacilli. While the amidocarboxy derivative could be stabilized in the observed conformation by a similar set of specific interactions, it would also sterically occlude crosslinked peptide stems. Diaminopimelic acid is thus likely to follow the same path predicted for lysine by forming a simple, extended conformation to project into solvent.
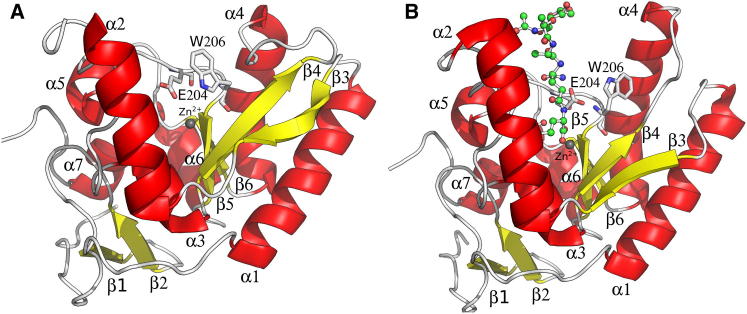
Several key structural changes occur on ligand binding in SpLdcB (Figure 7; Movie S1). First and foremost, substrates are occluded in the empty form of the enzyme by a loop between residues Gly163 and Glu171, which blocks access to the active site. Most of these residues (Asp167 to Glu171) move by 10–15 Å in the presence of ligand to open up the cleft by refolding into an additional turn at the N terminus of α-helix 4. The rearrangement of these residues is accompanied by further conformational changes in Trp206, the indole ring of which moves by up to 9 Å, which not only aids to open up the active site of the enzyme but also forces the side chain of Glu204 to rearrange to take up a position capable of interacting with the substrate. The importance of Glu204 and the zinc ion for catalysis is highlighted by the observation that tetra-D is a much poorer substrate for the E204A variant of SpLdcB (Figure 2A) and by the absence of activity in the presence of EDTA (Figures 2A and 2C). The conformational changes seen on ligand binding to SpLdcB contrasts with the absence of significant conformational changes described for VanXYG in complex with phosphinate (PDB ID 4muq) (Meziane-Cherif et al., 2014). Like LdcB, VanXYG maintains the H-X(3–6)-D-X(not conserved)-H motif, and as also observed in LdcB, the catalytic H-X-H motif is replaced by a glutamate. The resculpting of the N terminus of α-helix 4 in SpLdcB to accommodate the tetrapeptide is not necessary in VanXYG, because this enzyme hydrolyzes a much smaller substrate, D-Ala-D-Ala dipeptide, which is accommodated by the enzyme without significant rearrangement.
Figure 7.
Conformational Changes in SpLdcB on Ligand Binding
(A and B) Cartoon representations of (A) apo SpLdcB and (B) SpLdcB with bound MurNAc-Ala-D-γ-Gln-Lys-(D-Asn) using the same color scheme as in Figure 3. Key residues that move on ligand binding are shown in stick representation.
Discussion
The presence of tripeptides in the bacterial cell wall implies that LD-carboxypeptidase activity must be present in the extracellular milieu. In this study, we have shown that LdcB, from both S. pneumoniae and B subtilis, are active against tetrapeptides found within the bacterial cell wall and are specific for LD linkages, confirming the LD-carboxypeptidase classification with a role in cell wall maturation. The precise in vivo role for LdcB remains to be determined; one possibility is that the enzyme allows recycling of D-Ala from the cell wall to support further cell wall synthesis and, thus, growth. Alternatively, the cleavage of D-Ala from the cell wall might permit the differentiation between old and new cell walls, a potentially powerful way to ensure the correct localization of new cell wall synthesis. The correct localization of new PG synthesis is likely to be more important to bacteria that have polarized cell growth, such as Streptococci, in comparison to those that have dispersed growth along the length of a cylinder (e.g., Bacilli) or that utilize a division-only mechanism, such as Staphylococci. The cell morphological defect observed in the S. pneumoniae ldcB mutant, and the absence of defect in the B. subtilis ldcB mutant, could be consistent with this premise. Further studies should distinguish these possibilities.
We also present crystal structures of BaLdcB, BsLdcB, and SpLdcB, the latter also in complex with a PG-fragment analog that has enabled the identification of amino acids key to molecular recognition of the substrate and a potential tunnel through which crosslinked PG peptides may pass during proteolysis. LdcB belongs to the LAS group of zinc metalloproteases; several members of this family are active against PG, albeit with different substrate specificities to LdcB, including the Gly-Gly endopeptidase lysostaphin (Korndörfer et al., 2008) and the DD-/LD-endopeptidase MepA (Marcyjaniak et al., 2004). LdcB is dissimilar to other groups of LD-carboxypeptidases, e.g., Pseudomonas aeruginosa LD-carboxypeptidase LdcA (PDB ID 1ZRS) (Korza and Bochtler, 2005) that degrades cell wall tetrapeptides in the cytoplasm. LdcA utilizes a Ser-His-Glu catalytic triad in catalysis and belongs to the S66 serine protease family of enzymes, a group with which LdcB shares no similarity (Korza and Bochtler, 2005). Likewise, LdcB shares no sequence similarity with Csd6 and Pgp2, two recently discovered LD-carboxypeptidases that aid to maintain the helical shape of H. pylori (Sycuro et al., 2012) and C. jejuni (Frirdich et al., 2014). Csd6 and Pgp2 belong to an LD-transpeptidase family that utilizes a conserved Cys/His motif for catalysis (Biarrotte-Sorin et al., 2006), and are, therefore, distinct from the metalloprotease LdcB. It is interesting that Csd6 can only hydrolyze D-Ala from monomeric stem peptides, whereas LdcB is active against both monomeric and crosslinked (tetra-tetra) stem peptides (Sycuro et al., 2013), but the absence of structural similarity between these enzymes precludes useful comparison.
While LdcB shares no structural homology with previously studied LD-carboxypeptidases, DALI searches revealed a strong similarity to members of the LAS superfamily. Of the two consensus sequences that characterize the LAS family, LdcB lacks the second His-rich motif, which is replaced spatially by the invariant Glu204, which is oriented away from the zinc ion. Given the residual activity of the E204A mutant (Figure 2A), Glu204 probably plays an indirect role in catalysis. The zinc ion probably plays a more direct role in catalysis, a hypothesis that is supported by the absence of enzymatic activity in the presence of EDTA (Figures 2A and 2C). Further support for the role of the metal ion, and the indirect role for the glutamate, comes from studies of other zinc-dependent metalloproteases such as pancreatic carboxypeptidase A, which is of historical importance given its role in the demonstration of Koshland’s induced fit hypothesis (Christianson and Lipscomb, 1989; Koshland, 1958). Given the conformational changes seen on binding ligand (Figure 7; Movie S1), LdcB appears to be another example of induced fit. Though the two enzymes are structurally distinct, the active sites of LdcB and pancreatic carboxypeptidase A are, in essence, mirror images of one another. Both contain a zinc ion coordinated by two histidines (His69 and His196 in carboxypeptidase A; His153 and His207 in SpLdcB) and an acidic amino acid (Glu72 in carboxypeptidase A; Asp160 in SpLdcB). SpLdcB Arg120 and Glu204 straddle the nucleophilic water, but their positions relative to their counterparts in pancreatic carboxypeptidase A (Arg127 and Glu270) are reversed (Figure 6C). Consequently, the path of the substrate through the active site is also reversed. Given the retention of the metal coordination and key adjacent amino acids in LdcB, it is entirely plausible that the reaction mechanism of LdcB occurs in a similar fashion to that of pancreatic carboxypeptidase A, utilizing the metal ion to activate the nucleophilic water to attack the scissile peptide bond. The enzymes would thus appear to have converged on a common mechanism, despite sharing little overall sequence and structural homology. Confirmation of the catalytic mechanism utilized by this enzyme, and its precise role in vivo, is the focus of current research activities in our laboratories.
Experimental Procedures
Cloning
B. subtilis ldcB and S. pneumoniae ldcB were amplified by PCR from B. subtilis 168 and S. pneumoniae R6 genomic DNA, respectively, to generate fragments with NdeI and XhoI restriction sites. Both fragments were digested with NdeI and XhoI and ligated with NdeI/XhoI-digested pET28a. B. anthracis ldcB was PCR amplified from B. anthracis str. Ames ancestor genomic DNA and cloned into p15TV-LIC (Eschenfeldt et al., 2009) that codes for a N-terminal His6 tag, followed by a tobacco etch virus (TEV) protease cleavage site. In all cases, the recombinant constructs were designed to remove membrane-anchoring amino acids to result in final clones encoding SpLdcB amino acids 56–238; BsLdcB, 61–273; and BaLdcB, 55–243.
Strain Construction
S. pneumoniae R6ΔldcB was constructed by transforming strain R6 with a PCR product covering the ldcB-deletion region from strain IU3880 (D39 Δcps2A′(cps2BCDETFG)H′ΔdacB <> Pc-kan-rpsL) amplified with primers P136 and P137 (Barendt et al., 2011), followed by selection on tryptic soy broth blood agar plates containing 250 μg/ml kanamycin. All strains were grown in C + Y medium (Horne and Tomasz, 1993), containing kanamycin if required. Competent bacteria were obtained as described elsewhere, with the addition of competence-stimulating peptide (Vollmer and Tomasz, 2001).
B. subtilis strains were constructed by transformation of chromosomal DNA (Anagnostopoulos and Spizizen, 1961; Young and Spizizen, 1961) from strains bearing null mutations for dacA (MC01, trpC2 ΔdacA cat) or yodJ (MC05, trpC2 ΔyodJ neo) into strain RD180 (trpC2 ΔalrA zeo), selecting for the appropriate resistance markers (chloramphenicol or kanamycin at 5 μg/ml) in the presence of 40 μg/ml D-alanine, yielding strains KS03 and KS02, respectively.
Growth Curves
B. subtilis strains RD180, KS02, and KS03 were grown overnight at 30°C in Luria broth (LB) supplemented with 40 μg/ml D-Ala. The overnight cultures were diluted 1:10 with LB supplemented with 40 μg/ml D-Ala and grown at 37°C for 2 hr. The cells were collected by centrifugation and resuspended in D-Ala-free media at 37°C. This suspension was used to inoculate media with various concentrations of D-Ala to an optical density 600 (OD600) of 0.05, and the subsequent growth of each culture at 37°C was monitored by determining the optical density at 600 nm every 20 min for 3 hr. The growth rate was determined during the exponential growth phase only.
Mutagenesis
A modified QuikChange protocol, incorporating “touchdown” (Don et al., 1991), was used for site-directed mutagenesis. The annealing temperature of the first 10 cycles was reduced by one degree per cycle, from 68°C to 58°C, before a further ten cycles were performed at a constant annealing temperature of 58°C. The extension temperature was maintained at 72°C for 11 min per cycle. Mutagenic primers (0.3 μl) were mixed with 50 ng of template DNA, 0.5 mM dinucleotide triphosphates, and 2.5 U of Pfu DNA polymerase in a total reaction volume of 20 μl. The template plasmid was digested using DpnI.
Peptidoglycan Activity Assays
Cell wall and PG from S. pneumoniae and B. subtilis strains were isolated by published procedures (Bui et al., 2012; Garcia-Bustos et al., 1988). To test for LD-carboxypeptidase activity, PG from S. pneumoniae R6ΔldcB (1.6 mg/ml) or B. subtilis 168ΔldcB (3.1 mg/ml) was incubated in a total volume of 160 μl of 20 mM sodium phosphate, pH 4.8, with 0.13 mg/ml SpLdcB and 0.31 mg/ml BsLdcB, respectively, for 16 hr at 37°C. If required, 0.6 mM Zn2+ or 10 mM EDTA was added, and control samples contained no added enzyme. The PG was digested with 25 μg/ml of the muramidase cellosyl (a kind gift of Hoechst AG) for 24 hr at 37°C. The samples were boiled for 10 min and centrifuged for 10 min at 15,000 × g. The muropeptides present in the supernatant were reduced with sodium borohydride and analyzed by high-pressure liquid chromatography (HPLC) (Bui et al., 2012; Garcia-Bustos et al., 1988). S. pneumoniae and B. subtilis muropeptide peaks were assigned based on their published elution profile and retention times (Bui et al., 2012; Atrih et al., 1999). Fractions containing the reduced muropeptides Tetra and TetraTetra present in B. subtilis strain 168ΔldcB were collected and analyzed by mass spectrometry (Bui et al., 2009). We determined a neutral mass of 940.4353 atomic mass units (amu) for Tetra (theoretical: 940.4237 amu) and of 1862.8406 amu for TetraTetra (theoretical: 1862.8368 amu).
Thin-Layer Chromatography
Tetrapeptides Ala-D-Gln-L-Lys-D-Ala (Tetra-D; Activotec) or Ala-D-Gln-L-Lys-L-Ala (Tetra-L; Activotec) at 20 mM were incubated with 700 uM of zinc-loaded SpLdcB or SpLdcB-E153A for 2 hr at 37°C in a buffer of 10 mM HEPES/NaOH, pH 8.0, 100 mM NaCl in a reaction volume of 10 μl. One sample of SpLdcB contained 50 mM EDTA. Ethanol (5 μl) was added to the reactions before spotting on to silica gel thin-layer chromatography (TLC) aluminum foils (Sigma). The TLCs were developed in a running buffer of butan-1-ol, acetic acid, water in a 3:1:1 ratio for 40 min before staining with 0.1% ninhydrin solution in ethanol (Sigma). The TLC foils were dried and warmed with a domestic hairdryer for visualizing the results.
Protein Overexpression and Purification
BsLdcB and SpLdcB were prepared in the same way. The proteins were overexpressed in the methionine auxotrophic B834(DE3) strain of E. coli by growth in LB media (or in minimal medium supplemented with selenomethionine for selenomethionine-labeled protein) at 37°C up to an OD600 of 0.4 before induction with 1 mM IPTG for 18 hr at 16°C. Cells were harvested by centrifugation at 3000 g for 25 min at 4°C and the pellet was resuspended in 40 ml buffer A (50 mM HEPES.NaOH, pH 8.0, 300 mM NaCl, 20 mM imidazole) per liter of culture and lysed on ice for 3 min with a Sonopuls HD2070 (Bandelin) sonicator. The cell lysate was clarified at 33000 g for 20 min and the supernatant loaded onto a 5 ml Ni+-NTA column (GE Healthcare) pre-equilibrated with buffer A. The column was washed with buffer A before elution of bound proteins with buffer B (50 mM HEPES/NaOH, pH 8.0, 300 mM NaCl, 500 mM imidazole). The fractions containing LdcB were pooled and the N-terminal hexahistidine tag was removed by digestion with thrombin (Sigma-Aldrich) at 20°C for 16 hr at a ratio of 1 unit of enzyme per mg of LdcB. LdcB was concentrated to a volume of 1 ml before loading onto a Superdex S75 HiLoad 16/60 (GE Healthcare) gel filtration column pre-equilibrated in buffer C (10 mM HEPES/NaOH, pH 8.0, 100 mM NaCl).
For BaLdcB, freshly transformed E. coli BL21-CodonPlus (DE3)-RIPL cells were grown in 0.5 l of LB medium containing 100 μg/ml ampicillin. Cultures were grown at 37°C to an OD600 of 0.8 and induced for 16 hr at 16°C with 1 mM IPTG, and the cells were harvested by centrifugation and resuspended in 20 ml of buffer A (50 mM HEPES, pH 7.5, 300 mM NaCl, 10% [v/v] glycerol) supplemented with 10% (v/v) BugBuster 10× Protein Extraction Reagent (Novagen), 25 U Benzonase (Sigma-Aldrich), and 5 mM imidazole. The mixture was stirred for 10 min at room temperature and centrifuged at 20,000 × g for 45 min, and the supernatant was applied to a 1 ml HisTrap Fast Flow column (GE Healthcare) equilibrated with buffer A containing 40 mM imidazole. The protein was eluted with buffer A with a gradient of 40–500 mM imidazole over 20 ml. Fractions containing the recombinant His-tagged proteins were identified by SDS-PAGE; pooled; dialyzed overnight against 3 l of 50 mM HEPES, pH 7.5, 300 mM NaCl, 5% (v/v) glycerol, 1 mM TCEP; and stored at −80°C. The His6-tag was cleaved by TEV protease in 500 mM NaCl, 50 mM Tris-HCl, pH 7.5, 1 mM TCEP, 1 mM EDTA, 20% glycerol.
Crystallization
BsLdcB and SpLdcB were concentrated to 15 mg/ml, and two molar equivalents of zinc chloride were added. Crystallization conditions for BsLdcB and SpLdcB were obtained by sitting drop vapor diffusion at 20°C. The best SpLdcB crystals were obtained from a 0.1 M mixture of amino acids (glycine, L-glutamate, and racemates of alanine, serine, and lysine), 0.1 M Morpheus buffer system 2, pH 7.5, and 37.5% 2-Methyl-2,4-pentanediol (MPD)/polyethylene glycol (PEG) 1000/PEG 3350 (Gorrec, 2009). A single BsLdcB crystal grew from 0.1 M phosphate/citrate buffer, pH 4.2, 40% PEG 300 over a period of ∼2 months. Crystals of SpLdcB with bound MurNAc—L-Ala—D-γ-Gln—L-Lys—(D-Asn) (InvivoGen) were obtained by incubating the protein with the ligand at 2 mM final concentration for 30 min at room temperature prior to crystallization against a mother liquor of 0.1 M ammonium chloride, 0.1 M HEPES/NaOH, pH 8.0, and 20% PEG 6000. Crystals of BaLdcB (zinc free) were grown at 23°C using hanging-drop vapor diffusion by mixing 20 mg/ml protein with reservoir solution containing 0.3 M sodium chloride, 0.1 M HEPES, pH 7.5. As soaking of zinc chloride into BaLdcB apo-protein crystals did not provide sufficient occupancy of zinc into the active site, crystals of BaLdcB (zinc bound) were grown at 23°C by hanging-drop vapor diffusion by mixing 20 mg/ml protein and 5 mM zinc chloride prior to crystallization with 0.2 M ammonium acetate, 0.1 M sodium acetate, and 30% PEG 4000.
Data Collection and Processing
All crystals were cryoprotected with paratone-N before flash cooling in liquid nitrogen prior to data collection at 100 K. Diffraction data for BsLdcB and SpLdcB were on beamline IO2 of the Diamond Light Source. For selenomethionine-labeled SpLdcB, diffraction data were collected at a wavelength of 0.98 Å to permit phasing by selenomethionine-SAD (Se-SAD). Diffraction data were integrated with XDS (Kabsch, 2010) and reduced with SCALA (Evans, 2006). For BaLdcB, diffraction data were collected at on beamlines 21-ID-G at Life Sciences Collaborative Access Team, Advanced Photon Source, at 0.97856 Å (selenomethionine peak; zinc free) and 1.27696 Å (Zn2+ peak; zinc bound). The BaLdcB diffraction data were integrated and reduced with HKL-3000 (Minor et al., 2006).
Structure Solution
The structure of SpLdcB was solved using SHELX (Sheldrick, 2008) as implemented in the HKL2MAP interface (Pape and Schneider, 2004). An initial model was built by PHENIX.AUTOSOLVE (Adams et al., 2011). The completed SpLdcB structure was used as the molecular replacement search model in PHASER (McCoy et al., 2007) to solve BsLdcB and the SpLdcB-ligand complex. The BaLdcB Zn2+-bound structure was solved by SAD phasing using PHENIX.solve (Adams et al., 2011), which identified the positions of the two bound Zn2+ ions. The occupancies of the zinc atoms were refined during model refinement. The BaLdcB Zn2+-free structure was solved by molecular replacement using the D,D-peptidase domain of the apo structure of VanXYG (PDB ID 4f78) (Meziane-Cherif et al., 2014).
For all structures, manual building cycles in COOT (Emsley et al., 2010) were interspersed with restrained refinement in PHENIX.REFINE (Afonine et al., 2012). B factors were refined isotropically for all structures apart from BsLdcB, which was refined anisotropically. Noncrystallographic symmetry refinement was not used. Average B factor and geometric parameters were calculated using PHENIX and verified with PHENIX.refine and the Research Collaboratory for Structural Bioinformatics PDB Validation server. Data collection and final model refinement statistics for all structures can be found in Table 1. The graphics programs UCSF Chimera and PyMOL were used to generate all molecular figures presented.
Acknowledgments
We thank the staff at the Diamond Synchrotron Light Source for access to, and help with, its beamlines for X-ray diffraction data collection. We thank Arnaud Baslé, Joseph Newman, and Vincent Rao for useful discussions; Atikah Mohd Sukor for excellent technical assistance; Z. Wawrzak, S. Shatsman, and S.N. Peterson for assistance with data collection and refinement; and T. Skarina for assistance with purification and crystallization. We also thank the authors of Meziane-Cherif et al. (2014) for sharing results and atomic coordinates prior to publication. Finally, we thank Joe Gray of the Newcastle University Pinnacle-Proteomics and Biological Mass Spectrometry facility for mass spectrometry analysis of muropeptide fractions. This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, U.S. Department of Health and Human Services (contracts HHSN272200700058C and HHSN2722012000026C), and by the UK Biotechnology and Biological Sciences Research Council in an award to R.A.D., W.V., and R.J.L. (BB/G015902/1).
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
Accession Numbers
The PDB accession numbers for SpLdCB, BsLdcB, SpLdcB with bound ligand, apo BaLdcB, and BaLdcB with bound zinc reported in this paper are 4OX5, 4OX3, 4OXD, 4JID, and 4MPH, respectively.
Supplemental Information
Morph interpolation (UCSF Chimera) of the structural changes that occur upon ligand binding in SpLdcB. α-helices are shown in red, β-strands are in yellow, and loops between secondary structure elements are colored silver. The bound zinc ion is shown as a grey sphere. Key residues that move on ligand binding are shown in stick representation, and the movie was rendered in PyMOL.
References
- Adams P.D., Afonine P.V., Bunkóczi G., Chen V.B., Echols N., Headd J.J., Hung L.W., Jain S., Kapral G.J., Grosse Kunstleve R.W. The Phenix software for automated determination of macromolecular structures. Methods. 2011;55:94–106. doi: 10.1016/j.ymeth.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonine P.V., Grosse-Kunstleve R.W., Echols N., Headd J.J., Moriarty N.W., Mustyakimov M., Terwilliger T.C., Urzhumtsev A., Zwart P.H., Adams P.D. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 2012;68:352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arminjon F., Guinand M., Vacheron M.J., Michel G. Specificity profiles of the membrane-bound γ-D-glutamyl-(L)meso-diaminopimelateendopeptidase and LD-carboxypeptidase from Bacillus sphaericus 9602. Eur. J. Biochem. 1977;73:557–565. doi: 10.1111/j.1432-1033.1977.tb11351.x. [DOI] [PubMed] [Google Scholar]
- Atrih A., Bacher G., Allmaier G., Williamson M.P., Foster S.J. Analysis of peptidoglycan structure from vegetative cells of Bacillus subtilis 168 and role of PBP 5 in peptidoglycan maturation. J. Bacteriol. 1999;181:3956–3966. doi: 10.1128/jb.181.13.3956-3966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendt S.M., Sham L.T., Winkler M.E. Characterization of mutants deficient in the L,D-carboxypeptidase (DacB) and WalRK (VicRK) regulon, involved in peptidoglycan maturation of Streptococcus pneumoniae serotype 2 strain D39. J. Bacteriol. 2011;193:2290–2300. doi: 10.1128/JB.01555-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biarrotte-Sorin S., Hugonnet J.E., Delfosse V., Mainardi J.L., Gutmann L., Arthur M., Mayer C. Crystal structure of a novel β-lactam-insensitive peptidoglycan transpeptidase. J. Mol. Biol. 2006;359:533–538. doi: 10.1016/j.jmb.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Bishop B., Aricescu A.R., Harlos K., O’Callaghan C.A., Jones E.Y., Siebold C. Structural insights into hedgehog ligand sequestration by the human hedgehog-interacting protein HHIP. Nat. Struct. Mol. Biol. 2009;16:698–703. doi: 10.1038/nsmb.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochtler M., Odintsov S.G., Marcyjaniak M., Sabala I. Similar active sites in lysostaphins and D-Ala-D-Ala metallopeptidases. Protein Sci. 2004;13:854–861. doi: 10.1110/ps.03515704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui N.K., Gray J., Schwarz H., Schumann P., Blanot D., Vollmer W. The peptidoglycan sacculus of Myxococcus xanthus has unusual structural features and is degraded during glycerol-induced myxospore development. J. Bacteriol. 2009;191:494–505. doi: 10.1128/JB.00608-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui N.K., Eberhardt A., Vollmer D., Kern T., Bougault C., Tomasz A., Simorre J.P., Vollmer W. Isolation and analysis of cell wall components from Streptococcus pneumoniae. Anal. Biochem. 2012;421:657–666. doi: 10.1016/j.ab.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Bussiere D.E., Pratt S.D., Katz L., Severin J.M., Holzman T., Park C.H. The structure of VanX reveals a novel amino-dipeptidase involved in mediating transposon-based vancomycin resistance. Mol. Cell. 1998;2:75–84. doi: 10.1016/s1097-2765(00)80115-x. [DOI] [PubMed] [Google Scholar]
- Cho J.H., Kim D.H., Chung S.J., Ha N.C., Oh B.H., Yong Choi K. Insight into the stereochemistry in the inhibition of carboxypeptidase A with N-(hydroxyaminocarbonyl)phenylalanine: binding modes of an enantiomeric pair of the inhibitor to carboxypeptidase A. Bioorg. Med. Chem. 2002;10:2015–2022. doi: 10.1016/s0968-0896(01)00429-1. [DOI] [PubMed] [Google Scholar]
- Christianson D.W., Lipscomb W.N. Carboxypeptidase A. Acc. Chem. Res. 1989;22:62–69. [Google Scholar]
- Courtin P., Miranda G., Guillot A., Wessner F., Mézange C., Domakova E., Kulakauskas S., Chapot-Chartier M.P. Peptidoglycan structure analysis of Lactococcus lactis reveals the presence of an L,D-carboxypeptidase involved in peptidoglycan maturation. J. Bacteriol. 2006;188:5293–5298. doi: 10.1128/JB.00285-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R.H., Cox P.T., Wainwright B.J., Baker K., Mattick J.S. ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991;19:4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenfeldt W.H., Lucy S., Millard C.S., Joachimiak A., Mark I.D. A family of LIC vectors for high-throughput cloning and purification of proteins. Methods Mol. Biol. 2009;498:105–115. doi: 10.1007/978-1-59745-196-3_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 2006;62:72–82. doi: 10.1107/S0907444905036693. [DOI] [PubMed] [Google Scholar]
- Frirdich E., Biboy J., Adams C., Lee J., Ellermeier J., Gielda L.D., Dirita V.J., Girardin S.E., Vollmer W., Gaynor E.C. Peptidoglycan-modifying enzyme Pgp1 is required for helical cell shape and pathogenicity traits in Campylobacter jejuni. PLoS Pathog. 2012;8:e1002602. doi: 10.1371/journal.ppat.1002602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frirdich E., Vermeulen J., Biboy J., Soares F., Taveirne M.E., Johnson J.G., DiRita V.J., Girardin S.E., Vollmer W., Gaynor E.C. Peptidoglycan LD-carboxypeptidase Pgp2 influences Campylobacter jejuni helical cell shape and pathogenic properties and provides the substrate for the DL-carboxypeptidase Pgp1. J. Biol. Chem. 2014;289:8007–8018. doi: 10.1074/jbc.M113.491829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bustos J.F., Chait B.T., Tomasz A. Altered peptidoglycan structure in a pneumococcal transformant resistant to penicillin. J. Bacteriol. 1988;170:2143–2147. doi: 10.1128/jb.170.5.2143-2147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrec F. The MORPHEUS protein crystallization screen. J. Appl. Crystallogr. 2009;42:1035–1042. doi: 10.1107/S0021889809042022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L., Rosenström P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 2010;38(Web Server issue):W545–W549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D.S., Tomasz A. Possible role of a choline-containing teichoic acid in the maintenance of normal cell shape and physiology in Streptococcus oralis. J. Bacteriol. 1993;175:1717–1722. doi: 10.1128/jb.175.6.1717-1722.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korndörfer I.P., Kanitz A., Danzer J., Zimmer M., Loessner M.J., Skerra A. Structural analysis of the L-alanoyl-D-glutamate endopeptidase domain of Listeria bacteriophage endolysin Ply500 reveals a new member of the LAS peptidase family. Acta Crystallogr. D Biol. Crystallogr. 2008;64:644–650. doi: 10.1107/S0907444908007890. [DOI] [PubMed] [Google Scholar]
- Korza H.J., Bochtler M. Pseudomonas aeruginosa LD-carboxypeptidase, a serine peptidase with a Ser-His-Glu triad and a nucleophilic elbow. J. Biol. Chem. 2005;280:40802–40812. doi: 10.1074/jbc.M506328200. [DOI] [PubMed] [Google Scholar]
- Koshland D.E. Application of a theory of enzyme specificity to protein synthesis. Proc. Natl. Acad. Sci. USA. 1958;44:98–104. doi: 10.1073/pnas.44.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering A.L., Safadi S.S., Strynadka N.C.J. Structural perspective of peptidoglycan biosynthesis and assembly. Annu. Rev. Biochem. 2012;81:451–478. doi: 10.1146/annurev-biochem-061809-112742. [DOI] [PubMed] [Google Scholar]
- Marcyjaniak M., Odintsov S.G., Sabala I., Bochtler M. Peptidoglycan amidase MepA is a LAS metallopeptidase. J. Biol. Chem. 2004;279:43982–43989. doi: 10.1074/jbc.M406735200. [DOI] [PubMed] [Google Scholar]
- McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz R., Henning S., Hammes W.P. LD-carboxypeptidase activity in Escherichia coli. II. Isolation, purification and characterization of the enzyme from E. coli K 12. Arch. Microbiol. 1986;144:181–186. doi: 10.1007/BF00414732. [DOI] [PubMed] [Google Scholar]
- Meziane-Cherif D., Stogios P.J., Evdokimova E., Savchenko A., Courvalin P. Structural basis for the evolution of vancomycin resistance D,D-peptidases. Proc. Natl. Acad. Sci. USA. 2014;111:5872–5877. doi: 10.1073/pnas.1402259111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor W., Cymborowski M., Otwinowski Z., Chruszcz M. HKL-3000: the integration of data reduction and structure solution—from diffraction images to an initial model in minutes. Acta Crystallogr. 2006;D62:859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- Pape T., Schneider T.R. HKL2MAP: a graphical user interface for phasing with SHELX programs. J. Appl. Crystallogr. 2004;37:843–844. [Google Scholar]
- Royet J., Gupta D., Dziarski R. Peptidoglycan recognition proteins: modulators of the microbiome and inflammation. Nat. Rev. Immunol. 2011;11:837–851. doi: 10.1038/nri3089. [DOI] [PubMed] [Google Scholar]
- Sauvage E., Kerff F., Terrak M., Ayala J.A., Charlier P. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- Sheldrick G.M. A short history of SHELX. Acta Crystallogr. A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Sycuro L.K., Pincus Z., Gutierrez K.D., Biboy J., Stern C.A., Vollmer W., Salama N.R. Peptidoglycan crosslinking relaxation promotes Helicobacter pylori’s helical shape and stomach colonization. Cell. 2010;141:822–833. doi: 10.1016/j.cell.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sycuro L.K., Wyckoff T.J., Biboy J., Born P., Pincus Z., Vollmer W., Salama N.R. Multiple peptidoglycan modification networks modulate Helicobacter pylori’s cell shape, motility, and colonization potential. PLoS Pathog. 2012;8:e1002603. doi: 10.1371/journal.ppat.1002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sycuro L.K., Rule C.S., Petersen T.W., Wyckoff T.J., Sessler T., Nagarkar D.B., Khalid F., Pincus Z., Biboy J., Vollmer W., Salama N.R. Flow cytometry-based enrichment for cell shape mutants identifies multiple genes that influence Helicobacter pylori morphology. Mol. Microbiol. 2013;90:869–883. doi: 10.1111/mmi.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templin M.F., Ursinus A., Höltje J.V. A defect in cell wall recycling triggers autolysis during the stationary growth phase of Escherichia coli. EMBO J. 1999;18:4108–4117. doi: 10.1093/emboj/18.15.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer W., Tomasz A. Identification of the teichoic acid phosphorylcholine esterase in Streptococcus pneumoniae. Mol. Microbiol. 2001;39:1610–1622. doi: 10.1046/j.1365-2958.2001.02349.x. [DOI] [PubMed] [Google Scholar]
- Vollmer W., Blanot D., de Pedro M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- Vollmer W., Joris B., Charlier P., Foster S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 2008;32:259–286. doi: 10.1111/j.1574-6976.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- Young F.E., Spizizen J. Physiological and genetic factors affecting transformation of Bacillus subtilis. J. Bacteriol. 1961;81:823–829. doi: 10.1128/jb.81.5.823-829.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Morph interpolation (UCSF Chimera) of the structural changes that occur upon ligand binding in SpLdcB. α-helices are shown in red, β-strands are in yellow, and loops between secondary structure elements are colored silver. The bound zinc ion is shown as a grey sphere. Key residues that move on ligand binding are shown in stick representation, and the movie was rendered in PyMOL.