Abstract
The sleep EEG undergoes many changes during adolescence. We assessed whether sleep homeostasis is altered across adolescent development using two measures: the dissipation of slow-wave activity (SWA, 0.6 to 4.6 Hz) across the night and the rate of build-up of SWA in the first NREM sleep episode. Furthermore, we examined the association between homeostatic and circadian measures, by correlating the build-up of SWA in the first NREM sleep episode with circadian phase. Finally, we compared the dissipation of SWA in individuals with (PH+) and without (PH-) a parental history of alcohol abuse/dependence. Twenty children (8 PH+) and twenty-five teens (10 PH+) underwent two consecutive polysomnographic recordings at ages 9/10 and 15/16 years and again 1.5 to 3 years later. Thirteen young adults (ages 20 to 23; no PH+) were assessed one time. The decay of Process S was modeled for each individual at each assessment using data from both recordings. Four parameters of Process S were derived for EEG derivation C3/A2: time constant of the decay, lower asymptote (LA), the level of S at sleep onset (SSO), and SSO minus LA. We found no change in these parameters between assessments for the children and teen cohorts. Between-subjects analysis of the follow-up assessment for children (ages 11-13) and the initial assessment for teens (ages 15/16) showed no difference in these parameters, nor did follow-up assessment of teens (ages 17-19) compared to the single assessment of young adults (ages 20-23). Similarly, we observed no developmental changes in the rate of the build-up of SWA in the first NREM sleep episode for our within- and between-subjects analyses, or a correlation between this measure and circadian phase for either cohort. With regards to parental alcohol history, we found no difference in the dissipation of sleep pressure between PH+ and PH- children and teens. These results indicate that the dissipation of sleep pressure does not change across adolescent development, is not correlated to circadian phase, and does not differ between PH+ and PH- children and teens.
1. Introduction
Alterations in the timing and duration of sleep are among the biological and behavioral changes that accompany adolescent development. One of the most readily observable changes is a preference for later bedtimes in post-pubertal teens as compared to prepubertal children. Survey data from many samples indicate that the delay of bedtime is common for adolescent humans. Thus, this pattern may have a biological basis (reviewed in (Tarokh et al., 2010b)). Indeed, this sleep delay pattern is also found in juveniles of a number of nonhuman species (see (Hagenauer et al., 2009) for review). These maturational changes in sleep timing and duration may arise from alterations in circadian timing mechanisms or from mechanisms responsible for sleep regulation.
Our current understanding of sleep regulation comes from the two-process model proposed by Borbély in 1982 (Borbely, 1982) and later elaborated (Daan et al., 1984). According to this model, sleep-wake timing and duration are driven by an interaction between two independent processes – the circadian timing system and a sleep-wake homeostatic system. The circadian process, or Process C, is independent of prior sleep-wake distribution and operates in a clock-like fashion with a period of about 24 hours. Studies in humans and animals support the notion that changes to the circadian timing system underlie the delayed bedtimes observed in adolescents (reviewed in (Hagenauer et al., 2009)).
Unlike the circadian process, the homeostatic process (Process S) is sensitive to prior sleep-wake history. Sleep EEG spectral power in the delta (~0.75 – 4.5 Hz) frequency band is a marker of Process S and shows an increase with time awake and a decline over the course of sleep. Power in the delta band, i.e. slow-wave activity (SWA), is greatest in the first NREM sleep episode on average and declines over subsequent NREM sleep episodes. This decline in SWA over a sleep bout can be modeled using an exponential decay function (Achermann and Borbely, 1990, Rusterholz et al., 2008).
To date, only two cross-sectional studies (Jenni and Carskadon, 2004, Jenni et al., 2005) and one longitudinal study (Campbell et al., 2011) have examined the dynamics of Process S in adolescent humans. In both cross-sectional studies (Jenni and Carskadon, 2004, Jenni et al., 2005), participants kept a fixed sleep schedule for 10 days of 10 hours in bed prior to the in-lab. This design controls prior sleep history, which is known to affect absolute SWA. The first study examined the nocturnal dissipation of sleep pressure in eight pre-pubertal (mean age = 11.3 years, SD = ± 1.2) and eight post-pubertal (mean age = 14.1 years, SD = ± 1.3) adolescents at EEG derivation C3/A2 (Jenni and Carskadon, 2004). The estimated decay obtained from fitting data pooled across participants was 2.38 hours (95% confidence interval, 1.45 – 3.32 h) in pre-pubertal children and 2.14 hours (95% confidence interval, 1.34 – 2.95 h) in post-pubertal adolescents. The confidence intervals of the two functions were overlapping, indicating that the dissipation of sleep pressure did not differ between the two developmental groups.
A subsequent study confirmed this finding at the same EEG derivation for a different group of seven pre/early pubertal (mean age = 11.9 years, SD = ± 0.8) and six post-pubertal (mean age = 14.2 years, ± 1.4, 2 boys) adolescents (Jenni et al., 2005). This analysis estimated parameters for individual and mean group data, and statistical analysis of the time constant of the decay was performed using individual data. The time constant of the decay was estimated at 3.1 hours in pre/early pubertal children and 2.3 hours in post-pubertal children. As before, the two functions had overlapping confidence intervals. Both of these cross-sectional studies had small sample sizes, which limited the ability to detect group differences because of the individual variability in the time constant of the decay (reviewed in (Rusterholz et al., 2010)).
A recent longitudinal study by Campbell and colleagues (Campbell et al., 2011), examined sleep homeostasis in sixty-seven participants in two cohorts: one that started the study at the age of nine (n = 32) and the other at the age of twelve (n = 38). Participants were recorded semiannually in their homes for six years; thus, the analysis included an age range of nine to eighteen years. The authors used nonlinear mixed effects analysis to estimate the parameters of Process S for slow-wave activity (1 – 4 Hz) and theta activity (4 – 8 Hz). The estimated value for the time constant of the decay at age 9 was 1.56 hours for the delta band and 1.25 hours for the theta band, increasing by approximately one percent per year. This age-related change in decay time constant, however, was not statistically significant. On the other hand, a significant age-related decline of normalized delta and theta power at sleep onset was found for both frequency bands.
Rusterholz and colleagues (Rusterholz et al., 2010) have devised a new method for reliable estimation of Process S parameters for an individual. The approach is similar to that used by Jenni et al. (Jenni et al., 2005); however, the new approach constricts time constants to a physiologically meaningful range. We use this approach to model the dissipation of sleep pressure in two longitudinal cohorts and one cross-sectional cohort, which together span the ages of 9 to 24 years. Furthermore, in all of the studies reviewed above, parameters were estimated using one night of data. We aim to improve the reliability of the fits by estimating parameters at each assessment using data from two consecutive nights of sleep recording. In addition, we assess another measure of the homeostatic process by modeling the speed of the build-up of SWA in the first NREM sleep episode. This measure is sensitive to sleep pressure in adults, showing the fastest build-up when sleep pressure is at its highest (Achermann and Borbely, 1987) (Khatami et al., 2008). Finally, we were interested in whether measures of the circadian and homeostatic systems are correlated. We hypothesize that the longer the duration between dim light melatonin onset (DLMO) and sleep onset, the greater sleep pressure at sleep onset, and thus the faster the build-up of SWA in the first NREM sleep episode.
2. Experimental Procedure
2.1 Participants
Data from fifty-eight participants in three cohorts are presented here. EEG coherence, spectral, and trait analysis of a subset of these data have been published (Tarokh et al., 2010a) (Tarokh and Carskadon, 2010) (Tarokh et al., 2011). Two of the cohorts were assessed longitudinally with 1.5 to 3 years between recordings. The first longitudinal cohort included 20 children (seven females; 16 White, 2 Hispanic, 1 Black, and 1 Multiracial) ages 9 or 10 years at the initial recording; the second longitudinal cohort comprised 24 teens (13 females; 16 White, 4 Black, 3 Multiracial, and 1 Unknown) ages 15 or 16 years at the initial recording. A third cohort, 13 young adults (six females; ages 20 – 23 years; 8 White, 1 Hispanic, 2 Black, and 2 Unknown), was assessed one time. Mean and standard deviations of age for each cohort are shown in Table 1. Tanner staging (Tanner, 1962), a measure of external primary and secondary sex characteristics, was determined during a brief physical examination at each assessment for the children and teen cohorts. All children – except two who were Tanner 3 and 4 –were Tanner 1 or 2 (pre/early-pubertal) at the initial assessment and had advanced at least one Tanner stage at the time of the follow-up assessment. All teens were Tanner 5 (post-pubertal) at both assessments. Participants were screened by self- or parent-reports for a current or chronic illness, evidence of learning disability, sleep disorder, personal or family history of psychopathology and a pattern of insufficient sleep or excessive daytime sleepiness, indicated by reports of napping two or more times per week. The Lifespan Institutional Review Board approved all procedures, and participants and families were compensated for their time.
Table 1.
Sleep stage variables on the baseline night. Means (standard deviations) in minutes. P-values report statistical significance assessed using a paired t-test between initial and follow-up assessment for the children and teen cohorts. SWS = slow wave sleep; WASO = wake after sleep onset; Sleep Latency = first 1.5 consecutive minutes of stage 1 or first stage 2 sleep; REM Sleep Latency = sleep onset to first occurrence of REM sleep; Sleep Efficiency = total sleep time divided by time in bed.
| Sleep Variable | Children | Teens | Young Adults | ||||
|---|---|---|---|---|---|---|---|
| Initial | Follow- Up | P- Value | Initial | Follow- Up | P-Value | Initial | |
| Age (Years) | 10.1 (0.57) | 12.4 (0.73) | < 0.0001 | 15.7 (0.46) | 18.1 (0.63) | < 0.0001 | 22.5 (1) |
| Stage 1 (Min) | 34 (13) | 35 (14) | 0.86 | 34 (9) | 42 (12) | 0. 001 | 53 (19) |
| Stage 2 (Min) | 192 (54) | 233 (33) | 0.004 | 225 (35) | 262 (33) | 0.00002 | 252 (34) |
| SWS (Min) | 234 (52) | 180 (47) | 0.0005 | 157 (34) | 112 (34) | 0.000001 | 76 (32) |
| REM Sleep (Min) | 107 (20) | 116 (23) | 0.08 | 101 (17) | 108 (28) | 0.15 | 99 (15) |
| WASO (Min) | 17 (18) | 12 (15) | 0.39 | 10 (12) | 9 (7) | 0.58 | 17 (12) |
| Sleep Latency (Min) | 14 (11) | 16 (16) | 0.61 | 9 (5) | 9 (6) | 0.93 | 10 (9) |
| REM Sleep Latency (Min) | 156 (42) | 146 (51) | 0.43 | 153 (50) | 133 (44) | 0.11 | 122 (36) |
| Total Sleep Time (Min) | 567 (22) | 564 (20) | 0.53 | 516 (14) | 524 (27) | 0.18 | 480 (15) |
| Time in Bed (Min) | 603 (10) | 600 (<1) | 0.16 | 541 (6) | 546 (25) | 0.35 | 511 (4) |
| Sleep Efficiency (%) | 94 (4) | 94 (3) | 0.95 | 95 (2) | 96 (2) | 0.25 | 94 (3) |
2.2 Parental History of Alcoholism
A subset of the participants in this study had a past parental history of alcohol abuse or dependence, which was assessed using DSM-IV criteria applied to structured interviews (DIS-IV; (Robins et al., 2000)). Three females and five males in the children cohort had a positive parental history (PH+); five females and five males were PH+ in the teen cohort. None of the young adults met criteria for parental history of alcohol abuse or dependence. We used a mixed-models ANOVA with factors parental history and assessment to determine whether the parameters of Process S differed between PH+ and PH- participants; we found no main effect or an interaction of parental history for either cohort for any of the parameters of Process S ( τd , LA, and SSO; p > 0.1). Therefore, data for all participants were pooled in the subsequent analyses presented below.
2.3 Procedures
Participants slept on a stabilization sleep schedule with fixed bedtimes and rise times for at least one week before the in-lab polysomnography (PSG) recordings. Time in bed (TIB) for an individual was kept constant between assessments; however, TIB varied across cohorts. Participants in the children cohort had a schedule of at least 10 hours TIB, the teens were allotted at least 9 hours TIB, and the young adults a minimum of 8 hours TIB (Table 1). These at-home bed and rise times were maintained for the in-lab sessions. Participants spent two consecutive nights in the lab at each assessment (adaptation followed by a baseline night). During in-lab assessments, participants slept in individual darkened and temperature-controlled bedrooms while PSG was recorded.
2.4 PSG Recording
Baseline night recordings included two central (C3/A2 and C4/A1) and two occipital (O2/A1 and O1/A2) EEG derivations placed according to the international 10-20 system (Jasper, 1958), right and left electrooculgram (EOG), electromyogram (EMG; mentalis, submentalis), and electrocardiogram (ECG). On adaptation nights, EEG from derivations C3/A2 and O2/A1, EOG, EMG, and ECG were recorded. In addition, adaptation night recordings included screening for sleep-related breathing abnormalities and periodic limb movements using oral/nasal thermocouples and leg electromyogram (EMG), respectively. No sleep disorders were detected.
Recordings were performed on two data acquisition systems due to equipment upgrades midway through the study. All initial recordings, except three children, two teens and five young adults, were performed using the Albert Grass Heritage System (Astromed, Grass, West Warwick, RI) with GAMMA software. The EEG signals were digitized online (12 bit AD converter; Butterworth filter, -12 dB/octave; low-pass filter, -6dB at 35 Hz; time constant 1.0 s) with a sampling rate of either 100 or 128 Hz. The remaining participants’ initial recordings and all follow-up recordings were performed on the TWin system (Astromed, Grass, West Warwick, RI) using TWin AS40 bedside amplifiers (high-pass EEG filter 0.3 Hz; low-pass filter 35 Hz). These signals were collected digitally (storage resolution 400 Hz) and saved as EDF files. Impedance values were at or below 10 KΩ. We input a calibration signal simultaneously to both systems to test whether the signals from the two systems were comparable. The signals were in agreement between 0.3 to 16 Hz.
2.5 Data Processing and Signal Analysis
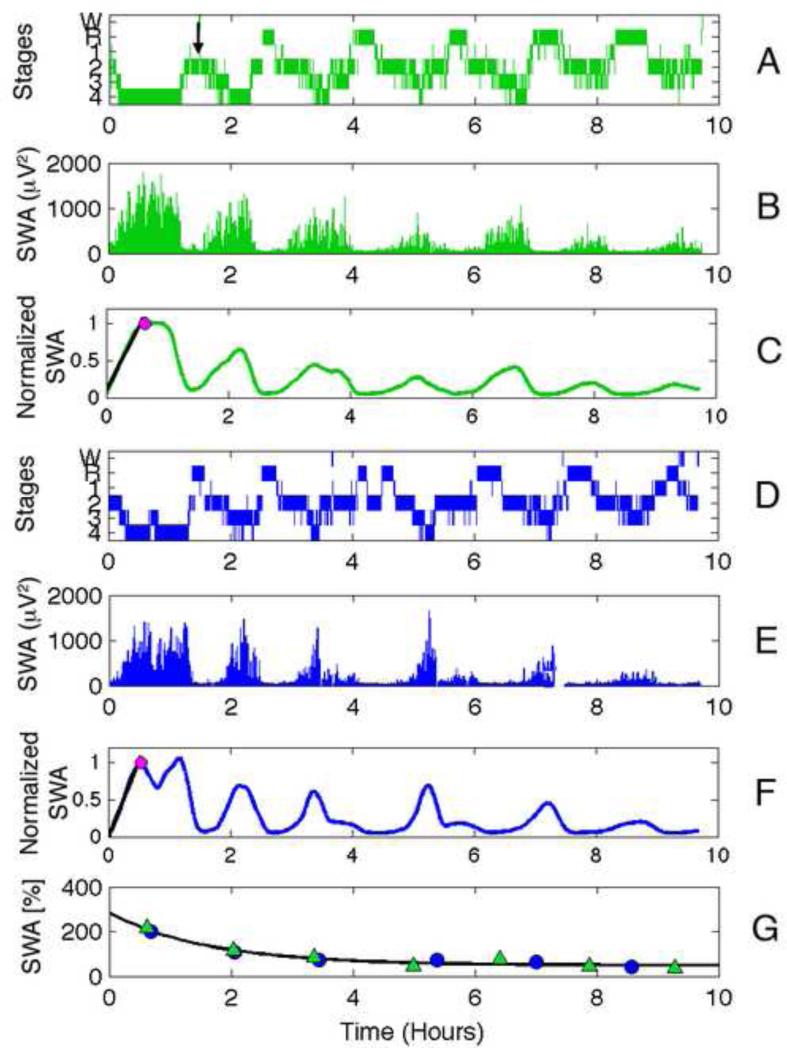
Sleep EEG data were scored according to the criteria of Rechtschaffen and Kales (Rechtschaffen and Kales, 1968), with inter- and intra-rater reliability of at least 86%. Sleep cycles were defined using a modified version of the criteria of Feinberg and Floyd (Feinberg and Floyd, 1979). The definition of Feinberg and Floyd requires that a REM sleep episode, with the exception of the first, be a minimum of 5 minutes in duration. We modified these criteria such that a REM sleep episode of any length signals the end of a NREM sleep episode. Furthermore, because children often “skip” their first REM sleep episode, resulting in a long first NREM sleep episode, we separated the first NREM sleep episode into two episodes when: (1) a continuous episode of stage 1, 2, awake or movement time lasting at least 12 minutes was preceded and followed by stage 3 or 4 (slow wave sleep; SWS) sleep (Jenni and Carskadon, 2004) or (2) two clear peaks of SWA plotted as a function of time separated by a trough were seen during the first NREM sleep episode (Campbell et al., 2011) (Figure 1A). The number of skipped REM sleep episodes applying these criteria were as follows: 13 children at the initial session and six at the follow-up session, 11 teens at the initial session and five at the follow-up session, and two young adults (one-time assessment). Only participants with an uninterrupted night of baseline sleep were included in the study. If sleep on an adaptation night was interrupted by a bout of waking greater than or equal to 30 minutes, only the NREM sleep episodes prior to that sleep disruption were used for that night. This occurred on adaptation nights in 4 children and 5 teens at one assessment and in 2 children at both assessments. The number of NREM sleep episodes used for analysis in these participants ranged from one to four.
Figure 1.
Illustration of methodology. Data are from the initial assessment of a boy in the children cohort. The data in green correspond to the adaptation night (Panels A and B), blue the baseline (panels D and E) night data. For each night, a hypnogram (Panels A and D), SWA (0.6 to 4.6 Hz; Panels B and E), and smoothed normalized SWA are shown (Panels C and F). The first REM sleep episode is skipped on the adaptation night (Panel A) and indicated with a black arrow. In the normalized SWA plots (Panels C and F), the maximum value in the first NREM sleep episode is depicted with a magenta dot and the fit to the first peak from which the rise-rate is derived are shown in black. Panel G shows the exponential decay fit using mean normalized SWA plotted at NREM sleep episode midpoint from both adaptation (green triangles) and baseline (blue circles) nights.
Power spectra were calculated for each 30-s epoch (Hanning window, average of six 5-s epochs) using MATLAB (The MathWorks Inc., Nantick, MA, USA), resulting in a frequency resolution of 0.2 Hz. Signal artifacts were rejected using a semi-automated procedure based on power in the 0.6 to 4.6 Hz and 20 to 40 Hz bands and confirmed by visual inspection. Slow-wave activity (SWA) was defined as power between 0.6 and 4.6 Hz.
2.6 Exponential Decay
An exponential decay function can be used to estimate Process S during sleep:
| (1) |
S(t) is the level of Process S at time t, τd is the time constant of the decaying exponential function, SSO is the level of S at sleep onset, and LA is the lower asymptote. For each night, mean SWA in each NREM sleep episode (stages 2 to 4) was calculated and then normalized by the mean SWA across NREM sleep (restricted to the minimal duration of NREM sleep across participants; Figure 1G). Normalized mean SWA within an episode was placed at the time of episode midpoint. As in previous studies (e.g., (Jenni et al., 2005) (Jenni and Carskadon, 2004) (Campbell et al., 2011)), we use the midpoint of each NREM sleep episode because it takes into account the duration of the NREM sleep episode. Thus shorter episodes result in a steeper decline (less total SWA; dissipation occurs more quickly) and longer episodes (more SWA; dissipation takes longer to achieve) result in a slower decline. A single night yields few data points (dependent on the number of NREM sleep episodes), therefore, we combined data from adaptation and baseline nights to estimate S(t) at each assessment to improve stability of the measures. Thus, a single exponential decay was fit to adaptation and baseline nights, resulting in one fit (i.e., τd, SSO, and LA) for each participant at each assessment (Figure 1G). The decay of Process S was modeled within an individual using the method of Rusterholz and τd colleagues (Rusterholz et al., 2010): the average time constant of the decay, , was determined by a fit to the pooled data across participants, and individual fits were restricted to hours. The lower asymptote, LA, was forced to be greater than zero due to the assumption that a certain level of SWA must be present to maintain sleep. Because the distance between SSO and LA determine the range of the exponential decline, we also report the difference between SSO and LA.
Goodness of fit was calculated for the fit at each assessment by computing the coefficient of determination, i.e., R2, as follows:
| (2) |
SSerr in this equation is the sum of squares of residuals and SStot is the total sum of squares. We consider R2 value less than 0.6 as poor fits and therefore exclude participants with low R2 values at either assessment.
2.7 Build-up of SWA in First NREM Sleep Episode
We used a moving average filter over fifty 30-second epochs to smooth SWA activity across the night (MATLAB function SMOOTH) as represented in Figure 1C and 1F and to identify the local maximum in the first NREM sleep episode. If more than one peak was found, the first peak was used unless it was less than 60% of the second peak in which case the second peak was used. Because sleep EEG power decreases with age (e.g., (Tarokh and Carskadon, 2010) (Kurth et al., 2010) (Campbell and Feinberg, 2009)) and affects our measure (i.e., greater power will result in steeper slope), we normalized the data by setting power at the peak to 1 (see Figure 1C and 1F). The slope between SWA in the first epoch of sleep onset and the peak of SWA in the first NREM sleep episode was computed and used to define the rising phase of sleep homeostasis (riserate). We also report rise-time as the time it takes for the build-up to occur (i.e., minutes from sleep onset to the peak of SWA; rise-time); however, we note that the rise-rate and rise-time are not independent. We performed this analysis for adaptation and baseline nights separately.
2.8 Statistical Analysis: Exponential Decay
Mixed model ANOVAs with within-subjects factor assessment (initial versus follow-up_ and between subjects factor sex (male versus female) were computed within children and teen cohorts to assess initial to follow-up differences in the parameters of Process S (i.e., SSO, LA, SSO – LA, and τd). In addition, between subjects ANOVAs were performed comparing the follow-up assessment of children (ages 11 to 13) and the initial assessment of the teens (ages 15 to 16) to assess changes in the parameters of Process S (i.e., SSO, LA, SSO – LA, and τd) between these ages (i.e., 2×2 ANOVA with factors cohort: children follow-up versus teens initial and sex: male versus female). Differences between the one-time assessment of the adult cohort (ages 20 to 23) and the follow-up assessment of the teens (ages 17 to 19) were also examined using a 2×2 ANOVA (factors cohort: teens follow-up versus adults initial and sex: male versus female). Four parameters (SSO, LA, SSO – LA, and τd) were tested; therefore, we use a Bonferroni correction, resulting in an alpha value of 0.0125 (α = 0.05/4).
2.9 Statistics: Build-up of SWA in First NREM Sleep Episode
A repeated measures within subjects’ 2×2 ANOVA with factors assessment (initial versus follow-up) and night (adaptation versus baseline) was used within longitudinal cohorts to assess whether the rise-rate and rise-time differed as a function of development. Similarly, we performed a between-subject analysis using a 2×2 ANOVA (factors: assessment and night) to compare the follow-up assessment of children to the initial assessment of teens. Similarly, we compared the follow-up assessment of teens to the one time assessment of young adults.
2.10 Circadian Phase Measurement
Dim light melatonin onset was assessed in-lab using serial saliva samples prior to bedtime on the adaptation nights at each assessment. Approximately 10 samples were collected every 30-minutes in dim light (< 50 lux) before scheduled bedtime. Saliva samples were frozen and assayed for melatonin using radioimmunoassay test kits (Alpco, Salem, NH). A threshold of 4 pg/mL was used as our threshold (Carskadon et al., 1997, Crowley et al., 2006) and time of DLMO phase was calculated by linear interpolation between the time points before and after the melatonin concentration increased above 4 pg/mL (Crowley et al., 2006). If the last sample did not exceed threshold (4pg/mL) and the value at the last sample was greater than half the threshold (≥ 2 pg/mL), the next time point was used as the estimate of DLMO. This DLMO estimation occurred in four children and two teens. Conversely, in two teens for whom the first sample was above threshold, but by no more than by 50% (6 pg/mL), the preceding time point was used as the estimate of DLMO. One teen and one child had melatonin values below 2 pg/mL at the last sample and one teen had a melatonin value above 6 pg/mL at the first sample, exceeding our rubric for estimating DLMO phase; these participants were not included in the analysis. The time between DLMO and sleep onset was then calculated and correlated with the build-up of SWA in the first NREM sleep episode on adaptation nights.
3. Results
3.1 Sleep Stage Analysis
In line with previous reports (Williams et al., 1972, Carskadon, 1982, Coble et al., 1984, Koessler et al., 2009, Tarokh and Carskadon, 2010) (Jenni and Carskadon, 2004), we observed a change in sleep architecture between assessments within the children and teen cohorts (Table 1). Baseline night data from both cohorts showed a significant increase in minutes of stage 2 sleep (children: t(19) = -3.25; teens: t(24) = -5.30) and a decline in minutes of slow wave sleep (children: t(19) = 4.17; teens: t(24) = 9.5) from the initial to the follow-up assessments. Minutes of stage 1 sleep increased across assessments for the teen cohort only (t(24) = 3.74). No maturational changes were observed in the other sleep stage variables as shown in Table 1.
3.2 Episode Length Analysis
Duration of NREM sleep episodes affects the estimation of the parameters of Process S; therefore, we assessed whether episode duration changed between assessments by performing a repeated measures ANOVA with factors assessment (initial versus follow-up) and NREM sleep episode (1 through 4) for the baseline nights. We found no significant change in NREM sleep episode duration between assessments (main effect of assessment; F(1) = 0.47; p = 0.50), nor was there a significant interaction with NREM sleep episode (F(3) = 1.24; p = 0.30).
3.3 Parameters of Process S: Within Subject Analyses of Children Cohort
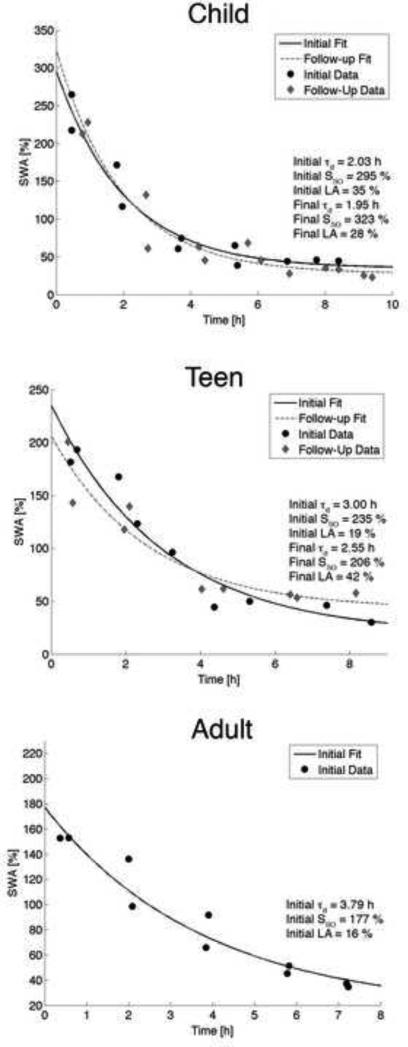
Two participants’ data were excluded from the analysis of the children cohort due to R2 values less than 0.6. Data and fits from one participant at both assessments are shown in Figure 2 (upper panel) to demonstrate the overall findings. We found no significant change between assessments for any of the parameters of the Process S ( τd, SSO, LA, and SSO - LA) or a difference between males and females in the children cohort (Table 2; Figure 3).
Figure 2.
Data and exponential declined fitted to the data for one exemplary child, teen and young adult at derivation C3/A2. Normalized mean SWA at episode midpoint is plotted as a black dot for the initial session and as a diamond for the follow-up session. The exponential fit to the decay is shown as a solid line for the initial session and a dashed line at the follow-up session. We observed no change between assessments for any derivation in the children and teen cohorts.
Table 2.
Parameter of the exponential decline and p-values for all cohorts and withinsubject analysis. τ d = the time constant of the decay; LA = Lower Asymptote and SSO = Level of S at sleep onset. Mean and standard deviations for all parameters for the three
| Initial | Follow-up | |||||
|---|---|---|---|---|---|---|
| τd (h) | LA (%) | Sso (%) | τd (h) | LA (%) | Sso (%) | |
| Children | ||||||
| Mean | ||||||
| Female | 2.06 | 41 | 270 | 1.80 | 41 | 304 |
| Male | 2.01 | 38 | 279 | 2.02 | 37 | 274 |
| SD | ||||||
| Female | 0.68 | 14 | 43 | 0.64 | 16 | 58 |
| Male | 0.63 | 11 | 46 | 0.43 | 16 | 64 |
| F | ||||||
| Assessment | 0.30 | 0.03 | 0.65 | |||
| Assessment*Sex | 0.35 | 0.006 | 1.14 | |||
| P-value | ||||||
| Assessment | 0.59 | 0.86 | 0.43 | |||
| Assessment*Sex | 0.56 | 0.94 | 0.30 | |||
| Teens | ||||||
| Mean | ||||||
| Female | 2.34 | 32 | 263 | 2.56 | 28 | 236 |
| Male | 2.11 | 43 | 250 | 2.32 | 43 | 226 |
| SD | ||||||
| Female | 0.60 | 11 | 50 | 0.62 | 11 | 51 |
| Male | 0.62 | 14 | 32 | 0.66 | 10 | 59 |
| F | ||||||
| Assessment | 1.62 | 0.32 | 5.11 | |||
| Assessment*Sex | 0.003 | 0.43 | 0.02 | |||
| P-value | ||||||
| Assessment | 0.22 | 0.58 | 0.04 | |||
| Assessment*Sex | 0.96 | 0.52 | 0.90 | |||
| Adults | ||||||
| Mean | ||||||
| Female | 2.97 | 25 | 200 | |||
| Male | 2.84 | 27 | 199 | |||
| SD | ||||||
| Female | 0.72 | 12 | 30 | |||
| Male | 0.34 | 14 | 14 | |||
Figure 3.
Individual parameters of Process S for all cohorts. Each color represents an individual within a cohort. Black circles and bars show the mean value across participants and standard deviations.
3.4 Parameters of Process S: Within Subject Analyses of Teen Cohort
Two participants’ data were excluded from the analysis of the teen cohort due to R2 values below our criterion. Similar to the children cohort, we found no change in the time constant of the decay, the lower asymptote, SSO or SSO - LA between assessments or between sexes (Figure 3). Means and standard deviations of the parameters of the fit, along with p-values are shown in Table 2. Fits and data from one teen are depicted in Figure 2 (middle panel).
3.5 Parameters of Process S: Across Cohort Analyses
Between subjects analysis of the follow-up session of the children (ages 11 to 13 years) and the initial session of the teens (ages 15 and 16 years) revealed no significant differences for any parameter and no effect of sex. Similarly, we found no difference between the follow-up session of teens (ages 17 to 19 years) and the one-time assessment of young adults (ages 20 to 23 years) for any parameter or an effect of sex (adult parameter means and standard deviations are presented in Table 2; individual values Figure 3).
3.6 Build-up of SWA in the First NREM sleep Episode
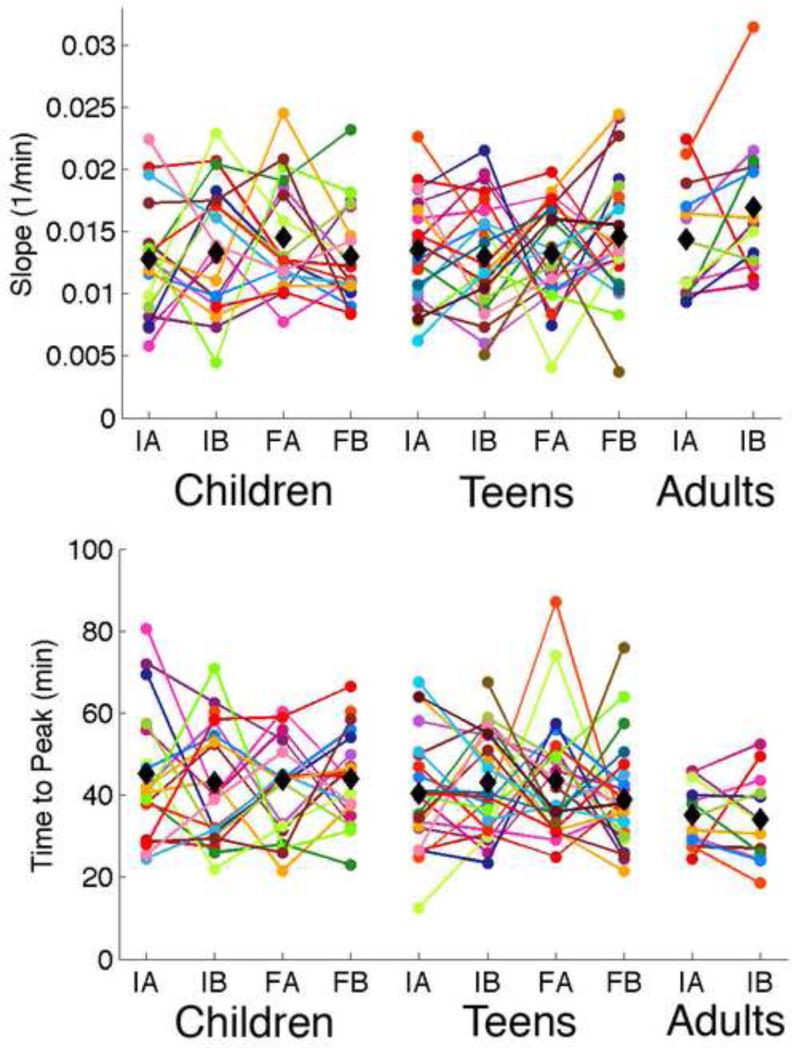
We did not find a difference in the rise-rate or rise-time between assessments (main effect: initial versus follow-up), consecutive nights (main effect: adaptation versus baseline), or an interaction of the two for either longitudinal cohort (Figure 4). Our between-subjects analysis of the follow-up assessment of children compared to the initial assessment of teens and the follow-up assessment of teens compared to the initial assessment of young adults was also not significant (Figure 4).
Figure 4.
Parameters obtained from the rise of SWA in the first NREM sleep episode. Each individual is shown in one color. The top plot shows the slope of the rise to the peak in the first NREM sleep episode. The bottom plots shows the time in minutes to the first peak. IA = Initial adaptation; IB = Initial baseline; FA = Final Adaptation; FB = Final Baseline. Mean values at each time point are shown in black diamonds.
3.7 Circadian Phase and Build-up of SWA
Dim light melatonin onset (DLMO) occurred at 20.08 (SD = 0.91) and time from DLMO to sleep onset (DLMO phase angle) was 1.66 hours (SD = 1.09) at the initial assessment in the child cohort. One and half to three years later at follow-up, DLMO was at 20.01 (SD = 0.82) and DLMO phase angle was 1.56 hours (SD = 0.78). In the teen cohort, DLMO and DLMO phase angle at the initial assessment were 20.32 (SD = 0.97), and 2.09 hours (SD = 0.9) respectively. At teen follow-up, DLMO was 21.32 (SD = 0.86) while DLMO phase angle was 1.51 hours (SD = 0.74). Circadian phase and build-up of SWA in the first NREM sleep cycle were not correlated for either assessment in the children (Initial: r = 0.046, p = 0.86; Follow-up: r = 0.11, p = 0.72) or teen (Initial: r = 0.35, p = 0.17; Follow-up: r = 0.46, p = 0.06) cohorts.
4. Discussion
This analysis included participants studied with a well-controlled prior sleep schedule and laboratory protocol to examine sleep homeostasis across the ages of 9 to 23 years. A combination of several methodological approaches sets this analysis apart from the previous work in this area. In the first place, we used a novel method developed by Rusterholz and colleagues (Rusterholz et al., 2010) to fit SWA using data from two nights in an individual. This procedure allowed us to assess goodness of fit at the individual level, to discard poor fits, and to perform more precise statistical testing. Second, we used two approaches to assess sleep homeostasis – parameters of Process S and the rise of SWA in the first NREM sleep episode. Finally, we combined a longitudinal approach with a level of control imposed on sleep before and during the study, which exceeds that of previous longitudinal studies (e.g., (Campbell et al., 2011)). Sleep duration prior to the study was held constant across assessments for at least one week, and compliance to the schedule was confirmed using sleep diaries, actigraphy, and daily phone calls to the lab. Furthermore, participants slept in a laboratory in individual darkened bedrooms that were temperature controlled with no intrusion of outside light or noise during the night. We feel that this level of control of sleep is important to avoid extrinsic factors that may disrupt sleep and artificially affect sleep structure. For example, sleep disruption of sufficiently long duration, may affect sleep pressure and intensify subsequent SWA affecting the fit to the data (e.g., (Dijk and Beersma, 1989)). Therefore, we exclude NREM sleep episodes following an extended period of waking (> 30 minutes) and feel it is essential that sleep be recorded in a controlled environment.
Despite the well-controlled environment for sleep-wake conditions and data acquisition, night-to-night variability in visually scored sleep and levels of normalized SWA across the night were striking in many participants. This night-to-night variability has previously been noted, and although in some participants consecutive nights are well aligned, this is not the case for all participants. For example, examining 14 consecutive nights on fixed sleep-wake schedules from three participants, Achermann and Borbély (Achermann and Borbely, 1987) observed considerable intra-individual and inter-individual variability in the distribution of SWA across the night. Given the sensitivity of exponential fits to the length of NREM sleep episodes, the duration of intervening REM sleep episodes, and the level of SWA within episodes, we expect the quality of fits differ across consecutive nights if these variables differ. Furthermore, unlike the averaged sleep EEG spectrum, which is based on an average of hundreds of values and is stable within adolescence (Tarokh et al., 2011) and young adults (Buckelmuller et al., 2006), each exponential fit for the homeostatic analysis is based on a limited number of data points (restricted to the number of NREM sleep episodes). We were able to increase the number of data points used for each fit by pooling both adaptation and baseline nights; however, considerable individual variability in the trajectory of these parameters over time is seen in our estimated parameters (Figure 3).
Our analysis of the dissipation of sleep pressure across adolescent development in two longitudinal and one cross-sectional cohort indicated no difference between males and females and no developmental progression. This finding aligns well with previous longitudinal and cross-sectional studies of sleep homeostasis in adolescence (Jenni and Carskadon, 2004, Jenni et al., 2005) (Campbell et al., 2011). The participants in our within subjects analysis of the children cohort are comparable in age to two cross-sectional studies reported by Jenni and colleagues comparing sleep homeostasis in pre/early and post-pubertal participants (Jenni and Carskadon, 2004, Jenni et al., 2005). In these studies the pre/early pubertal groups were, on average, 11.3 years (Jenni and Carskadon, 2004) and 11.9 years (Jenni et al., 2005) while the post-pubertal groups were, on average, 14.1 and 14.2 years respectively. Our results in this within subject assessment of a larger sample are in line with findings of no change in the parameters of the decline of Process S across this age range.
A recent extensive study by Campbell and colleagues (Campbell et al., 2011) examined the parameters of the decline of Process S in a longitudinal study of cohorts spanning the ages of 9 to 18 years using derivation C3/A2 or C4/A1 (as available from recordings). These authors observed a developmental decline in the percent of delta energy in the first NREM sleep episode and in normalized delta power at the start of the night. We did not find a decline in normalized delta power at sleep onset in either cohort; however we detected a trend (F(1,19) = 5.11, p = 0.036) for a decline in SSO for the teen cohort. The physiological significance of such a developmental change, if present, remains to be determined, though likely related to anatomical changes in the brain. Similar to the Campbell et al. study (Campbell et al., 2011), we found no evidence of a developmental change in the time constant of the decay or the lower asymptote. If the time constant of the decay, τd, reflects sleep's recuperative process, one interpretation of this finding is that the need for sleep does not change across adolescent development.
In addition to the exponential fits, we measured the rate of SWA build-up in the first NREM sleep episode to examine sleep homeostasis. Studies have shown that the slope of the build-up to the peak of SWA on a single sleep episode is greater if sleep pressure is higher, and thus, this measure provides another index of sleep homeostasis (Achermann and Borbely, 1987, Khatami et al., 2008). Similar to our exponential fits, we observed no developmental change in this measure. Thus, our two measures of sleep homeostasis are in agreement, indicating that the dissipation of sleep pressure does not change across adolescence.
4.1 Circadian Phase and Sleep Homeostasis
We hypothesized that a longer duration between DLMO and sleep onset would result in greater sleep pressure and thus may be reflected in the sleep EEG as a faster build-up of SWA in the first NREM sleep episode. We did not, however, find a correlation between our circadian and homeostatic measures. One possibility is that although the circadian and homeostatic systems work in concert, the association between the outputs from these two systems is complex and cannot be captured by a simple correlation. An alternate hypothesis is that our sample lacks the variability necessary to make meaningful correlations. In fact, a study by Carskadon and colleagues (Carskadon et al., 1997) found less variability in DLMO when participants slept on fixed sleep schedules as compared to self-selected schedules or under constant routine conditions. Thus self-selected schedules may be better suited for such studies.
4.2 Parental Alcohol History
Our sample consisted of children and teens with and without a parental history of alcohol abuse or dependence. The risk for developing alcoholism is affected by genetic and environmental factors, and individuals with a parental history of alcoholism are at an increased risk for themselves developing alcohol dependence (Merikangas et al., 1998, Lieb et al., 2002). Several studies have shown that sleep homeostasis is disrupted in alcoholics. For example, Irwin and colleagues showed that, unlike healthy controls, alcoholics do not show an increase in SWA after sleep deprivation, indicating impaired sleep homeostasis (Irwin et al., 2002). Similarly, a recent study by Brower and colleagues reported slower dissipation of SWA percent across the night in alcohol dependent men compared to healthy controls (Brower et al., 2011). Since children with a family history of alcoholism are at an increased risk for developing alcoholism due to the contribution of genes and environment, it is possible that impaired sleep regulation may be a marker that precedes the onset of alcohol use. We found no differences in the dissipation of sleep pressure between children and teens with and without a parental history of alcoholism, suggesting that the impairments in sleep regulation observed in adult alcoholics may be a consequence of long-term alcohol consumption. On the other hand, our sample was small and parents were not necessarily alcohol dependent at the time of the study making this conclusion tentative.
This study has several limitations: EEG recordings were analyzed at only one derivations, which limits our ability to examine regional differences; the modest sample size; differential times in bed between cohorts; the longer duration between assessments as compared to the study of Campbell and colleagues (Campbell et al., 2011); and only two data points for individual participants. One limitation of all human studies examining sleep homeostasis is the use of one, or in this case two, recording nights. Future studies should examine the stability of these fits by analyzing data from multiple nights. Furthermore, this analysis was able to address only one aspect of Process S – the dissipation of sleep pressure. The build-up of Process S should be examined longitudinally, since cross-sectional data indicate that this process is altered in adolescence (Jenni et al., 2005).
We note, however, that these longitudinal and cross-sectional data show that the dissipation of Process S was not modified in our group of late adolescence, a finding that may have implications for public health. Epidemiological data show that sleep duration decreases across adolescence, with 12th graders getting on average 1.5 hours less sleep than 6th grader students (Foundation, 2006). Yet a well-controlled study examining sleep “need” showed that late teens given the same opportunity will sleep for just as long as early adolescents (Carskadon, 1982). Our finding lends support to the behavioral observation that sleep need does not decrease across this age range.
Highlights.
We find no change in the dissipation of sleep pressure across adolescence
Sleep homeostasis comparable in teens with/without a parental history of alcoholism
No association between DLMO phase and rise of SWA in the first NREM sleep episode
Acknowledgments
The authors are grateful to Dr. Thomas Rusterholz for computation assistance. We also thank Drs. Ronald Seifer, Christine Acebo, Tracy Rupp, Oskar Jenni, Monique LeBourgeois, Eliza Van Reen, Gahan Fallone, and Margaret Borkowski for assistance with recruitment, screening, recording and evaluation of the participants. Drs. Elizabeth Forbes and Judith Owens performed the Tanner staging. William Coon and Henry Arantes scored sleep stages and we are grateful for their contribution. We also thank our research staff, laboratory technicians, and participants.
This work was supported by grant AA13252 (to M.A.C.) and the Swiss National Science Foundation (320030-130766 to P.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
L. Tarokh, M.A. Carskadon and P. Achermann declare no conflicts of interest.
References
- Achermann P, Borbely AA. Dynamics of EEG slow wave activity during physiological sleep and after administration of benzodiazepine hypnotics. Human neurobiology. 1987;6:203–210. [PubMed] [Google Scholar]
- Achermann P, Borbely AA. Simulation of human sleep: ultradian dynamics of electroencephalographic slow-wave activity. Journal of biological rhythms. 1990;5:141–157. doi: 10.1177/074873049000500206. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Human neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- Brower KJ, Hoffmann R, Conroy DA, Arnedt JT, Armitage R. Sleep homeostasis in alcohol-dependent, depressed and healthy control men. European archives of psychiatry and clinical neuroscience. 2011 doi: 10.1007/s00406-011-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckelmuller J, Landolt HP, Stassen HH, Achermann P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006;138:351–356. doi: 10.1016/j.neuroscience.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Darchia N, Higgins LM, Dykan IV, Davis NM, de Bie E, Feinberg I. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during NREM sleep. Sleep. 2011;34:83–91. doi: 10.1093/sleep/34.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5177–5180. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA. The second decade. In: Guilleminault C, editor. Sleep and Waking Disorders: Indications and Techniques. Addison Wesley; Menlo Park: 1982. pp. 99–125. [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. Journal of biological rhythms. 1997;12:278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- Coble PA, Kupfer DJ, Taska LS, Kane J. EEG sleep of normal healthy children. Part I: Findings using standard measurement methods. Sleep. 1984;7:289–303. doi: 10.1093/sleep/7.4.289. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, Fallone G, Carskadon MA. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep. 2006;29:1632–1641. doi: 10.1093/sleep/29.12.1632. [DOI] [PubMed] [Google Scholar]
- Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. The American journal of physiology. 1984;246:R161–183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG. Effects of SWS deprivation on subsequent EEG power density and spontaneous sleep duration. Electroencephalography and clinical neurophysiology. 1989;72:312–320. doi: 10.1016/0013-4694(89)90067-9. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–291. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- Foundation NS. 2006 Sleep In America Poll Summary Findings. 2006 [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Developmental neuroscience. 2009;31:276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biological psychiatry. 2002;51:632–641. doi: 10.1016/s0006-3223(01)01304-x. [DOI] [PubMed] [Google Scholar]
- Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroenceph Clin Neurophysiol. 1958;10:370–371. [Google Scholar]
- Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–783. [PubMed] [Google Scholar]
- Khatami R, Landolt HP, Achermann P, Adam M, Retey JV, Werth E, Schmid D, Bassetti CL. Challenging sleep homeostasis in narcolepsy-cataplexy: implications for non-REM and REM sleep regulation. Sleep. 2008;31:859–867. doi: 10.1093/sleep/31.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koessler L, Maillard L, Benhadid A, Vignal JP, Felblinger J, Vespignani H, Braun M. Automated cortical projection of EEG sensors: anatomical correlation via the international 10-10 system. NeuroImage. 2009;46:64–72. doi: 10.1016/j.neuroimage.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Kurth S, Jenni OG, Riedner BA, Tononi G, Carskadon MA, Huber R. Characteristics of sleep slow waves in children and adolescents. Sleep. 2010;33:475–480. doi: 10.1093/sleep/33.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb R, Merikangas KR, Hofler M, Pfister H, Isensee B, Wittchen HU. Parental alcohol use disorders and alcohol use and disorders in offspring: a community study. Psychological medicine. 2002;32:63–78. doi: 10.1017/s0033291701004883. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O'Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Archives of general psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. UCLA Brain Information Service/Brain Research Institute; Los Angeles: 1968. [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W, North C, Rourke K. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) 2000 [Google Scholar]
- Rusterholz T, Durr R, Achermann P. Inter-individual differences in the dynamics of sleep homeostasis. Sleep. 2010;33:491–498. doi: 10.1093/sleep/33.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusterholz T, Huber R, Massimini M, Durr R, Achermann P. Distinct topographical patterns in the dynamics of sleep homeostasis. Journal of sleep research. 2008;17:225. [Google Scholar]
- Tanner J. Growth at Adolescence. Oxford: Blackwell. 1962 [Google Scholar]
- Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep ePUB ahead of print. 2010 doi: 10.1093/sleep/33.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA, Achermann P. Developmental changes in brain connectivity assessed using the sleep EEG. Neuroscience. 2010a;171:622–634. doi: 10.1016/j.neuroscience.2010.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA, Achermann P. Trait-like characteristics of the sleep EEG across adolescent development. J Neurosci. 2011;31:6371–6378. doi: 10.1523/JNEUROSCI.5533-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Raffray T, Van Reen E, Carskadon MA. Physiology of normal sleep in adolescents. Adolescent medicine: state of the art reviews. 2010b;21:401–417. vii. [PubMed] [Google Scholar]
- Williams RL, Karacan I, Hursch CJ, Davis CE. Sleep patterns of pubertal males. Pediatric research. 1972;6:643–648. doi: 10.1203/00006450-197208000-00001. [DOI] [PubMed] [Google Scholar]