Abstract
In the Old World, cutaneous leishmaniasis (CL) is zoonoses and natural vertebrate hosts of CL parasites are mammals. This study was carried out on natural infection rates of Leishmania parasites in reservoir hosts in one new focus of zoonotic cutaneous leishmaniasis (ZCL) and in suspected reservoir in an old focus of ACL in Iran. The sampling of rodents using Sherman traps was carried out and PCR technique was used for detection and identification of Leishmania species in Bahreman district, Kerman province, southeast of Iran. In addition, the smears were taken from suspicious lesions in stray dogs in the city of Kerman, the center of Kerman province. Simultaneously, pieces of lesion (1 × 1×1 cm) were taken for further histopathological examination. Overall, 25 rodents were collected and identified, including Meriones libycus and Rhombomys opimus. Amastigotes were observed in 33 % of the R. opimus by microscopic examination and indentified as Leishmania major by PCR technique. Four suspicious dogs out of 391 stray dogs showed no Leishmania species. To the best of our knowledge, this is the first isolation and identification of L. major from R. opimus in Kerman province, where ZCL has been present in recent years. Therefore, R. opimus is considered as the main animal reservoir host in Bahreman ZCL focus. In ACL focus such as the city of Kerman, dogs had no role in CL infection as reservoir host.
Keywords: Reservoir host, Cutaneous leishmaniasis, Kerman, Iran
Introduction
Cutaneous leishmaniasis (CL) is caused by several species of Leishmania, with diverse clinical manifestations and epidemiological characteristics. In the Old World CL is zoonosis and the human infection is incidental. In zoonotic cutaneous leishmaniasis (ZCL), this relationship involves the parasite, the sandfly, and the mammalian host(s), which is considered as a self-sustaining and independent system. Natural vertebrate hosts of CL parasites are mammals including Rodentia (e.g., rats, gerbils) and Carnivora (e.g., dogs, cats) (Ashford 1996; Gramiccia and Gradoni 2005; Saliba and Oumeish 1999). Gerbils have been found naturally infected by different species of Leishmania, such as Leishmania (Leishmania) major (Ashford 1996; Javadian et al. 1998; Yaghoobi-Ershadi et al. 1996; Yaghoobi-Ershadi and Javadian 1996). Although L. (Leishmania) tropica is anthroponotic causing CL in many countries in Western Asia, it has been suggested that it could be also zoonotic. The parasite has been isolated from dogs and some believe that the animals are more victims than reservoirs (Dereure et al. 1991).
Zoonotic cutaneous leishmaniasis is an important health problem in Iran and there are several foci of the disease in the country. Rhombomys opimus is considered the most important in central, north and east of Iran and the secondary in importance is Meriones libycus (Javadian et al. 1998; Mohebali et al. 2004; Nadim and Faghih 1968; Rassi et al. 2008; Seyedi-Rashti and Nadim 1967). While in the west and southwest of Iran, Tatera indica is primary and Nesokia indica and M. libycus are the secondary reservoirs of the disease (Javadian et al. 1998; Mohebali et al. 2004). In the southeast of country (Baluchistan), M. hurrianae plays as a main and T. indica as a secondary reservoir host of disease (Seyedi-Rashti and Nadim 1984).
This study was carried out on natural infection rates of Leishmania parasites in reservoir host in one new focus of ZCL and in suspected reservoir in an old focus of ACL in Iran. This baseline information is needed for future control programs.
Materials and methods
Study area
The study on gerbils was carried out in three villages; Javadieh, Ahmad Abad, Daghogh Abad, in the rural district of Bahreman, Rafsanjan county, Kerman province, in the southeast of Iran. This rural district is currently introduced as a new focus of ZCL (Yaghoobi-Ershadi et al. 2010). Rafsanjan county (29°45′N, 54°50′–56°45′E) is located in the southeast of Iran, 100 km northwest of Kerman at an altitude of 1,460 m above sea level. It has a desert climate, very hot and dry in summer and cold and dry in winter. It is Iran’s center for Pistachio cultivation.
In addition, the city of Kerman was selected as a focus of ACL (Sharifi et al. 2012). Kerman (30°17′N, 57°5′E) is the capital city of Kerman province, southeast of Iran. It is located on a large, flat plain, 1,036 km south of Tehran, the capital of Iran. The mean elevation of the city is about 1,755 m above sea level. Kerman city has a moderate climate and the average annual rainfall is 135 mm. Because it is located close to the Kavir-e lut, Kerman has hot summers and in the spring, it often has violent sand storms. Otherwise, its climate is relatively cool.
Rodent collection
Specimens were collected from the colonies of rodents located about 500 m around the selected villages, in Bahreman district. The active colonies of rodents were identified and the rodents were trapped alive in various parts of these areas. Around 17 live traps (Sherman) baited with cucumber were used for 3 weeks. The genus and species of the rodents were determined by external characteristics (Etemad 1978): color, body measurements, ears, tail, feet, teeth and cranium.
For detecting the CL infection, two impression smears were taken from the ears of each rodent (Edrissian et al. 1982; Mohebali et al. 1998). The smears were fixed in methanol, stained by standard Giemsa methods and examined for parasites by a light microscope at high magnification (1,000×). The samples from infected rodents were cultured in Novy–MacNeal–Nicolle (NNN) medium and sub-cultured in RPMI1640 medium (Gibco Life Technologies, NY, USA) containing 10 % heat-inactivated fetal calf serum and penicillin (200 IU/ml) and streptomycin (200 μl/ml). The cultures were checked for promastigotes twice a week for a period of 6 weeks.
Inoculation of susceptible mice
Male BALB/c mice (1 month-old) were obtained from the breeding stock maintained at the Pasteur Institute of Iran (Tehran). Samples from infected rodents were injected subcutaneously into the base of the tail of three BALB/c mice. The mice were examined weekly for appearance of lesion in the injection site up to 6 months. After appearance of the lesion, parasite was reisolated and cultured in NNN and then in RPMI1640 for further identification of Leishmania species.
Extraction and PCR amplification of kDNA
Parasites from a 15-ml mid-logarithmic phase of bulk culture were harvested by centrifugation (700 g for 20 min at 4 °C) and washed three times in ice cold sterile PBS, pH 7.2. The pellet was resuspended in 1 ml sterile cell lysis buffer (125 mM NaCl, 125 mM EDTA, 2.5 % w/v SDS, 125 mM Tris, pH 8.0) with 100 μg/ml proteinase K, and incubated at 56 °C for 3 h. DNA was freed from contaminants in the lysate by phenol/chloroform extraction and ethanol precipitation (Kelly 1993). PCR was performed using a pair of primers of 5′ TCGCAGAACGCCCCT-ACC 3′and 5′ AGGGGTTGGTGTAAAATAGG 3′. DNA (1 μl) was added to a PCR mixture (30 μl) contained 10 μ PCR buffer, 0.2 mM dNTP, 1.5 mM MgCl2, 1 unit of Taq DNA polymerase (Roche Diagnostic) and 10 pM of each primer. The following amplification program was used: primary denaturation was performed at 95 °C for 3 min, followed by 35 cycles of 93 °C for 40 s, 57 °C for 30 s and 72 °C for 1 min. This was followed by final extension cycle at 72 °C for 10 min (Tashakori et al. 2003). PCR products were analyzed by 1.5 % agarose gel electrophoresis with 8 μl of the reaction mixture. Positive and negative controls were included in all tests and finally the bands were compared with the positive controls.
Dogs’ specimen collection and histopathological study
In order to control the population of stray dogs we carried out another survey in line with municipalities. Due to numerous populations, stray dogs were killed by shooting or baited with strychnine in rural and urban areas of the city of Kerman. A whole body physical examination was performed. If lesions were present, smears were taken from suspicious lesions in hairless parts including snout, tip of nose, claws and the end of tail. The smears were fixed in methanol, stained by standard Giemsa method and examined for amastigotes by a light microscope. In addition, small pieces of tissue (1 × 1 × 1 cm dimensions slices) were prepared as soon as possible after the death of the animal, preserved in 10 % formalin and embedded in paraffin. Four micrometer thick tissue sections were stained with haematoxylin and eosin (H&E) for further histopathological examination. Also, the same experiments were carried out randomly on suspected stray and household dogs, in ZCL focus of the three mentioned villages of Bahreman district.
Results
Overall, 25 rodents consisting of M. libycus (11 males and 5 females) and R. opimus (5 males and 4 females) were collected and identified in three villages of Bahreman district, a focus of recently emerged ZCL (Table 1). Gerbils were examined for parasites infection by two diagnostic techniques: direct smear preparations and confirmed by PCR technique.
Table 1.
Frequency and species of captured rodents in Bahreman district, Rafsanjan county, Kerman province, southeast of Iran
| Villages | Javadieh | Daghugh Abad | Ahmad Abad | Infected no. | Total |
|---|---|---|---|---|---|
| Rodents | |||||
| Meriones libycus | 8 (50 %) | 5 (31.3 %) | 3 (18.7 %) | – | 16 |
| Rhombomys opimus | 9 (100 %) | – | – | 3 | 9 |
Amastigotes were observed in 33 % (2 males and 1 female) of the R. opimus by direct high magnification microscopic examination. Amastigotes from infected gerbils were injected subcutaneously at the base of the tail of nine BALB/c mice. Nodules and ulcers containing numerous amastigotes, appeared within 2 months in one mouse (11.1 %). Parasite of one infected BALB/c was cultured into NNN, then sub-cultured in monophasic medium (RPMI1640) and indentified as L. major using PCR technique with a fragment of 600 bp in gel electrophoresis (Fig. 1). Fifteen suspicious stray and household dogs were also examined for leishmanial infection, although no infection was detected by direct smear preparation and histopathological examination.
Fig. 1.
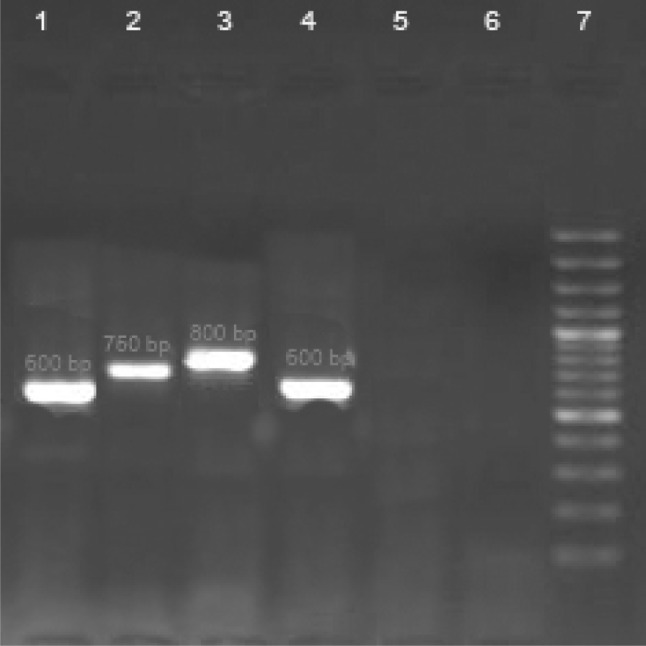
Gel electrophoresis of kDNA-PCR. Lane 1 L. major (positive control 600 bp), Lane 2 L. infantum (positive control 750 bp), Lane 3 L. tropica (positive control 800 bp), Lane 4 sample detected from R.opimus, Lanes 5, 6 negative control, Lane 7 DNA size marker 100 bp
The results of four suspicious dogs out of 391 dogs (169 females and 222 males) in Kerman county, an old focus of ACL showed no leishmanial infection.
Discussion
The small mammals consist of R. opimus, the great gerbil (36 %) and M. libycus (64 %) were trapped from the study area. Leishmanial infection was found only in R. opimus (33 %) and L. major was isolated from naturally infected R. opimus. To the best of our knowledge, this is the first isolation and identification of L. major from R. opimus in Kerman province, where ZCL has been present in recent years in the studied area as the main animal reservoir host. This is essentially a disease of gerbils, transmitted by sand flies that live and breed in the gerbil burrows. The human disease is secondary to the infection of gerbils and is seen only in places where the infected gerbils live.
Rhombomys opimus (great gerbil) is the principal reservoir host of ZCL in the central and north-east parts of the country. M. libycus (libyan jird) was also found to be infected and can act as a secondary reservoir host in the absence of R. opimus (Mohebali et al. 2004; Yaghoobi-Ershadi et al. 1996). Our finding showed that R. opimus is probably the principal reservoir host of ZCL and the source of human infection in this focus.
Appropriate conditions such as vegetation established with Haloxylon for sand dune stabilization, storage of waste construction materials and waste of Pistachio around villages, margins of narrow roads that are constructed between gardens, shallow under grounded water table and adequate moisture, made this environment suitable for rodent biology and breeding of the sand fly vectors and consequently the transmission of the CL disease. Construction of buildings nearby rodent burrows has increased contact between human and parasite transmission cycle in rodents.
According to the results of this study, the best way for control of the CL disease in Bahreman district is to improve the environmental conditions, which provide the growth and development of rodents and their nests. Because rodent colonies are mostly on the fringes of Pistachio gardens in this region, they can be destroyed by using zinc phosphide bait relevant guidelines and eliminate disease animal reservoir by preventing the accumulation of garbage, construction debris and waste pistachios around the villages. In the study carried out by Yaghoobi-Ershadi et al. (2000) it was shown that the control program reduced the incidence of ZCL 12-fold in the treated village compared to the control at the end of the first year of operation, and to more than one-fifth of its original level after 2 years (Yaghoobi-Ershadi et al. 2000).
Cases of natural infection of dogs by L. tropica, (Dereure et al. 1991; Guessous-Idrissi et al. 1997) have been documented. Although dogs have been found naturally infected by several species of Leishmania, their role as reservoir hosts of these parasites is probably negligible. In some instance [e.g., L. (L.) tropica in central to southwest Asia and L. (L.) donovani in northeast India], the man is the only source of infection for the vector (Ashford 2000). Our study confirmed these results in the city of Kerman, in an ACL focus of southeast of Iran.
In conclusion, R. opimus is probably the principal reservoir host of ZCL and the source of human infection in Bahreman ZCL focus and dogs had no role in CL infection as reservoir host in both of foci.
Acknowledgments
We thank School of Public Health, Tehran University of Medical Sciences for supporting this study, Pasteur Institute of Iran and Rafsanjan Health Center for their close collaborations. We are grateful to Mr. Arandian and Mr. Abdoli for helping us in carrying out this program.
References
- Ashford RW. Leishmaniasis reservoirs and their significance in control. Clin Dermatol. 1996;14:523–532. doi: 10.1016/0738-081X(96)00041-7. [DOI] [PubMed] [Google Scholar]
- Ashford RW. The leishmaniases as emerging and reemerging zoonoses. Int J Parasitol. 2000;30:1269–1281. doi: 10.1016/S0020-7519(00)00136-3. [DOI] [PubMed] [Google Scholar]
- Dereure J, Rioux JA, Gallego M, Perieres J, Pratlong F, Mahjour J, Saddiki H. Leishmania tropica in Morocco: infection in dogs. Trans R Soc Trop Med Hyg. 1991;85:595. doi: 10.1016/0035-9203(91)90356-4. [DOI] [PubMed] [Google Scholar]
- Edrissian GH, Zovein Z, Nadim A. A simple technique for preparation of smears from the ear of Rhombomys opimus for the detection of leishmanial infection. Trans R Soc Trop Med Hyg. 1982;76:706. doi: 10.1016/0035-9203(82)90255-3. [DOI] [PubMed] [Google Scholar]
- Etemad E. Mammals of Iran. Tehran###, National Society of Guardianship of Natural Resources and Human Environment: Rodents and their Identification Keys; 1978. [Google Scholar]
- Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol. 2005;35:1169–1180. doi: 10.1016/j.ijpara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Guessous-Idrissi N, Chiheb S, Hamdani A, Riyad M, Bichichi M, Hamdani S, Krimech A. Cutaneous leishmaniasis: an emerging epidemic focus of Leishmania tropica in north Morocco. Trans R Soc Trop Med Hyg. 1997;91:660–663. doi: 10.1016/S0035-9203(97)90511-3. [DOI] [PubMed] [Google Scholar]
- Javadian E, Dehestani M, Nadim A, Rassi Y, Tahvildar-Bidruni GH, Seyedi-Rashti MA, Shadmehr A. Confirmation of Tatera indica (Rodentia: Gerbilidae) as the main reservoir host of zoonotic cutaneous leishmaniasis in the west of Iran. Iran J Public Health. 1998;27:55–60. [Google Scholar]
- Kelly JM. Isolation of DNA and RNA from Leishmania. Methods Mol Biol. 1993;21:123–131. doi: 10.1385/0-89603-239-6:123. [DOI] [PubMed] [Google Scholar]
- Mohebali M, Poormohammadi B, Kanani A, Hajjaran H, Edrissian GH. Rodents: another group of animal hosts of visceral leishmaniasis in Meshkin-Shahr district, Islamic Republic of Iran. East Mediterr Health J. 1998;4(2):376–378. [Google Scholar]
- Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjaran H, Abaei MR. Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. East Mediterr Health J. 2004;10:591–599. [PubMed] [Google Scholar]
- Nadim A, Faghih M. The epidemiology of cutaneous leishmaniasis in the Isfahan province of Iran: I. The reservoir II. The human disease. Trans R Soc Trop Med Hyg. 1968;62:534–542. doi: 10.1016/0035-9203(68)90140-5. [DOI] [PubMed] [Google Scholar]
- Rassi Y, Sofizadeh A, Abai MR, Oshaghi MA, Rafizadeh S, Mohebail M, Mohtarami F, Salahi R. Molecular detection of Leishmania major in the vectors and reservoir hosts of cutaneous leishmaniasis in Kalaleh district, Golestan province, Iran. Iran J Arthropod Borne Dis. 2008;2(2):21–27. [Google Scholar]
- Saliba EK, Oumeish OY. Reservoir hosts of cutaneous leishmaniasis. Clin Dermatol. 1999;17:275–277. doi: 10.1016/S0738-081X(99)00045-0. [DOI] [PubMed] [Google Scholar]
- Seyedi-Rashti MA, Nadim A. Epidemiology of cutaneous leishmaniasis in Iran. B. Khorassan area. I. The reservoirs. Bull Soc Pathol Exot Filiales. 1967;60:510–514. [PubMed] [Google Scholar]
- Seyedi-Rashti MA, Nadim A (1984) Cutaneous leishmaniasis in Baluchistan, Iran and Poster vol. XI. International Congress for Tropical Medicine and Malaria. Calgary, Sep, p 124
- Sharifi F, Sharifi I, Zarean M, Hakimi Parizi M, Aflatoonian M, Fasihi Harandi M, Zahmatkesh R, Mashayekhi M, Kermanizadeh AR. Spatial distribution and molecular identification of Leishmania species from endemic foci of south-eastern Iran. Iran J Parasitol. 2012;7(1):45–52. [PMC free article] [PubMed] [Google Scholar]
- Tashakori M, Ajdary S, Kariminia A, Mahboudi F, Alimohammadian MH. Characterization of Leishmania species and L. major strains in different endemic areas of cutaneous leishmaniasis in Iran. Iran Biomed J. 2003;7:43–50. [Google Scholar]
- Yaghoobi-Ershadi MR, Javadian E. Epidemiological study of reservoir hosts in an endemic area of zoonotic cutaneous leishmaniasis in Iran. Bull World Health Organ. 1996;74(6):587–590. [PMC free article] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Akhavan AA, Mohebali M. Meriones libycus and Rhombomys opimus (Rodentia: Gerbillidae) are the main reservoir hosts in a new focus of zoonotic cutaneous leishmaniasis in Iran. Trans R Soc Trop Med Hyg. 1996;90:503–504. doi: 10.1016/S0035-9203(96)90295-3. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Akhavan AA, Zahraei-Ramazani AR, Javadian E, Motavalli-Emami M. Field trial for the control of zoonotic cutaneous leishmaniosis in Badrood, Iran. Ann Saudi Med. 2000;20:386–389. doi: 10.5144/0256-4947.2000.386. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Hakimiparizi M, Zahraei-Ramazani AR, Abdoli H, Aghasi M, Arandian MH, Ranjbar AA. Sandfly surveillance within an emerging epidemic focus of cutaneous leishmaniasis in southeastern Iran. Iran J Arthropod Borne Dis. 2010;4:17–23. [PMC free article] [PubMed] [Google Scholar]


