Abstract
Over the last decade, a few cases of visceral leishmaniasis (VL) have been reported in some districts of the province of Golestan, in north-eastern Iran. The aim of the present study was to investigate the prevalence of Leishmaniainfantum infection among humans and domestic dogs by using direct agglutination test (DAT) and PCR assays in the eastern zone of the province. Between 2011 and 2012, blood samples were randomly collected from 450 humans and 50 domestic dogs, in the eastern zone of Golestan Province including 7 villages from Marave-tappeh district where new cases of human VL had been recorded there. Each of these samples was tested for anti-Leishmania antibodies, in DAT, and for L. infantum kinetoplast DNA on whole blood, in PCR-based assays. A total of 450 human samples, 6 (1.33 %) were found seropositive and 13 (2.8 %) was found PCR-positive. Of the 50 dog samples, 16 (32 %) were found seropositive and 15 (30 %) were PCR-positive. All PCR-positive dogs were found seropositive except one as well as 6 (46.2 %) PCR-positive humans were also found seropositive. Moreover, the species of L. infantum was detected in all PCR–positive samples. The high prevalence of VL in the study areas offer it has emerged as an endemic focus in the province. Further investigations on the vectors, reservoirs and human population are recommended.
Keywords: Zoonotic visceral leishmaniasis, Leishmania infantum, Emergence, Endemic focus, Iran
Introduction
Visceral leishmaniasis (VL) or Kala-azar, is a systemic parasitic disease that is caused by Leishmania donovani complex which is transmitted by different species of sandflies. The annual occurrence of human visceral leishmaniasis (HVL) cases worldwide is estimated to be 500,000 and accounts for 75,000 deaths annually (WHO 2000, 2010a). Nevertheless, these diseases are still considered as neglected tropical diseases (WHO 2010b). Leishmaniasis still constitutes a major public health problem and burden of the disease is being increased in the World (Desjeux 2001). In addition, Leishmania-HIV co-infections in the adult population are being reported with increasing frequency (WHO 2000). The predominant clinical signs and symptoms of HVL include prolonged fever, hepatosplenomegaly, substantial weight loss, progressive anemia, and death. The disease is fatal in left treated cases (Caldas et al. 2006). Leishmania infantum is responsible cause for visceral leishmaniasis among children in the Mediterranean regions including Iran and domestic dogs are considered a main reservoir hosts for the disease (Mazloumi Gavgani et al. 2002; Edrissian et al. 1993). At the present time, VL is known as an endemic disease in at least five provinces of Iran and sporadic cases of VL are reported from other areas of the country (Mohebali et al. 2001, 2011; Edrissian et al. 1999; Fakhar et al. 2004, 2006).
VL is considered to be a emerging disease globally and in the Eastern Mediterranean Region (Rathor 1996). Recent outbreaks of VL in India and the epidemic of human immunodeficiency virus (HIV) make VL a re-emerging problem in India (Redhu et al. 2006; Sharma et al. 2007), Brazil (Arias et al. 1996) and Israel (Yaari et al. 2004). Outbreaks of VL have also been related with deforestation, environmental changes and population migrations (Ashford 2000; Sharma et al. 2007). Individual risk factors such as malnutrition, HIV, genetic factors, etc. are also responsible for epidemiologic trend diversity of VL (Desjeux 2004).
The unpublished records of the provincial health service in the province of Golestan indicate a worrying recent increase in the incidence of VL in the eastern district of the province where each year nomadic tribes are settling in summer there. To our knowledge, no study concerning VL has been carried out in the province so far. Therefore, the aim of our study was to investigate the prevalence of L.infantum infection among humans and domestic dogs in the Marave-tappeh district of the province, by using direct agglutination test (DAT) and polymerase chain reaction (PCR)-based assays to check blood samples from human subjects and dogs for evidence of recent or current infection.
Materials and methods
Study area
The study was conducted throughout 2011–2012 in the eastern zone of Golestan Province including 7 villages from Marave-tappeh district where cases of human VL had previously been reported. Golestan Province is located in the north-east of Iran (54°26′E, 36°50′N) and also has a moderate and humid climate with an annual mean rainfall of 556 mm (Saeedian 2009).
Sample collection and testing
Blood samples were collected in EDTA- coated tubes from children under 12 years old and 10 % of their parents as well as of domestic dogs from 7 villages. All the samples were collected by cluster sampling methods. Overall, 450 human and 50 dog blood samples were collected. All the samples centrifuged at 1,000 g for 5 min and their plasma and buffy coat were separated. All the human plasma samples were tested in DAT (Harith et al. 1989) and buffy coat were examined by PCR assay. DAT antigen was prepared in Leishmaniasis lab, at the School of Public Health, Tehran University of Medical Sciences and stored at 4 °C until used according to the method described by Harith et al. (1989). For primary screening in human samples; dilutions were made at 1:800. Samples with titers 1:800 were diluted further to give end-point titers of 1:102,400. All the dog plasma samples were tested by DAT according to the method as described by Mohebali et al. (2006). Specific anti-Leishmania antibodies at a titer of ≥1:3,200 in human plasma and ≥1:320 in dog plasma were considered as positive based on our previous studies (Fakhar et al. 2006; Mohebali et al. 2006, 2011).
Total DNA was extracted from blood buffy coat as described by Motazedian et al. (2002). Briefly, 200 μl of buffy coat was homogenized with 200 μl lyses buffer [50 mM Tris–HCl (pH = 7.6), 1 mM EDTA and 1 % Tween 20] and 10 μl of proteinase K solution (containing 19 mg of the enzyme/ml), in a 1.5 ml micro centrifuge tube. The homogenate was then incubated at 37 °C overnight before 200 μl of a phenol: chloroform: isoamyl alcohol mixture was added. After being shaken vigorously, the tube holding the mix was centrifuged (10,000×g for 10 min) and then the DNA in the supernatant solution was precipitated with 400 μl cold, pure ethanol, re-suspended in 50 μl double-distilled water and then stored at 4 °C until it could be tested. It was re-suspended in 100 μl sterile distillated water and stored at 4 °C until it could be tested in a modified genous-specific PCR for a sequence from the kinetoplast DNA (k DNA) of Leishmania. The primers used, RV1 (5′-CTT TTC TGG TCC CGC GGG TAG G-3′) and RV2 (5′-CCA CCT GCG CTA TTT TAC ACC A-3′) amplify a 145-bp sequence from the Leishmania kDNA minicircles, according to the methods as described by Fakhar et al. (2008).
The PCR products were separated by electrophoresis in a 2 % agarose gel, stained with ethidium bromide, visualized under ultra-violet trans-illumination, and sized by comparison with a 100 bp ladder. Each sample found PCR-positive for leishmanial DNA was then investigated using the PCR described by Fakhar et al. (2008), which is based on the species-specific primers LINR4 and LIN17 to confirm that the DNA detected was that of L. infantum. Data analyses were performed with SPSS (version 13.5; SPSS Inc, Chicago, IL, USA), with a probability (P) value of <0.05 were considered as statistically significant.
Results
DAT screening
Of the 450 subjects (214 males and 236 females) investigated, 48, 46.9 and 5.1 % were aged <5, 5–10 and >10 years respectively. Two (0.44 %) of the subjects had suffered from VL, at least one year before their blood was sampled for the present study.
Overall, 6 (1.33 %) of the subjects were found seropositive. Male and female subjects were found serpositive as equal. All 6 seropositive cases were children aged ≤10 years and three of them had clinical sings of VL including prolonged fever, hepato-splenomegaly and weight loss. Although a 5–10 years subjects was more likely to be found seropositive than a <5 one, the difference was not statistically significant (1.1 vs. 0.2 %; P = 0.55). Although all suspected subjects (at 1:1,600 titers) have been followed-up for at least 11 months but none have yet shown any symptoms of VL.
A total of 50 dogs (44 males and 6 females) examined, 64 and 36 % were aged 0–2 and >2 years respectively. Sixteen (32 %) of them were found seropositive. Most (13) of cases were aged 0–2 years and only one of them had clinical sings including alopecia, skin lesions and weight loss and also the remained ones were asymptomatic. Although a male dog was more likely to be found seropositive than a female one, the difference was not statistically significant (26 vs. 6 %; P = 0.07).
PCR screening
Overall, 13 (2.8 %) of the subjects were found PCR-positive in the Leishmania- specific PCR based on the RV1/RV2 primer set (see Fig. 1). Only 6 (46.1 %) of the PCR-positive subjects were also found seropositive. There was no significant difference between male and female in PCR- positive. All 13 PCR-positive subjects were children aged ≤10 years. Only three of them had clinical sings of VL and they also were seropositive by DAT. Although 10 of the PCR positive cases have now been followed-up for at least 11 months, none has yet shown any symptoms of VL.
Fig. 1.
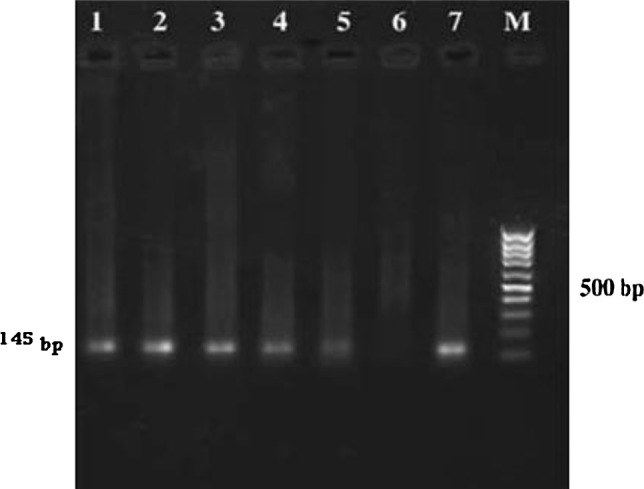
2 % agarose gel electrophoresis of PCR products from buffy coat DNA of humans. M standard marker (100 bp), Lanes 1–5 positive human samples, Lane 6 negative control, Lane 7 standard L. infantum (145 bp)
Overall, 15 (30 %) of the dogs were found PCR-positive. All 15 PCR-positive dogs were also found seropositive. Most (14) of them were aged 0–2 years and only one of them had clinical sings. Although a male dog was more likely to be found PCR-positive than a female one, the difference was not statistically significant (24 vs. 6 %; P = 0.07). All 13 subjects and 15 dogs were subsequently found positive for L. infantum DNA, using species-specific PCR based on the LINR4/LIN17 primer set.
Discussion
For the first time, our preliminary study was carried out in Golestan Province. Overall, 1.33 and 32 % of the human and dogs investigated in the present study were found seropositive in a DAT based assay respectively. DAT is a simple, cost-effective and field-applicable method that is often suggested for the field diagnosis of VL in endemic regions and frequently used for that reason in Iran (Mohebali et al. 2001, 2011; Edrissian et al. 1999; Fakhar et al. 2006, 2008). The sensitivity and specificity of this method varies in different studies between 90–100 % and 72–100 %, respectively (Sundar and Rai 2002; Singh 2006; Fakhar et al. 2012a). Our DAT results showed that 50 % (3/6), 93.7 % (15/16) of human and dogs were asymptomatic, respectively. Recent epidemiological reports from Iran (Fakhar et al. 2008; Alborzi et al. 2008) have shown that asymptomatic HVL infection with L. infantum occurs more frequently than was previously believed. Fakhar et al. (2008) suggested that those with asymptomatic infection can act as reservoirs for HVL in southern Iran. Asymptomatic human carriers also pose a threat to blood banks in those areas were L. infantum is endemic, since the parasite can be transmitted by transfusion. Also they clearly pose a problem to VL-control programmes, especially when, as in Golestan Province (present study), they are fairly common (Fakhar et al. 2008). Moreover, epidemiological investigations in L. infantum endemic regions of Iran indicate that more than 60–70 % of seropositive dogs are asymptomatic (Mohebali et al. 2011; Edrissian et al. 1999; Fakhar et al. 2012b; Moshfea et al. 2009).
In our study, 2.8 and 30 % of the human and dogs were found PCR positive respectively. Gao et al. (2006) and Lachaud et al. (2001) described that the primer pairs RV1–RV2 were most sensitive (with sensitivity equal to 0.0001 parasite ml−1 blood) and suitable for detection the asymptomatic and symptomatic infections of L. infantum in human and dogs in the endemic area, so, we used RV1–RV2 primers set in the present study.
Recently, the use of PCR with high sensitivity (70–100 %) and specificity (100 %) has been used in different parts of the World (Sundar and Rai 2002; Singh 2006; Riera et al. 2004). Based on our previous study that carried out in Fars Province, the sensitivity and specificity of PCR for detection of HVL were determined 82.1 and 100 %, respectively (Fakhar et al. 2012a). The PCR technique has several advantages including the ability to work with small amounts of target material, fast detection of Leishmania in symptomatic patients and asymptomatic carriers as well as Leishmania/HIV co-infected patients, the follow up of treatment and the assessment of the successful cure of visceral leishmaniasis (Cascio et al. 2002; Bossolasco et al. 2003; Riera et al. 2004; Maurya et al. 2005). It is also useful to complement the serological results (Fakhar et al. 2012a).
Our PCR results showed that 76.9 % (3/13), 93.3 % (14/15) of human and dogs were asymptomatic, respectively. These finding are same as other studies in Iran that described above such as Fakhar et al. (2008, 2012b). Since the majority of PCR-positive dogs were asymptomatic, indicating the presence of L. infantum amastigotes in the peripheral blood of the subjects, it clear that these asymptomatic dogs are common in the study areas and may act as reservoir hosts in the transmission of L. infantum, to humans and to other dogs, by sandflies.
Visceral leishmaniasis is considered as an emerged and neglected parasitic disease in the Middle East, Americas and African regions (Ashford 2000; WHO 2010a, b). Because of the high prevalence of L. infantum infection in the area of Golestan Province and establishment of nomadic tribes in this province, it is considered as a new endemic focus in Iran and that this could have an impact on tourisms and emigrants as well as cause a high public health burden for local residents specially children aged under 10 years old.
Moreover, we believe that the VL is one of the most neglected of the neglected parasitic diseases in the province. As a whole, according to aforementioned and our investigations look like VL has been emerged in the area. The main reasons of the emergence of VL may be including migrating of tribes to the province, ecological and whether changes, mass screening the individuals using valuable serological and rapid tests such as DAT and the increasing number of infants suffer from malnutrition condition.
In conclusion, our study confirmed that asymptomatic L. infantum infections are more common than symptomatic one in the areas. Also, asymptomatic dogs as well as symptomatic ones, have an important role in the maintenance of L. infantum infection. Our results could increase the awareness of local physicians and alertness Provincial Health centers for planning VL control programmes in the area. Further investigations on other epidemiological aspects of VL regarding to the vectors, reservoirs and human population are recommended in the province.
Acknowledgments
The author’s are grateful to the staff of the Marave-tappeh and Kalaleh Health Centers for their kindly cooperation to perform this study. They also would like to thank of financially supported by Vice Chancellors for Research of Mazandaran University of Medical Sciences (project number: 90-149).
References
- Alborzi A, Pourabbas B, Shahian F, Mardaneh J, Pouladfar GhR, Ziaeyan M. Detection of Leishmania infantum kinetoplast DNA in the whole blood of asymptomatic individuals by PCR-ELISA and comparison with other infection markers in endemic areas, southern Iran. Am J Trop Med Hyg. 2008;79:839–842. [PubMed] [Google Scholar]
- Arias JR, Monteiro PS, Zicker F. The reemergence of visceral leishmaniasis in Brazil. Emerg Infect Dis. 1996;2(2):145–146. doi: 10.3201/eid0202.960213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford RW. The leishmaniases as emerging and reemerging zoonoses. Int J Parasitol. 2000;30:1269–1281. doi: 10.1016/S0020-7519(00)00136-3. [DOI] [PubMed] [Google Scholar]
- Bossolasco S, Gaiera G, Olchini D, Gulletta M, Martello L, Bestetti A. Real-time PCR assay for clinical management of human immunodeficiency virus-infected patients with visceral leishmaniasis. J Clin Microbiol. 2003;41:5080–5084. doi: 10.1128/JCM.41.11.5080-5084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldas AJMBB, Costa J, Aquino D, Silva AAM, Barral-Netto M, Barral A. Are there differences in clinical and laboratory parameters between children and adults with American leishmaniasis? Acta Trop. 2006;97:252–258. doi: 10.1016/j.actatropica.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Cascio A, Calattini S, Colomba C, Scalamonga C, Galazzi M, Pizzuto M, Camilli R, Gramiccia M, Titone L, Corbellino M, Antinori S. Polymerase chain reaction in the diagnosis and prognosis of Mediterranean visceral leishmaniasis in immunocompetent children. Pediatrics. 2002;109:1–5. doi: 10.1542/peds.109.2.e27. [DOI] [PubMed] [Google Scholar]
- Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95:239–243. doi: 10.1016/S0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Edrissian GhH, Ahanchin AR, Gharachahi AM. Seroepidemiological studies of visceral leishmaniasis and search for animal reservoirs in Fars Province, southern Iran. Iran J Med Sci. 1993;18:99–105. [Google Scholar]
- Edrissian GH, Nadim A, Alborzi AV, Ardehali S. Visceral leishmaniasis: the Iranian experiences. Arch Iran Med. 1999;1:22–26. [Google Scholar]
- Fakhar M, Mohebali M, Barani M. Identification of endemic focus of Kala-azar and seroepidemiological study of visceral Leishmania infection in human and canine in Qom Province. Armaghan-Danesh. 2004;9:43–52. [Google Scholar]
- Fakhar M, Motazedian MH, Asgari Q, Mohebali M, Mehrabani D. A new endemic focus of visceral leishmaniosis in southern Iran. Armaghane-Danesh. 2006;42:103–114. [Google Scholar]
- Fakhar M, Motazedian MH, Hatam GR, Asgar Q, Kalantari M, Mohebali M. Asymptomatic human carriers of Leishmania infantum: possible reservoirs for Mediterranean visceral leishmaniasis in southern Iran. Ann Trop Med Parasitol. 2008;102:577–583. doi: 10.1179/136485908X337526. [DOI] [PubMed] [Google Scholar]
- Fakhar M, Motazedian MH, Hatam GhR, Asgari Q, Monabati A, Keighobadi M. Comparative performance of DAT, IFAT, PCR and bone marrow aspiration methods for diagnosis of Mediterranean visceral leishmaniasis (MVL) Afr J Microbiol Res. 2012;6:5777–5781. [Google Scholar]
- Fakhar M, Motazedian MH, Kalantari M, Asgari Q. Asymptomatic domestic dogs are carriers of Leishmania infantum: possible reservoirs host for human visceral leishmaniasis in southern Iran. Comp Clin Pathol. 2012;21:801–807. doi: 10.1007/s00580-011-1179-6. [DOI] [Google Scholar]
- Gao CH, Wang JY, Yang YT, Bao YF. Study on PCR method for detecting the asymptomatic infection of Leishmania infantum. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2006;24:92–96. [PubMed] [Google Scholar]
- Harith A, Salappendel RJ, Reiter I, Knapen F, Korte P, Huigen E, Kolk RHG. Application of a direct agglutination test for detection of specific anti-leishmania antibodies in the canine reservoir. J Clin Microbiol. 1989;27:2252–2257. doi: 10.1128/jcm.27.10.2252-2257.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaud L, Chabbert E, Dubessay P, Reynes J, Lamothe J, Bastien P. Comparison of various sample preparation methods for PCR diagnosis of visceral leishmaniasis using peripheral blood. J Clin Microbiol. 2001;39:613–617. doi: 10.1128/JCM.39.2.613-617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya R, Singh RK, Kumar B, Salotra P, Rai M, Sundar S. Evaluation of PCR for diagnosis of Indian kala-azar and assessment of cure. J Clin Microbiol. 2005;43:3038–3041. doi: 10.1128/JCM.43.7.3038-3041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazloumi Gavgani AS, Mohite H, Edrissian GH, Mohebali M, Davies CR. Domestic dog ownership in Iran is a risk factor for human infection with Leishmania infantum. Am J Trop Med Hyg. 2002;67:511–515. doi: 10.4269/ajtmh.2002.67.511. [DOI] [PubMed] [Google Scholar]
- Mohebali M, Hamzavi Y, Edrissian GH, Forouzani A. Seroepidemiological study of visceral leishmaniasis among humans and animal reservoirs in Bushehr Province. Islamic Republic of Iran. East Mediterr Health J. 2001;7:912–917. [PubMed] [Google Scholar]
- Mohebali M, Edrissian GH, Nadim A, Hajjaran H, Akhoundi B, Hooshmand B, Zarei Z, Arshi S, Mirsamadi N, Manouchehri-Naeini K, Mamishi S, Sanati AA, Moshfe AA, Charehdar S, Fakhar M. Application of direct agglutination test (DAT) for the diagnosis and seroepidemiological studies of visceral leishmaniasis in Iran. Iran J Parasitol. 2006;1:15–25. [Google Scholar]
- Mohebali M, Edrissian GhH, Shirzadi MR, Akhoundi B, Hajjaran H, Zarei Z, Molaei S, Sharifi I, Mamishi S, Mahmoudvand H, Torabi V, Moshfe AA, Malmasi AA, Motazedian MH, Fakhar M. An observational study on the current distribution of visceral leishmaniasis in different geographical zones of Iran and implication to health policy. Travel Med Infect Dis. 2011;9:67–74. doi: 10.1016/j.tmaid.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Moshfea AA, Mohebali M, Edrissiana GhH, Zarei Z, Akhoundi B, Kazemid B, Jamshidi Sh, Mahmoodi M. Canine visceral leishmaniasis: asymptomatic infected dogs as a source of L. infantum infection. Acta Trop. 2009;112:101–105. doi: 10.1016/j.actatropica.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Motazedian H, Karamian M, Noyes HA, Ardehali S. DNA extraction and amplification of Leishmania from archived, Giemsa-stained slides, for the diagnosis of cutaneous leishmaniasis by PCR. Ann Trop Med Parasitol. 2002;96:31–34. doi: 10.1179/000349802125000484. [DOI] [PubMed] [Google Scholar]
- Rathor HR. The role of vectors in emerging and re-emerging diseases in the Eastern Mediterranean Region. East Mediterr J. 1996;2:61–67. [Google Scholar]
- Redhu NS, Dey A, Balooni V, Singh S. Leishmania-HIV co-infection: an emerging problem in India. AIDS. 2006;20:1213–1215. doi: 10.1097/01.aids.0000226971.42622.4e. [DOI] [PubMed] [Google Scholar]
- Riera C, Fisa R, Udina M, Gállego M, Portus M. Detection of Leishmania infantum cryptic infection in asymptomatic blood donors living in an endemic area (Eivissa, Balearic Islands, Spain) by different diagnostic methods. Trans R Soc Trop Med Hyg. 2004;98:102–110. doi: 10.1016/S0035-9203(03)00015-4. [DOI] [PubMed] [Google Scholar]
- Saeedian AH. Encyclopedia of the land and population of Golestan Province. Tehran: Aram Press; 2009. [Google Scholar]
- Sharma U, Redhu NS, Mathur P, Singh S. Re-emergence of visceral leishmaniasis in Gujarat, India. J Vector Borne Dis. 2007;44:230–232. [PubMed] [Google Scholar]
- Singh S. New developments in diagnosis of leishmaniasis. Indian J Med Res. 2006;123:311–330. [PubMed] [Google Scholar]
- Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diag Lab Immunol. 2002;9:951–958. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2000) Leishmaniasis and Leishmania/HIV Co-infection. Document WHO/CDS/CSR/ISR/2000.1. WHO, Geneva
- World Health Organization (WHO) (2010a) Technical Report Series. Control of leishmaniases. Report of a meeting of the WHO expert committee on the control of leishmaniases, Geneva
- World Health Organization (WHO) (2010b) First WHO report on neglected tropical diseases: working to overcome the global impact of neglected tropical diseases WHO/HTM/NTD/2010.1, pp 91–96
- Yaari A, Jaffe CL, Garty BZ. Viseceral leishmaniasis in Israel, 1960–2000. IMAJ. 2004;6:205–208. [PubMed] [Google Scholar]


