Abstract
AIM: To evaluate the utility of the innovative fecal tumor M2-Pyruvate kinase (M2-PK) test in our daily clinical routine, as a marker for the pre-selection of patients who should subsequently undergo colonoscopy for the diagnosis or exclusion of colorectal cancer.
METHODS: Fecal tumor M2-PK was measured in stool samples of 96 study participants (33 patients with colorectal cancer, 21 patients with rectal carcinoma and 42 controls) who all underwent total colonoscopy.
RESULTS: In 39 of 42 individuals in the control group, fecal tumor M2-PK was below 4.0 kU/L (93% specificity). Colorectal tumors were accompanied by a highly significant increase (P < 0.001) in fecal tumor M2-PK levels (median: colon carcinoma, 23.1 kU/L; rectal carcinoma, 6.9 kU/L; colorectal carcinoma, 14.7 kU/L), which correlated with Duke’s staging and T-classification. The overall sensitivity was 78% for colorectal cancer, increasing from 60% for stage T1 to 100% for stage T4 and from 60% for Duke’s A to 90% for Duke’s D tumors.
CONCLUSION: Fecal tumor M2-PK is an appropriately sensitive tool to pre-select those patients requiring colonoscopy for the further diagnostic confirmation or exclusion of colorectal cancer.
Keywords: Tumor M2-Pyruvate kinase, Pyruvate kinase type M2, Colon cancer, Rectal cancer, Adenoma, Feces, Cancer screening
INTRODUCTION
In Germany, about 70 000 people are diagnosed with colorectal cancer each year[1]. This figure is about 1 million worldwide, with approximately 528 000 deaths from colorectal cancer each year[2]. The gold standard for the early detection of colorectal cancer is colonoscopy. However, the acceptance of this costly and invasive method is low. Only 1.7% of people entitled to colo-noscopy under the German national colorectal cancer screening program actually undergo the procedure[3].
In order to increase the participation in colorectal cancer screening programs, an easy, fast and economical initial screening method, with good patient compliance, is absolutely necessary. This allows identification of those patients most likely to have colorectal cancer, who require further investigation by colonoscopy.
The guaiac-based fecal occult blood test (FOBT), which is based on the premise that polyps and cancer bleed more than normal mucosa[4], is currently the most commonly used test for colorectal cancer screening. Guaiac-based FOBTs have been investigated in a number of large studies and shown to reduce mortality by about 15%-33% in screened populations[5,6]. However, they have limited sensitivity. For example, Lieberman et al[7] and Koss et al[8] found their sensitivity was less than 30% for colorectal cancer and less than 15% for advanced adenomas. Newer immunological FOBTs showed higher sensitivities[9,10] with the advantage of no dietary restrictions. In most studies with immunological FOBTs to date, however, colonoscopy has been performed only in FOBT-positive cases. Non-bleeding colorectal tumors and those not consistently discharging sufficient blood into the gut lumen are not detected by either guaiac or immunological FOBTs.
Recently a new screening test for the early detection of adenomas and colorectal tumors has been described. The tumor M2-Pyruvate kinase (M2-PK) stool test is based on the measurement of a key enzyme involved in tumor metabolism[8,11-14].
Tumor M2-PK is the dimeric form of the glycolytic pyruvate kinase isoenzyme type M2[15]. The enzyme catalyzes the last reaction step within the glycolytic sequence from phosphoenolpyruvate (PEP) to lactate and is responsible for net ATP production within this pathway. Enzymatic characterization of a wide range of different tumors revealed that tumorigenesis is accompanied by an increase in total pyruvate kinase v-max activities. There is also a shift towards the expression of the pyruvate kinase isoenzyme type M2 (M2-PK) and away from the tissue-specific isoenzymes (L-PK in liver and kidney, M1-PK in muscle and brain and R-PK in erythrocytes)[16-18]. The increased expression of M2-PK is under the control of ras, and the transcription factors SP1 and HIF-1. Ras and HIF-1 are both consistently altered in gastrointestinal tumors[19-22]. M2-PK can occur in a tetrameric form which is characterized by a high affinity to its substrate PEP and in a dimeric form with a low PEP affinity. The tetramer: dimer ratio of M2-PK determines the proportion of glucose carbons used for glycolytic energy production (tetrameric form) or channeled into synthetic processes (dimeric form). In tumor cells, M2-PK is mainly found in the dimeric form (tumor M2-PK) due to direct interaction with various oncoproteins, i.e. pp60v-src kinase and HPV-16 E7[15,21,23]. Tumor M2-PK is released into the blood, and in the case of adenomas and tumors in the lower gastrointestinal tract also into the stool of patients. An increase in tumor M2-PK in EDTA plasma samples is found in gastrointestinal cancers, as well as a wide range of other tumors such as lung, renal, breast and cervical cancer. The EDTA plasma test is highly suitable for patient monitoring[24-30].
The fecal tumor M2-PK test has been described as a promising new screening tool for adenomas and colorectal cancer[8,11-14]. Therefore, the aim of our study was to evaluate the utility of the tumor M2-PK test in our own daily clinical routine as a marker for the pre-selection of patients requiring subsequent diagnostic colonoscopy.
MATERIALS AND METHODS
Patients
Our study consisted of 96 participants who underwent complete colonoscopy. The control group consisted of 42 healthy individuals (15 male and 27 female; median age: 58 years; range: 25-79 years) without any findings at colonoscopy, who were participating in the national screening colonoscopy program provided by the German health insurance system. All screening colonoscopies were conducted between September 2005 and April 2006 in a primary care gastroenterology and hepatology medical center. Healthy individuals were included in the control group.
The 54 participants with colorectal cancer underwent diagnostic colonoscopy at the Offenbach Municipal Hospital between January 2003 and April 2006. Rectal carcinomas were diagnosed in 21 patients (15 male and 6 female; median age: 70 years; range: 52-84 years). Colonic adenocarcinomas were diagnosed in 33 patients (24 male and 9 female; median age: 70 years; range: 43-84 years).
All participants received a stool sample collection pot and were instructed to collect a single stool sample (naturally produced, walnut sized) one day prior to the laxative administration in preparation for colonoscopy. No special diet was recommended. Paper collecting devices were used to avoid stool contact with water in the toilet bowl. Stool samples were initially stored at room temperature by the participants until the day of colonoscopy. Thereafter, these pre-colonoscopy stool samples were stored at -20°C at the medical center or hospital until analyzed for tumor M2-PK.
Measurement of fecal tumor M2-PK concentrations
Fecal tumor M2-PK concentrations were determined using a commercially available sandwich ELISA based on two different monoclonal antibodies which specifically recognize the dimeric form of M2-PK (ScheBo® • Biotech AG, Giessen, Germany). A positive test result was defined as > 4.0 kU/L, as indicated by the manufacturer.
Statistical analysis
Since the data were skewed to the right, the statistical analysis was conducted using the Kruskall-Wallis ANOVA test (Statistica, StatSoft® Inc., Tulsa, USA).
RESULTS
This study evaluated 54 patients with colorectal cancer and 42 healthy controls with no indication of gastrointestinal diseases at colonoscopy. In the control group, fecal tumor M2-PK levels were below 4.0 kU/L in 39 of the 42 subjects (median: 0.8 kU/L), resulting in 93% specificity (Figure 1). In two of the three control samples which were above the cut-off value only a slight increase of tumor M2-PK (4.4 kU/L and 5.3 kU/L) was measured.
Figure 1.
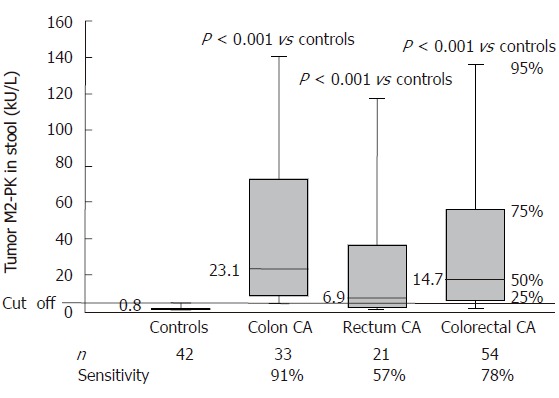
Tumor M2-PK levels in stool samples of healthy control individuals and patients with colon or rectal cancer.
Colorectal tumors were accompanied by a highly significant increase in fecal tumor M2-PK levels. The median value was 23.1 kU/L for colon carcinoma (P < 0.001), 6.9 kU/L for rectal carcinoma (P < 0.001) and 14.7 kU/L (P < 0.001) for colorectal carcinoma when both groups were combined.
At a cut-off level of 4.0 kU/L, the sensitivity was 91% for colon carcinoma, 57% for rectal carcinoma and 78% when both groups were combined. Both T classification and Duke’s staging of the colorectal tumors revealed a strong correlation between fecal tumor M2-PK levels and staging. The sensitivities increased from 60% for stage T1 to 100 % for stage T4 and from 60% for Duke´s A to 90% for Duke’s D (Figures 2 and 3; Table 1).
Figure 2.
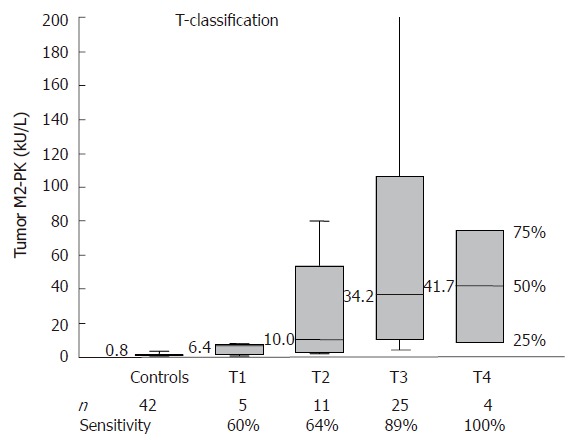
Correlation between fecal tumor M2-PK levels and TNM staging.
Figure 3.
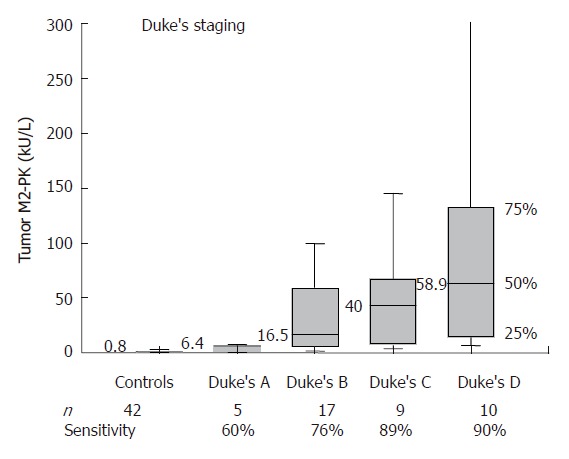
Correlation between fecal tumor M2-PK and Duke's staging.
Table 1.
Correlation between fecal tumor M2-PK levels and TNM classification or duke's staging
| Median | Mean | SE | Range | ||
| Classification | n | (kU/L) | (kU/L) | (kU/L) | (kU/L) |
| Controls | 42 | 0.8 | 1.5 | 0.4 | 0.1-17.3 |
| T1 | 5 | 6.4 | 4.5 | 1.6 | 0.3-7.7 |
| T2 | 11 | 10.0 | 30.5 | 10.1 | 1.5-100 |
| T3 | 25 | 34.2 | 106.3 | 35.2 | 1.7-620 |
| T4 | 4 | 41.7 | 41.4 | 19.2 | 6.6-76 |
| Duke's A | 5 | 6.4 | 4.5 | 1.6 | 0.3-7.7 |
| Duke's B | 17 | 16.5 | 63.5 | 34.7 | 0.2-604 |
| Duke's C | 9 | 40.0 | 50.0 | 17.8 | 1.9-176 |
| Duke's D | 10 | 58.9 | 138.5 | 65.3 | 4.5-620 |
DISCUSSION
Tumor M2-PK is the synonym for the dimeric form of the glycolytic pyruvate kinase isoenzyme type M2[15]. M2-PK is the pyruvate kinase isoenzyme which is characteristic of all proliferating cells and can occur in a tetrameric form as well as a dimeric form. Previous studies describe that tumor M2-PK is released into the stool of patients with adenomas and colorectal tumors and can easily be quantified with a commercially available sandwich ELISA[8,11-14].
In order to evaluate whether tumor M2-PK is a practical tool for the pre-selection of patients with colorectal cancer in our daily routine, we measured fecal tumor M2-PK in a cohort of 96 individuals. All 42 healthy control individuals, 33 patients with colon carcinoma and 21 patients with rectal carcinoma underwent total colonoscopy in order to confirm or exclude colorectal cancer. Our study revealed a highly significant increase (P < 0.001) in tumor M2-PK in the stool samples of those patients with colorectal cancer, whereby fecal tumor M2-PK values correlated well with Duke’s staging and T-classification (Figures 1-3; Table 1). Even stage T1 or Duke’s A showed 60% sensitivity, increasing to 100% in stage T4 and to 90% in Duke’s D tumors.
At a cut-off value of 4.0 kU/L, our overall sensitivity for colorectal carcinoma was 78 %. These data correspond well with the results of Hardt et al[13] who reported a sensitivity of 78% in 60 colorectal cancer patients, and those of Naumann et al[31] who found a sensitivity of 85.2% in a cohort of 27 colorectal cancer patients. A higher sensitivity was reported by McLoughlin et al[14] (92% in 25 colorectal cancer patients and 67% in 30 patients with adenomas) and by Koss et al[8] (92.3 % in 26 colorectal cancer patients and 60% for adenomas > 1 cm in ten patients).
The most commonly used fecal test in current screening programs is the guaiac-based FOBT[32,33]. Liebermann et al[7] and Koss et al[8] have reported an overall sensitivity for guaiac-based FOBTs of less than 30% for colorectal cancer and less than 15% for advanced adenomas. Results with newer, immunological FOBTs showed higher sensitivities than guaiac-based FOBTs for colorectal cancers[9,10] but in most studies colonoscopy was performed only in FOBT-positive cases.
The overall sensitivity of tumor M2-PK is increased if a higher proportion of late stage patients are included, but this is also true for FOBTs[10]. Nevertheless, McLoughlin et al[14] reported a sensitivity of 67% for adenomas with the fecal tumor M2-PK test. Similarly, Koss et al[8] found a sensitivity of 60% for adenomas > 1 cm.
In a head-to-head comparison of fecal tumor M2-PK and the commonly used guaiac-based FOBT, Koss et al[8] demonstrated a sensitivity for colorectal cancer of 92.3% for fecal tumor M2-PK and 20% for FOBT. No comparative study of fecal tumor M2-PK and immunochemical FOBTs is currently available.
The high sensitivity of the tumor M2-PK test is due to its ability to detect bleeding and non-bleeding tumors. From a practical viewpoint, the use of a single random formed stool sample for tumor M2-PK analysis, without requiring dietary restrictions, might be of greater patient convenience compared with the need to collect stool on three consecutive days for the guaiac FOBT.
In our study, the control group consisted of individuals without any signs of gastrointestinal diseases at colonoscopy. The median tumor M2-PK value in this group was 0.8 kU/L. In 39 of 42 subjects, tumor M2-PK levels were below the cut-off value; in two further control samples tumor M2-PK levels were only slightly increased (4.4, 5.3 kU/L). The resulting specificity at a cut off value of 4.0 kU/L is 93%, which is in general accordance with the studies of Hardt et al[13], Koss et al[8] and McLoughlin et al[14] who report specificities between 78% and 98%. Naumann et al[31] found increased fecal tumor M2-PK levels in cases of active Crohn’s disease and ulcerative colitis in which increased cell proliferation is expected. In addition, patients with inflammatory bowel disease have an increased risk of developing colorectal cancer, probably linked to frequent cycles of damage and regeneration of the colonic mucosa associated with flares of active disease.
Another new approach for pre-selective colorectal cancer screening is the determination of mutated oncogenes and anti-oncogenes[22,34-38]. These tests have the advantage of very high specificities. However, due to high genetic heterogeneity within colorectal cancers, a panel of different targets (k-ras, p53, APC genes, as well as microsatellite instability marker) must be measured in order to reach acceptable sensitivity which makes the test extensive and expensive. Using a 21-target multipanel, sensitivities between 44% and 91% and specificities between 93% and 100% are described[36-38]. Furthermore, to assure the stability of DNA within the stool, samples have to be frozen at -80°C within 12 h after defecation. Fecal tumor M2-PK is stable for 48 h at room temperature and for up to one year when frozen at -20°C (manufacturer’s
data sheet), which makes it practical for routine use. In addition, the tumor M2-PK test could be conducted in virtually all hospital and private diagnostic laboratories because it can either be run manually combined with an ELISA plate reader or automated using existing commercially available equipment.
Overall, our results are in general agreement with previous studies which have demonstrated that fecal tumor M2-PK is an appropriate tool to achieve a sensitive pre-selection by identifying those patients with the greatest need to undergo diagnostic colonoscopy to confirm or exclude colorectal cancer.
Footnotes
S- Editor Wang GP L- Editor Ma JY E- Editor Ma WH
References
- 1.Krebs in Deutschland, Häufigkeit und Trends, Robert Koch Institut, 5. überarbeitete, aktualisierte Ausgabe, Saarbrücken 2006. Available from: http://www.rki.de/cln_006/nn_226978/DE/Content/GBE/DachdokKrebs/Broschuere/kid2006,templateId=raw,property=publicationFile.pdf/kid2006.
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3. Darmkrebs–wenig Resonanz auf Angebot zur Früherkennung, Ärztezeitung, 2004-10-27. Available from: http://www.aerztezeitung.de/docs/2004/10/27/195a0401.asp.
- 4.Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82:891–898. [PubMed] [Google Scholar]
- 5.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 6.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91:434–437. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman DA, Weiss DG. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345:555–560. doi: 10.1056/NEJMoa010328. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman DA, Weiss DG. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345:555–560. doi: 10.1056/NEJMoa010328. [DOI] [PubMed] [Google Scholar]
- 9.Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med. 1996;334:155–159. doi: 10.1056/NEJM199601183340304. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005;129:422–428. doi: 10.1016/j.gastro.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 11.Ewald N, Toepler M, Akinci A, Kloer HU, Bretzel RG, Hardt PD. [Pyruvate kinase M2 (tumor M2-PK) as a screening tool for colorectal cancer (CRC). A review of current published data] Z Gastroenterol. 2005;43:1313–1317. doi: 10.1055/s-2005-858657. [DOI] [PubMed] [Google Scholar]
- 12.Hardt PD, Toepler M, Ngoumou B, Rupp J, Kloer HU. Measurement of fecal pyruvate kinase type M2 (tumor M2-PK) concentrations in patients with gastric cancer, colorectal cancer, colorectal adenomas and controls. Anticancer Res. 2003;23:851–853. [PubMed] [Google Scholar]
- 13.Hardt PD, Mazurek S, Toepler M, Schlierbach P, Bretzel RG, Eigenbrodt E, Kloer HU. Faecal tumour M2 pyruvate kinase: a new, sensitive screening tool for colorectal cancer. Br J Cancer. 2004;91:980–984. doi: 10.1038/sj.bjc.6602033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLoughlin R, Shiel E, Sebastian S, Ryan B, O´Connor HJ, O´Morain C. Tumor M2-PK, a novel screening tool for colorectal cancer. Poster Abstracts and Trade Exhibition Book: NCRI Cancer Conference; 2005 Oct 2-5; Birmingham, UK. London: Callisto; 2005. p. 202. [Google Scholar]
- 15.Eigenbrodt E, Reinacher M, Scheefers-Borchel U, Scheefers H, Friis R. Double role for pyruvate kinase type M2 in the expansion of phosphometabolite pools found in tumor cells. Crit Rev Oncog. 1992;3:91–115. [PubMed] [Google Scholar]
- 16.Reinacher M, Eigenbrodt E. Immunohistological demonstration of the same type of pyruvate kinase isoenzyme (M2-Pk) in tumors of chicken and rat. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37:79–88. doi: 10.1007/BF02892557. [DOI] [PubMed] [Google Scholar]
- 17.Board M, Humm S, Newsholme EA. Maximum activities of key enzymes of glycolysis, glutaminolysis, pentose phosphate pathway and tricarboxylic acid cycle in normal, neoplastic and suppressed cells. Biochem J. 1990;265:503–509. doi: 10.1042/bj2650503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hacker HJ, Steinberg P, Bannasch P. Pyruvate kinase isoenzyme shift from L-type to M2-type is a late event in hepatocarcinogenesis induced in rats by a choline-deficient/DL-ethionine-supplemented diet. Carcinogenesis. 1998;19:99–107. doi: 10.1093/carcin/19.1.99. [DOI] [PubMed] [Google Scholar]
- 19.Discher DJ, Bishopric NH, Wu X, Peterson CA, Webster KA. Hypoxia regulates beta-enolase and pyruvate kinase-M promoters by modulating Sp1/Sp3 binding to a conserved GC element. J Biol Chem. 1998;273:26087–26093. doi: 10.1074/jbc.273.40.26087. [DOI] [PubMed] [Google Scholar]
- 20.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 21.Mazurek S, Zwerschke W, Jansen-Dürr P, Eigenbrodt E. Metabolic cooperation between different oncogenes during cell transformation: interaction between activated ras and HPV-16 E7. Oncogene. 2001;20:6891–6898. doi: 10.1038/sj.onc.1204792. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa T, Maemura K, Hirata I, Matsuse R, Morikawa H, Toshina K, Murano M, Hashimoto K, Nakagawa Y, Saitoh O, et al. A simple method of detecting K-ras point mutations in stool samples for colorectal cancer screening using one-step polymerase chain reaction/restriction fragment length polymorphism analysis. Clin Chim Acta. 2002;318:107–112. doi: 10.1016/s0009-8981(01)00806-3. [DOI] [PubMed] [Google Scholar]
- 23.Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Oremek GM, Teigelkamp S, Kramer W, Eigenbrodt E, Usadel KH. The pyruvate kinase isoenzyme tumor M2 (Tu M2-PK) as a tumor marker for renal carcinoma. Anticancer Res. 1999;19:2599–2601. [PubMed] [Google Scholar]
- 25.Wechsel HW, Petri E, Bichler KH, Feil G. Marker for renal cell carcinoma (RCC): the dimeric form of pyruvate kinase type M2 (Tu M2-PK) Anticancer Res. 1999;19:2583–2590. [PubMed] [Google Scholar]
- 26.Lüftner D, Mesterharm J, Akrivakis C, Geppert R, Petrides PE, Wernecke KD, Possinger K. Tumor type M2 pyruvate kinase expression in advanced breast cancer. Anticancer Res. 2000;20:5077–5082. [PubMed] [Google Scholar]
- 27.Schneider J, Morr H, Velcovsky HG, Weisse G, Eigenbrodt E. Quantitative detection of tumor M2-pyruvate kinase in plasma of patients with lung cancer in comparison to other lung diseases. Cancer Detect Prev. 2000;24:531–535. [PubMed] [Google Scholar]
- 28.Kaura B, Bagga R, Patel FD. Evaluation of the Pyruvate Kinase isoenzyme tumor (Tu M2-PK) as a tumor marker for cervical carcinoma. J Obstet Gynaecol Res. 2004;30:193–196. doi: 10.1111/j.1447-0756.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 29.Ventrucci M, Cipolla A, Racchini C, Casadei R, Simoni P, Gullo L. Tumor M2-pyruvate kinase, a new metabolic marker for pancreatic cancer. Dig Dis Sci. 2004;49:1149–1155. doi: 10.1023/b:ddas.0000037803.32013.aa. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, Chen JY, Chen DD, Wang GB, Shen P. Tumor type M2 pyruvate kinase expression in gastric cancer, colorectal cancer and controls. World J Gastroenterol. 2004;10:1643–1646. doi: 10.3748/wjg.v10.i11.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naumann M, Schaum B, Oremek GM, Hanisch E, Rösch W, Mössner J, Caspary WF, Stein J. [Faecal pyruvate kinase type M2--a valid screening parameter for colorectal cancer Preliminary results from a multicenter comparative study] Dtsch Med Wochenschr. 2004;129:1806–1807. doi: 10.1055/s-2004-829033. [DOI] [PubMed] [Google Scholar]
- 32.Schmiegel W, Selbmann HK. Leitlinie “Kolorektales Karzinom: Prävention, Diagnostik und Therapie 2004” Im Auftrag der Deutschen Gesellschaft für Verdauungs- und Stoffwechselkrankheiten mit Unterstützung der Deutschen Krebshilfe. Available from: http://www.dgvs.de/322.php#Leitlinie_Kolorektales_Karzinom_Prvention_Diagnostik_und_Therapie_2004.
- 33.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 2006;56:11–25; quiz 49-50. doi: 10.3322/canjclin.56.1.11. [DOI] [PubMed] [Google Scholar]
- 34.Rengucci C, Maiolo P, Saragoni L, Zoli W, Amadori D, Calistri D. Multiple detection of genetic alterations in tumors and stool. Clin Cancer Res. 2001;7:590–593. [PubMed] [Google Scholar]
- 35.Traverso G, Shuber A, Olsson L, Levin B, Johnson C, Hamilton SR, Boynton K, Kinzler KW, Vogelstein B. Detection of proximal colorectal cancers through analysis of faecal DNA. Lancet. 2002;359:403–404. doi: 10.1016/S0140-6736(02)07591-8. [DOI] [PubMed] [Google Scholar]
- 36.Ahlquist DA, Skoletsky JE, Boynton KA, Harrington JJ, Mahoney DW, Pierceall WE, Thibodeau SN, Shuber AP. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology. 2000;119:1219–1227. doi: 10.1053/gast.2000.19580. [DOI] [PubMed] [Google Scholar]
- 37.Dong SM, Traverso G, Johnson C, Geng L, Favis R, Boynton K, Hibi K, Goodman SN, D'Allessio M, Paty P, et al. Detecting colorectal cancer in stool with the use of multiple genetic targets. J Natl Cancer Inst. 2001;93:858–865. doi: 10.1093/jnci/93.11.858. [DOI] [PubMed] [Google Scholar]
- 38.Tagore KS, Lawson MJ, Yucaitis JA, Gage R, Orr T, Shuber AP, Ross ME. Sensitivity and specificity of a stool DNA multitarget assay panel for the detection of advanced colorectal neoplasia. Clin Colorectal Cancer. 2003;3:47–53. doi: 10.3816/CCC.2003.n.011. [DOI] [PubMed] [Google Scholar]


