Abstract
AIM: To study the effect of bilirubin on the oxidative liver status and the activity and expression of heme oxygenase-1 (HO-1) in rat liver injury induced by prehepatic portal hypertension.
METHODS: Wistar male rats, weighing 200-250 g, were divided at random into two groups: one group with prehepatic portal hypertension (PH) induced by regulated prehepatic portal vein ligation (PPVL) and the other group corresponded to sham operated rats. Portal pressure, oxidative stress parameters, antioxidant enzymes, HO-1 activity and expression and hepatic sinusoidal vasodilatation were measured.
RESULTS: In PPVL rats oxidative stress was evidenced by a marked increase in thiobarbituric acid reactive substances (TBARS) content and a decrease in reduced glutathione (GSH) levels. The activities of liver antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) were also diminished while activity and expression of HO-1 were enhanced. Administration of bilirubin (5 μmol/kg body weight) 24 h before the end of the experiment entirely prevented all these effects. Pretreatment with Sn-protoporphyrin IX (Sn-PPIX) (100 μg/kg body weight, i.p.), a potent inhibitor of HO, completely abolished the oxidative stress and provoked a slight decrease in liver GSH levels as well as an increase in lipid peroxidation. Besides, carbon monoxide, another heme catabolic product, induced a significant increase in sinusoidal hepatic areas in PPVL group. Pretreatment of PPVL rats with Sn-PPIX totally prevented this effect.
CONCLUSION: These results suggest a beneficial role of HO-1 overexpression in prehepatic portal hypertensive rats.
Keywords: Heme oxygenase-1, Portal hypertensive rats, Liver oxidative stress
INTRODUCTION
Portal hypertension (PH) constitutes a major complication in chronic liver diseases including cirrhosis. It is associated with a hyperdynamic splanchnic circulation in response to the resistance of portal blood flow to reach the liver. This complication is associated with collateralization of the portal system, leading to the development of varix at various locations in the upper gastrointestinal tract[1].
Oxidative stress is the result of excessive generation of reactive oxygen species (ROS), depletion of intracellular antioxidant defences or a combination of both, leading to an imbalance in the redox status of the cell. Reactive oxygen species induce cell, tissue or organ damage and ROS have been proposed as a major factor responsible for several diseases, and have been implicated in portal hypertension[2].
ROS occur in tissues and may damage DNA, proteins, carbohydrates, and lipids. These potentially deleterious reactions are controlled by a system of antioxidant defences, which eliminate pro-oxidants and scavenge free radicals. Various intracellular compounds such as glutathione, and antioxidant enzymes including catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px)[3] provide protection against oxidation. Heme oxygenase (HO) is the key microsomal enzyme in heme degradation to carbon monoxide (CO), iron and biliverdin, the latter being converted into bilirubin by the cytosolic biliverdin reductase[4,5]. Heme oxygenase, the rate limiting enzyme in heme degradation pathway, is induced in animal tissues, particularly in liver by many factors including its own substrate heme, several heme-proteins, heavy metals, UVA radiation, hypoxia, hyperoxia and others[6-9]. Induction of HO is entirely prevented by administration of several antioxidants such as α-tocopherol and allopurinol[9]. In recent years good evidence has been accumulated showing that bilirubin can act as a highly effective antioxidant and free radical scavenger, and it has been proposed that it can play a physiological and key role as an endogenous protective agent against oxidant mediated injury[10-14].
Antioxidant effects suggest that oxidant species play a major part in the induction of HO either directly or by GSH depletion[9,10,15]. An increase in HO activity will enhance bilirubin formation and because unconjugated bilirubin is an efficient scavenger of ROS, its increase would be the cellular response to oxidative stress[10,12-14]. Besides, similar to nitric oxide, CO derived from heme oxygenase reaction also acts as a neurotransmitter and regulator of vascular tone[16].
Of the two known mammal liver HO isoenzymes, HO-1 and HO-2[17], only HO-1 is inducible, herewith we will only refer to HO-1.
The aim of this work was to investigate the effect of HO-1 overexpression in rat livers with prehepatic portal hypertension.
MATERIALS AND METHODS
Animals and surgical procedures
Wistar male rats (200-250 g) were housed separately and acclimatised before use under conditions of controlled temperature (25 ± 2°C) and illumination (12 h light/dark cycle). Rats were fed with standard rat chow and water ad libitum. After 1 wk of acclimatisation, rats were randomised and separated into two groups: (1) Sham operated (n = 24) and (2) Prehepatic portal vein ligated rats (n = 24) (PPVL). Portal hypertension was induced by a calibrated portal vein stenosis according to the procedure of Chojkier et al[18]. In brief, rats were anaesthetised with sodium pentobarbital (50 mg/kg body weight, i.p.) and then a midline abdominal incision was made. The portal vein was located and isolated from the surrounding tissues. A ligature of 3-0 silk was placed around the vein and snugly tied it to a 20 gauge blunt end needle placed along side the portal vein. The needle was subsequently removed to yield a calibrated stenosis of the portal vein. Sham rats underwent an identical procedure except that portal vein was isolated but not stenosed. Fourteen days after the operation rats of group 2 developed portal hypertension. Then, one day before the experiment, portal hypertensive and Sham rats were divided into three subgroups. One of each subgroup received one bolus injection of bilirubin (5 μmol/kg body weight, i.p) or one bolus injection of Sn-protoporphyrin IX (Sn-PPIX; 100 μmol/kg body weight, i.p). Then, six groups of animals were used (n = 8): Sham group (Sham), PPVL, Sham pretreated with bilirubin group (Sham + bilirubin), PPVL pretreated with bilirubin group (PPVL + bilirubin), sham pretreated with Sn-PPIX group (Sham + Sn-PPIX) and PPVL pretreated with Sn-PPIX group (PPVL + Sn-PPIX). Bilirubin and Sn-PPIX were prepared as follows: solutions in 0.1 mol/L NaOH were freshly prepared before administration, adjusted to pH 7.4 with phosphate buffer and diluted with saline. In each subgroup portal pressure was measured immediately before the sacrifice. Fourteen days after the corresponding operation and before sacrifice, the rats were anaesthetised with sodium pentobarbital (50 mg/kg), intraperitoneally (i.p.). Portal pressure was measured through a needle placed in the splenic pulp, and maintained in place by cyanoacrylate gel. The needle was cannulated to a polyethylene catheter (50) filled with a heparinized saline solution (25 U/mL) and connected to a Statham Gould P23ID pressure transducer (Statham, Hato Rey, Puerto Rico) coupled to a Grass 79D polygraph (Grass Instruments, Quincy, MA). Animals were treated in accordance with guidelines established by the Animal Care and Use Committee of the Argentine Association of Specialists in Laboratory Animals (AADEALC), and were in accordance with the Guide to the Care and Use of Experimental Animals published by the Argentine Council on Animal Care.
Enzyme preparations and assays
Rats were anaesthetised with sodium pentobarbital (50 mg/kg body weight, i.p.) and killed 14 d after surgery. Livers were excised and perfused "in situ" with ice-cold saline solution (0.9% NaCl), then excised and homogenised in a Potter-Elvehejm homogenizer using different solutions. For heme oxygenase assay the homogenate was prepared using 4 vol of ice-cold 0.25 mol/L sucrose solution containing 1 mmol/L phenylmethyl sulfonyl fluoride, 0.2 mmol/L EDTA and 50 mmol/L potassium phosphate buffer (pH 7.4). Homogenates were centrifuged at 20 000 g for 20 min and supernatant fractions centrifuged at 150 000 g for 90 min. The microsomal pellet obtained was washed and resuspended in 20 mmol/L potassium phosphate buffer (pH 7.4), containing 135 mmol/L KCl, 1 mmol/L phenyl-methylsulfonyl fluoride and 0.2 mmol/L EDTA to a protein concentration of 10 mg/mL. Microsomal HO-1 was obtained as described elsewhere[17]. The 150 000 g supernatants obtained from the microsomal preparation were fractionated by addition of ammonium sulfate (AS), and the 40%-60% AS fraction dissolved in 10 mmol potassium phosphate buffer (pH 7.4) and dialyzed against the same buffer using this preparation as biliverdin reductase. Heme oxygenase activity was determined as described elsewhere[10]. The standard incubation mixture in a final volume of 200 μL contained 10 μmol potassium phosphate buffer (pH 7.4), 60 nmol NADPH, 50 μL HO-1 (0.5 mg protein), 50 μL biliverdin reductase (0.42 mg protein), and 200 nmol hemin. Incubations were carried out at 37°C for 30 min. Activity was determined by measuring bilirubin formation, which was calculated as the difference in absorbance measured at 455 and 520 nm, employing an ε value of 50 mM-1 cm-1 (vismax 455 nm)[11]. CAT, SOD and GSH-Px activities were determined spectrophotometrically in liver homogenates prepared in a medium consisting of 140 mmol/L KCl and 25 mmol/L potassium phosphate buffer (pH 7.4), and centrifuged at 600 g for 10 min. The supernatant, a suspension of preserved organelles, was used as homogenate. Catalase activity was determined by measuring the decrease in absorbance at 240 nm[19] glutathione peroxidase activity following NADPH oxidation at 340 nm[20], and superoxide dismutase activity by inhibition of adrenochrome formation rate at 480 nm[21]. One unit in the SOD assay is defined as the amount of enzymatic protein required to inhibit 50% of epinephrine auto-oxidation.
Lipid peroxidation
Lipid peroxidation in liver was determined by measuring the rate of production of thiobarbituric acid reactive substances (TBARS), expressed as malondialdehyde equivalents[22]. One volume of homogenate was mixed with 0.5 volume TCA (15% w/v) and centrifuged at 2000 g for 10 min. The supernatant (1 mL) was mixed with 0.5 mL thiobarbituric acid (0.7% w/v) and boiled for 10 min. After cooling, sample absorbance was read spectrophotometrically at 535 nm. Malondialdehyde concentration was calculated using an ε value of 1.56 × 105 M-1cm-1.
Endogenous hepatic GSH content
Total glutathione (GSH plus GSSG) was determined in liver homogenates after precipitation with 2% perchloric, and using yeast-glutathione reductase, 5, 5’ dithio-bis- (2-nitrobenzoic acid) (DTNB) and NADPH and reading at 340 nm. GSSG was determined by the same method in the presence of 2-vinylpyridine. GSH was calculated from the difference between total glutathione and GSSG[23].
Western-blot analysis of HO-1 expression
Samples of homogenate obtained for HO-1 activity assays were also analyzed by Western immunoblot technique as previously described[24]. An amount of protein (50 μg) from homogenates of control and treated rats was run in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis using a 12% acylamide resolving gel (Mini Protean II System, BioRad, Hertz, UK). Separated proteins were transferred to nitrocellulose membranes and non-specific binding of antibodies was blocked with 3% non-fat dried milk in PBS, pH 7.4 for 1 h at room temperature. Membranes were then probed with polyclonal goat anti-HO-1 antibody (Santa Cruz, BioTech, California)(1:300 dilution in Tris-buffered saline, pH 7.4) overnight at 4°C. Immune complexes were detected using donkey anti goat secondary antibody (1:1500) (Santa Cruz, BioTech, California), and were visualized using ECL reagent (Amersham, Pharmacia). Intensity of bands was analyzed with Gel-Pro® analyzer 3.1 version, Media Cybernetics.
Microscopy and image analysis
Hepatic tissue was fixed in buffered formalin and stained with routine techniques (HE, Reticulin and Masson’s trichromic) for light microscopy. For high resolution optic microscopy (HROM) tissue was fixed in 3% glutaraldehyde buffered with sodium cacodylate, embedded in epoxy and stained with toluidine blue. Images from light microscopy were captured and digitised (Cap view) and standardised (PhotoShop 7.0); then the selected sinusoidal areas of the pericentral vein areas were extracted with auto level function. Threshold in red images were obtained through Scion Image B4.02 analyser. Selected areas for quantification were measured as pixels per area (square inches). Standard referenced area utilized was 8.33 square inches[25].
Protein determination
Protein concentration was measured following Lowry et al[26] using bovine serum albumin as standard.
Statistical analysis
Results are expressed as mean ± SE. Data were analysed statistically by factorial analysis of variance (ANOVA) and followed by the Neuman-Keuls' test for comparison of means. Differences were considered significant at P < 0.05.
RESULTS
Portal pressure measurement
The portal pressure results are shown in Table 1. Differences between Sham vs PPVL rats at d 14 without and with bilirubin and Sn-PPIX were significant (P < 0.001). No differences were found either in Sham or in PPVL animals when they were treated with bilirubin and Sn-PPIX.
Table 1.
Effect of different treatments on portal pressure (mean ± SE)
| Treatment | Portal pressure (PP) |
| (mmHg) | |
| Sham | 7.9 ± 0.6b |
| PPVL | 14.4 ± 1.9 |
| Sham + bilirubin | 7.6 ± 0.9b |
| Sham + Sn-PPIX + | 7.3 ± 0.7b |
| PPVL + Bilirubin | 14.0 ± 1.8 |
| PPVL + Sn-PPIX | 13.8 ± 1.6 |
P < 0.001 between sham groups and PPVL, PPVL + Bilirubin and PPVL + Sn-PPIX groups, according to Neuman-Keuls' test.
Oxidative stress generation
Reactive oxygen species are responsible for peroxidative cell damage. TBARS were 150% increased in PPVL rat livers (Figure 1).
Figure 1.
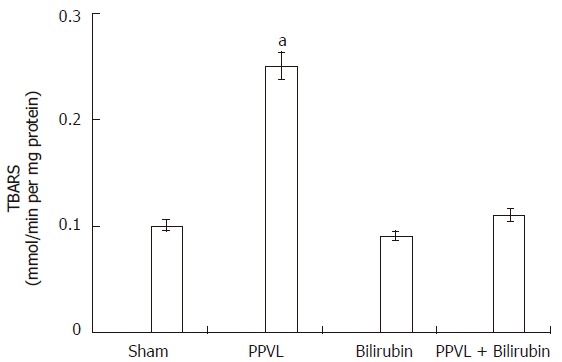
Effect of PPVL and bilirubin on lipid peroxidation. Rats were treated as described in Methods. Values are the means of three different experiments using three rats each time, and bars indicate SE. aP < 0.05 vs sham.
GSH is a leading substrate for enzymatic antioxidant functions and is also a known radical scavenger. Therefore, if PPVL treatment induced the formation of ROS, it could be expected that GSH levels be affected. It can be seen in Figure 2 that in the liver of PPVL animals GSH concentration was about 50% decreased when compared to Sham group. When rats were pretreated with bilirubin both TBARS and GSH levels were equal to Sham animals (Figures 1 and 2).
Figure 2.
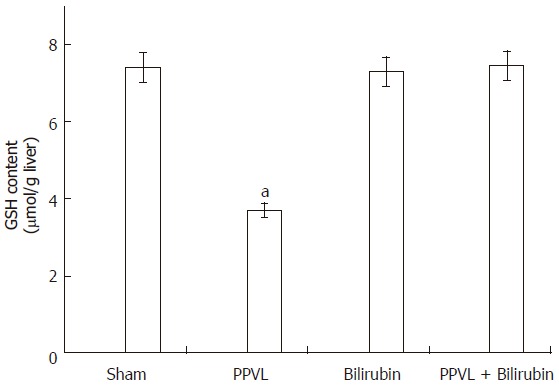
Effect of PPVL and bilirubin on GSH liver content. Rats were treated as described in Methods. Values are the means of three different experiments using three rats each time, and bars indicate SE. aP < 0.05 vs sham.
Enzymatic defence system
Table 2 shows the activities of CAT, SOD and GSH-Px in the liver of Sham and PPVL animals pretreated with bilirubin as well as in both groups without bilirubin. These enzymes were significantly inhibited (CAT 38%, SOD 50% and GSH-Px 23%) in PPVL rats. Pretreatment with bilirubin had no effect in Sham animals, but in PPVL rats the inhibition of antioxidant enzymes was totally prevented.
Table 2.
Effect of PPVL and bilirubin on antioxidant enzyme activities (mean ± SE)
| Group | Catalase (pmol/mg protein) | Total superoxide dismutase (U/mg protein)1 | Glutathione peroxidase (U/mg protein)2 |
| Sham | 10.5 ± 1.1a | 9.0 ± 0.2b | 0.129 ± 0.004a |
| PPVL | 6.5 ± 0.5 | 4.5 ± 0.1 | 0.100 ± 0.004 |
| Sham + Bilirubin | 10.7 ± 0.9a | 9.3 ± 0.1b | 0.132 ± 0.006a |
| PPVL + Bilirubin | 9.4 ± 0.9a | 8.9 ± 0.2b | 0.131 ± 0.004a |
Enzymatic activities were assayed as described in the text.
One unit of SOD activity is defined as the amount of enzyme required to inhibit 50% of the epinephrine autooxidation.
One unit of the enzyme represents the decrease of 1 mmol of NADPH/min under the assay conditions.
P < 0.05 between Catalase and Glutathione Peroxidase of Sham groups, PPVL + Bilirubin and PPVL group,
P < 0.01 between Superoxide Dismutase of group of Sham groups, PPVL + Bilirubin and PPVL group and PPVL group according to Neuman-Keuls' test.
Heme oxygenase activity and expression
As it is shown in Figure 3, PPVL treatment increased liver HO-1 activity by 80%. Bilirubin pretreatment did not have any effect in Sham rats, but it completely prevented HO-1 induction provoked by PPVL. As occurred with HO-1 activity, a similar increment in HO-1 expression was obtained in liver of PPVL rats (Figure 4A and B).
Figure 3.
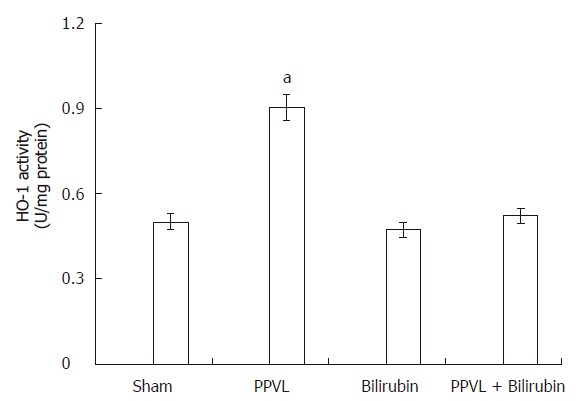
Effect of PPVL and bilirubin on HO-1 activity. Rats were treated as described in Methods. One unit of HO-1 is defined as the amount of enzyme producing 1 nmol of bilirubin per 30 min under the standard incubation conditions. Values are the means of three different experiments using three rats each time, and bars indicate SE. aP < 0.05 vs sham.
Figure 4.
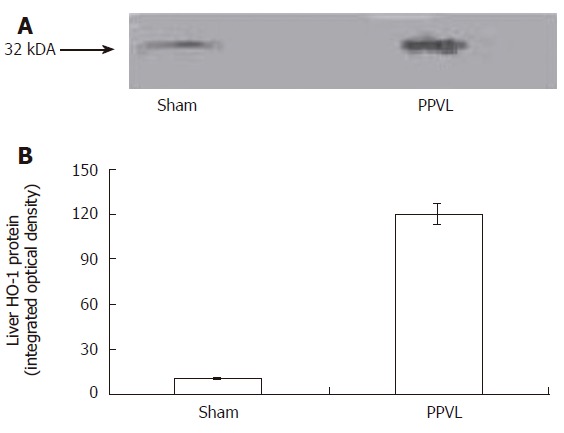
Western blot analysis of HO-1 expression in PPVL rat liver (A). Densitometry was done to quantify HO-1 protein expression (B). The blot is representative of 3 blots with a total of 4-5 samples/group between the 3 blots. P < 0.01 sham vs PPVL group.
Previous results using acute intoxication with different drugs demonstrated that as a consequence of ROS production, liver GSH levels rapidly decreased and then HO-1 was induced. It was also observed that HO-1 induction appeared to occur once the ROS was increased and the antioxidant defence system decreased. Bilirubin action as a protective agent against oxidative stress suggests that inhibition of HO-1 could enhance even more the oxidative stress induced by PPVL model. Therefore, the effect of Sn-PPIX, a strong inhibitor of HO-1 was assayed 24 h before the end of the experiment. Table 3 shows that a single administration of Sn-PPIX decreased to half HO-1 activity in Sham rats. PPVL animals pretreated with the inhibitor of HO-1 (Sn-PPIX), slightly decreased hepatic GSH levels (20%) and significantly increased TBARS production (70%) over the values obtained by PPVL treatment.
Table 3.
Effect of Sn-PPIX administration on the HO-1 induction and on TBARS and GSH contents in PPVL rat livers (mean ± SE)
| Treatment | HO-1 | TBARS | GSH content |
| (U/mg protein)1 | (nmol/min per mg protein) | (μmol/g liver) | |
| Sham | 0.50 ± 0.01b | 0.10 ± 0.01d | 7.5 ± 0.2d |
| PPVL | 0.90 ± 0.02 | 0.25 ± 0.01 | 3.7 ± 0.1 |
| Sham + Sn-PPIX | 0.25 ± 0.01 | 0.11 ± 0.01d | 7.2 ± 0.3d |
| PPVL + Sn-PPIX | 0.48 ± 0.04 | 0.32 ± 0.02 | 2.3 ± 0.2 |
Sn-PPIX was administered as described in Materials and Methods. Sham animals were injected with saline solution. Enzymatic activity was assayed as described in the text.
One unit of the enzyme forms 1 nmol of bilirubin/30 min under assay conditions. Different letters within columns indicate significant differences according to Neuman-Keuls' test. Differences in HO-1 were of
P < 0.01 between Sham group and PPVL, Sham + Sn-PPIX groups. Differences in TBARS and in GSH content were of
P < 0.01 between Sham groups and PPVL groups.
Effect of CO on hepatic sinusoidal vasodilatation
As it is shown in Table 4 and Figure 5, CO produced a significant increase in the sinusoidal hepatic areas of PPVL rats when compared to sham animals. Pretreatment of PPVL animals with Sn-PPIX totally prevented this effect and similar values to Sham group were obtained (Table 4). Groups Sham and PPVL + Sn-PPIX showed normal histological features when focusing on sinusoidal space (similar Figure 5A), while group PPVL showed sinusoidal dilation (Figure 5B).
Table 4.
Effect of different treatments on vasodilatation of hepatic sinusoids (mean ± SE)
| Treatment | Sinusoidal area |
| Sham (n = 258) | 3.64 ± 0.08 |
| PPVL (n = 245) | 4.20 ± 0.02b |
| Sham + Sn-PPIX (n = 257) | 3.45 ± 0.01 |
| PPVL + Sn-PPIX (n = 249) | 3.48 ± 0.04 |
The selected area for quantification was measured as pixels per area (square inch). The area standard used was 8.33 square inch.
P < 0.01 between PPVL and the other three groups, according to Neuman-Keuls' test.
Figure 5.
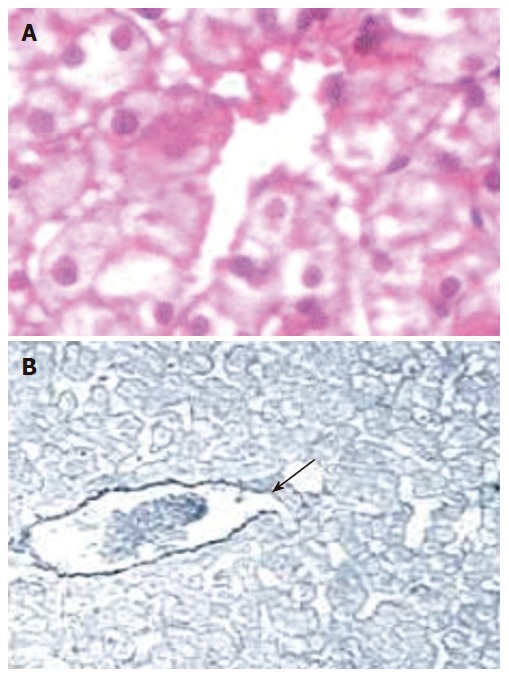
Hepatic centrolobular zone of Sham operated rats (A). Sinusoidal area spread normal histological features (HE, 100 x magnification). Hepatic centrolobular zone corresponding to PPVL group (B). Sinusoidal area is increased (arrows) (Reticulin, enhanced image, 40 x magnification).
DISCUSSION
Aerobic organisms are continuously exposed to oxygen, which renders them prone to damage generated by ROS. There are a number of cellular mechanisms to protect the cell from oxidative stress. Antioxidant enzymes such as CAT, SOD and GSH-Px play critical roles in oxidative stress protection by converting ROS into less harmful products[27]. Recently, HO-1 and its product bilirubin have gained attention in cytoprotection against oxidant mediated injury[10-14,28,29].
Our results showed that the administration of bilirubin in rats decreased PPVL-induced lipid peroxidation, restored GSH content and activity of the antioxidant enzymatic system to normal levels. These results open possibilities for considering the use of bilirubin as an efficient antioxidant and free radical scavenger (Figures 1 and 2, Table 2).
Bilirubin administration totally prevented the decrease of antioxidant enzyme activities and induction of HO-1 activity provoked by PPVL (Table 2, Figure 3). These findings are in agreement with our own previous reports on the protective effect of bilirubin against oxidative stress caused by different physical and chemical agents[28].
Administration of Sn-PPIX, a known competitive inhibitor of HO-1 expectedly decreased HO-1 activity (Table 3). When the inhibitor was given to PPVL rats, HO-1 activity remained at control levels, but an increase in TBARS content and a decrease in GSH levels were observed (Table 3).
CO is, like nitric oxide, an endogenous compound that activates guanylate cyclase[30], leading to the generation of cyclic guanosine monophosphate, which in turn mediates various physiological functions, and excessive production of CO, a consequence of HO-1 overexpression, could play an important role in modulating vascular tone under different pathological situations[16,31]. Besides, it has been demonstrated that administration of zinc protoporphyrin IX, a strong inhibitor of heme oxygenase, elicits a marked increase in the vascular resistance as a consequence of sinusoidal constriction[32]. These results are in agreement with the effects observed in PPVL rats pretreated with Sn-PPIX (Table 4, Figure 5). Increased production of CO, as a consequence of HO-1 induction, may be beneficial in some pathological situations, including hypertension[33], but can also be detrimental in other disease conditions[34]. For instance, in PH it would be detrimental because an increased production of CO in splanchnic organs may contribute to the development and maintenance of the splanchnic hyperdynamic circulation associated with the PH syndrome. But at this point, we can also speculate that an enhanced release of the vasodilator CO may contribute to the modulation of hepatic blood flow.
It has been demonstrated that HO-1 is induced in splanchnic organs of PH rats [35-37], and it was also reported that patients with portal hypertensive diseases had significantly greater the activity and expression of liver HO-1 than those from normal individuals[38]. HO-1 activity in the liver is not related to systemic vascular resistance; however this organ may show high activity of HO due to that reticuloendothelial cell-rich tissues are involved in the removal of senescent erythrocytes and plasmatic hemoglobin from the circulation.
The precise mechanisms whereby HO-1 expression is induced in PH rats are still unknown, but the results here obtained lead us to believe that this induction was provoked as a consequence of oxidative stress generation. Besides, it is noteworthy that HO-1 is transcriptionally activated by several chemical and physical factors that may be increased during PH[1], including cytokines, endotoxin and shear stress[39]. For these reasons, in the present study we suggest a beneficial role of HO-1 overexpression in a rat model of prehepatic portal hypertension.
In this study, no differences in portal pressure were observed in PPVL rats when compared this group with PPVL + Bilirubin and PPVL + Sn-PPIX groups. This fact could be explained by the persistence of some amount of portal systemic shunting and by the hepatic blood resistance that was not modified despite the pericentral sinusoidal dilation documented here.
In conclusion, our data suggest that ROS generation by portal hypertension situation alter heme degradation. They also evidenced that the cell possesses an inducible pathway against oxidative stress finally leading to increased bilirubin formation, which then exerts its antioxidant protective action. These results shed light on the response of heme degradation to PPVL-induced oxidative stress, and further support the involvement of bilirubin as a physiological protective agent against PPVL-induced oxidative cell injury. The potential use of bilirubin as an antioxidant in combination with other antioxidants or with conventional treatments, as a new therapeutic approach for portal hypertensive patients, can be considered.
Footnotes
Supported by Grants from the University of Buenos Aires, Buenos Aires, Argentina and CONICET, Buenos Aires, Argentina
S- Editor Wang J L- Editor Zhu LH E- Editor Ma WH
References
- 1.Bosch J, Pizcueta P, Feu F, Fernández M, García-Pagán JC. Pathophysiology of portal hypertension. Gastroenterol Clin North Am. 1992;21:1–14. [PubMed] [Google Scholar]
- 2.Evelson P, Llesuy S, Filinger E, Rodriguez RR, Lemberg A, Scorticati C, Susemihl M, Villareal I, Polo JM, Peredo H, et al. Decreased oxidative stress in prehepatic portal hypertensive rat livers following the induction of diabetes. Clin Exp Pharmacol Physiol. 2004;31:169–173. doi: 10.1111/j.1440-1681.2004.03963.x. [DOI] [PubMed] [Google Scholar]
- 3.Di Mascio P, Murphy ME, Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr. 1991;53:194S–200S. [PubMed] [Google Scholar]
- 4.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kutty RK, Maines MD. Purification and characterization of biliverdin reductase from rat liver. J Biol Chem. 1981;256:3956–3962. [PubMed] [Google Scholar]
- 6.Maines MD, Kappas A. Studies on the mechanism of induction of haem oxygenase by cobalt and other metal ions. Biochem J. 1976;154:125–131. doi: 10.1042/bj1540125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 9.Tomaro ML, Frydman J, Frydman RB. Heme oxygenase induction by CoCl2, Co-protoporphyrin IX, phenylhydrazine, and diamide: evidence for oxidative stress involvement. Arch Biochem Biophys. 1991;286:610–617. doi: 10.1016/0003-9861(91)90088-z. [DOI] [PubMed] [Google Scholar]
- 10.Llesuy SF, Tomaro ML. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim Biophys Acta. 1994;1223:9–14. doi: 10.1016/0167-4889(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 11.Ossola JO, Tomaro ML. Heme oxygenase induction by UVA radiation. A response to oxidative stress in rat liver. Int J Biochem Cell Biol. 1998;30:285–292. doi: 10.1016/s1357-2725(97)00109-x. [DOI] [PubMed] [Google Scholar]
- 12.Clark JE, Foresti R, Green CJ, Motterlini R. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem J. 2000;348 Pt 3:615–619. [PMC free article] [PubMed] [Google Scholar]
- 13.Stocker R. Induction of haem oxygenase as a defence against oxidative stress. Free Radic Res Commun. 1990;9:101–112. doi: 10.3109/10715769009148577. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T, Terakado M, Horio F, Aoki K, Tanaka M, Nakajima H. Role of bilirubin as an antioxidant in an ischemia-reperfusion of rat liver and induction of heme oxygenase. Biochem Biophys Res Commun. 1996;223:129–135. doi: 10.1006/bbrc.1996.0857. [DOI] [PubMed] [Google Scholar]
- 15.Ewing JF, Maines MD. Glutathione depletion induces heme oxygenase-1 (HSP32) mRNA and protein in rat brain. J Neurochem. 1993;60:1512–1519. doi: 10.1111/j.1471-4159.1993.tb03315.x. [DOI] [PubMed] [Google Scholar]
- 16.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 17.Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- 18.Chojkier M, Groszmann RJ. Measurement of portal-systemic shunting in the rat by using gamma-labeled microspheres. Am J Physiol. 1981;240:G371–G375. doi: 10.1152/ajpgi.1981.240.5.G371. [DOI] [PubMed] [Google Scholar]
- 19.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 20.Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 21.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 22.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 23.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 24.Foresti R, Clark JE, Green CJ, Motterlini R. Thiol compounds interact with nitric oxide in regulating heme oxygenase-1 induction in endothelial cells. Involvement of superoxide and peroxynitrite anions. J Biol Chem. 1997;272:18411–18417. doi: 10.1074/jbc.272.29.18411. [DOI] [PubMed] [Google Scholar]
- 25.Masseroli M, Caballero T, O'Valle F, Del Moral RM, Pérez-Milena A, Del Moral RG. Automatic quantification of liver fibrosis: design and validation of a new image analysis method: comparison with semi-quantitative indexes of fibrosis. J Hepatol. 2000;32:453–464. doi: 10.1016/s0168-8278(00)80397-9. [DOI] [PubMed] [Google Scholar]
- 26.LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 2nd Ed. Oxford: Clarendon Press; 1989. pp. 105–161. [Google Scholar]
- 28.Tomaro ML, Batlle AM. Bilirubin: its role in cytoprotection against oxidative stress. Int J Biochem Cell Biol. 2002;34:216–220. doi: 10.1016/s1357-2725(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 29.Noriega GO, Tomaro ML, del Batlle AM. Bilirubin is highly effective in preventing in vivo delta-aminolevulinic acid-induced oxidative cell damage. Biochim Biophys Acta. 2003;1638:173–178. doi: 10.1016/s0925-4439(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Wang Z, Wu L. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br J Pharmacol. 1997;121:927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 32.Suematsu M, Goda N, Sano T, Kashiwagi S, Egawa T, Shinoda Y, Ishimura Y. Carbon monoxide: an endogenous modulator of sinusoidal tone in the perfused rat liver. J Clin Invest. 1995;96:2431–2437. doi: 10.1172/JCI118300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motterlini R, Gonzales A, Foresti R, Clark JE, Green CJ, Winslow RM. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ Res. 1998;83:568–577. doi: 10.1161/01.res.83.5.568. [DOI] [PubMed] [Google Scholar]
- 34.Lores-Arnaiz S, Perazzo JC, Prestifilippo JP, Lago N, D'Amico G, Czerniczyniec A, Bustamante J, Boveris A, Lemberg A. Hippocampal mitochondrial dysfunction with decreased mtNOS activity in prehepatic portal hypertensive rats. Neurochem Int. 2005;47:362–368. doi: 10.1016/j.neuint.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez M, Bonkovsky HL. Vascular endothelial growth factor increases heme oxygenase-1 protein expression in the chick embryo chorioallantoic membrane. Br J Pharmacol. 2003;139:634–640. doi: 10.1038/sj.bjp.0705272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erario MA, Gonzales S, Romay S, Eizayaga FX, Castro JL, Lemberg A, Tomaro ML. Role of heme oxygenase/carbon monoxide pathway on the vascular response to noradrenaline in portal hypertensive rats. Clin Exp Pharmacol Physiol. 2005;32:196–201. doi: 10.1111/j.1440-1681.2005.04171.x. [DOI] [PubMed] [Google Scholar]
- 37.Eizayaga F, Scorticati C, Prestifilippo JP, Romay S, Fernandez MA, Castro JL, Lemberg A, Perazzo JC. Altered blood-brain barrier permeability in rats with prehepatic portal hypertension turns to normal when portal pressure is lowered. World J Gastroenterol. 2006;12:1367–1372. doi: 10.3748/wjg.v12.i9.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makino N, Suematsu M, Sugiura Y, Morikawa H, Shiomi S, Goda N, Sano T, Nimura Y, Sugimachi K, Ishimura Y. Altered expression of heme oxygenase-1 in the livers of patients with portal hypertensive diseases. Hepatology. 2001;33:32–42. doi: 10.1053/jhep.2001.21161. [DOI] [PubMed] [Google Scholar]
- 39.Zuckerbraun BS, Billiar TR. Heme oxygenase-1: a cellular Hercules. Hepatology. 2003;37:742–744. doi: 10.1053/jhep.2003.50139. [DOI] [PubMed] [Google Scholar]


