Abstract
AIM: To investigate the clinicopathological features of gastrointestinal stromal tumor (GIST) and to study the reference indexes for malignancy.
METHODS: Fifty-two cases of primary GIST were distinguished from a group of gastrointestinal mesenchymal tumors using a panel of antibodies such as CD117 and CD34 by immunohistochemical SP method. Their biological behaviors were analyzed using the expression of p21WAF1 and Bax in 52 cases of GIST.
RESULTS: Grossly, the tumor size was between 1.5 cm and 13 cm (mean: 5.5 cm). Focal areas of hemorrhage, necrosis, or small cyst formation could be seen. Microscopically, the tumor was composed of spindle cells (20 cases), epithelioid cells (20 cases) and mixed cells (12 cases). Immunohistochemically, CD117 and CD34 showed diffuse strong positive expressions, the positive rates were 98.1% and 92.3%. SMA, S-100, NSE, NF and MBP showed focal positive expressions, the positive rates were 48.1%, 28.8%, 25%, 21.2% and 42.3% respectively. Vimentins were all positive desmin and CgA were all negative. In normal adult stomach and intestine, the immunoreactive staining for CD117 and CD34 showed immunoreactive interstitial cells of Cajal in myenteric neuroplexus. Among the 52 cases of GIST, 27 were positive for p21WAF1 (51.9%), 29 for Bax (55.8%). The expression of p21WAF1 and Bax had no significent difference with the localization, size, histological subtype of GIST, but had a significent difference with the histological grade (P = 0.000, respectively). p21WAF1 expression had a positive correlation to Bax expression (r = 0.461, P = 0.001, κ = 0.459).
CONCLUSION: GIST has complicated arrangements and various cell types. Positivity of CD117 and CD34 is the most valuable factor in diagnosing GIST. Expression of p21WAF1 and Bax plays an important role in potential malignancy and malignancy rather than in benign GIST. p21WAF1 and Bax may be used as the markers in the assessment of GIST malignant potential.
Keywords: Gastrointestinal stromal tumor, p21WAF1, Bax
INTRODUCTION
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor in the digestive tract[1]. Mutational activation of c-kit has been found to be associated with the pathogenesis of GIST[2], CD117 positivity is the most valuable marker in diagnosing GIST. But the biological behaviors of GIST are difficult to predict[3], some metastasize whereas others remain asymptomatic for years[4]. In this study, we detected CD117, CD34, SMA, S-100, NSE, NF, MBP, vimentin, desmin, CgA, p21WAF1 and Bax using immunohistochemical staining method to explore the expression of p21WAF1 and Bax and its correlation to clinicopathologic characteristics of GIST.
MATERIALS AND METHODS
Specimens
Fifty-two cases of GIST were selected from the Department of Pathology, First Hospital of Harbin Medical University in 2001 to 2004. The slides stained with hematoxylin and eosin were reviewed. Based on the diagnostic criteria proposed by Haber et al[5], among the 52 cases of GIST, 20 were cases of benign GIST, 12 and 20 were cases of potentially malignant and malignant GIST respectively. Age ranged 34-78 years (mean: 54.3 years).
Immunohischemistry
Resected specimens were fixed in 40 g/L formaldehyde and embedded in paraffin. Four-μm thick sections were dewaxed, rehydrated in graded alcohols, and processed using immunohistochemical SP method. All antibodies were purchased from Beijing Zhongshan Biotechnology CO. LTD (Table 1). Tissues positive for all the purchased antibodies were used as positive controls, sections prepared with PBS instead of the primary antibody were used as negative controls. When the number of positive cells was < 10%, 10%-50%, or > 50%, the immunoreactivity for p21WAF1 and Bax was scored as 1 +, 2 +, 3 +, respectively. When the number of positive cells was ≤ 50% and > 50%, the immunoreactivity for other antibodies was scored as 1 +, 2 +, and 3 +, respectively.
Table 1.
Primary antibody used for immunohistochemical study
| Antibody | Clone | Dilution | Pretreatment |
| CD117 | polyclonal | instant | Microwave |
| CD34 | QBEnd/10 | instant | Microwave |
| SMA | 1A4 | instant | None |
| Vimentin | V9 | instant | None |
| Desmin | ZC18 | instant | None |
| S-100 | 4C4.9 | instant | None |
| NSE | E27 | instant | None |
| NF | DA2/FNP7 | instant | Microwave |
| MBP | polyclonal | instant | None |
| CgA | LK2H10 | instant | Microwave |
| p21WAF1 | 4D10 | 1:25 | Microwave |
| Bax | 2D2 | 1:50 | Microwave |
Statistical analysis
Statistical analyses were performed using SPSS 11.5 software. Pearson χ2 test, Fisher’s exact test, Spearman rank correlation test and Kappa test were used when appropriate. P < 0.05 was considered statistically significant.
RESULTS
Grossing findings
The tumor size was 1.5-13 cm (mean: 5.5 cm). Tumors were generally round or oval in shape with pink-white and firm well-circumscribed and cut surface. Focal areas of hemorrhage, necrosis, or small cyst formation could be seen. Submucosal or subserosal tumors sometimes extended into the gastrointestinal lumen, leading to ulceration in mucosa.
Light microscopic findings
Among the 52 cases of GIST, spindle cell type was found in 20, epithelioid cell type in 20 (Figure 1A) and mixed type in 12. The tumor cells arranged in interlacing fascicles or formed whirls. The tumor cells were spindle, oral or round in shape, sometimes signet-ring like cells could be observed with a clear cytoplasm (Figure 1B). Hemorrhage and/or necrosis and/or hyaline degeneration (Figure 1C) could be found in some cases. Mucosal or serosal invasion (Figure 1D) sometimes could be seen in some malignant GISTs.
Figure 1.
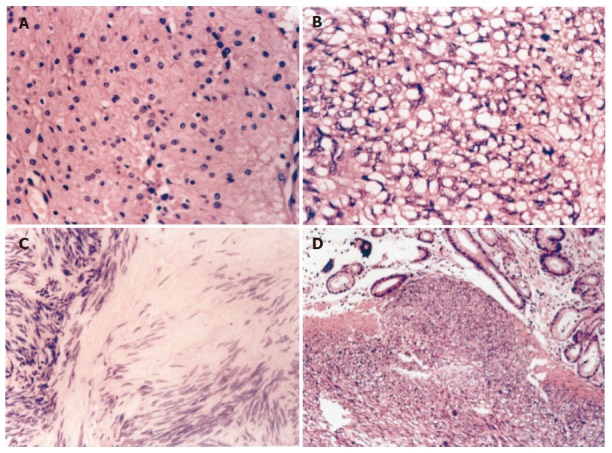
Epithelioid cells (A), signet-ring like cells (B), hyaline degeneration (C), and mucosal invasion (D) in GISTs.
Immunohistochemical findimgs
CD117 and CD34 showed diffuse positive expressions, the positive rates were 98.1% (Figure 2A) and 92.3%. SMA, S-100, NSE, NF and MBP showed focal positive expressions, the positive rates were 48.1%, 28.8%, 25%, 21.2% and 42.3%, respectively. Vimentins were all positive while desmin and CgA were all negative. In normal adult stomach and intestine, the immunoreactive staining for CD117 and CD34 showed immunoreactive interstitial cells of Cajal in myenteric neuroplexus (Figure 2B). Among the 52 cases of GIST, 27 were positive for p21WAF1(51.9%, Figure 2C), 29 for Bax (55.8%, Figure 2D).
Figure 2.
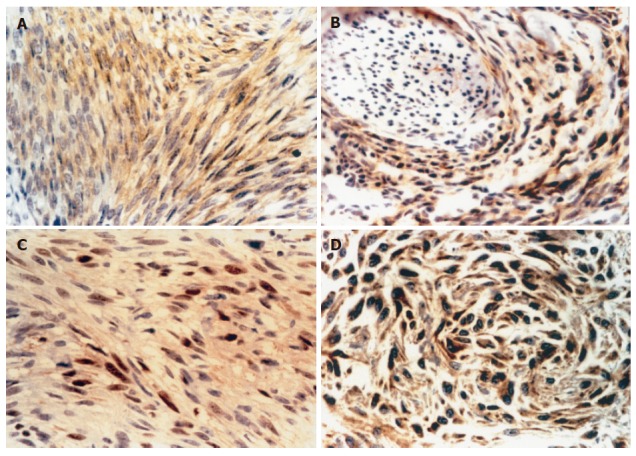
Expressions of CD117 (A), CD34 (B), p21WAF1 (C), and Bax (D) in GIST.
According to the χ2 test, the expression of p21WAF1 and Bax had no significant differences in the localization, size, and histological subtype of GIST, but there was a significant difference in the histological grade (P = 0.000, Table 2). There was a significant difference between benign and potentially malignant or malignant GISTs, but the difference in p21WAF1 and Bax expression between potentially malignant and malignant GISTs was not significent. According to Spearman rank correlation test and Kappa test, p21WAF1 expression had a positive correlation to Bax expression (r = 0.461, P = 0.001, κ = 0.459, Table 3).
Table 2.
Relation between p21WAF1, Bax expression and clinicopathological features
| Items | n |
p21WAF1 |
Bax |
|||||
| positive | % | P | positive | % | P | |||
| Localization | Stomach/ | 36 | 17 | 47.2 | 0.309 | 18 | 50 | 0.209 |
| Intestine | ||||||||
| Others | 16 | 10 | 62.5 | 11 | 68.8 | |||
| Tumor size | < 5 cm | 21 | 12 | 57.1 | 0.535 | 13 | 61.9 | 0.463 |
| ≥ 5 cm | 31 | 15 | 48.4 | 16 | 51.6 | |||
| Histological subtype | Spindle | 20 | 11 | 55 | 0.720 | 9 | 45 | 0.086 |
| Epitheloid | 20 | 9 | 45 | 10 | 50 | |||
| Mixed | 12 | 7 | 58.3 | 10 | 83.3 | |||
| Histological grade | Benign | 20 | 1 | 5 | 0.000 | 2 | 10 | 0.000 |
| Potentially malignant | 12 | 9 | 75b | 11 | 91.7b | |||
| Malignant | 20 | 17 | 85 | 16 | 80 | |||
bP < 0.01.
Table 3.
Correlation between expressions of p21WAF1 and Bax
| p21WAF1 | n |
Bax |
r | P | |
| + | - | ||||
| + | 27 | 21 | 6 | 0.461 | 0.001 |
| - | 25 | 8 | 17 | ||
DISCUSSION
In 1983, Mazur and Clark[6] first introduced the vague term ‘gastrointestinal stromal tumor'. Under light microscope, the morphology of stromal tumors looks sometimes like a leiomyoma, sometimes like a Schwannoma[7]. Most gastrointestinal mesenchymal tumours, previously classified as leiomyomas, schwannomas or leiomyosarcomas, are today classified as GISTs on the basis of molecular and immunohistological features[8]. GIST derives from the interstital cells of Cajal (ICC), or from a common precursor of ICC and smooth muscle cells of the digestive tract[9]. Immunoperoxidase staining can show both c-kit and CD34-positive cells surrounding the Auerbach ganglia plexus in the gastrointestinal tract[10]. The great majority of GISTs occur in the stomach (60%-70%) and small intestine (25%-35%)[11]. In this study, 46.1% GISTs occurred in stomach and 23.1% in small intestine. In addition, the tumors arose in the esophagus, mesentery. The 52 cases of GISTs were subclassified as spindle or epithelioid type stromal tumors based on the predominant pattern[12]. Among them, spindle cell type was found in 20, epithelioid cell type in 20 and mixed type in 12.
To make a diagnosis of GISTs, immunohistochemical staining of the CD117 and CD34 is required, because they can characteristically express CD34 and CD117[13]. In this study, CD117 and CD34 showed diffuse positive expressions, the positive rates were 98.1% and 92.3%. Eighteen cases with focal immunoreactivity for SMA were diagnosed as GISTs with smooth muscle differentiation. S-100, NSE, NF and MBP showed focal positive expressions, which could be used as the diagnostic criteria of GISTs with nerve differentiation.
As a sensitive and specific marker of GIST, c-kit seems to be a useful antibody in diagnosis and differential diagnosis of GIST, but it may not be used as a prognostic index[14]. Coagulative necrosis, mitotic activity over 10/50HPF, high cellularity with obvious pleomorphism are also helpful parameters for diagnosis of malignancy aside from metastasis and invasion. Adhesion over 5 cm in diameter and mitotic activity over 5/50HPF but less than 10/50HPF might be the potentially malignant parameters[15]. But the effective and reproducible diagnostic parameters for differentiating benign from malignant gastrointestinal stromal tumors (GISTs) are still not clear[16].
p21WAF1 is a cyclin-dependent kinase inhibitor (CDKI) which contributes to the regulation of cell cycle progression by controlling CDK activity and induces a G1 arrest[17,18]. Thus, it is a tumor suppressor gene and likely plays an important role in tumor development. Moreover, reduced expression of p21WAF1 has been reported to have a prognostic value in several human malignancies[19]. Pindzola et al[18] reported that malignant gastrointestinal stromal tumor expresses p21WAF1/CIP1. In this study, p21WAF1 expression was not associated with the localization, size and histological subtype of GISTs, except for the tumor grade showing a higher frequency of p21WAF1 expression in potential malignancy and malignancy than that in benign GISTs ( 75%, 85% and 5%, respectively), indicating that p21WAF1 expression plays an important role in potential malignancy and malignancy rather than in benign GISTs. However, there was no statistical significance between potentially malignant and malignant GISTs, suggesting that overexpression of p21WAF1 is associated with increasing malignant potential, and that p21WAF1 overexpression may be another useful marker in the assessment of the malignant potential in GIST.
The Bcl-2 protein family plays an important role in the regulation of apotosis. This family contains both proapoptotic members (Bax, Bid, Bad, and Bak) and antiapoptotic members (Bcl-2 and Bcl-xl[20]. Overexpression of Bax protein increases apoptosis[21]. Previous studies have shown that Bax expression might be involved in differentiation/histological types of colorectal cancer[22]. Chao et al[23] reported that in endometrial carcinoma, the positive rate of Bax overexpression increases correspondingly with increase in histological grade. Although apoptosis is associated with the tumor grade of various carcinomas, little is understood about the association of apoptosis in mesenchymal tumors[24]. Noguchi et al[25] reported that there is no statistically significant difference in Bax expression between benign and malignant tumors. In our study, Bax expression was associated with tumor grade showing a higher frequency of Bax expression in potential malignancy and malignancy than in benign GISTs (91.7%, 80%, 10%, respectively), suggesting that Bax expression plays an important role in potential malignancy and malignancy rather than in benign GISTs. However, there was no statistical significance between potential malignancy and malignancy, Suggesting that overexpression of Bax is associated with increasing malignant potential. Thus Bax overexpression may be another useful marker in assessment of the malignant potential in GISTs.
Yang et al[26] reported that p21WAF1/CIP1 could inhibit proliferation and induce apoptosis of hepatocellular carcinoma cells, and that inhibition of VSMC growth by overexpression of human p21 gene is accompanied with induction of apoptosis. These results suggest that regulation of cell cycle by p21 may be closely linked to programmed cell death /apoptosis in human vascular smooth muscle cells[27], but a number of recent studies have pointed out that in addition to being an inhibitor of cell proliferation, p21WAF1 acts as an inhibitor of apoptosis[28,29]. In our study, a positive correlation was found between p21WAF1 and Bax (r = 0.461, κ = 0.459), demonstrating that p21WAF1 is closely linked to Bax. In conclusion, p21 gene induces apoptosis by increasing Bax expression and plays an important role in potential malignant and malignant GISTs. Moreover, other factors besides p21WAF1 may regulate Bax.
Footnotes
S- Editor Li WZ L- Editor Wang XL E- Editor Bai SH
References
- 1.Duensing A, Heinrich MC, Fletcher CD, Fletcher JA. Biology of gastrointestinal stromal tumors: KIT mutations and beyond. Cancer Invest. 2004;22:106–116. doi: 10.1081/cnv-120027585. [DOI] [PubMed] [Google Scholar]
- 2.Kim TW, Lee H, Kang YK, Choe MS, Ryu MH, Chang HM, Kim JS, Yook JH, Kim BS, Lee JS. Prognostic significance of c-kit mutation in localized gastrointestinal stromal tumors. Clin Cancer Res. 2004;10:3076–3081. doi: 10.1158/1078-0432.ccr-03-0581. [DOI] [PubMed] [Google Scholar]
- 3.Gelen T, Elpek GO, Aksoy NH, Ogüş M, Keleş N. p27 Labeling index and proliferation in gastrointestinal stromal tumors: correlations with clinicopathologic factors and recurrence. Jpn J Clin Oncol. 2003;33:346–352. doi: 10.1093/jjco/hyg071. [DOI] [PubMed] [Google Scholar]
- 4.Tornóczky T, Kövér E, Pajor L. Frequent occurrence of low grade cases among metastatic gastrointestinal stromal tumours. J Clin Pathol. 2003;56:363–367. doi: 10.1136/jcp.56.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haber MH, Gattuso P, Spitz DJ, David O. Differential diagnosis in surgical pathology. 1st ed. Beijing: Health Science Asia, Elsevier Science; 2002. pp. 188–192. [Google Scholar]
- 6.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507–519. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Liu P, Na J, Wang Y, He Q, Zhang Y, Tang X, Zou W. Study of gastrointestinal stromal tumors by light microscopy, electron microscopy and immunohistochemistry. Zhonghua Binglixue Zazhi. 2002;31:199–203. [PubMed] [Google Scholar]
- 8.Bucher P, Villiger P, Egger JF, Buhler LH, Morel P. Management of gastrointestinal stromal tumors: from diagnosis to treatment. Swiss Med Wkly. 2004;134:145–153. doi: 10.4414/smw.2004.10530. [DOI] [PubMed] [Google Scholar]
- 9.Ray-Coquard I, Le Cesne A, Michallet V, Boukovinas I, Ranchere D, Thiesse P, Baty V, Blay JY. [Gastro-intestinal stromal tumors: news and comments] Bull Cancer. 2003;90:69–76. [PubMed] [Google Scholar]
- 10.Wang L, Vargas H, French SW. Cellular origin of gastrointestinal stromal tumors: a study of 27 cases. Arch Pathol Lab Med. 2000;124:1471–1475. doi: 10.5858/2000-124-1471-COOGST. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39–S51. doi: 10.1016/s0959-8049(02)80602-5. [DOI] [PubMed] [Google Scholar]
- 12.Ma CK, Amin MB, Kintanar E, Linden MD, Zarbo RJ. Immunohistologic characterization of gastrointestinal stromal tumors: a study of 82 cases compared with 11 cases of leiomyomas. Mod Pathol. 1993;6:139–144. [PubMed] [Google Scholar]
- 13.Boggino HE, Fernandez MP, Logroño R. Cytomorphology of gastrointestinal stromal tumor: diagnostic role of aspiration cytology, core biopsy, and immunochemistry. Diagn Cytopathol. 2000;23:156–160. doi: 10.1002/1097-0339(200009)23:3<156::aid-dc3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Ma D, Wu L, Bai C, Hu H. [Expression and clinical significance of c-kit oncogene in gastrointestinal stromal tumors] Zhonghua Waike Zazhi. 2002;40:277–279. [PubMed] [Google Scholar]
- 15.Hou Y, Wang J, Zhu X, Du X, Sun M, Zheng A. [A clinicopathologic and immunohistochemical study on 76 cases of gastrointestinal stromal tumors] Zhonghua Binglixue Zazhi. 2002;31:20–25. [PubMed] [Google Scholar]
- 16.Kim MK, Lee JK, Park ET, Lee SH, Seol SY, Chung JM, Kang MS, Yoon HK. [Gastrointestinal stromal tumors: clinical, pathologic features and effectiveness of new diagnostic criteria] Korean J Gastroenterol. 2004;43:341–348. [PubMed] [Google Scholar]
- 17.Okamoto K, Kato S, Arima N, Fujii T, Morimatsu M, Imaizumi T. Cyclin-dependent kinase inhibitor, p21Waf1, regulates vascular smooth muscle cell hypertrophy. Hypertens Res. 2004;27:283–291. doi: 10.1291/hypres.27.283. [DOI] [PubMed] [Google Scholar]
- 18.Pindzola JA, Palazzo JP, Kovatich AJ, Tuma B, Nobel M. Expression of p21WAF1/CIP1 in soft tissue sarcomas: a comparative immunohistochemical study with p53 and Ki-67. Pathol Res Pract. 1998;194:685–691. doi: 10.1016/s0344-0338(98)80127-1. [DOI] [PubMed] [Google Scholar]
- 19.Migaldi M, Sgambato A, Garagnani L, Ardito R, Ferrari P, De Gaetani C, Cittadini A, Trentini GP. Loss of p21Waf1 expression is a strong predictor of reduced survival in primary superficial bladder cancers. Clin Cancer Res. 2000;6:3131–3138. [PubMed] [Google Scholar]
- 20.van der Woude CJ, Kleibeuker JH, Tiebosch AT, Homan M, Beuving A, Jansen PL, Moshage H. Diffuse and intestinal type gastric carcinomas differ in their expression of apoptosis related proteins. J Clin Pathol. 2003;56:699–702. doi: 10.1136/jcp.56.9.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheaton S, Netser J, Guinee D, Rahn M, Perkins S. Bcl-2 and bax protein expression in indolent versus aggressive B-cell non-Hodgkin's lymphomas. Hum Pathol. 1998;29:820–825. doi: 10.1016/s0046-8177(98)90451-8. [DOI] [PubMed] [Google Scholar]
- 22.Jansson A, Sun XF. Bax expression decreases significantly from primary tumor to metastasis in colorectal cancer. J Clin Oncol. 2002;20:811–816. doi: 10.1200/JCO.2002.20.3.811. [DOI] [PubMed] [Google Scholar]
- 23.Chao H, Sun J, Lu S. [Bax gene expression in endometrial carcinoma] Zhonghua Zhongliu Zazhi. 2001;23:214–216. [PubMed] [Google Scholar]
- 24.Liu Y, Chen C, Chen C, Hsieh H, Chang C, Shyu J, Yen C, Harn H. Apoptosis and Fas-ligand expression correlate to the histopathological grade of gastric smooth muscle tumors. J Surg Res. 2001;95:92–98. doi: 10.1006/jsre.2000.6023. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi T, Sato T, Takeno S, Uchida Y, Kashima K, Yokoyama S, Müller W. Biological analysis of gastrointestinal stromal tumors. Oncol Rep. 2002;9:1277–1282. [PubMed] [Google Scholar]
- 26.Yang F, Wang W. [Effects of overexpression of p21WAF1/CIP1 on the malignant phenotype and apoptosis of human hepatocellular carcinoma cells] Zhonghua Zhongliu Zazhi. 1999;21:99–101. [PubMed] [Google Scholar]
- 27.Matsushita H, Morishita R, Kida I, Aoki M, Hayashi S, Tomita N, Yamamoto K, Moriguchi A, Noda A, Kaneda Y, et al. Inhibition of growth of human vascular smooth muscle cells by overexpression of p21 gene through induction of apoptosis. Hypertension. 1998;31:493–498. doi: 10.1161/01.hyp.31.1.493. [DOI] [PubMed] [Google Scholar]
- 28.Rau B, Sturm I, Lage H, Berger S, Schneider U, Hauptmann S, Wust P, Riess H, Schlag PM, Dörken B, et al. Dynamic expression profile of p21WAF1/CIP1 and Ki-67 predicts survival in rectal carcinoma treated with preoperative radiochemotherapy. J Clin Oncol. 2003;21:3391–3401. doi: 10.1200/JCO.2003.07.077. [DOI] [PubMed] [Google Scholar]
- 29.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]


