Abstract
AIM: To assess the utility of contrast-enhanced ultrasonography (CEUS) with a second-generation contrast medium in the differential diagnosis between mass-forming pancreatitis and pancreatic carcinoma.
METHODS: From our radio-pathology database, we retrieved all the patients affected by mass-forming pancreatitis or pancreatic carcinoma who underwent CEUS. We evaluated the results of CEUS in the study of the 173 pancreatic masses considering the possibilities of a differential diagnosis between mass-forming pancreatitis and pancreatic tumor by identifying the “parenchymographic” enhancement during the dynamic phase of CEUS, which was considered diagnostic for mass-forming pancreatitis.
RESULTS: At CEUS, 94% of the mass-forming pancreatitis showed intralesional parenchymography. CEUS allowed diagnosis of mass-forming pancreatitis with sensitivity of 88.6%, specificity of 97.8%, positive predictive value of 91.2%, negative predictive value of 97.1%, and overall accuracy of 96%. CEUS significantly increased the diagnostic confidence in the differential diagnosis between mass-forming pancreatitis and pancreatic carcinoma, with receiver operating characteristic curve areas from 0.557 (P = 0.1608) for baseline US to 0.956 (P < 0.0001) for CEUS.
CONCLUSION: CEUS allowed diagnosis of mass-forming pancreatitis with diagnostic accuracy of 96%. CEUS significantly increases the diagnostic confidence with respect to basal US in discerning mass-forming pancreatitis from pancreatic neoplasm.
Keywords: Contrast-enhanced ultrasonography, Pancreatitis, Pancreatic neoplasm, Mass-forming pancreatitis
INTRODUCTION
Mass-forming pancreatitis usually arises in patients with a history of chronic pancreatitis[1]. The main characteristic feature at pathology is progressive interstitial fibrosis with chronic inflammatory infiltrate. Differential diagnosis with a neoplastic disease may be difficult because mass-forming pancreatitis and pancreatic tumor may present with the same symptoms and signs[2]. Autoimmune pancreatitis is a rare type of chronic pancreatitis which has been proposed as a separate clinical entity in 1995 and later defined[3,4]. As opposed to the other forms of chronic pancreatitis, in the autoimmune form, the pancreas is increased in volume, usually in a diffuse way with the typical “sausage” look, and Wirsung duct is compressed by glandular parenchyma or string-like[4]. At ultrasonography (US), mass-forming pancreatitis is often very similar to pancreatic carcinoma[1,5], presenting in most cases as a hypoechoic mass in a limited sector of the gland, usually at the head, often with enlargement or lumpiness of the gland contour. Contrast-enhanced ultrasonography (CEUS), thanks to the real-time continuous visualization of blood perfusion of the pancreas and its masses, has been recently used in the evaluation of the vascularization of solid pancreatic lesions, with results superior to single-slice spiral CT[6,7]. The CEUS features of autoimmune pancreatitis have also been evaluated[8]. In this study, we aimed to assess the utility of CEUS with a second-generation contrast medium in the differential diagnosis between mass-forming pancreatitis and pancreatic carcinoma.
MATERIALS AND METHODS
From our radio-pathology database, we retrieved all the patients affected by mass-forming pancreatitis or pancreatic ductal adenocarcinoma who underwent CEUS at our Institution between January 2002 and January 2005. Our Institutional Review Board does not require any informed consent for retrospective studies. This study included 35 patients (26 males, 9 females, mean age 49.1 years) affected by mass-forming pancreatitis (19 chronic alcohol-related pancreatitis, 15 chronic autoimmune pancreatitis, 1 genetic pancreatitis) and 138 patients affected by pancreatic tumors (78 males, 60 females, mean age 62.4 years). A total of 173 pancreatic masses were enrolled. All the pancreatic masses underwent cytological or histological diagnosis. All the patients with cytological diagnosis of pancreatitis were followed up at least for one year. All CEUS examinations were performed by radiologists on a Sequoia 512 6.0 (Acuson, Mountain View, CA, USA) ultrasound system, with harmonic microbubble-specific imaging with low acoustic ultrasound pressure (2-4 MHz Coherent Contrast Imaging or Cadence Contrast Pulse Sequencing; Mechanical Index 0.2; 12-13 frames/s). A 2.4 mL bolus of a second-generation contrast medium, SonoVue® (Bracco, Milan, Italy), was intravenously injected, followed by a 5 mL bolus of saline solution. All CEUS examinations were performed by the same radiologist and recorded on videotape/VHS or magneto optical disk/MOD systems to have the possibility to immediately review the dynamic study. Insonation of the pancreatic lesion was continuous with dynamic observation of the shift from the unenhanced phase to the contrast-enhanced phase. The enhancement pattern of the lesions was compared to that of the adjacent normal parenchyma. Definition of the arterial phase is possible when observing hyperechogenity of the aorta or other big perilesional arteries. The venous phase is defined when the splenomesenteric-portal tree becomes hyperechoic. The lesions were classified according to the lesional enhancement in the enhanced phases as hypovascular/hypoechoic (lesions almost without enhancement or with enhancement lower than that of the adjacent parenchyma), isovascular/isoechoic (lesions with slight continuous enhancement or enhancement similar to that of the adjacent parenchyma) and hypervascular/hyperechoic (lesions with bright enhancement or enhancement superior to that of the adjacent parenchyma). The presence of a slight continuous enhancement inside the pancreatic masses, isovascular with the adjacent pancreatic parenchyma, was defined as “parenchymographic enhancement”. We evaluated the results of CEUS in the study of the 173 pancreatic masses considering the possibilities of a differential diagnosis between mass-forming pancreatitis and pancreatic tumor by identifying the parenchymographic enhancement during the dynamic phase of CEUS, which was considered diagnostic for mass-forming pancreatitis. The reports of all the CEUS examinations were reviewed retrospectively, but all the CEUS studies had been interpreted in a prospective manner by the attending radiologist and so utilized for the data analysis. The sensitivity, specificity, positive and negative predictive values and diagnostic accuracy of CEUS in the characterization of the pancreatic masses were then calculated. Moreover, to compare baseline US and CEUS, each mass-forming pancreatitis was evaluated at baseline US and at CEUS with a 3 level diagnostic score: 0 = absence of malignancy (isoechoic lesions); 1 = indeterminate (hyperechoic lesions); and 2 = presence of malignancy (hypoechoic lesions). Diagnostic confidences of baseline US and of CEUS in the characterization of mass-forming pancreatitis were represented by means of the ROC (receiver operating characteristic) curves; diagnostic advantage of CEUS on baseline US was calculated by comparing the areas under the ROC curves obtained by using a computer software package (Analise-it; Analise-it-software, Leeds, England).
RESULTS
All the contrast-enhanced ultrasound examinations were technically adequate allowing the dynamic observation of the shift from the unenhanced to the contrast-enhanced arterial and venous phases.
Pancreatic tumors
Pancreatic carcinomas were hypoechoic to the adjacent parenchyma at CEUS during the dynamic phases in 91% (126/138) of the cases (Figure 1), while hyperechoic and isoechoic in 7% (9/138) and 2% (3/138) of the cases, respectively.
Figure 1.
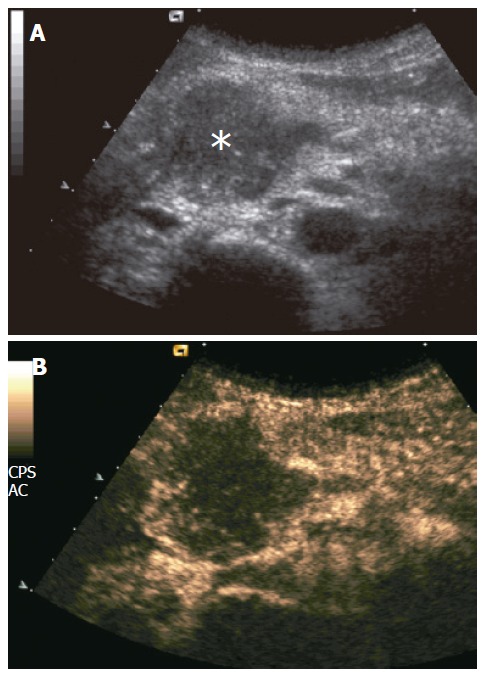
Ductal adenocarcinoma. A: US showing slightly hypoechoic pancreatic head mass (asterisk); B: CEUS showing poor enhancement of the mass, appearing hypoechoic to the rest of pancreatic parenchyma in the contrast-enhanced phases.
Mass-forming pancreatitis
Mass-forming pancreatitis involved diffusely the pancreatic parenchyma in 8.5% (10/35) of the cases, while 71.5% (25/35) of the cases were focally localized. Of the 25 focally localized cases, 16 were at the pancreatic head, 5 at the uncinate process and 4 at the pancreatic body. The main pancreatic duct was dilated in 10 cases, the common bile duct was dilated in 11, and dilation of both ducts was observed in 6 cases. Lesional calcifications were observed in 5 (14%) patients. One patient was positive for the SPINK-1 genetic marker. Five patients had significantly higher levels of serum tumoral markers (CA 125 in 1 and CA 19-9 in 4 cases). At CEUS, 94% of the mass-forming pancreatitis showed intralesional glandular parenchymography (Figure 2), while 6% of the mass-forming pancreatitis remained hypoechoic during the dynamic phase. CEUS allowed diagnosis of mass-forming pancreatitis, assuming isoechogenicity as significant for pancreatitis, with sensitivity of 88.6%, specificity of 97.8%, positive predictive value of 91.2%, negative predictive value of 97.1%, and overall accuracy of 96%. The presence of parenchymographic enhancement or hypoechogenicity in the examined pancreatic masses in the dynamic phases of CEUS significantly increased the diagnostic confidence in the differential diagnosis between mass-forming pancreatitis and pancreatic tumor, with receiver operating characteristic curve areas from 0.557 (P = 0.1608) for baseline US to 0.956 (P < 0.0001) for CEUS (Figure 3).
Figure 2.
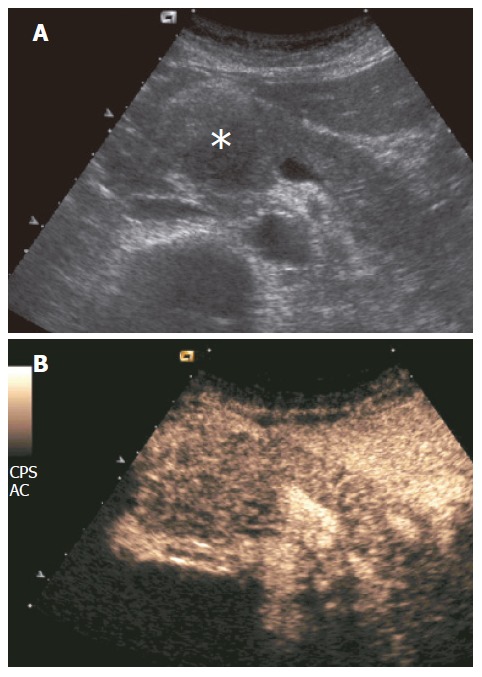
Mass-forming chronic autoimmune pancreatitis. A: US showing hypoechoic head pancreatic mass (asterisk); B: CEUS showing parenchymographic enhancement of the pancreatic lesion in the head of the pancreas during the contrast-enhanced phases.
Figure 3.
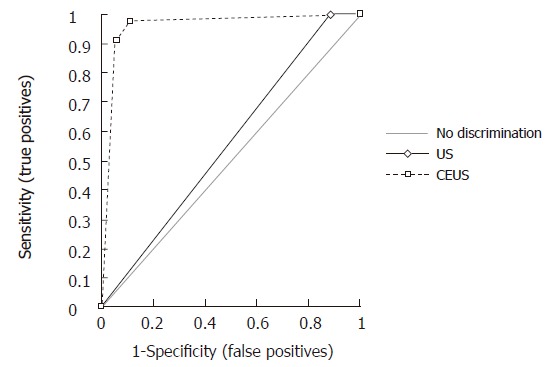
Receiver operating characteristic curves of baseline ultrasound and contrast-enhanced ultrasound in the characterization of 173 pancreatic masses, discerning between benignancy (pancreatitis) and malignancy (tumor).
DISCUSSION
Mass-forming pancreatitis is caused by various etiopathogenetic factors. However, at least two distinct categories have been recognized: alcohol-induced and autoimmune-related[9]. Differential diagnosis between mass-forming pancreatitis and pancreatic tumor is a crucial point for the correct management of patients affected by pancreatic masses. However, differential diagnosis can be difficult in clinical practice[10,11]. In fact, mass-forming pancreatitis and pancreatic tumors may present with the same symptoms and signs[2]. Several clinical and imaging features have been proposed to be helpful for the differential diagnosis. In case of mass-forming chronic pancreatitis, the presence of small calcifications in the lesion may suggest its inflammatory nature[12], but is surely poorly specific. The identification of perfusion features similar to those of the normal pancreatic parenchyma (i.e., glandular parenchymography) is strongly suggestive of inflammatory mass, while ductal carcinomas typically display a low/absent enhancement due to the scirrhous content of this tumor. Contrast-enhanced ultrasonography (CEUS) has recently been used for pancreatic lesions study with good results[6,13]. The CEUS finding consistent with an inflammatory origin of a pancreatic mass is the presence of a parenchymographic enhancement, defined as slight continuous enhancement inside the pancreatic mass with isovascularity to the adjacent pancreatic parenchyma. In this study, considering the presence of this sign, CEUS diagnosis of mass-forming pancreatitis was possible with sensitivity of 88.6%, specificity of 97.8%, positive predictive value of 91.2%, negative predictive value of 97.1% and an overall diagnostic accuracy of 96%. The intensity of this parenchymographic enhancement is surely related to the length of the underlying inflammatory process. It has been observed that the more the inflammatory process is chronic and long-standing, the less intense is the intralesional parenchymography, probably in relation to the entity of the associated fibrosis. As opposed to this, in mass-forming pancreatitis of more recent onset, the enhancement is usually more intense and prolonged[5]. The characteristic findings of autoimmune pancreatitis have been well defined: increased levels of serum gammaglobulin or IgG; presence of autoantibodies; enlargement of the pancreas; diffusely irregular narrowing of the main pancreatic duct and occasional stenosis of the intrapancreatic bile duct; fibrotic changes with lymphocyte infiltration; absent or mild symptoms; rare pancreatic calcifications and cysts; occasional association with other autoimmune diseases and effective steroid therapy[14]. Autoimmune pancreatitis is also reported to be characterized by periductal flogosis, mainly sustained by lymphocytic infiltration, with evolution to fibrosis[3,4]. The exact incidence and prevalence of this disease are not known, but previous studies have shown a male preponderance (ratio of 2:1) and a predominant involvement of the elderly age group[15]. The association with other autoimmune diseases has been reported, although data about the exact incidence of this association are not available[14]; the most commonly associated diseases are Sjogren syndrome, diabetes mellitus, inflammatory bowel disease (especially Crohn’s disease), primary biliary cirrhosis, primary sclerosing cholangitis and systemic lupus erythematosus[15,16]. Response to steroid therapy is reported in the literature, but dosage and duration of the therapy are not standardized. However, pancreatitis is the most common benign condition that mimics pancreatic neoplasm, and in recent experience at Johns Hopkins, lymphoplasmacytic sclerosing pancreatitis is the most common form of pancreatitis in patients who are subjected to pancreaticduodenectomy for suspected neoplasm[17]. Ultrasonographic features of autoimmune pancreatitis are very similar to those of focal pancreatitis, even though autoimmune pancreatitis may interest more frequently the entire gland or present a larger extension and ubiquitous localization. US findings are characteristic in the diffuse form when the entire gland is involved. Echogenicity is markedly reduced, gland volume is increased and Wirsung duct is compressed by glandular parenchyma. Focal autoimmune pancreatitis at the pancreatic head is often characterized by the sole dilation of the common bile duct[8]. The vascularization of autoimmune pancreatitis can be demonstrated by CEUS, which shows most often a moderate or marked[8] enhancement in the early contrast-enhanced phase, though inhomogeneous due to the thinning of the glandular vessels as the consequence of the thick lymphocytic infiltration and fibrosis. In our study, parenchymographic enhancement, defined as slight continuous enhancement inside the pancreatic masses with isovascularity to the adjacent pancreatic parenchyma, was shown in 94% of the mass-forming pancreatitis. These CEUS findings have been reported to be especially useful in the study of focal forms of autoimmune chronic pancreatitis, in which differential diagnosis with ductal carcinoma is a priority[11]. The ability of EUS as well other imaging modalities to differentiate pancreatic cancer from pseudotumorous chronic pancreatitis is reported in literature[18]. Our data suggests that contrast-enhanced ultrasonography should be used in the characterization of pancreatic masses as complementary to CT and MRI. The sensitivity of CEUS in the identification of inflammatory masses allows to propose to obtain a diagnosis with fine needle percutaneous cytology in pancreatic focal masses that show glandular parenchymography at the examination. However, our study is surely limited by the retrospective evaluation.
In conclusion, CEUS allows diagnosis of mass-forming pancreatitis with diagnostic accuracy of 96%. CEUS significantly increases the diagnostic confidence in respect to basal US in discerning mass-forming pancreatitis from pancreatic neoplasm.
Footnotes
S- Editor Wang J L- Editor Kumar M E- Editor Liu Y
References
- 1.Kim T, Murakami T, Takamura M, Hori M, Takahashi S, Nakamori S, Sakon M, Tanji Y, Wakasa K, Nakamura H. Pancreatic mass due to chronic pancreatitis: correlation of CT and MR imaging features with pathologic findings. AJR Am J Roentgenol. 2001;177:367–371. doi: 10.2214/ajr.177.2.1770367. [DOI] [PubMed] [Google Scholar]
- 2.van Gulik TM, Reeders JW, Bosma A, Moojen TM, Smits NJ, Allema JH, Rauws EA, Offerhaus GJ, Obertop H, Gouma DJ. Incidence and clinical findings of benign, inflammatory disease in patients resected for presumed pancreatic head cancer. Gastrointest Endosc. 1997;46:417–423. doi: 10.1016/s0016-5107(97)70034-8. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa N, Muranaka T, Yasumori K, Matsubayashi R, Hayashida K, Arita Y. Autoimmune pancreatitis: radiologic findings in three histologically proven cases. J Comput Assist Tomogr. 1998;22:880–883. doi: 10.1097/00004728-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Koito K, Namieno T, Nagakawa T, Morita K. Inflammatory pancreatic masses: differentiation from ductal carcinomas with contrast-enhanced sonography using carbon dioxide microbubbles. AJR Am J Roentgenol. 1997;169:1263–1267. doi: 10.2214/ajr.169.5.9353439. [DOI] [PubMed] [Google Scholar]
- 6.D'Onofrio M, Malagò R, Zamboni G, Vasori S, Falconi M, Capelli P, Mansueto G. Contrast-enhanced ultrasonography better identifies pancreatic tumor vascularization than helical CT. Pancreatology. 2005;5:398–402. doi: 10.1159/000086540. [DOI] [PubMed] [Google Scholar]
- 7.Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, Nakaoka R, Kawasaki T. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut. 2004;53:854–859. doi: 10.1136/gut.2003.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Numata K, Ozawa Y, Kobayashi N, Kubota T, Akinori N, Nakatani Y, Sugimori K, Imada T, Tanaka K. Contrast-enhanced sonography of autoimmune pancreatitis: comparison with pathologic findings. J Ultrasound Med. 2004;23:199–206. doi: 10.7863/jum.2004.23.2.199. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi T, Kawaura Y, Satomura Y, Watanabe H, Motoo Y, Okai T, Sawabu N. Clinical and imaging features of autoimmune pancreatitis with focal pancreatic swelling or mass formation: comparison with so-called tumor-forming pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2679–2687. doi: 10.1111/j.1572-0241.2003.08727.x. [DOI] [PubMed] [Google Scholar]
- 10.Kamisawa T, Egawa N, Nakajima H. Autoimmune pancreatitis is a systemic autoimmune disease. Am J Gastroenterol. 2003;98:2811–2812. doi: 10.1111/j.1572-0241.2003.08758.x. [DOI] [PubMed] [Google Scholar]
- 11.Koga Y, Yamaguchi K, Sugitani A, Chijiiwa K, Tanaka M. Autoimmune pancreatitis starting as a localized form. J Gastroenterol. 2002;37:133–137. doi: 10.1007/s005350200009. [DOI] [PubMed] [Google Scholar]
- 12.Remer EM, Baker ME. Imaging of chronic pancreatitis. Radiol Clin North Am. 2002;40:1229–1242, v. doi: 10.1016/s0033-8389(02)00044-1. [DOI] [PubMed] [Google Scholar]
- 13.D'Onofrio M, Mansueto G, Falconi M, Procacci C. Neuroendocrine pancreatic tumor: value of contrast enhanced ultrasonography. Abdom Imaging. 2004;29:246–258. doi: 10.1007/s00261-003-0097-8. [DOI] [PubMed] [Google Scholar]
- 14.Okazaki K, Chiba T. Autoimmune related pancreatitis. Gut. 2002;51:1–4. doi: 10.1136/gut.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klöppel G, Lüttges J, Löhr M, Zamboni G, Longnecker D. Autoimmune pancreatitis: pathological, clinical, and immunological features. Pancreas. 2003;27:14–19. doi: 10.1097/00006676-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki K. Autoimmune pancreatitis: etiology, pathogenesis, clinical findings and treatment. The Japanese experience. JOP. 2005;6:89–96. [PubMed] [Google Scholar]
- 17.Kawamoto S, Siegelman SS, Hruban RH, Fishman EK. Lymphoplasmacytic sclerosing pancreatitis with obstructive jaundice: CT and pathology features. AJR Am J Roentgenol. 2004;183:915–921. doi: 10.2214/ajr.183.4.1830915. [DOI] [PubMed] [Google Scholar]
- 18.Dancygier H, Lightdale CJ. Endoscopic ultrasonography of the upper gastrointestinal tract and colon. In: Stevens PD, ed , editors. Endosonography in Gastroenterology: principles, Techniques, Findings. New York: Thieme;; 1999. pp. 13–173. [Google Scholar]


