Abstract
AIM: To study the anti-tumor effect of caffeic acid phenethyl ester (CAPE) and the influence of CAPE on β-catenin associated signaling pathway in SW480 colorectal cancer (CRC) cells.
METHODS: SW480 cells were treated with CAPE at serial concentrations. The proliferative status of cells was measured by methabenzthiazuron (MTT) assay. Cell cycle and cell apoptosis were analyzed using flow cytometry (FCM). Western blotting assay was used to evaluate the protein level of β-catenin, c-myc and cyclinD1. β-catenin localization was determined by indirect immunofluorescence.
RESULTS: CAPE displayed a strong inhibitory effect in a significant dose- and time-dependent manner on SW480 cell growth. FCM analysis showed that the ratio of G0 /G1 phase cells increased, S phase ratio decreased and apoptosis rate increased after SW480 cells were exposed to CAPE for 24 h. Pretreatment of SW480 cells with CAPE significantly suppressed β-catenin, c-myc and cyclinD1 protein expression. CAPE treatment was associated with decreased accumulation of β-catenin protein in nucleus and cytoplasm, and concurrently increased its accumulation on the surface of cell membrane.
CONCLUSION: CAPE can inhibit SW480 cell proliferation by inducing cell cycle arrest and apoptosis. Decreased β-catenin and the associated signaling pathway target gene expression may mediate the anti-tumor effects of CAPE.
Keywords: Caffeic acid phenethyl ester, Colorectal cancer, Proliferation, β-catenin, Signaling pathway
INTRODUCTION
Caffeic acid phenethyl ester (CAPE) is a phenolic antioxidant. As an active component of propolis, CAPE has many biological and pharmacological functions including immunoregulation, anti-inflammatory, antiviral, antibacterial and antitumor activities. Several studies have demonstrated that CAPE has antiproliferative effect, apoptosis-inducing effect in various tumor cells in vitro[1-4] and in vivo[5,6]. CAPE also inhibited the development of azoxymethane-induced aberrant crypts in the colon of rats[7]. Furthermore, a number of studies reported that CAPE elicits apoptosis and suppresses the growth of transformed cells, and the cytotoxicity of CAPE to transformed cells is sensitive and selective[8-10]. Multiple molecular mechanisms seem to be involved in the tumor suppressive effects of CAPE. It was reported that CAPE could inhibit NFκB and induce apoptosis via Fas signal activation in human breast cancer MCF-7 cells[11]. Additionally, Carrasco et al[12] found an 85% decrease in nuclear localization of the p65 subunit of NF-kappa B. While in C6 glioma cells, the tumor suppressor proteins P53 and p38 mitogen-activated protein kinase (p38 MAPK) are involved in CAPE-induced apoptotic cell death[13].
Colorectal cancer (CRC) is the third most common cancer and the fourth most frequent cause of cancer death worldwide. Recent studies have indicated that in most of CRC, there are aberrant β-catenin associated signaling pathways[14]. The β-catenin associated signaling pathway plays critical roles in cell proliferation and differentiation, and the β-catenin is the central component in the pathway. Aberrant β-catenin associated signaling pathway, which generally results from inactivating mutation of adenomatous polyposis coli (APC) or activating mutation of β-catenin, leads to the accumulation of β-catenin in the nucleus of cells, which is subsequently complexed with T-cell factor (TCF) and promotes transcription of a variety of target genes, such as c-myc, cyclinD1, ultimately leading to cell aberrant proliferation and tumor formation. Dysregulation of β-catenin associated signaling and hence β-catenin expression is believed to be central to the early stages of sporadic colorectal carcinogenesis in humans[15-17]. Therefore, control of β-catenin and/or control of the downstream target gene expression represents an opportunity for rational colorectal cancer therapy.
Here we report the anti-tumor effect of CAPE in association with β-catenin associated signaling in SW480 colorectal cancer cells. These studies have important implications for CAPE to be used as a potential therapeutic agent for colorectal cancer.
MATERIALS AND METHODS
Chemicals
CAPE, dimethyl sulphoxide (DMSO), PI, metha-benzthiazuron (MTT), mouse anti-human β-catenin, c-myc, cyclinD1 and β-actin monoclonal antibody were purchased from Sigma-Aldrich (USA). RPMI-1640 medium, fetal calf serum and trypsin-EDTA were purchased from Hyclone (USA). FITC and horseradish peroxidase-conjugated secondary antibody were obtained from Pierce Biotechnology (USA). Annexin-V and PI double staining kit and Tripure were purchased from Roche (Germany). Protein assay kits were obtained from Bio-Rad Labs (USA). Enhanced chemiluminescence (ECL) system was purchased from Amersham Life Science (UK). CAPE was dissolved with DMSO and adjusted to final concentrations with culture medium prior to use.
Cell culture
The human CRC cell line SW480 was purchased from the American Type Culture Collection (ATCC). The cells were cultured in RPMI-1640 medium supplemented with penicillin G (100 U/mL), streptomycin (100 μg/mL) and 10% fetal calf serum. Cells were grown at 37°C in a humidified atmosphere of 50 mL/L CO2 and were routinely sub-cultured using 0.25% (w/v) trypsin-EDTA solution.
MTT assay
The logarithmically growing SW480 cells were plated into a 96-well plate at a density of 4 × 103 cells/well. After 24 h, the cells were treated with CAPE at designated concentrations (2.5, 5, 10, 20, 40 and 80 mg/L) for 24, 48, 72 and 96 h, respectively. Control wells were treated with 0.1% DMSO alone. Then, 20 μL MTT (5 g/L) was added to each well and incubated for an additional 4 h, and then culture media were discarded followed by addition of 0.15 mL DMSO and vibration for 10 min. The absorbance was measured at 490 nm. The inhibitory rates (IR) were calculated as follows: IR (%) = [(1-absorbance of the treated wells)/(absorbance of the control wells) ] × 100%. The 50% inhibitory concentration (IC50) value was determined using CalcuSyn software.
Flow cytometry analysis
Cell density was adjusted to 0.3-1.0 × 107 cells/mL. Cells were serum-starved for 24 h and then treated with different concentrations of CAPE (2.5, 5 and 10 mg/L) for 24 h. Then the cells were harvested with trypsin-EDTA to produce a single cell suspension. The cells were pelleted by centrifugation and washed twice with phosphate-buffered saline (PBS). Then, the cell pellets were resuspended in 0.5 mL PBS and fixed in 5 mL ice-cold 70% ethanol at 4°C. The fixed cells were spun down by centrifugation and the pellets were washed with PBS. After resuspension with 1 mL PBS, the cells were incubated with RNase A (20 mg/L) and PI (50 mg/L) and shaken for 1 h at 37°C in the dark. The stained cells were analyzed using a FACScan flow cytometer in combination with BD analysis II software (Becton Dickinson).
Annexin-V and PI double-staining flow cytometry analysis
The cells were treated and harvested in the same way as mentioned above. Analysis of apoptosis was performed using the Annexin-V and PI double staining kit according to the manufacturer’s protocol. After treatment with CAPE, the cells were immediately analyzed by flow cytometry. The apoptotic cells were localized in the right lower quadrant of a dot-plot graph by using Annexin-V-fluorescein versus PI.
Western blot analysis
Cells were placed in serum-free medium with or without CAPE (2.5, 5 and 10 mg/L) for 24 and 48 h, then were lysed in solubilization buffer containing sodium dodecyl sulfate(SDS) and β-mercaptoethanol. After centrifugation, the supernatant was collected and the protein concentration determined by the BioRad DC kit. Equal amounts of protein (50 μg) in each sample were resolved in 10% SDS polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane. After blocked with 2% skim milk, the membranes were incubated with primary antibodies at appropriate dilution (β-actin, 1/1000; β-catenin, c-myc and cyclinD1, 1/500) overnight at 4°C. Horseradish peroxidase-conjugated secondary antibody was diluted 1/5000 and incubated for 1 h at 20°C. Immunoreactive proteins were detected with an ECL system, and light emission was captured on Kodak X-ray films. Product bands were quantitated by scanning densitometry.
Indirect immunofluorescence
SW480 cells grown on glass coverslips were treated for 48 h with CAPE (2.5, 5 and 10 mg/L) or an equivalent dilution of DMSO, under standard culture conditions as described above. Then cells were washed with PBS and fixed with methanol for 20 min. Incubation with monoclonal anti-β-catenin antibody (1:200) was carried out overnight at 4°C. This was followed by incubation with FITC-conjugated secondary antibody (1:200) for 1 h at room temperature. Omission of the primary antibody was used as a negative control. Images were collected using laser scanning confocal microscopy.
Statistical analysis
Data were expressed as mean ± SD. Analysis of data was performed using one-way ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Effect of CAPE on cell proliferation
SW480 cells were treated with various concentrations (2.5, 5, 10, 20, 40 and 80 mg/L) of CAPE for 24, 48, 72 and 96 h, respectively. Then the cell viability was measured by MTT assay. CAPE showed a significant dose-dependent and time-dependent inhibition of cell growth (Figure 1). The IC50 value for CAPE at 24, 48, 72 and 96 h after treatment was 20.27, 11.38, 6.15 and 5.44 mg/L, respectively.
Figure 1.
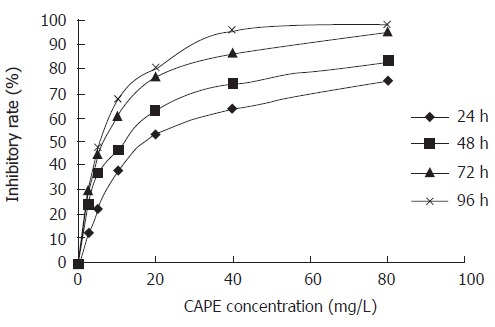
Effect of CAPE on SW480 cell proliferation. SW480 cells were treated with CAPE at indicated concentrations for 24, 48, 72 and 96 h. The cell viability was determined by MTT assay.
Effects of CAPE on cell cycle progression
In order to examine the effects of CAPE on cell cycle progression, SW480 cells were treated with designated concentrations of CAPE for 24 h. The distributions of cells in each of the cell cycle phases were determined by FCM. As shown in Figures 2 and 3, the percentage of cell population of the G0/G1 phase significantly increased, while those in S and G2/M phase decreased. CAPE caused cell cycle arrest in G0/G1 phase in a dose-dependent manner.
Figure 2.
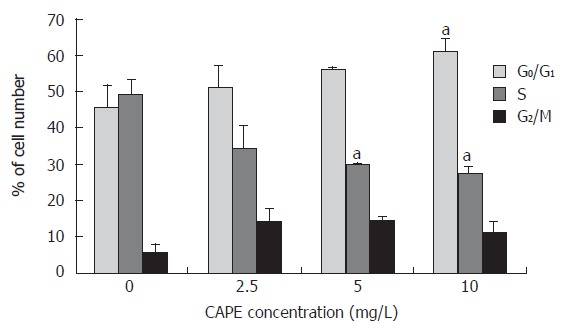
Effect of CAPE on SW480 cell cycle distribution. The percentage of cells in each phase of the cell cycle, G0/G1, S, and G2/M was determined by FCM after cells were treated with indicated concentrations (2.5, 5 and 10 mg/L) of CAPE for 24 h. aP < 0.05 vs the control group, treated with vehicle, DMSO only.
Figure 3.
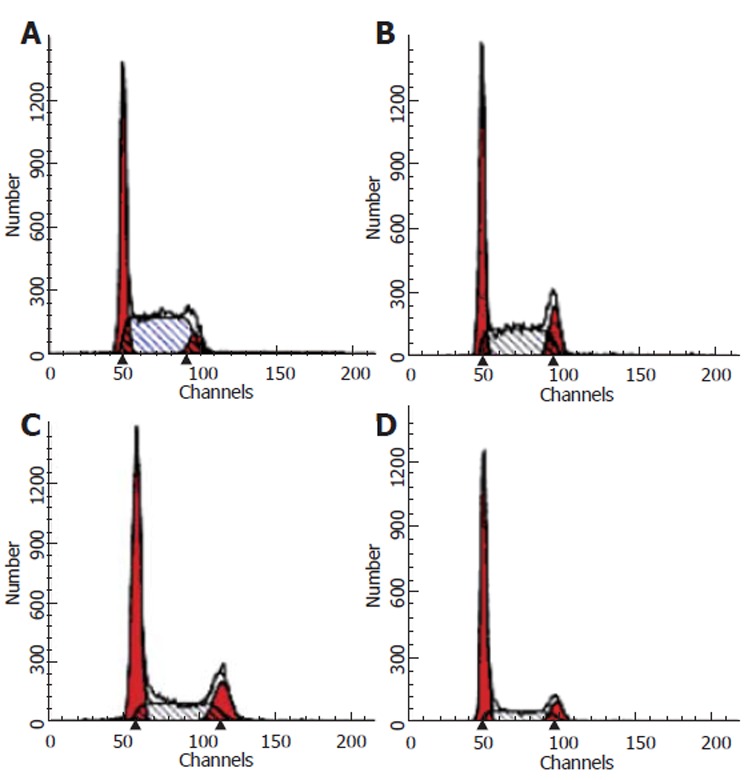
Flow cytometry analysis of SW480 cells following 24 h incubation with CAPE (A) 0 mg/L, (B) 2.5 mg/L, (C) 5 .0 mg/L, (D) 10 mg/L.
Effect of CAPE on apoptosis
After SW480 cells were exposed to CAPE at various concentrations (2.5, 5 and 10 mg/L) for 24 h, Annexin-V and PI double-staining flow cytometry analysis showed that the apoptosis rates were 9.1% ± 0.7%, 15.23% ± 0.6% and 21.1% ± 1.44%, respectively, which were significantly higher than that in the control group (3.7% ± 0.9%). CAPE induced apoptosis of SW480 cells in a dose-dependent manner (Figures 4 and 5).
Figure 4.
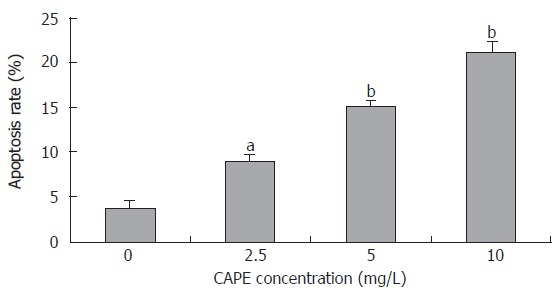
Effect of CAPE on SW480 cell apoptosis. After treated with CAPE at indicated concentrations for 24 h, the SW480 cell apoptosis rates were analyzed by Annexin-V and PI double-staining flow cytometry. aP < 0.05, bP < 0.01 vs the control group.
Figure 5.
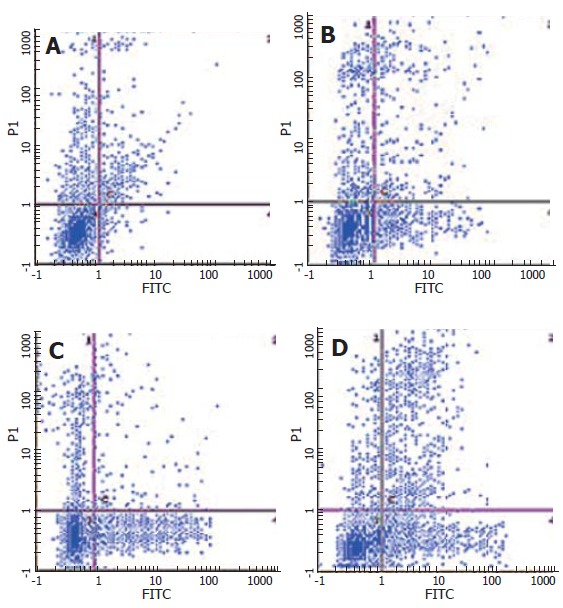
Annexin-V and PI double-staining flow cytometry analysis of SW480 cells following 24 h incubation with CAPE (A) 0 mg/L, (B) 2.5 mg/L, (C) 5.0 mg/L, (D) 10 mg/L.
Effect of CAPE on the expression of β-catenin, c-myc and cyclinD1
To investigate the mechanisms underlying CAPE induced apoptosis, we examined the effect of CAPE on the expression of β-catenin signaling pathway related genes. After SW480 cells were exposed to CAPE (2.5, 5 and 10 mg/L) for 24 and 48 h, Western blot analysis showed that CAPE significantly suppressed β-catenin protein expression in a dose and time-dependent manner. CAPE treatment after 48 h markedly decreased the expression of c-myc and cyclin D1, two of the β-catenin associated signaling pathway target genes (Figure 6).
Figure 6.
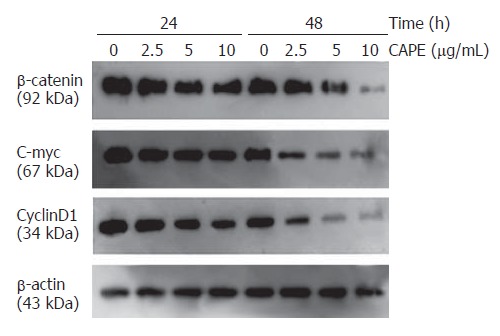
Western blot analysis of β-catenin, c-myc and cyclinD1 protein expression in SW480 cells following 24 and 48 h incubation with indicated concentrations of CAPE. β-actin served as a loading control.
Indirect immunofluorescence studies of β-catenin localization in SW480 cells revealed that CAPE treatment decreased the accumulation of β-catenin protein in nucleus and cytoplasm, and concurrently increased its accumulation on the surface of cell membrane (Figure 7).
Figure 7.
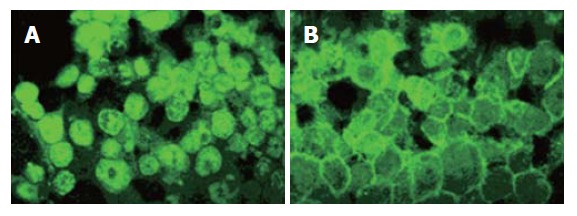
Indirect immunofluorescence analysis of β-catenin protein localization in SW480 cells following 48 h incubation with CAPE (A) 0 mg/L, (B) 10 mg/L.
DISCUSSION
Propolis has been used since ancient times in folk medicine. Researches indicate that propolis exhibits immunoregulation, anti-bacterial, anti-inflammatory and anti-tumor activities[18-20]. As an active component of propolis, CAPE is receiving much attention in the medical research community because of its potential for the treatment of a number of disorders, including cancer. Recent studies demonstrated that CAPE has antiproliferative and apoptosis-inducing effects in various tumor cells. An in vivo study showed that CAPE decreased the growth of the xenografts of C6 glioma cells in nude mice[6]. Also, dietary intake of CAPE decreased tumor formation in the enterocytes of the Min/+ mouse[21].
In the present study, we investigated the effect of CAPE on cell proliferation, cell cycle and apoptosis of SW480 CRC cells. Our data demonstrated that CAPE treatment could significantly inhibit cell growth in a dose- and time-dependent manner, along with induction of G0/G1 arrest and apoptosis in SW480 cells.
Multiple molecular mechanisms seem to be involved in the tumor suppressive effect of CAPE. Recently, it was reported that CAPE could inhibit NFкB, inducing apoptosis via Fas signal activation in human tumor cells[11,12]. Hung et al[22] reported that down-regulation of Mcl-1 gene expression and activation of caspase-8 are associated with CAPE-triggered cell apoptosis. Moreover, tumor suppressor proteins P53 and p38 MAPK play a prominent role in the CAPE-induced apoptotic cell death in C6 glioma cells, which might contribute to the anti-tumor effect in these cells[13].
Our results suggest that the anti-tumor effects of CAPE on SW480 cells are associated with down-regulation of the β-catenin associated signaling pathways. β-catenin plays a dual role in cells. It is a structural component of cell-cell adherent junctions as well as a key player in β-catenin associated signaling pathway[23]. The signaling function of β-catenin is particularly important in colorectal cancer since mutations of APC or tumor-associated mutations of β-catenin lead to its stabilization[24,25]. Here we showed that CAPE treatment inhibited β-catenin in a dose-dependent manner in SW480 cells. More interestingly, CAPE changed the localization of β-catenin in cells. As shown in Figure 7, nuclear accumulated β-catenin in SW480 cells mainly relocalized on the cell membrane at cell-cell contacts after CAPE treatment. Two of target genes of the activated β-catenin associated signaling pathways have been reported to be c-myc and cyclin D1[26,27]. Oncogenes c-myc and cyclinD1 are a kind of effector protein of the karyomitosis signal, which can trigger and regulate the transcription of the genes related with proliferation. They are frequently overexpressed in several human tumors, including colorectal cancer. Our results showed that CAPE treatment markedly decreased the expression of c-myc and cyclinD1. This indicates that CAPE induced cell cycle arrest and apoptosis might be associated with down-regulation of β-catenin associated signaling pathways.
In summary, we have shown that CAPE can inhibit the growth of human SW480 colorectal cancer cells by inducing cell cycle arrest and apoptosis. Decreased β-catenin and the β-catenin associated signaling pathway target gene expression might mediate the anti-tumor effects of CAPE. These findings have strong implications for CAPE as a potential therapeutic agent for colorectal cancer.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30100228
S- Editor Wang J L- Editor Zhu LH E- Editor Ma WH
References
- 1.Chen YJ, Shiao MS, Wang SY. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs. 2001;12:143–149. doi: 10.1097/00001813-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Lee YJ, Liao PH, Chen WK, Yang CY. Preferential cytotoxicity of caffeic acid phenethyl ester analogues on oral cancer cells. Cancer Lett. 2000;153:51–56. doi: 10.1016/s0304-3835(00)00389-x. [DOI] [PubMed] [Google Scholar]
- 3.Usia T, Banskota AH, Tezuka Y, Midorikawa K, Matsushige K, Kadota S. Constituents of Chinese propolis and their antiproliferative activities. J Nat Prod. 2002;65:673–676. doi: 10.1021/np010486c. [DOI] [PubMed] [Google Scholar]
- 4.Nagaoka T, Banskota AH, Tezuka Y, Saiki I, Kadota S. Selective antiproliferative activity of caffeic acid phenethyl ester analogues on highly liver-metastatic murine colon 26-L5 carcinoma cell line. Bioorg Med Chem. 2002;10:3351–3359. doi: 10.1016/s0968-0896(02)00138-4. [DOI] [PubMed] [Google Scholar]
- 5.Orsolić N, Terzić S, Mihaljević Z, Sver L, Basić I. Effects of local administration of propolis and its polyphenolic compounds on tumor formation and growth. Biol Pharm Bull. 2005;28:1928–1933. doi: 10.1248/bpb.28.1928. [DOI] [PubMed] [Google Scholar]
- 6.Kuo HC, Kuo WH, Lee YJ, Lin WL, Chou FP, Tseng TH. Inhibitory effect of caffeic acid phenethyl ester on the growth of C6 glioma cells in vitro and in vivo. Cancer Lett. 2006;234:199–208. doi: 10.1016/j.canlet.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 7.Borrelli F, Izzo AA, Di Carlo G, Maffia P, Russo A, Maiello FM, Capasso F, Mascolo N. Effect of a propolis extract and caffeic acid phenethyl ester on formation of aberrant crypt foci and tumors in the rat colon. Fitoterapia. 2002;73 Suppl 1:S38–S43. doi: 10.1016/s0367-326x(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 8.Chiao C, Carothers AM, Grunberger D, Solomon G, Preston GA, Barrett JC. Apoptosis and altered redox state induced by caffeic acid phenethyl ester (CAPE) in transformed rat fibroblast cells. Cancer Res. 1995;55:3576–3583. [PubMed] [Google Scholar]
- 9.Liao HF, Chen YY, Liu JJ, Hsu ML, Shieh HJ, Liao HJ, Shieh CJ, Shiao MS, Chen YJ. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J Agric Food Chem. 2003;51:7907–7912. doi: 10.1021/jf034729d. [DOI] [PubMed] [Google Scholar]
- 10.Lee YT, Don MJ, Hung PS, Shen YC, Lo YS, Chang KW, Chen CF, Ho LK. Cytotoxicity of phenolic acid phenethyl esters on oral cancer cells. Cancer Lett. 2005;223:19–25. doi: 10.1016/j.canlet.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 11.Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFkappaB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem. 2004;279:6017–6026. doi: 10.1074/jbc.M306040200. [DOI] [PubMed] [Google Scholar]
- 12.Carrasco-Legleu CE, Márquez-Rosado L, Fattel-Fazenda S, Arce-Popoca E, Pérez-Carreón JI, Villa-Treviño S. Chemoprotective effect of caffeic acid phenethyl ester on promotion in a medium-term rat hepatocarcinogenesis assay. Int J Cancer. 2004;108:488–492. doi: 10.1002/ijc.11595. [DOI] [PubMed] [Google Scholar]
- 13.Lee YJ, Kuo HC, Chu CY, Wang CJ, Lin WC, Tseng TH. Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells. Biochem Pharmacol. 2003;66:2281–2289. doi: 10.1016/j.bcp.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 15.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 16.Behrens J. Control of beta-catenin signaling in tumor development. Ann N Y Acad Sci. 2000;910:21–33; discussion 33-5. doi: 10.1111/j.1749-6632.2000.tb06698.x. [DOI] [PubMed] [Google Scholar]
- 17.Behrens J, Lustig B. The Wnt connection to tumorigenesis. Int J Dev Biol. 2004;48:477–487. doi: 10.1387/ijdb.041815jb. [DOI] [PubMed] [Google Scholar]
- 18.Orsolić N, Saranović AB, Basić I. Direct and indirect mechanism(s) of antitumour activity of propolis and its polyphenolic compounds. Planta Med. 2006;72:20–27. doi: 10.1055/s-2005-873167. [DOI] [PubMed] [Google Scholar]
- 19.Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem Toxicol. 1998;36:347–363. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 20.Orsolić N, Knezević AH, Sver L, Terzić S, Basić I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J Ethnopharmacol. 2004;94:307–315. doi: 10.1016/j.jep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, Newmark HL, Bertagnolli MM. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 2000;21:921–927. doi: 10.1093/carcin/21.5.921. [DOI] [PubMed] [Google Scholar]
- 22.Hung MW, Shiao MS, Tsai LC, Chang GG, Chang TC. Apoptotic effect of caffeic acid phenethyl ester and its ester and amide analogues in human cervical cancer ME180 cells. Anticancer Res. 2003;23:4773–4780. [PubMed] [Google Scholar]
- 23.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 24.Bright-Thomas RM, Hargest R. APC, beta-Catenin and hTCF-4; an unholy trinity in the genesis of colorectal cancer. Eur J Surg Oncol. 2003;29:107–117. doi: 10.1053/ejso.2002.1331. [DOI] [PubMed] [Google Scholar]
- 25.Gottardi CJ, Wong E, Gumbiner BM. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J Cell Biol. 2001;153:1049–1060. doi: 10.1083/jcb.153.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karim R, Tse G, Putti T, Scolyer R, Lee S. The significance of the Wnt pathway in the pathology of human cancers. Pathology. 2004;36:120–128. doi: 10.1080/00313020410001671957. [DOI] [PubMed] [Google Scholar]


