Abstract
AIM: To study the role of an insertion/deletion polymorphism in the pepsinogen C (PGC) gene, an effective marker for terminal differentiation of the stomach mucosa, in the susceptibility to the development of gastric lesions.
METHODS: The study was performed with 99 samples of known gastric lesions and 127 samples without evidence of neoplastic disease. PCR was employed and the 6 polymorphic alleles were amplified: Allele 1 (510 bp), Allele 2 (480 bp), Allele 3/4 (450/460 bp), Allele 5 (400 bp) and Allele 6 (310 bp).
RESULTS: Our results revealed that Allele 6 carriers seemed to have protection against the development of any gastric lesion (OR = 0.34; P < 0.001), non-dysplastic lesions associated with gastric adenocarcinoma such as atrophy or intestinal metaplasia (OR = 0.28; P < 0.001) or invasive GC (OR = 0.39; P = 0.004).
CONCLUSION: Our study reveals that the Allele 6 carrier status has a protective role in the development of gastric lesions, probably due to its association with higher expression of PGC. Moreover, the frequency of Allele 6 carriers in the control group is far higher than that obtained in Asian populations, which might represent a genetic gap between Caucasian and Asian populations.
Keywords: Gastric adenocarcinoma, Pepsinogen C, Polymorphism
INTRODUCTION
Gastric adenocarcinoma (GC) is a major public health problem worldwide and the third cause of cancer-related mortality in Europe[1-3]. In Portugal, gastric cancer represents a sixth of all cancer related deaths, with twice the average mortality of European Union and the highest among Western European countries 25/100 000 and 12/100 000 persons × year in men and women, respectively[4,5]. Portuguese inhabitants show a life-time risk for gastric cancer of approximately 2% (95% CI 1.9-2.1), with half the decline observed at other European countries during the last decade[6].
Lauren’s classification is most commonly used for gastric adenocarcinoma because of its epidemiologic importance[2,7]. It defines two main morphological types: ‘diffuse type’ and ‘intestinal type’. The latter is characterized by a stepwise transformation from normal mucosa through atrophic gastritis, atrophy, intestinal metaplasia and dysplasia to invasive gastric adenocarcinoma[2,8]. This type shows a male:female ratio of 2:1 and it has been related mostly to environmental factors such as H pylori infection and diet[2,6].
However, recently several host genetic variations have been regarded as potential risk markers[9-18]. Pepsinogen is an effective marker of terminal differentiation of the stomach mucosa[19,20]. Pepsinogen C (PGC) is mainly secreted by chief cells of gastric gland, while also by cardiac, pyloric and Brunner glands. Its serum levels have been related with atrophic changes in gastric mucosa and extension of intestinal metaplasia[21,22]. The pepsinogen C (PGC) gene, localized on chromosome 6 between regions 6p11-6p21.3, encodes the PGC, also known as progastricsin, which is the precursor of pepsin C or gastricsin++[20]. Taggart et al have described an insertion/deletion polymorphism of about 100 bp located between exons 7 and 8[23-25], which was regarded as a susceptibility marker for the development of gastric adenocarcinoma[26]. However, the role of this polymorphism has not been completely established and it has never been measured in Caucasian populations.
The aim of this control study was to evaluate the role of the PGC polymorphism in the development of GC within a southern European population from the north region of Portugal.
MATERIALS AND METHODS
Patients
A cross-sectional study was performed among healthy individuals without clinical evidence of cancer (n = 127 as control group) and consecutive patients with known gastric lesions (n = 99), both from the northern region of Portugal attending at the Portuguese Institute of Oncology of Porto (Portugal).
Patients were further divided according to the type of lesions presented upon histopathological diagnosis after endoscopic multiple biopsies. It included patients who displayed lesions as severe as high-grade dysplasia and intestinal type invasive gastric adenocarcinoma (n = 52) and patients with non-dysplastic but associated lesions with gastric adenocarcinoma such as atrophy or intestinal metaplasia (n = 47), who belonged to a standardized follow-up since 2001.
All individuals included in this study gave their informed consent before their inclusion in the study, according to the Declaration of Helsinki.
Sample collection and DNA extraction
Blood samples were obtained with a standard venipuncture technique using EDTA containing tubes, and the genomic DNA was extracted from the white blood cell fraction of each sample, using a standard salting out protocol[27].
PGC polymorphism analysis
The analysis of PGC polymorphism (insertion/deletion of approximately 100 bp) located between exons 7 and 8 was carried out by polymerase chain reaction (PCR), as described by Yamagata et al[15]. The PCR reaction was performed with the antisense (PGCa: 5’-AGCCCTAAGCCTCTTTTTGG-3’) and sense primers (PGCb: 5’-GGCCAGATCTGCGTGTTTTA-3’) in a 50 μL PCR reaction mixture containing: 1 × Taq buffer, 1.5 mmol/L of MgCl2, 0.2 mmol/L of dNTPs, 0.25 μmol/L of each primer and 1 U Taq DNA polymerase (Amersham Bioscience, USA). Cycling parameters are: 95°C for 5 min for Taq DNA polymerase activation, followed by 35 cycles of denaturation for 60 s at 94°C, annealing for 60 s at 55°C, extension for 60 s at 72°C and a final extension step at 72°C for 7 min.
PCR amplification identified the following products: 510 bp (Allele 1), 480 bp (Allele 2), 460 bp (Allele 3), 450 bp (Allele 4), 400 bp (Allele 5) and 310 bp (Allele 6); which were analyzed by electrophoresis in a 3% MetaP® Agarose gel (Cambrex Bio Science Rockland Inc, USA) stained with 5% ethidium bromide (Figure 1).
Figure 1.
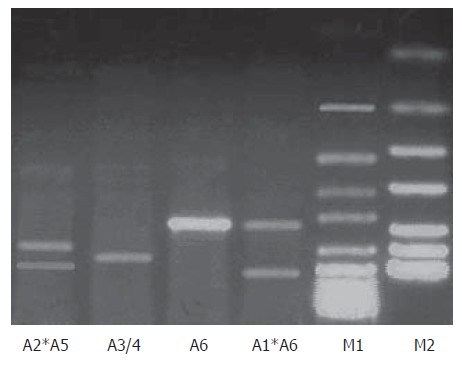
Analysis of PGC polymorphism by PCR. M1: 50 bp DNA ladder; M2: pUC DNA ladder; Allele 1 (510 bp); Allele 2 (480 bp); Allele 3/4 (450/460 bp); Allele 5 (400 bp) and Allele 6 (310 bp).
Variables
The study variables included gastric type of lesion: gastric adenocarcinoma or atrophy or intestinal metaplasia or its absence and PGC alleles (1-6).
Statistical analysis
Data analysis was performed using the computer software Statistical Package for Social Sciences - SPSS for Windows (version 11.5). Chi-square analysis was used to compare categorical variables, using a 5% level of significance. Logistic regression was used to estimate odds ratio (OR) and its 95% CI as a measure of the association between PGC Allele 6 carrier and risk for the development of gastric lesions.
RESULTS
Allelic distribution of PGC polymorphism
The allelic distribution of the PGC polymorphism is shown in Table 1. No significant differences between controls and patients with known gastric lesions were observed as far as Alleles 1 to 6 were concerned.
Table 1.
Allelic distribution of PGC polymorphism according to the type of gastric lesion or its absence n (%)
| Allele 1 carrier | Allele 2 carrier | Allele 3/4 carrier | Allele 5 carrier | Allele 6 carrier | |
| Controls (n = 127) | 2 (1.6) | 31 (24.4) | 39 (30.7) | 37 (19.1) | 92 (72.4) |
| All cases (n = 99) | 1 (1.0) | 30 (30.3) | 33 (33.3) | 39 (39.4) | 47 (47.5) |
| AIM (n = 42) | - | 16 (38.1) | 14 (33.3) | 16 (38.1) | 18 (42.9) |
| GC (n = 57) | 1 (1.8) | 14 (24.6) | 19 (33.3) | 23 (40.4) | 29 (50.9) |
AIM: Atrophy or intestinal metaplasia; GC: Gastric adenocarcinoma.
Risk estimates for associated lesions and invasive gastric adenocarcinoma
Table 2 describes the risk estimation for the development of different gastric lesions considering Allele 6 carrier status. Significant differences were found in controls and patients with known gastric lesions concerning Allele 6 carrier status (P ≤ 0.001). Moreover, significant differences were also found for Allele 6 carriers when comparing the different types of gastric lesions with healthy individuals (P ≤ 0.001 and P = 0.004).
Table 2.
Association of PGC Allele 6 carriers and risk for development of associated lesions and invasive gastric adenocarcinoma
| Allele 6 carrier | P | OR | 95% CI | |
| n (%) | ||||
| Controls (n = 127) | 92 (72.4) | 1.00 | Reference | |
| All cases (n = 99) | 47 (47.5) | < 0.001 | 0.34 | 0.20-0.60 |
| AIM (n = 42) | 18 (42.9) | < 0.001 | 0.28 | 0.14-0.59 |
| GC (n = 57) | 29 (50.9) | 0.004 | 0.39 | 0.21-0.75 |
AIM: Atrophy or intestinal metaplasia; GC: Gastric adenocarcinoma; P: Pearson Chi-Square; OR: Odds ratio; CI: Confidence interval.
In addition, the results showed that Allele 6 carrier status had protection against the development of gastric lesions (OR = 0.34; 95% CI 0.20-0.60). This was also true when stratifying the analysis based upon the type of gastric lesions, such as atrophy or intestinal metaplasia (OR = 0.28; 95% CI 0.14-0.59) or invasive GC (OR = 0.39; 95% CI 0.21-0.75).
DISCUSSION
Gastric adenocarcinoma (GC) has distinct geographical distribution with the highest incidence rates in Asian countries, and it remains as the third cause of cancer-related mortality in Europe[1-3]. Portugal has the highest incidence and mortality rates of Western Europe[6]. On the other hand, it is well accepted that cancer is a multifactorial disease and that genetic polymorphism may influence the genetic susceptibility to the development of several cancers[9-18].
Pepsinogen, an effective marker of terminal differentiation of the stomach mucosa, has been used as a serological test for more than 20 years[21]. An insertion/deletion polymorphism in the PGC gene has been described as a susceptibility marker for the development of gastric adenocarcinoma. However, only a few studies have been published on pepsinogen C[24-26,28,29]. Up to our knowledge, this is the first study within a southern European population, from the north region of Portugal.
Our results suggest that Allele 6 carriers have protec-tion against the development of gastric lesions such as atrophy, intestinal metaplasia or invasive carcinoma. These results are not in concordance with the results in Asian populations (Table 3). By comparison with the results from Asian populations we observed that carriers of this allele were more frequent in our control group, which might represent a genetic gap between Caucasian and Asian populations. However, when we compared the Allele 6 distribution in the Chinese control population, no homogeneous frequencies were reported. Furthermore, the Allele 6 distribution we found within the Portuguese population is not significantly different from one of the Chinese reports[26].
Table 3.
Frequencies of PGC Allele 6 carriers within populations
| Population | n | Allele 6 Carriers n (%) | |
| Our Study | Caucasian (Porto, Portugal) | 127 | 92 (72.4) |
| Ohtaki et al, 1997 | Asian (Fukui, Japan) | 177 | 82 (46.3) |
| Liu et al, 2003 | Asian (Shenyang, China) | 42 | 24 (57.1) |
| Asian (Zhuanghe, China) | 113 | 69 (61.0) |
Even though our study included a small sample of cases, both allelic distribution and risk estimate seemed to exclude a type I error. A cohort study would seem ideal to estimate accurately risks, however, only a few if any cohort studies in this field are published. The recruitment of controls and the follow-up of patients with atrophy and intestinal metaplasia and with the absence of Allele 6 will probably give us more information in the near future.
The role of the PGC polymorphism in the carcino-genesis of GC is not clear. It was reported that PGC was not only a digestive enzyme, but also a growth factor under strict conditions, whereby the levels of serum expression of PGC might play important roles. This PGC polymorphism is located between exons 7 and 8, and by the analysis of the genomic sequence (Genome USCS NM_002630; Ref. NM_002630.1) with the Discovery Studio Gene v1.5 (Accelrys Inc), it is possible to identify, within the polymorphic region, an extensive number of TATA-box sequences. TATA-box sequences are extremely important in the activation of gene expression.
We hypothesize that this insertion/deletion polymorphism interferes directly with the number of TATA-box accessible for the activation of PGC expression. Usually as many TATA-boxes are available, as many expressions we could achieve. Although, in the presence of a great number of TATA-boxes, altogether in sequence, they might function as a confounder for the transcriptional activation factors. Thus, if a deletion occurs in a site like this, it would stabilize the activation of gene expression, increasing its levels. Evidences of higher levels of PGC associated with pre-neoplastic lesions could be correlated with this gene expression stabilization[22,30].
Although further studies are required to establish the role of this polymorphism in the development of GC, our study reveals that PGC Allele 6 (shorter allele) is associated with protection against development of gastric lesions in a Caucasian population.
Footnotes
Supported by the Portuguese League Against Cancer (Liga Portuguesa Contra o Cancro-Núcleo Regional do Norte) and Astra Zeneca Foundation
S- Editor Wang J L- Editor Zhu LH E- Editor Ma N
References
- 1.Parkin DM, Läärä E, Muir CS. Estimates of the worldwide frequency of sixteen major cancers in 1980. Int J Cancer. 1988;41:184–197. doi: 10.1002/ijc.2910410205. [DOI] [PubMed] [Google Scholar]
- 2.Stoicov C, Saffari R, Cai X, Hasyagar C, Houghton J. Molecular biology of gastric cancer: Helicobacter infection and gastric adenocarcinoma: bacterial and host factors responsible for altered growth signaling. Gene. 2004;341:1–17. doi: 10.1016/j.gene.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 4.Levi F, La Vecchia C, Lucchini F, Negri E. Cancer mortality in Europe, 1990-92. Eur J Cancer Prev. 1995;4:389–417. doi: 10.1097/00008469-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Black RJ, Bray F, Ferlay J, Parkin DM. Cancer incidence and mortality in the European Union: cancer registry data and estimates of national incidence for 1990. Eur J Cancer. 1997;33:1075–1107. doi: 10.1016/s0959-8049(96)00492-3. [DOI] [PubMed] [Google Scholar]
- 6.Pinheiro PS, Tyczyński JE, Bray F, Amado J, Matos E, Parkin DM. Cancer incidence and mortality in Portugal. Eur J Cancer. 2003;39:2507–2520. doi: 10.1016/s0959-8049(03)00570-7. [DOI] [PubMed] [Google Scholar]
- 7.LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 9.Medeiros R, Morais A, Vasconcelos A, Costa S, Pinto D, Oliveira J, Lopes C. Endothelial nitric oxide synthase gene polymorphisms and genetic susceptibility to prostate cancer. Eur J Cancer Prev. 2002;11:343–350. doi: 10.1097/00008469-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Pinto D, Vasconcelos A, Costa S, Pereira D, Rodrigues H, Lopes C, Medeiros R. HER2 polymorphism and breast cancer risk in Portugal. Eur J Cancer Prev. 2004;13:177–181. doi: 10.1097/01.cej.0000130015.91525.c7. [DOI] [PubMed] [Google Scholar]
- 11.Catarino R, Matos A, Pinto D, Pereira D, Craveiro R, Vasconcelos A, Lopes C, Medeiros R. Increased risk of cervical cancer associated with cyclin D1 gene A870G polymorphism. Cancer Genet Cytogenet. 2005;160:49–54. doi: 10.1016/j.cancergencyto.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Duarte I, Santos A, Sousa H, Catarino R, Pinto D, Matos A, Pereira D, Moutinho J, Canedo P, Machado JC, et al. G-308A TNF-alpha polymorphism is associated with an increased risk of invasive cervical cancer. Biochem Biophys Res Commun. 2005;334:588–592. doi: 10.1016/j.bbrc.2005.06.137. [DOI] [PubMed] [Google Scholar]
- 13.Sousa H, Santos AM, Catarino R, Pinto D, Vasconcelos A, Lopes C, Breda E, Medeiros R. Linkage of TP53 codon 72 pro/pro genotype as predictive factor for nasopharyngeal carcinoma development. Eur J Cancer Prev. 2006;15:362–366. doi: 10.1097/00008469-200608000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Craveiro R, Costa S, Pinto D, Salgado L, Carvalho L, Castro C, Bravo I, Lopes C, Silva I, Medeiros R. TP73 alterations in cervical carcinoma. Cancer Genet Cytogenet. 2004;150:116–121. doi: 10.1016/j.cancergencyto.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Medeiros R, Soares R, Vasconcelos A, Schmitt F, Lopes C. Glutathione S-transferase genotype GSTM1 as a predictor of elevated angiogenic phenotype in patients with early onset breast cancer. Angiogenesis. 2004;7:53–58. doi: 10.1023/B:AGEN.0000037330.20121.d8. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro R, Vasconcelos A, Costa S, Pinto D, Morais A, Oliveira J, Lobo F, Lopes C, Medeiros R. Overexpressing leptin genetic polymorphism (-2548 G/A) is associated with susceptibility to prostate cancer and risk of advanced disease. Prostate. 2004;59:268–274. doi: 10.1002/pros.20004. [DOI] [PubMed] [Google Scholar]
- 17.Santos AM, Sousa H, Portela C, Pereira D, Pinto D, Catarino R, Rodrigues C, Araújo AP, Lopes C, Medeiros R. TP53 and P21 polymorphisms: response to cisplatinum/paclitaxel-based chemotherapy in ovarian cancer. Biochem Biophys Res Commun. 2006;340:256–262. doi: 10.1016/j.bbrc.2005.11.176. [DOI] [PubMed] [Google Scholar]
- 18.Vasconcelos A, Medeiros R, Veiga I, Pereira D, Carrilho S, Palmeira C, Azevedo C, Lopes CS. Analysis of estrogen receptor polymorphism in codon 325 by PCR-SSCP in breast cancer: association with lymph node metastasis. Breast J. 2002;8:226–229. doi: 10.1046/j.1524-4741.2002.08407.x. [DOI] [PubMed] [Google Scholar]
- 19.Miki K, Ichinose M, Kawamura N, Matsushima M, Ahmad HB, Kimura M, Sano J, Tashiro T, Kakei N, Oka H. The significance of low serum pepsinogen levels to detect stomach cancer associated with extensive chronic gastritis in Japanese subjects. Jpn J Cancer Res. 1989;80:111–114. doi: 10.1111/j.1349-7006.1989.tb02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagata Z, Zhang Y, Shinozaki S, Miyamura T, Iijima S, Asaka A, Kobayashi K. Influence of pepsinogen gene polymorphisms on serum pepsinogen. Ann Hum Genet. 1997;61(Pt 2):93–97. doi: 10.1046/j.1469-1809.1997.6120093.x. [DOI] [PubMed] [Google Scholar]
- 21.Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, Barbosa J, Guilherme M, Moreira-Dias L, Lomba-Viana H, Silva R, Abreu N, Lomba-Viana R. Validity of serum pepsinogen I/II ratio for the diagnosis of gastric epithelial dysplasia and intestinal metaplasia during the follow-up of patients at risk for intestinal-type gastric adenocarcinoma. Neoplasia. 2004;6:449–456. doi: 10.1593/neo.03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ning PF, Liu HJ, Yuan Y. Dynamic expression of pepsinogen C in gastric cancer, precancerous lesions and Helicobacter pylori associated gastric diseases. World J Gastroenterol. 2005;11:2545–2548. doi: 10.3748/wjg.v11.i17.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taggart RT, Mohandas TK, Bell G. Assignment of the human preprogastricsin (PGC) to chromosome 6 and regional localization of PGC (6pter-p21.1), prolactin PRL (6pter-p21.1) Cytogenet Cell Genet. 1987;46:701–702. [Google Scholar]
- 24.Azuma T, Pals G, Taggart RT. RFLP for the human pepsinogen C gene (PGC) Nucleic Acids Res. 1988;16:9372. doi: 10.1093/nar/16.19.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taggart RT, Azuma T, Wu S, Bell GI, Bowcock AM. A highly informative polymorphism of the pepsinogen C gene detected by polymerase chain reaction. Adv Exp Med Biol. 1991;306:95–99. doi: 10.1007/978-1-4684-6012-4_10. [DOI] [PubMed] [Google Scholar]
- 26.Liu HJ, Guo XL, Dong M, Wang L, Yuan Y. Association between pepsinogen C gene polymorphism and genetic predisposition to gastric cancer. World J Gastroenterol. 2003;9:50–53. doi: 10.3748/wjg.v9.i1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müllenbach R, Lagoda PJ, Welter C. An efficient salt-chloroform extraction of DNA from blood and tissues. Trends Genet. 1989;5:391. [PubMed] [Google Scholar]
- 28.Ohtaka Y, Azuma T, Konishi J, Ito S, Kuriyama M. Association between genetic polymorphism of the pepsinogen C gene and gastric body ulcer: the genetic predisposition is not associated with Helicobacter pylori infection. Gut. 1997;41:469–474. doi: 10.1136/gut.41.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkateshwari A, Vidyasagar A, Prasad R, Pratap B, Pratibha N. Pepsinogen polymorphism in the Indian population and its association with duodenal ulcer. Hum Genet. 1997;101:201–204. doi: 10.1007/s004390050614. [DOI] [PubMed] [Google Scholar]
- 30.Wells M, Brown B, Hall J. Pepsinogen C expression in intestinal IEC-6 cells. Cell Physiol Biochem. 2003;13:301–308. doi: 10.1159/000074545. [DOI] [PubMed] [Google Scholar]


