Abstract
AIM: To explore whether a co-culture of cynomolgus monkey embryonic stem (cES) cells with embryonic liver cells could promote their differentiation into hepatocytes.
METHODS: Mouse fetal liver-derived cells (MFLCs) were prepared as adherent cells from mouse embryos on embryonic d (ED) 14, after which undifferentiated cES cells were co-cultured with MFLCs. The induction of cES cells along a hepatic lineage was examined in MFLC-assisted differentiation, spontaneous differentiation, and growth factors (GF) and chemicals-induced differentiations (GF-induced differentiation) using retinoic acid, leukemia inhibitory factor (LIF), FGF2, FGF4, hepatocyte growth factor (HGF), oncostatin M (OSM), and dexamethasone.
RESULTS: The mRNA expression of α-fetoprotein, albumin (ALB), α-1-antitrypsin, and hepatocyte nuclear factor 4α was observed earlier in the differentiating cES cells co-cultured with MFLCs, as compared to cES cells undergoing spontaneous differentiation and those subjected to GF-induced differentiation. The expression of cytochrome P450 7a1, a possible marker for embryonic endoderm-derived mature hepatocytes, was only observed in cES cells that had differentiated in a co-culture with MFLCs. Further, the disappearance of Oct3/4, a representative marker of an undifferentiated state, was noted in cells co-cultured with MFLCs, but not in those undergoing spontaneous or GF-induced differentiation. Immunocytochemical analysis revealed an increased ratio of ALB-immunopositive cells among cES cells co-cultured with MFLCs, while glycogen storage and urea synthesis were also demonstrated.
CONCLUSION: MFLCs showed an ability to induce cES cells to differentiate toward hepatocytes. The co-culture system with MFLCs is a useful method for induction of hepatocyte-like cells from undifferentiated cES cells.
Keywords: Primate embryonic stem cells, Fetal liver, Hepatic differentiation, Co-culture
INTRODUCTION
Embryonic stem (ES) cells are self-renewing, pluripotent cells derived from the inner cell masses of preimplantation blastocysts[1,2]. They can be expanded without limit and retain a potential to differentiate into various somatic cell types of the three germ layers. We previously reported the differentiation of mouse ES (mES) cells into insulin-producing cells[3,4], intestinal tract-related cells[5], dopamine-producing cells[6], photoreceptor-like cells[7], and hepatocytes[8-10].
Several of the characteristics of mES and primate ES cells are different[11-13]. To better understand the differentiation ability and therapeutic potential of human ES (hES) cells, the use of primate ES cells is indispensable. Although the differentiation of hepatocyte-like cells from hES cells has also been reported by some researchers, the use of hES cells for basic and clinical research is regulated in many countries, because of bio-ethical issues. Thus, monkey ES cells might be useful as a substitute model for basic and preclinical research using hES cells.
Herein, we report promoted differentiation of cynomolgus monkey ES (cES) cells into hepatocyte-like cells by use of a co-culture system with mouse fetal liver-derived cells (MFLCs). The expression of α-fetoprotein (AFP) mRNA expression was not observed until d 21 and 14 in spontaneous and growth factor (GF)-induced differentiations, respectively, and that of cytochrome P450 7a1 (CYP7A1), a possible marker of embryonic endoderm-derived mature hepatocytes[14], was undetectable even on d 28 in both cultures. Further, Oct3/4, a marker of an undifferentiated state, never disappeared throughout the experimental period. In contrast, in the present study of cES cells that were co-cultured with MFLCs on a membrane with 0.4-μm sized pores, the expressions of AFP, albumin (ALB), hepatocyte nuclear factor 4α (HNF4α), and CYP7A1 were detected as early as d 10, while the expression of Oct3/4 was detected only faintly on d 14 and not at all by d 21. Immunocytochemical analysis revealed an increased ratio of ALB-immunopositive cells among cES cells co-cultured with MFLCs, while glycogen storage and urea synthesis were also demonstrated.
These results show that MFLCs provide a conductive environment for the differentiation of cES cells toward hepatocyte-like cells, and suggest that the present co-culture system of cES cells with MFLCs may be useful for preparation of a cell replacement source that is rich with hepatocyte-like cells and does not retain undifferentiated cES cells.
MATERIALS AND METHODS
Cynomolgus monkey ES cells
A cynomolgus monkey ES (cES) cell line (CMK6) was obtained from Asahi Techno Glass Corp., Chiba, Japan[15]. Undifferentiated cES cells were maintained on a feeder layer of 40 Gy-irradiated mouse embryonic fibroblasts (MEF) in DMEM/F-12 (Asahi Techno Glass Corp.), supplemented with 20% Knockout Serum Replacement (KSR; GIBCO-Invitrogen, Carlsbad, CA, USA), 0.1 mmol/L 2-mercaptoethanol, 1 mmol/L sodium pyruvate, 2 mmol/L L-glutamine, 0.1 mmol/L nonessential amino acids, and a mixture of penicillin (25 U/mL) and streptomycin (25 μg/mL). The medium was changed daily. Cell colonies composed of closely packed cells were split every 3-4 d by incubation in a 0.25% trypsin-EDTA solution for 5 min at 37°C before transfer by pipetting onto the 40-Gy-irradiated MEF cells.
Preparation of mouse fetal liver-derived cells (MFLCs)
Embryonic liver tissues were collected from C57BL/6CrSlc mice (E14.0) and minced, then dissociated in 0.1% trypsin-EDTA solution for 20 min at 37°C followed by hemolysis with hypotonic buffer. Dissociated cells were suspended in culture media composed of DMEM supplemented with 10% fetal calf serum (FCS), 2 mmol/L L-glutamine, 1x non-essential amino acid solution, and a mixture of penicillin (25 U/mL) and streptomycin (25 μg/mL), after which 1 × 107 cells were plated onto a 0.1% gelatin-coated tissue culture dish sized 100 mm in diameter. After 24 h, contaminating hematopoietic cells and cell debris were removed by extensive washing with culture media, and the adherent cells were then cultured for 48 h to reach the confluent growth stage.
Spontaneous and GF-induced differentiation of cES cells
Spontaneous differentiation of undifferentiated cES cells was carried out as follows. Undifferentiated cES colonies were incubated with a 0.25% trypsin-EDTA solution for 5 min at 37°C, which obtained clusters of closely packed cells. Approximately 1 × 106 of these cells were then cultured in a 60-mm dish in DMEM supplemented with 10% FCS, 2 mmol/L L-glutamine, and 1 × non-essential amino acid solution (DMEM basic medium) for 28 d without any defined chemical factors (Figure 1A). The medium was exchanged with fresh medium every 2-3 d.
Figure 1.
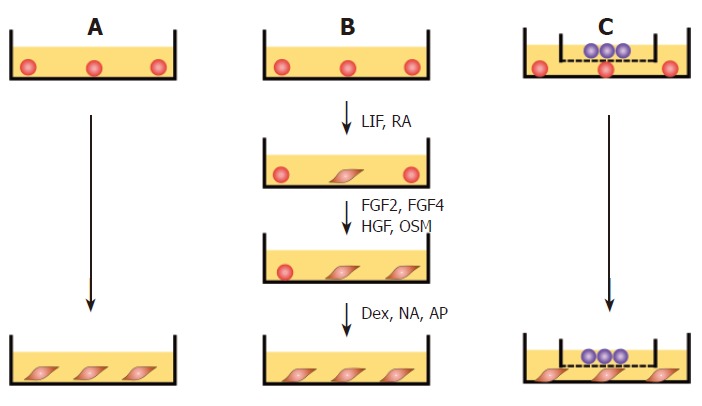
Protocol for differentiation of cES cells. A: Spontaneous differentiation. Undifferentiated cES cells were cultured in gelatin-coated dishes in DMEM basic medium for 28 d without additional feeders or growth factors; B: GF-induced differentiation. Undifferentiated cES cells were cultured in DMEM basic medium containing LIF (100 units/mL) and RA (10-8 mol/L) for 3 d, followed by a 5-d culture in DMEM basic medium containing FGF2 (10 mg/L), FGF4 (20 mg/L), HGF (25 mg/L), and OSM (10 mg/L). Thereafter, the cells were cultured for 20 d in DMEM basic medium containing 10-7 mol/L dexamethasone (Dex), 0.2 mmol/L, L-ascorbic-2-phosphate (AP), and 10 mmol/L nicotinamide (NA); C: Promoted differentiation by co-culture with MFLCs. Undifferentiated cES cells were plated onto 60-mm dishes and co-cultured with MFLCs in a 35-mm culture insert across a 0.4-μm Millicell CM membrane for 28 d.
For GF-induced differentiation toward hepatocyte-like cells, undifferentiated cES cells were treated stepwise with combinations of defined chemicals and growth factors, according to a method reported previously[16], with some modifications. Approximately 1 × 106 undifferentiated cES cells, prepared as cellular clusters by enzymatic digestion of undifferentiated cES colonies, were seeded in a 60-mm dish and cultured in DMEM basic medium containing leukemia inhibitory factor (LIF, 100 units/mL; GIBCO BRL, Rockville, MD, USA) and 10-8 mol/L all-trans retinoic acid (RA; Sigma) for 3 d. The cells were passaged in DMEM basic medium containing fibroblast growth factor 2 (FGF2, 10 mg/L; Genzyme/Techne, Minneapolis, MN, USA), FGF4 (20 mg/L, Genzyme/Techne), hepatocyte growth factor (HGF, 25 mg/L; Genzyme/Techne), and Oncostatin M (OSM, 10 mg/L; Genzyme/Techne). After 5 d, the cells were further cultured in DMEM basic medium containing 10-7 mol/L dexamethasone (Dex; Sigma), 0.2 mmol/L L-ascorbic-2-phosphate (AP; Wako, Osaka, Japan), and 10 mmol/L nicotinamide (NA; Sigma) until d 28 (Figure 1B).
Co-culture of cES cells with MFLCs
MFLCs showing confluent growth were digested with a 0.25% trypsin-EDTA solution for 5 min at 37°C. One million undifferentiated cES cells, prepared as cell clusters by enzymatic digestion of undifferentiated cES colonies, were co-cultured in a 60-mm dish with the same number (1 × 106) of MFLCs in a 35-mm culture insert across a 0.4-μm Millicell CM membrane (Millipore Corp., Bedford, MA USA) for 28 d (Figure 1C). A half volume of medium in the 60-mm dish was discarded with floating cells and replaced with fresh medium every 2 d.
RT-PCR
For RNA extraction and RT-PCR analysis, total RNA was purified using Trizol (Invitrogen) following the protocol of the manufacturer. One microgram of DNase-treated total RNA was used for the first-strand cDNA reaction, which was performed using a random primer (Invitrogen) and M-MLV reverse transcriptase (Promega, Madison, WI, USA). cDNA samples were subjected to PCR amplification with specific primers. The cycling parameters were as follows; denaturation at 94°C for 1 min, annealing at 55°C-60°C for 1 min (depending on the primer), and elongation at 72°C for 1 min (40 cycles). The PCR primers and length of the amplified products are shown in Table 1.
Table 1.
The PCR primers and length of the amplified products
| For detection | Gene | Primer sequence | Products [bp] | GenBank |
| Annealing temp.[°C] | accession No. | |||
| Cynomolgus | OCT3/4 | Sense: 5’-ACCACAGTCCATGCCATCAC-3’ | 660 | Z11898 |
| Antisense: 5’-TCCACCACCCTGTTGCTGTA-3’ | 60 | |||
| Albumin | Sense: 5’-GCATCCTGATTACTCTGACATG-3’ | 229 | AB158629 | |
| Antisense: 5’-CTTGGTGTAACGAACTAATTGC-3’ | 60 | |||
| AFP | Sense: 5’-TGCAGCCAAAGTGAAGAGGGAAGA-3’ | 217 | NM_001134 | |
| Antisense: 5’-CATAGCGAGCAGCCCAAAGAAGAA-3’ | 60 | |||
| HNF4α | Sense: 5’-CCGGATCAGCACTCGAA-3’ | 411 | NM_178849 | |
| Antisense: 5’-AGCTCGTCAAGGATGCGTATG-3’ | 60 | |||
| CYP7A1 | Sense: 5’-ATTTGGTGCCAATCCTCTTG-3’ | 312 | NM_000780 | |
| Antisense: 5’-CGTTGGAGGTTTTCCATCAT-3’ | 60 | |||
| Cynomolgus, mouse | GAPDH | Sense: 5’-ACCACAGTCCATGCCATCAC-3’ | 452 | NM_002046 |
| Antisense: 5’-TCCACCACCCTGTTGCTGTA-3’ | 60 | |||
| mouse | OCT3/4 | Sense: 5’-CGCCCGCATACGAGTTCTG-3’ | 678 | X52437 |
| Antisense: 5’-GGTGTCCCTGTAGCCTCAT-3’ | 60 | |||
| AFP | Sense: 5’-CTTTGGACCCTCTTCTGTGA-3’ | 909 | NM_007423 | |
| Antisense: 5’-CACTGCTGCAACTCTTCGTA-3’ | 55 | |||
| Albumin | Sense: 5’-TGAACTGGCTGACTGCTGTG-3’ | 718 | AJ457860 | |
| Antisense: 5’-CATCCTTGGCCTCAGCATAG-3’ | 60 | |||
| α1AT | Sense: 5’-TGGGGTCTACTGCTTCTGG-3’ | 693 | M25529 | |
| Antisense: 5’-TCATGGGCACCTTCACCGT-3’ | 60 | |||
| HNF4α | Sense: 5’-CTAAGCTGTCCCCACAAGGCTATGCA-3’ | 864 | NM_008261 | |
| Antisense 5’-CAGAGCTCCACCTGGAAAGGTGTTTG-3’ | 60 | |||
| TDO | Sense: 5’-AGAGCCAGCAAAGGAGGAC-3’ | 500 | BC018390 | |
| Antisense: 5’-CTGTCTGCTCCTGCTCTGAT-3’ | 60 | |||
| PEPCK | Sense: 5’-TCTGCCAAGGTCATCCAGG-3’ | 290 | AF009605 | |
| Antisense: 5’-GTTTTGGGGATGGGCACTG-3’ | 60 | |||
| CYP7A1 | Sense: 5’-AGGACTTCACTCTACACC-3’ | 453 | AK050260 | |
| Antisense: 5’-GCAGTCGTTACATCATCC-3’ | 56 | |||
| Desmin | Sense: 5’-ATGACCGCTTCGCCAACTA-3’ | 461 | NM_010043 | |
| Antisense: 5’-CATACTGAGCCCGGATGTC-3’ | 60 | |||
| Vimentin | Sense: 5’-TCAAGAACACCCGCACCAACGA-3’ | 463 | NM_011701 | |
| Antisense: 5’-GTTTGACACCTGCTTGGCCTGG-3’ | 60 |
AFP: Alpha-fetoprtein; HNF4α: Hepatic nuclear factor 4α; CYP7a1: Cytochrome P450 7A1; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; α1AT: Alpha-1 antitrypsin; TDO: Tryptophan 2, 3-dioxygenase; PEPCK: phosphoenolpyruvate carboxykinase.
In vitro immunofluorescence analysis
Immunofluorescence analysis was carried out using standard protocols. Briefly, the cells were fixed in 4% paraformaldehyde and incubated with cell specific marker antibodies in blocking serum at 4°C overnight. After incubation in species-specific IgG conjugated with Alexa Fluor 488 (donkey anti-sheep IgG; Invitrogen) or RITC (goat anti-mouse IgG; Biomeda Foster City, CA, USA), the cells were washed with PBS and examined under a microscope. All nuclei were stained with DAPI (Dojindo, Kumamoto, Japan). The primary antibodies and dilutions used were as follows: sheep polyclonal anti-human ALB (Biomeda), 1:100; mouse monoclonal anti-human AFP (Biomeda), 1:100; rabbit polyclonal anti-human HNF4α (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 1:100; and mouse monoclonal anti-human alpha-1 antitrypsin (Biomeda), 1:100.
To examine the immunological similarities between cES-derived hepatocyte-like cells and human hepatocytes, a monoclonal antibody mouse anti-human hepatocyte clone OCH1E5 (HepPar1; DAKO) was used[17,18]. HepPar1 reacts with both normal and neoplastic hepatocytes, but not with cholangiocytes.
In some experiments, cultured cES cells were trypsinized into single cells in suspension. After reattachment to the culture dish by an overnight culture, the cells were subjected to immunofluorescence analysis to determine the ratio of ALB-immunopositive cells. The numbers of total cells and ALB-immunopositive cells in 3 different microscopic fields were then counted.
Periodic acid Schiff (PAS) staining
Staining of glycogen was performed using a PAS reaction. For negative controls, fixed cells in 4% paraformaldehyde were treated with 1 mg/mL of α-amylase (3000 U/mg protein, Sigma) in 0.1 mol/L sodium phosphate buffer (pH 6.2) at 37°C for 30 min before PAS staining.
Measurement of urea
To examine urea synthesis, cES cells were subjected to spontaneous, GF-induced, or MFLC-co-cultured differentiation for 14 and 28 d. Then they were incubated in serum-free α-MEM medium in the presence of ammonium chloride (20 mmol/L) for 60 min. The level of urea nitrogen in the incubation medium was determined using a colorimetric assay (Determiner LUN kit, Kyowa Medix, Tokyo), after removal of endogenous ammonium by treatment with glutamate dehydrogenase.
Analysis
For qualitative analysis, all cES differentiation experiments were performed in duplicate and repeated. P < 0.05 was taken as significant.
RESULTS
Spontaneous differentiation of cES cells
Undifferentiated cES cell clusters were cultured in basic DMEM for 28 d and differentiation toward hepatocyte-like cells was analyzed by RT-PCR (Figure 2). AFP mRNA expression was not observed until d 14, while ALB remained undetectable on d 21. On d 28, ALB and HNF4α were both detected, whereas CYP7A1 was never detected throughout the experimental period. Further, immunocytochemical results demonstrated that ALB-immunopositive cells on d 28 comprised fewer than 1% of the total cultured cells. The expression of Oct3/4, a marker of an undifferentiated state, was distinctly detected throughout the experimental period.
Figure 2.
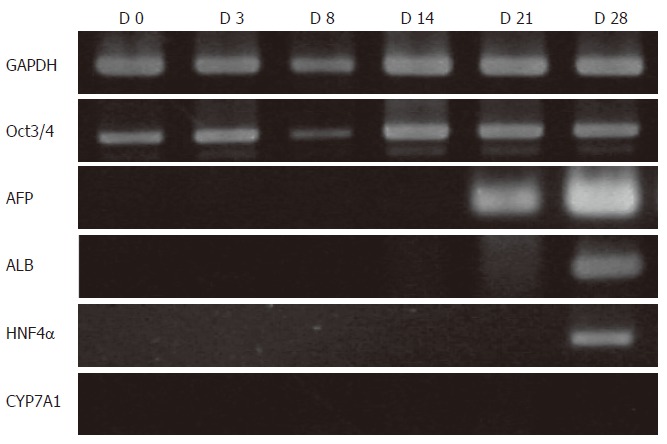
RT-PCR analysis of spontaneous differentiation of cES cells. AFP mRNA expression was not observed prior to d 14, while ALB was detected on d 21. On d 28, ALB and HNF4α expression became detectable. CYP7A1 was not detected throughout the experimental period, while Oct3/4 was distinctly detected at all time points.
GF-induced differentiation toward hepatocyte-like cells
Efficient induction of mouse ES cells into hepatocyte-like cells by sequential treatments with defined chemicals and without the use of embryoid bodies (EBs) has been reported[16]. Therefore, we applied that method to the present cES cells with some modifications. Undifferentiated cES cells were first treated with RA and LIF for 3 d, followed by 5 d of treatment with FGF2, FGF4, and HGF, and finally subjected to culture medium containing OSM and Dex.
AFP mRNA expression was not detected following the 3-d treatment with RA and LIF (Figure 3, lane D3), and was faintly detected on d 8 at the end of the 5-d treatment with FGF2, FGF4, and HGF (Figure 3, lane D8). Further, the expression of ALB and HNF4α became detectable after the differentiating cES cells were exposed to OSM and Dex (Figure 3, lanes D14, D21 and D28). Although ALB and HNF4α were clearly detected on d 21 and 28, no expression of CYP7A1 was observed. The immunocytochemical results demonstrated that about 7.2% ± 0.7% of the cultured cells were ALB-immunopositive on d 28. As for the presence of undifferentiated cES cells, Oct3/4 mRNA was still detected in the differentiating cES cells on d 28 of the induction culture oriented for hepatocyte-like cells.
Figure 3.
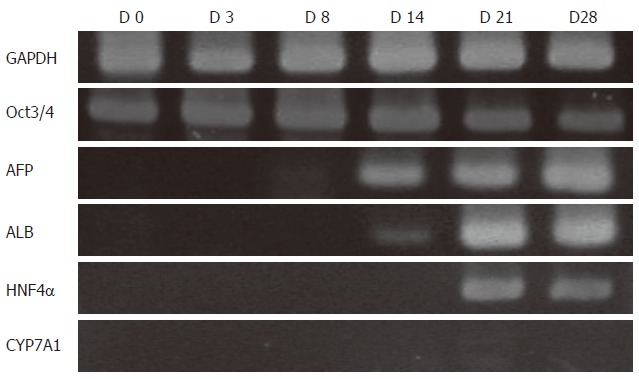
RT-PCR analysis of GF-induced differentiation of cES cells. AFP mRNA expression was not detected following a 3-d treatment with RA and LIF, though it was detected on d 8, and at the end of 5 d of treatment with FGF2, FGF4, and HGF. ALB and HNF4α were detected after the differentiating cES cells were exposed to OSM and Dex. Although ALB and HNF4α were strongly detected on d 21 and 28, no expression of CYP7A1 was observed. Oct3/4 mRNA expression remained up to d 28.
Characterization of mouse fetal liver-derived cells (MFLCs)
Although the cES cells that underwent GF-induced differentiation into hepatocyte-like cells showed an accelerated gene expression pattern as compared to those that underwent spontaneous differentiation, the prolonged detection of Oct3/4 mRNA and lack of CYP7A1 expression in both spontaneous and GF-induced differentiations suggested the presence of undifferentiated cES cells and insufficient differentiation into mature hepatocyte-like cells, respectively. We performed a co-culture of cES cells with MFLCs to search for epigenetic cues for the in vitro differentiation of cES cells toward hepatocyte-like cells. However, before performing that co-culture experiment, we examined the characteristics of MFLCs.
MFLCs were derived from E14.0 mouse livers and prepared as adherent cells by removal of the floating cell fraction from the culture of dissociated liver cells. They showed various morphologies, including cuboidal and stellate-shaped cells (Figure 4A). RT-PCR analysis demonstrated a distinctly different expression pattern as compared to that of adult liver tissues (Figure 4B). The MFLCs expressed AFP mRNA, but not ALB or other markers for differentiated hepatocytes, suggesting the presence of fetal hepatocytes. Further, the expressions of desmin and vimentin suggested the co-existence of cell types different from fetal hepatocytes, such as stellate cell-like cells, in accordance with the presence of cells with various shapes.
Figure 4.
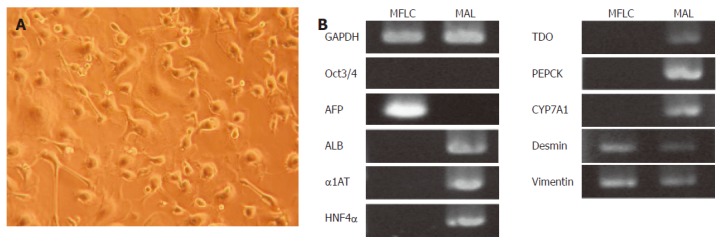
Characterization of MFLCs. A: Optical microscope image of MFLCs (× 100). MFLCs were prepared from E14.0 mouse livers and cultured for 48 h. After the floating cell fraction was discarded from the culture, resting adherent cells were further cultured until semi-confluent. MFLCs showed various morphologies, including cuboidal and stellate-shaped cells; B: RT-PCR analysis of MFLCs. AFP, desmin, and vimentin were expressed, whereas ALB was not. MAL: Mouse adult liver cells.
Promoted differentiation by co-culture with MFLCs
Undifferentiated cES cells were co-cultured with MFLCs across a membrane with 0.4-μm sized pores for 28 d and the gene expression of the differentiating cES cells were analyzed (Figure 5). There was no expression of AFP, ALB, or HNF4α detected in the differentiating cES cells on d 7, however, each was detectable within the next few days and all by d 10, which remained throughout the experimental period. Further, CYP7A1 mRNA expression, which was undetectable in the spontaneous and GF-induced differentiated cES cells, was observed on d 10. Immunocytochemical results demonstrated a high ratio of ALB-immunopositive cells (28.6% ± 4.2% as early as d 14), though the ratio did not greatly increase throughout the culture period (32.8% ± 5.6% on d 28). Despite the prompt induction of ALB mRNA, AFP mRNA was detected throughout the experimental period, suggesting an incapability of full maturation by the entire fraction of cES-derived hepatocyte-like cells by MFLCs alone. The expression pattern of Oct3/4 in cES cells co-cultured with MFLCs was quite different from that of cES cells subjected to spontaneous or GF-induced differentiation, as Oct3/4 mRNA, which never disappeared in the spontaneous and GF-induced differentiation experiments, was detected only faintly on d 14 and became undetectable by d 21. Without MFLCs on the Millicell CM membrane, no promoted differentiation of cES cells was observed.
Figure 5.
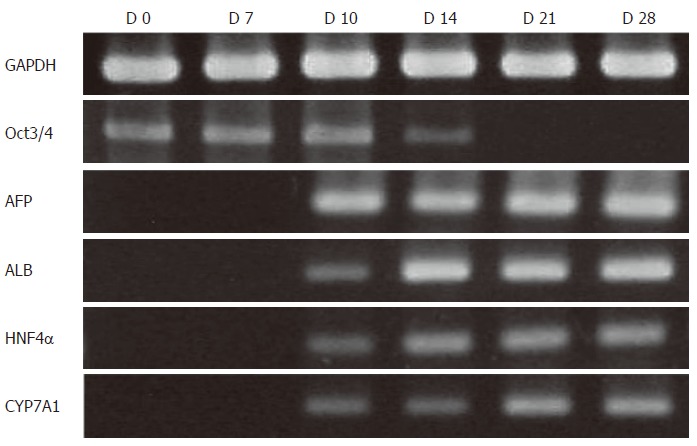
RT-PCR analysis of cES cells co-cultured with MFLCs. cES cells co-cultured with MFLCs across a membrane with 0.4-µm sized pores for 28 d were analyzed. AFP, ALB, HNF4α, and CYP7A1 mRNA expression was detected on d 10. Oct3/4 expression was faint on d 14 and disappeared by d 21.
Immunocytochemical analysis
cES cells differentiated in the co-culture with MFLCs were examined immunocytochemically on d 28 (Figure 6). Immunopositive reactions to AFP, ALB, and α1-AT were demonstrated as scattered colonized foci among the adherent cells. Representative areas of immunopositive distribution are shown in Figure 6A. In addition, HNF4α, an important transcription factor for mature hepatocytes, was also clearly detected in the cES cells co-cultured with MFLCs. We also examined immunoreactivity to the anti-human hepatocyte antibody Hep Par1. Cells immunopositive for human-albumin were also immunopositive for Hep Par1 (Figure 6B).
Figure 6.
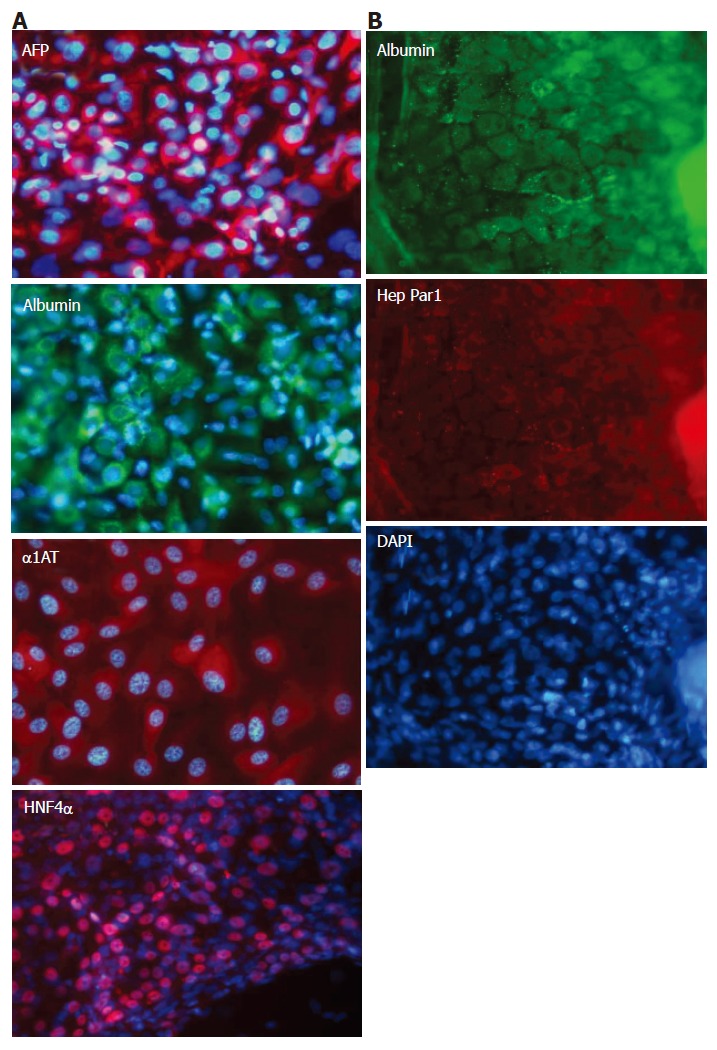
Immunocytochemical analysis of cES cells co-cultured with MFLCs. A: After being co-cultured with MFLCs for 28 d, cES cells were immunostained with anti-AFP, anti-ALB, anti-alpha-1-antitrypsin, and anti-HNF4α antibodies; B: Immunoreactivity to the anti-human hepatocyte antibody Hep Par1 was shown by the albumin-positive cells. Original magnification × 100.
Presence of glycogen in differentiated cES cells co-cultured with MFLCs
We performed PAS staining of undifferentiated cES cells and cES cells co-cultured with MFLCs to examine whether glycogen was stored, and both showed violet colored stains (Figure 7A and 7B). Similar staining results were obtained with undifferentiated cES cells pretreated with α-amylase (Figure 7C), while such staining did not appear following pretreatment with α-amylase in the differentiated cES cells co-cultured with MFLCs (Figure 7D). These results suggest that undifferentiated cES cells have an α-amylase-insensitive carbohydrate, while cES cells differentiated by MFLCs have an α-amylase-sensitive carbohydrate, which is probably glycogen.
Figure 7.
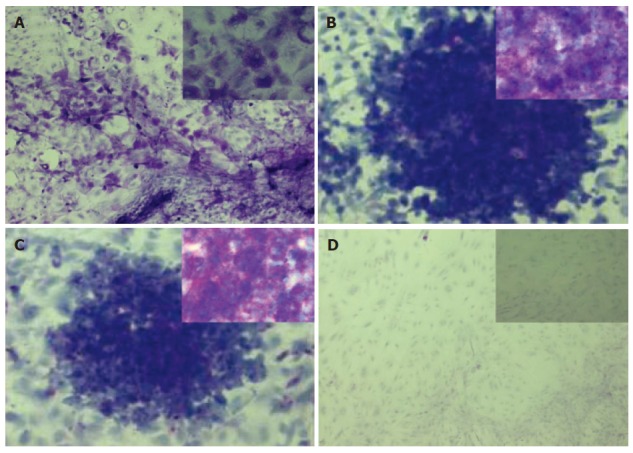
Histochemical staining of glycogen. Periodic acid Schiff (PAS) staining was performed on cES cells co-cultured with MFLCs for 28 d (A) and undifferentiated cES cells (B), as well as those following treatment with α-amylase (C and D, respectively). Both the undifferentiated cES cells and those co-cultured with MFLCs showed violet staining (A, B). Violet staining was apparent in the undifferentiated cES cells (C), while such staining did not appear in the differentiated cES cells pretreated with α-amylase(D). Original magnification ×100 (A-D), × 400 (insets in A-D).
Urea synthesis in differentiated cES cells co-cultured with MFLCs
We also examined ammonia metabolization in cES cells subjected to spontaneous, GF-induced, and MFLC-assisted differentiation after 14 and 28 d (Figure 8). The urea nitrogen level in the medium from cES cells co-cultured with MFLCs showed a high level as compared to that from cES cells under GF-induction. In a comparison between the levels on d 14 and 28, the amount of synthesized urea in cES cells co-cultured with MFLCs did not increase significantly. Further, spontaneously differentiated cES cells showed nearly no synthesized urea.
Figure 8.
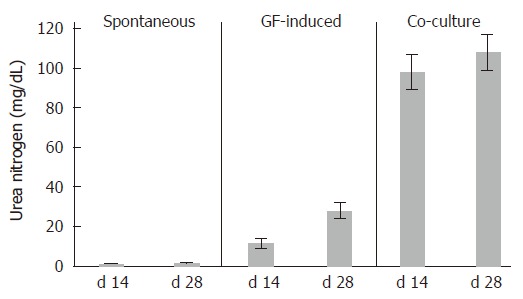
Urea synthesis by differentiated cES cells. The level of urea nitrogen in the culture medium of cES cells cultured with MFLCs was greater than that in the medium of cES cells subjected to GF-induction, though amounts of urea synthesized by cES cells co-cultured with MFLCs scarcely increased from d 14 to 28. Spontaneously differentiated cES cells showed nearly no ability to synthesize urea.
DISCUSSION
A number of studies have investigated the differentiation of mouse and human ES cells into hepatocyte-like cells[8-10,14,16,19-32]. In general, the methods used in those studies can be divided into spontaneous and directed differentiation. For spontaneous differentiation, the formation of EBs has been mostly utilized[8,14,19-23]. As for directed differentiation, a process of enrichment of a specific differentiated cell type that uses elements to promote the differentiation of ES cells into an endodermal lineage, such as the addition of growth factors (GFs) and hormones[16,24-31], and the constitutive expression of hepatic transcription factors[9,10,32], has been utilized.
In the present study, we used MFLCs to promote the differentiation of cES cells into hepatocyte-like cells. We co-cultured undifferentiated cES cells with MFLCs across a membrane with 0.4-μm sized pores and found early expression of AFP, ALB, and HNF4α mRNA, as compared to cES cells subjected to non-EB-mediated spontaneous or GF-induced differentiation. Further, the expression of CYP7A1 was detected in MFLC-assisted differentiated cES cells, but not in those undergoing non-EB-mediated spontaneous or GF-induced differentiation. In mice, CYP7A1 has been reported to be expressed in the liver, but not in yolk sac tissues[14], and suggested to be a definitive marker for endoderm-derived mature hepatocytes. The detection of CYP7A1 in the present cES cells co-cultured with MFLCs seems to indicate the usefulness of MFLCs in induction toward hepatocytes, though it has not been concluded whether CYP7A1 is a suitable marker for hepatocytes in primate cells.
We confirmed the promoted differentiation of cES cells along a hepatic lineage by immunocytochemistry. The ratio of ALB-immunopositive cells on d 28 was significantly higher with MFLC-assisted differentiation (32.8%) than non-EB-mediated spontaneous (less than 1%) or GF-induced (7.2%) differentiation. Similarly, cells immunopositive for alpha 1 antitrypsin and HNF4α were more abundant among those subjected to MFLC-assisted differentiation (data not shown). We further examined an immunoreactivity of differentiated cES cells to HepPar1. Although the target antigen recognized by HepPar1 has not been identified, HepPar1 is known to react in a restricted manner to adult and fetal human hepatocytes[17,18]. We found that the cES cells immunopositive to human-albumin were also immunopositive to Hep Par1, suggesting that cES-derived hepatocyte-like cells are immunologically very similar to human hepatocytes.
Both glycogen storage and ammonia metabolization are representative functions of hepatocytes. Periodic acid-Shiff (PAS) staining detected glycogen as α-amylase-sensitive carbohydrate in the cES cells differentiated by MFLCs. Although the undifferentiated cES cells were stained violet by PAS staining, they seemed to produce α-amylase-insensitive carbohydrate, not glycogen, which was in accordance with our previous observation of PAS staining of mES cells and mES-derived hepatocyte-like cells[10]. As for the ability to metabolize ammonia, the cES cells co-cultured with MFLCs produced urea more abundantly than those subjected to GF-induced differentiation.
We also evaluated the presence of undifferentiated cES cells among the differentiating cES cells by examining the mRNA expression of Oct3/4. In contrast to the continuous and stable detection of Oct3/4 mRNA throughout the entire 28-d experimental periods in the spontaneous and GF-induced differentiation experiments, the expression of Oct3/4 became faint on d 14 and was undetectable by d 21 in MFLC-assisted differentiation, indicating the absence of undifferentiated cES cells in cES cells co-cultured with MFLCs for more than 21 d. Since contamination by undifferentiated cES cells in transplants is critical for the development of tumors, cES cells co-cultured with MFLCs may be useful as a source rich in hepatocyte-like cells that is less risky for the development of cES-derived tumors.
The precise mechanisms by which MFLCs promote the differentiation of undifferentiated cES cells along a hepatic lineage were not investigated in the present study. However, it is known that non-parenchymal cells are required for differentiation of primitive hepatic endodermal cells and hepatoblasts into mature hepatocytes[33-38]. The MFLCs used in the present study were composed of heterogeneous cells, based on their various morphologies that showed cuboidal and stellate shapes, and on the results of PCR analysis demonstrating the expression of AFP, a hepatocyte-related marker, and desmin and vimentin, which are stellate cell-related markers. It is conceivable that the non-parenchymal cells among the MFLCs were involved in the differentiation of cES cells toward a hepatic lineage.
In the present study, MFLCs were prepared as adherent cells from E14.0 mouse fetal livers. The fetal liver is the major organ of hematopoiesis, and stromal cells there, like those in adult bone marrow, create a hematopoietic microenvironment and promote embryonic hematopoiesis[39]. Fetal liver-derived cells may also induce hematopoietic differentiation of ES cells, as in the case of stromal cells from bone marrow[40-45]. Indeed, a previous report documented the efficient induction of human ES cells into hematopoietic cells by fetal liver-derived cells[46]. In our experiments, MFLC-assisted differentiation of cES cells might have generated a number of hematopoietic cells in the culture as non-adherent floating cells, though they were removed from the culture by repeated media exchanges.
As the process of gestation proceeds from mid-gestation to birth, the fetal liver increases dramatically in size and switches to a metabolic organ from a hematopoietic organ at around the time of birth. Therefore, we anticipated that MFLCs might provide a conductive environment for cES cells to differentiate along a hepatic lineage. However, prior to starting the present experiments, we speculated that any promotion effect by MFLCs toward the hepatic differentiation of cES cells may be not distinguished or exerted only minimally. First, the species from which the cES cells and MFLCs originated were different. Further, the cES cells that were subjected to a co-culture with MFLCs were used in an undifferentiated state and they had not been previously induced toward an endoderm or hepatic lineage. Also, the undifferentiated cES cells were cultured separately from MFLCs by use of a 0.4-μm Millicell CM membrane, which did not allow direct cellular contact between the cES cells and MFLCs, and only permitted the transfer of diffusible factors. However, in contrast to our expectation, the co-culture of undifferentiated cES cells with MFLCs led to the promoted differentiation of cES cells toward a hepatic lineage. These results suggest that diffusible factors from MFLCs worked effectively in a cross-species manner on undifferentiated cES cells and seemed to be sufficient to stimulate the induction of hepatic differentiation of cES cells, though complete maturation of all cES-derived hepatocytes was not achieved, as shown by the prolonged detection of AFP mRNA throughout the experimental period.
In conclusion, the present results demonstrate that cynomolgus monkey ES cells can be induced into hepatocyte-like cells using a co-culture method with mouse fetal liver-derived cells. This co-culture system has been found to be an efficient method to obtain hepatocyte-like cells and may be useful for preparation of cellular grafts that do not contain undifferentiated ES cells, which would provide a limited risk for the development of tumors.
Footnotes
S- Editor Wang J L- Editor Zhu LH E- Editor Liu WF
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiroi A, Yoshikawa M, Yokota H, Fukui H, Ishizaka S, Tatsumi K, Takahashi Y. Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone. Stem Cells. 2002;20:284–292. doi: 10.1634/stemcells.20-4-284. [DOI] [PubMed] [Google Scholar]
- 4.Shiroi A, Ueda S, Ouji Y, Saito K, Moriya K, Sugie Y, Fukui H, Ishizaka S, Yoshikawa M. Differentiation of embryonic stem cells into insulin-producing cells promoted by Nkx2.2 gene transfer. World J Gastroenterol. 2005;11:4161–4166. doi: 10.3748/wjg.v11.i27.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada T, Yoshikawa M, Takaki M, Torihashi S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y. In vitro functional gut-like organ formation from mouse embryonic stem cells. Stem Cells. 2002;20:41–49. doi: 10.1634/stemcells.20-1-41. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura F, Yoshikawa M, Kanda S, Nonaka M, Yokota H, Shiroi A, Nakase H, Hirabayashi H, Ouji Y, Birumachi J, et al. Potential use of embryonic stem cells for the treatment of mouse parkinsonian models: improved behavior by transplantation of in vitro differentiated dopaminergic neurons from embryonic stem cells. Stem Cells. 2003;21:171–180. doi: 10.1634/stemcells.21-2-171. [DOI] [PubMed] [Google Scholar]
- 7.Sugie Y, Yoshikawa M, Ouji Y, Saito K, Moriya K, Ishizaka S, Matsuura T, Maruoka S, Nawa Y, Hara Y. Photoreceptor cells from mouse ES cells by co-culture with chick embryonic retina. Biochem Biophys Res Commun. 2005;332:241–247. doi: 10.1016/j.bbrc.2005.04.125. [DOI] [PubMed] [Google Scholar]
- 8.Yamada T, Yoshikawa M, Kanda S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20:146–154. doi: 10.1634/stemcells.20-2-146. [DOI] [PubMed] [Google Scholar]
- 9.Kanda S, Shiroi A, Ouji Y, Birumachi J, Ueda S, Fukui H, Tatsumi K, Ishizaka S, Takahashi Y, Yoshikawa M. In vitro differentiation of hepatocyte-like cells from embryonic stem cells promoted by gene transfer of hepatocyte nuclear factor 3 beta. Hepatol Res. 2003;26:225–231. doi: 10.1016/s1386-6346(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 10.Ishizaka S, Shiroi A, Kanda S, Yoshikawa M, Tsujinoue H, Kuriyama S, Hasuma T, Nakatani K, Takahashi K. Development of hepatocytes from ES cells after transfection with the HNF-3beta gene. FASEB J. 2002;16:1444–1446. doi: 10.1096/fj.01-0806fje. [DOI] [PubMed] [Google Scholar]
- 11.Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 12.Ulloa-Montoya F, Verfaillie CM, Hu WS. Culture systems for pluripotent stem cells. J Biosci Bioeng. 2005;100:12–27. doi: 10.1263/jbb.100.12. [DOI] [PubMed] [Google Scholar]
- 13.Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872–884. doi: 10.1038/nrm1744. [DOI] [PubMed] [Google Scholar]
- 14.Asahina K, Fujimori H, Shimizu-Saito K, Kumashiro Y, Okamura K, Tanaka Y, Teramoto K, Arii S, Teraoka H. Expression of the liver-specific gene Cyp7a1 reveals hepatic differentiation in embryoid bodies derived from mouse embryonic stem cells. Genes Cells. 2004;9:1297–1308. doi: 10.1111/j.1365-2443.2004.00809.x. [DOI] [PubMed] [Google Scholar]
- 15.Suemori H, Tada T, Torii R, Hosoi Y, Kobayashi K, Imahie H, Kondo Y, Iritani A, Nakatsuji N. Establishment of embryonic stem cell lines from cynomolgus monkey blastocysts produced by IVF or ICSI. Dev Dyn. 2001;222:273–279. doi: 10.1002/dvdy.1191. [DOI] [PubMed] [Google Scholar]
- 16.Teratani T, Yamamoto H, Aoyagi K, Sasaki H, Asari A, Quinn G, Sasaki H, Terada M, Ochiya T. Direct hepatic fate specification from mouse embryonic stem cells. Hepatology. 2005;41:836–846. doi: 10.1002/hep.20629. [DOI] [PubMed] [Google Scholar]
- 17.Wennerberg AE, Nalesnik MA, Coleman WB. Hepatocyte paraffin 1: a monoclonal antibody that reacts with hepatocytes and can be used for differential diagnosis of hepatic tumors. Am J Pathol. 1993;143:1050–1054. [PMC free article] [PubMed] [Google Scholar]
- 18.Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA. Identification of bipotential progenitor cells in human liver development. Hepatology. 1996;23:476–481. doi: 10.1002/hep.510230312. [DOI] [PubMed] [Google Scholar]
- 19.Abe K, Niwa H, Iwase K, Takiguchi M, Mori M, Abé SI, Abe K, Yamamura KI. Endoderm-specific gene expression in embryonic stem cells differentiated to embryoid bodies. Exp Cell Res. 1996;229:27–34. doi: 10.1006/excr.1996.0340. [DOI] [PubMed] [Google Scholar]
- 20.Jones EA, Tosh D, Wilson DI, Lindsay S, Forrester LM. Hepatic differentiation of murine embryonic stem cells. Exp Cell Res. 2002;272:15–22. doi: 10.1006/excr.2001.5396. [DOI] [PubMed] [Google Scholar]
- 21.Miyashita H, Suzuki A, Fukao K, Nakauchi H, Taniguchi H. Evidence for hepatocyte differentiation from embryonic stem cells in vitro. Cell Transplant. 2002;11:429–434. [PubMed] [Google Scholar]
- 22.Chinzei R, Tanaka Y, Shimizu-Saito K, Hara Y, Kakinuma S, Watanabe M, Teramoto K, Arii S, Takase K, Sato C, et al. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36:22–29. doi: 10.1053/jhep.2002.34136. [DOI] [PubMed] [Google Scholar]
- 23.Kumashiro Y, Asahina K, Ozeki R, Shimizu-Saito K, Tanaka Y, Kida Y, Inoue K, Kaneko M, Sato T, Teramoto K, et al. Enrichment of hepatocytes differentiated from mouse embryonic stem cells as a transplantable source. Transplantation. 2005;79:550–557. doi: 10.1097/01.tp.0000153637.44069.c6. [DOI] [PubMed] [Google Scholar]
- 24.Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacham AM, Zon LI, Terada N. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett. 2001;497:15–19. doi: 10.1016/s0014-5793(01)02423-1. [DOI] [PubMed] [Google Scholar]
- 25.Kuai XL, Cong XQ, Li XL, Xiao SD. Generation of hepatocytes from cultured mouse embryonic stem cells. Liver Transpl. 2003;9:1094–1099. doi: 10.1053/jlts.2003.50207. [DOI] [PubMed] [Google Scholar]
- 26.Hu AB, Cai JY, Zheng QC, He XQ, Shan Y, Pan YL, Zeng GC, Hong A, Dai Y, Li LS. High-ratio differentiation of embryonic stem cells into hepatocytes in vitro. Liver Int. 2004;24:237–245. doi: 10.1111/j.1478-3231.2004.00910.x. [DOI] [PubMed] [Google Scholar]
- 27.Kania G, Blyszczuk P, Jochheim A, Ott M, Wobus AM. Generation of glycogen- and albumin-producing hepatocyte-like cells from embryonic stem cells. Biol Chem. 2004;385:943–953. doi: 10.1515/BC.2004.123. [DOI] [PubMed] [Google Scholar]
- 28.Jochheim A, Hillemann T, Kania G, Scharf J, Attaran M, Manns MP, Wobus AM, Ott M. Quantitative gene expression profiling reveals a fetal hepatic phenotype of murine ES-derived hepatocytes. Int J Dev Biol. 2004;48:23–29. doi: 10.1387/ijdb.15005571. [DOI] [PubMed] [Google Scholar]
- 29.Shirahashi H, Wu J, Yamamoto N, Catana A, Wege H, Wager B, Okita K, Zern MA. Differentiation of human and mouse embryonic stem cells along a hepatocyte lineage. Cell Transplant. 2004;13:197–211. doi: 10.3727/000000004783984016. [DOI] [PubMed] [Google Scholar]
- 30.Imamura T, Cui L, Teng R, Johkura K, Okouchi Y, Asanuma K, Ogiwara N, Sasaki K. Embryonic stem cell-derived embryoid bodies in three-dimensional culture system form hepatocyte-like cells in vitro and in vivo. Tissue Eng. 2004;10:1716–1724. doi: 10.1089/ten.2004.10.1716. [DOI] [PubMed] [Google Scholar]
- 31.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 32.Levinson-Dushnik M, Benvenisty N. Involvement of hepatocyte nuclear factor 3 in endoderm differentiation of embryonic stem cells. Mol Cell Biol. 1997;17:3817–3822. doi: 10.1128/mcb.17.7.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagai H, Terada K, Watanabe G, Ueno Y, Aiba N, Shibuya T, Kawagoe M, Kameda T, Sato M, Senoo H, et al. Differentiation of liver epithelial (stem-like) cells into hepatocytes induced by coculture with hepatic stellate cells. Biochem Biophys Res Commun. 2002;293:1420–1425. doi: 10.1016/S0006-291X(02)00406-0. [DOI] [PubMed] [Google Scholar]
- 34.Nitou M, Sugiyama Y, Ishikawa K, Shiojiri N. Purification of fetal mouse hepatoblasts by magnetic beads coated with monoclonal anti-e-cadherin antibodies and their in vitro culture. Exp Cell Res. 2002;279:330–343. doi: 10.1006/excr.2002.5615. [DOI] [PubMed] [Google Scholar]
- 35.Koike T, Shiojiri N. Differentiation of the mouse hepatic primordium cultured in vitro. Differentiation. 1996;61:35–43. doi: 10.1046/j.1432-0436.1996.6110035.x. [DOI] [PubMed] [Google Scholar]
- 36.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 37.Hoppo T, Fujii H, Hirose T, Yasuchika K, Azuma H, Baba S, Naito M, Machimoto T, Ikai I. Thy1-positive mesenchymal cells promote the maturation of CD49f-positive hepatic progenitor cells in the mouse fetal liver. Hepatology. 2004;39:1362–1370. doi: 10.1002/hep.20180. [DOI] [PubMed] [Google Scholar]
- 38.Ishii T, Yasuchika K, Fujii H, Hoppo T, Baba S, Naito M, Machimoto T, Kamo N, Suemori H, Nakatsuji N, et al. In vitro differentiation and maturation of mouse embryonic stem cells into hepatocytes. Exp Cell Res. 2005;309:68–77. doi: 10.1016/j.yexcr.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 39.Martin MA, Bhatia M. Analysis of the human fetal liver hematopoietic microenvironment. Stem Cells Dev. 2005;14:493–504. doi: 10.1089/scd.2005.14.493. [DOI] [PubMed] [Google Scholar]
- 40.Deryugina EI, Müller-Sieburg CE. Stromal cells in long-term cultures: keys to the elucidation of hematopoietic development. Crit Rev Immunol. 1993;13:115–150. [PubMed] [Google Scholar]
- 41.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 42.Umeda K, Heike T, Yoshimoto M, Shiota M, Suemori H, Luo HY, Chui DH, Torii R, Shibuya M, Nakatsuji N, et al. Development of primitive and definitive hematopoiesis from nonhuman primate embryonic stem cells in vitro. Development. 2004;131:1869–1879. doi: 10.1242/dev.01065. [DOI] [PubMed] [Google Scholar]
- 43.Mitjavila MT, Filippi MD, Cohen-Solal K, Le Pesteur F, Vainchenker W, Sainteny F. The Mpl-ligand is involved in the growth-promoting activity of the murine stromal cell line MS-5 on ES cell-derived hematopoiesis. Exp Hematol. 1998;26:124–134. [PubMed] [Google Scholar]
- 44.Tian X, Kaufman DS. Hematopoietic development of human embryonic stem cells in culture. Methods Mol Med. 2005;105:425–436. doi: 10.1385/1-59259-826-9:425. [DOI] [PubMed] [Google Scholar]
- 45.Tian X, Morris JK, Linehan JL, Kaufman DS. Cytokine requirements differ for stroma and embryoid body-mediated hematopoiesis from human embryonic stem cells. Exp Hematol. 2004;32:1000–1009. doi: 10.1016/j.exphem.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Qiu C, Hanson E, Olivier E, Inada M, Kaufman DS, Gupta S, Bouhassira EE. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp Hematol. 2005;33:1450–1458. doi: 10.1016/j.exphem.2005.09.003. [DOI] [PubMed] [Google Scholar]


