Abstract
The MDR1 gene is an attractive candidate gene for the pathogenesis of inflammatory bowel disease (IBD) and perhaps response to therapy, with evidences at both functional and genetic levels. Its product, the P-glycoprotein (P-gp) functions as a transmembrane efflux pump thus influencing disposition and response of many drugs, some of whom (i.e. glucocorticoids) central to IBD therapy. In addition P-gp is highly expressed in many epithelial surfaces, included gastrointestinal tract (G-I) with a putative role in decreasing the absorption of endogenous or exogenous toxins, and perhaps host-bacteria interaction. Many genetic variations of MDR1 gene has been described and in some instances evidences for different P-gp expression as well drugs metabolism have been provided. However data are often conflicting due to genetic heterogeneity and different methodologies employed. Perhaps the greatest piece of evidence of the physiological importance of P-gp in the G-I tract has come from the description of the mdr1 knock-out mice model, which develops a spontaneous colitis in a specific pathogen-free environment. Studies investigating MDR1 gene polymorphism and predisposition to IBD have also shown conflicting results, owing to the known difficulties in complex diseases, especially when the supposed genetic contribution is weak. In this study we have undertaken a meta-analysis of the available findings obtained with two SNPs polymorphism (C3435T and G2677T/A) in IBD; a significant association of 3435T allele and 3435TT genotype has been found with UC (OR = 1.17, P = 0.003 and OR = 1.36, P = 0.017, respectively). In contrast no association with CD and the G2677T/A polymorphism could be demonstrated.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Crohn’s disease, P-glycoprotein, Multidrug resistance 1 gene, Meta-analysis
INTRODUCTION
The role of P-glycoprotein (P-gp), the encoded product of the MDR1 gene, has been extensively studied as a mediator of multidrug resistance phenotype associated with certain types of cancers. Constitutive expression of this transporter in normal tissues has been shown to play an important role in drug disposition and response. More recently, genetic heterogeneity in terms of single nucleotide polymorphisms (SNPs) in this transporter has received significant attention as potential determinant of susceptibility to inflammatory bowel disease (IBD), and variability in drug efficacy in this condition.
The goal of this review is to summarize the available knowledge regarding the role of P-gp in the human gastrointestinal tract and on drug disposition process, and to focus specifically on the putative effects of the MDR1 gene polymorphisms in IBD by means of a meta-analysis of the available studies.
P-GP FUNCTION
P-gp is a member of the adenosine triphosphate (ATP)-binding cassette family that is encoded by the human ABCB1 gene (ATP-binding cassette, subfamily B), also called MDR1[1]. Human P-gp is a phosphorylated and glycosilated transmembrane protein consisting of 1280 amino acids and 2 homologous and symmetric sequences, each containing 6 transmembrane domains and an ATP-binding motif. P-gp functions as a transmembrane efflux pump, thereby moving drugs from the intracellular to the extra cellular domain; it may also interact with drug molecules trapped within the cell membrane lipid bilayer. ATP hydrolysis provides the energy for active drug transport against steep concentration gradient (like a “vacuum cleaner”)[2]. P-gp was first isolated from colchicine-resistant Chinese hamster ovary cells[3]. Subsequently the gene coding for P-gp (MDR1) was identified owing to its over expression in tumour cells associated with an acquired cross-resistance to multiple cytotoxic anticancer agents[4]. In humans, two MDR genes, MDR1 and MDR3 (also called MDR2), have been described, whereas in rodents three genes mdr1a, mdr1b, mdr2, have been identified. Subsequently, P-gp was also recognized to be expressed in many normal tissues, such as the canalicular surface of hepatocytes, the apical surface of proximal tubular cells in kidneys, and the brush borders of the enterocytes[5]. In addition, P-gp is also found in the epithelium of the brain choroids plexus (where constitutes the blood-cerebrospinal fluid barrier) as well as on the luminal surface of blood capillaries of the brain (blood-brain barrier)[6]. P-gp is also expressed in other tissues known to have blood-tissues barriers, such as placenta, ovaries, and testes[7]. Moreover, P-gp has been detected in haematopoietic stem cells, peripheral blood mononuclear cells, mature macrophage, natural killer cells, antigen-presenting dendritic cells, and T and B-lymphocytes[8].
The function and especially the anatomic localization of P-gp suggest that this transporter acts as a protective barrier to keep toxins out of the body by secreting them into bile, urine, and intestinal lumen, and thereby preventing their accumulation in critical organs[9].
P-gp IN THE GASTROINTESTINAL TRACT
In the human gastrointestinal tract (GI), P-gp is found at high concentrations on apical surfaces of superficial columnar epithelial cells of the colon and distal small bowel. High levels are also found in small biliary ductules and small pancreatic ductules[5]. In the GI tract there is a regional variation in P-gp expression with a maximal expression in the epithelial cells of the ileum and a gradual decline proximally to the jejunum, duodenum, and stomach; variations across the colon are less well defined[10]. The putative role of the P-gp in the GI tract is to decrease the absorption of endogenous and exogenous hydrophobic amphipathic toxins[11,12]. A great insight into the physiological role of P-gp protein in the GI tract has derived from the phenotype of the mdr1a gene knockout mice. This model was prompted by the finding that the MDR1 gene is present in a region of the human genome (7q21.1) that may harbour a disease gene involved in susceptibility to IBD[13]. Interestingly, the mdr1a knockout mice also develop a spontaneous colitis when maintained under specific pathogen-free conditions[14]. The colitis is prevented and reversed by the administration of antibiotics, suggesting that the intestinal flora is necessary to initiate and perpetuate the inflammation. This model implies that loss of the xenobiotic efflux mechanism may promote the development of the colitis. At histology, presence of long and dysregulated crypts, crypt abscess, and superficial mucosal inflammation has been described, a picture reminiscent of ulcerative colitis. However, transmural infiltration resembling Crohn’s disease has also been observed. More recently it has been described that Helicobacter bilis infection accelerated while Helicobacter hepaticus infection delayed the development of spontaneous colitis[15], thus suggesting that specific luminal bacteria may critically influence the development of colitis in genetically susceptible animals.
P-gp SUBSTRATES
The structure-activity relationship for P-gp substrates has yet to be clearly defined. Table 1 summarizes a comprehensive list of compounds that act as substrates, inhibitors or inducers of P-gp (modified from Marzolini et al)[9]. The range of substrates is broad and it also evident that many drugs are also substrates of the cytochrome P450 (CYP) 3A4. The overlap between CYP3A4 and P-gp is also emphasized by the genomic proximity of both genes (7q22.1 and 7q21.1 for CYP3A4 and MDR1, respectively)[16]. The co-localization of the P-gp and CYP3A4 in the small intestine and liver suggests that this transporter also plays a significant role in the oral bioavailability, distribution, and excretion of drugs. Evidence supporting such a role was obtained from animal models. A mouse strain naturally deficient in mdr1a demonstrated a marked sensitivity to the neurotoxic effects of ivermectin, an antiparasiticide[17]. Experiments with mdr1a knockout mice have revealed the P-gp limits the central nervous system entry of drugs like cyclosporine, digoxin, vinblastine[18], and the oral absorption of drug like paclitaxel[19]. Of broad clinical relevance is the interaction between digoxin and other cardiac drugs such as verapamil, quinidine, and amiodarone[20]. Of interest, several drugs central to IBD therapy are also MDR1 substrates like glucocorticoids[21,22], cyclosporine[23] and methotrexate[24].
Table 1.
Substrates (S), inhibitors (I-) and inducers (I+) of P-glycoprotein (Modified from ref. 9)
| DRUG | S | I- | I+ | DRUG | S | I- | I+ |
| Anticancer | Antiviral | ||||||
| Actinomycin D | √ | Amprenavir | √ | √ | |||
| Daunorubicin | √ | Indinavir | √ | √ | √ | ||
| Mitomicyn C | √ | Nelfinavir | √ | √ | √ | ||
| Mitoxantrone | √ | Antibiotics | |||||
| Vinblastine | √ | Claritromycin | √ | ||||
| Vincristine | √ | Erythromycin | √ | √ | |||
| Antihypertensive | Levofloxacin | √ | |||||
| Diltiazem | √ | Rifampin | √ | √ | |||
| Losartan | √ | Tetracycline | √ | ||||
| Nicardipine | √ | Antimycotics | |||||
| Talinolol | ± | Itraconazole | √ | √ | |||
| Antiarrhythmics | Ketoconazole | √ | |||||
| Amiodarone | √ | Immunosup. | |||||
| Digoxin | √ | Cyclosporine | √ | √ | |||
| Quinidine | √ | √ | Tacrolimus | √ | √ | ||
| Verapamil | √ | √ | Methotrexate | √ | |||
| Glucocorticoids | Antidepressants | ||||||
| Aldosterone | √ | Amitriptyline | √ | ||||
| Cortisol | √ | Fluoxetine | √ | ||||
| Dexamethasone | √ | √ | Neuroleptics | ||||
| Methylprednisolone | √ | Chlorpromazine | √ | ||||
| Others | Phenotiazine | √ | |||||
| Colchicine | √ | Phenobarbital | √ | ||||
| Dipyridamole | √ | Opioids | |||||
| Loperamide | √ | Methadone | √ | ||||
| Progesterone | √ | Morphine | √ | ||||
| Spironolactone | √ | Antacids | |||||
| Domperidone | √ | Ranitidine | √ | ||||
| Ondansetron | √ | Cimetidine | √ |
POLYMORPHISM IN THE HUMAN MDR1 GENE
MDR1 is located in the human chromosome 7 band p21-21.1 on a 600 kb NruI fragment[25], and the MDR1 coding region is contained on a 120 kb XhoI fragment[26]. The gene extends over more than 100 kb containing 28 introns, 26 of them interrupting the protein-coding sequence. MDR1 mRNA has a size of 4.7 kDa, thus its coding region accounts for less than 5% of the total. The first report of MDR1 polymorphism was presented in 1989[27]. To date, genetic variations of the human MDR1 gene has been extensively studied (reviewed in ref.28, 29) with 50 SNPs and 3 insertion/deletion polymorphisms reported. Moreover, several preclinical and clinical studies have provided evidence for naturally occurring polymorphisms of MDR1 gene and their effects on drug absorption, distribution, and elimination[30]. Hoffmeyer et al performed the first systematic screening for MDR1 polymorphisms in 2000[31]; in that study a synonymous SNP in exon 26 (C3435T) was the first variation to be associated with altered protein expression, although the SNP does not change the encoded amino acid (Ile). In addition, P-gp expression in the duodenum of individuals with the homozygous T allele (variant) was decreased when compared with individuals with the C allele (wild type). Other studies have shown that SNPs at exon 21 (G2677T/A) and at exon 1b (T129C) may also be associated with altered transport function or expressions[32,33]. However, a disequilibrium exists between SNPs in exon 26 (C3435T) and exon 21 (G2677T/A), suggesting that the observed differences in P-gp, initially attributed to the exon 26 SNP, may be the result of the associated polymorphism in exon 21[32]. Furthermore, several groups have subsequently confirmed the existence of such distinct haplotypes[34,35], with three common haplotypes found in more than 70% of individuals. It has also been recently shown that a synonymous SNP in exon 12 (C1236T) is linked to the C3435T and G2677T/A SNPs[36,37]. Available data indicate that the allele frequencies of these three main variants differ considerably in the various populations. Of note, marked differences of the C3435T SNP allele frequency have been observed with an increased frequency of the C allele (wild type) in African populations compared with Caucasian-Asian populations[38,39]. Similarly, the 2677A genotype of the G2677T/A SNP is significantly more common in Japanese subjects[40], while the variant 1236T of the C1236T SNP is significantly more frequent in Asian subjects compared to Caucasian subjects[41].
The study of Hoffmeyer et al[31] was the first to demonstrate a 2-fold reduction in P-gp expression in duodenal biopsy samples among healthy Caucasian subjects homozygous for the exon 26 3435T allele. Those subjects were also shown to have increased digoxin plasma concentrations after oral administration, suggesting greater drug absorption in individuals with low intestinal P-gp levels. However, the replication of this finding has been controversial with consistent[42,43] and conflicting studies[44-46]. In a study quantifying MDR1 mRNA in the duodenum, Nakamura et al[47] showed, in contrast to the Hoffmeyer’s findings, greater levels in healthy Japanese subjects carrying the 3435T allele as compared with subjects with the C3435T allele of the exon 26. This controversy, however, is not limited to the Asian population[34], as conflicting data have been noted also for others P-gp substrates including fexofenadine[32,48] cyclosporine[49-53] and tacrolimus[36,54-57]. Similarly conflicting data have also been reported for the exon 21 polymorphisms concerning the digoxin[44,45], tacrolimus[35,54,55-57], and multiple drugs[58,59] pharmacokinetics. Furthermore, conflicting data have also been obtained when evaluating the P-gp or mRNA mucosal expression with studies demonstrating reduced[31,60,48,61], increased[47] or unchanged[33,34,36,58,62-65] P-gp function for mutant C3435T SNP. Functional studies investigating the contribution of G2677T/A polymorphism also accounted for conflicting results[32-34,36,40,58,59,63-65]. Possible reasons for such discrepancies are the existence of gene-gene interaction, linkage disequilibrium between C3435T SNP and other MDR1 SNPs, and environmental factors influencing also the CYP enzyme activity. Moreover, it should be noted that regulation of P-gp expression might significantly differ from tissue to tissue. For example, the ligand-activated nuclear receptor PXR is though to be critical to P-gp expression in the liver and intestine, whereas in sites such as blood-brain barrier, PXR is unlikely to display regulatory functions. Finally, the methodology used to measure P-gp expression widely differs between studies. In addition different substrate drugs have been used in various studies, with different route of administration and potential different extent of metabolism relative to P-gp-mediated transport. For example, cyclosporine not only is transported by P-gp but also it is a substrate of CYP3A4, thus a potential P-GP effect may be masked by CYP3A4 activity.
MDR1 AND INFLAMMATORY BOWEL DISEASE
The MDR1 gene is an attractive candidate gene for the pathogenesis of IBD and perhaps response to therapy, with evidences at both functional and genetic levels[66]. In a German case-control study Schwab et al investigating the C3435T polymorphism firstly reported an increase of the T allele and TT genotype frequencies in 149 patients with ulcerative colitis, but not Crohn’s disease, compared with controls (P = 0.049, OR = 1.4; P = 0.005, OR = 2.1, respectively)[67]. Subsequent studies, however, have gained conflicting results: Glas et al[68] in a small group of 123 patients with UC found results in partial accordance with a trend towards an increased frequency of T allele compared to healthy controls, but a statistical difference was obtained only in one of two different control groups (T allele, P = 0.018; TT genotype, P = 0.016), thus suggesting the key factor of patients and controls recruitment. In a small Greek study[69] no difference was found in both UC and CD patients, but this study has been criticized because the investigated populations were not in Hardy-Weinberg equilibrium. A large study[70] performed in a German and British cohort investigating 307 UC and 564 CD patients by using both a case-control strategy and a transmission disequilibrium test based analysis (which is more resilient to latent effects such as population stratification), failed to demonstrate an association, even after stratifying individuals on the basis of CARD15 gene variants. More recently Ho et al[71] confirmed the association in UC patients (P = 0.04, OR 1.6 for TT genotype), especially those with extensive colitis (P = 0.003, OR = 2.64), but in contrast completely negative findings have been reported in large studies from North America[72], Slovenia[73] and Italy[74]. In particular in the latter study from our Institution, about one thousand IBD patients have been genotyped (Tables 2 and 3) with negative results. In addition, Urcelay et al[75] paradoxically found a significant association of the CC3435 genotype (wild) in CD patients (P = 0.007), but data were not in Hardy-Weinberg equilibrium.
Table 2.
Distribution of C3435T alleles and genotypes (cases and controls) in the existing literature
| C | T | CC | CT | TT | |
| Glas, 2003 | |||||
| UC = 1231 | 111 (45) | 135 (55) | 19 (15) | 73 (60) | 31 (25) |
| CD = 135 | 130 (48) | 140 (52) | 26 (19) | 78 (58) | 31 (23) |
| IBD = 258 | 241 (47) | 275 (53) | 45 (17) | 151 (59) | 62 (24) |
| HC = 265 | 272 (51) | 258 (49) | 70 (26) | 132 (50) | 63 (24) |
| Schwab, 2003 | C | T | CC | CT | TT |
| UCa = 149 | 129 (43) | 169 (57) | 26 (17) | 77 (52) | 46 (31) |
| HC = 149 | 154 (52) | 144 (48) | 39 (26) | 76 (51) | 34 (23) |
| CD = 126 | 134 (53) | 118 (47) | 33 (26) | 68 (54) | 25 (20) |
| HC = 126 | 128 (51) | 124 (49) | 35 (28) | 58 (46) | 33 (26) |
| IBD = 275 | 263 (48) | 287 (52) | 59 (21) | 145 (53) | 71 (26) |
| HC = 275 | 282 (51) | 268 (49) | 74 (27) | 134 (49) | 67 (24) |
| Ho, 2005 | C | T | CC | CT | TT |
| UCc = 335 | 280 (42) | 390 (58) | 61 (18) | 158 (47) | 116 (35) |
| CD = 268 | 252 (47) | 284 (53) | 56 (21) | 140 (52) | 72 (27) |
| IBD = 603 | 532 (44) | 674 (56) | 117 (19) | 298 (50) | 188 (31) |
| HC = 370 | 354 (48) | 386 (52) | 82 (22) | 190 (51) | 98 (27) |
| Palmieri, 2005 | C | T | CC | CT | TT |
| UC = 468 | 488 (52) | 448 (48) | 124 (27) | 240 (51) | 104 (22) |
| CD = 478 | 503 (53) | 453 (47) | 125 (26) | 253 (53) | 100 (21) |
| IBD = 946 | 991 (52) | 901 (48) | 249 (26) | 493 (52) | 204 (22) |
| HC = 450 | 470 (52) | 430 (48) | 115 (26) | 240 (53) | 95 (21) |
| Potocnik, 2004 | C | T | CC | CT | TT |
| UC = 144 | 134 (47) | 154 (53) | - | - | - |
| CD = 163 | 161 (49) | 165 (51) | - | - | - |
| IBD = 307 | 295 (48) | 319 (52) | |||
| HC = 355 | 376 (53) | 334 (47) | - | - | - |
| Urcelay, 2006 | |||||
| UC = 311 | 317 (51) | 305 (49) | 87 (28) | 143 (46) | 81 (26) |
| CDb = 3031 | 369 (61) | 237 (39) | 122 (40) | 125 (41) | 56 (19) |
| IBD = 614 | 686 (56) | 542 (44) | 209 (34) | 268 (44) | 137 (22) |
| HC = 324 | 344 (53) | 304 (47) | 97 (30) | 150 (46) | 77 (24) |
UC = Ulcerative colitis, CD = Crohn’s disease, HC = healthy controls. aP = 0.045 (T vs C); P = 0.049 (TT vs CC). cP = 0.02 (T vs C); P = 0.04 (TT vs CC). bP = 0.006 (T vs C); P = 0.01 (TT vs CC). 1 Subjects not in Hardy-Weinberg equilibrium.
Table 3.
Distribution of G2677T/A alleles and genotypes (cases and controls) in the existing literature
| A | G | T | AA | GG | GT | TT | GA | TA | |
| Brant, 2003 | |||||||||
| IBD_NJa = 211 | 2 (0.5) | 254 (60.2) | 166 (39.3) | - | 76 (36) | 101 (48) | 32 (15) | 1 (0.5) | 1 (0.5) |
| HC_NJ = 392 | 20 (2.6) | 411 (52.4) | 353 (45) | - | 108 (27) | 183 (47) | 81 (21) | 12 (3) | 8 (2) |
| IBD_J = 114 | 1 (0.5) | 143 (62.7) | 84 (36.8) | - | 41 (36) | 60 (53) | 12 (10) | 1 (1) | 0 |
| HC_J = 219 | 13 (3) | 265 (60.5) | 160 (36.5) | - | 85 (39) | 89 (41) | 32 (15) | 6 (2) | 7 (3) |
| Ho, 2005 | A | G | T | AA | GG | GT | TT | GA | TA |
| UC = 335 | - | 366 (54.6) | 304 (45.4) | - | 95 (28.3) | 176 (52.5) | 64 (19.1) | - | - |
| CD = 268 | - | 283 (52.8) | 253 (47.2) | - | 75 (27.9) | 133 (47.8) | 60 (22.4) | - | - |
| IBD = 603 | - | 649 (53.8) | 557 (46.2) | - | 170 (28.2) | 309 (51.2) | 124 (20.6) | - | - |
| HC = 370 | - | 378 (51.2) | 362 (49.8) | - | 102 (27.6) | 174 (47.0) | 94 (25.4) | - | - |
| Palmieri, 2005 | A | G | T | AA | GG | GT | TT | GA | TA |
| UC = 468 | 23 (2.5) | 494 (52.8) | 419 (44.7) | 1 (0.2) | 127 (27.1) | 229 (49.0) | 90 (19.2) | 11 (2.4) | 10 (2.1) |
| CD = 478 | 15 (1.6) | 534 (55.9) | 407 (42.5) | 0 (0.0) | 143 (29.9) | 237 (49.6) | 83 (17.4) | 11 (2.3) | 4 (0.8) |
| IBD = 946 | 38 (2) | 1028 (54) | 826 (44) | 1 (0.1) | 270 (29.5) | 466 (49.2) | 173 (18.3) | 22 (2.3) | 14 (1.5) |
| HC = 450 | 19 (2.1) | 500 (55.6) | 381 (42.3) | 0 (0.0) | 126 (28.0) | 236 (52.4) | 69 (15.3) | 12 (2.7) | 7 (1.6) |
| Potocnik, 2004 | A & G | T | |||||||
| UC = 144 | - | 157 (54) | 131 (46) | - | - | - | - | - | - |
| CD = 163 | - | 187 (57) | 139 (43) | - | - | - | - | - | - |
| IBD = 307 | - | 344 (56) | 270 (44) | - | - | - | - | - | - |
| HC = 355 | - | 424 (59.7) | 286 (40.3) | - | - | - | - | - | - |
| Urcelay, 2006 | |||||||||
| UC = 311 | 4 (0.7) | 372 (62.8) | 216 (36.5) | 0 (0) | 118 (39.9) | 133 (44.9) | 41 (13.9) | 3 (1) | 1 (0.3) |
| CD = 303 | 6 (0.9) | 403 (63.2) | 229 (35.9) | 0 (0) | 139 (43.6) | 122 (38.3) | 52 (16.3) | 3 (0.9) | 3 (0.9) |
| IBD = 615 | 10 (0.8) | 775 (63) | 445 (36.2) | 0 (0) | 257 (41.8) | 255 (41.5) | 93 (16.1) | 6 (0.9) | 4 (0.7) |
| HC = 352 | 8 (1.1) | 426 (60.5) | 270 (38.4) | 0 (0) | 140 (39.8) | 142 (40.3) | 62 (17.6) | 4 (1.1) | 4 (1.1) |
aP = 0.03 (G vs T); P = 0.08 (GG vs GT TT).
In a multicenter North American cohort with 444 IBD trios, Brant et al[72] investigated also another polymorphism of MDR1, the tri-allelic G2677T/A SNP (Ala893Ser/Thr). They found a significant association of the Ala893 variant (G2677), known to decrease transporter function, with IBD in both case-control analysis (P = 0.002) and the pedigree disequilibrium test (PDT) (P = 0.0002), especially in non-Jewish subjects. The association was confirmed by PDT analysis within the CD subset (P = 0.001) with a similar, non-significant trend in the small subset of UC patients. Curiously, an association with another allele (T allele, 893Ser variant) of this SNP was found in UC patients (P = 0.029) by Potočnik et al[73]. Ho et al[71], did not find an association between this polymorphism and IBD; however, a 2-locus haplotype (3435T/G2677) was significantly associated with UC in this study (P = 0.03). Urcelay et al[75] similarly did not found a correlation between this polymorphism and IBD, however, a trend towards an increased frequency of 2-locus 2677T/C3435 haplotype was found in CD patients. In contrast, more recently Palmieri et al[74] did not found any association of this polymorphism and 2-locus haplotype with IBD patients (Table 3). Reasons for this discrepancy may lie in population heterogeneity, sample size, selection of control population, incomplete phenotype description, and difficulty to replicate results in case control study when the contribution of the genetic predisposing factor under investigation is weak. Accordingly, when looking for example at the existing data on C3435T polymorphism, it is apparent that the 5%-8% difference of the “risk” allele and genotype frequencies in UC patients and their significance (P values ranging from 0.02 to 0.049) are rather modest. More importantly, there is a significant heterogeneity in patients (T allele frequency ranging from 42.3% up to 65.3% in UC) and especially control populations (T allele frequency ranging from 35.7% up to 63%).
The hypothesis that altered PGP expression in IBD patients could modify the response to medical therapy was put forward by Farrell et al[76]. Peripheral blood lymphocytes (PBL) from patients with active Crohn’s disease (CD) and Ulcerative colitis (UC) in whom medical therapy had failed and surgical intervention had been necessary were shown to have higher expression of P-gp glycoprotein compared with those from patients who had inactive disease and required surgery for obstruction or dysplasia. Based on this observation the authors speculated that poor response to glucocorticoids in IBD might relate in part to constitutive MDR1 expression. We have recently challenged this hypothesis in a large cohort of IBD patients[74] using steroids, by evaluating both C3435T and G2677T/A polymorphisms. Allele and genotype frequencies were compared in 594 patients using systemic steroids, sub-grouped in 320 responders, 76 non-responders and 198 steroid-dependent. No significant differences were found within subgroups and between subgroups and 450 healthy subjects. Moreover, 297 patients taking immunosuppressive drugs (included azathioprine, 6-mercaptopurine, methotrexate, and cyclosporine) were also evaluated. No influence of MDR1 genotypes was found with respect to response to therapy. Similarly, in a recent study by Mc Govern et al in which the need for colectomy in UC patients was used as a surrogate marker for glucocorticoids resistance, no association was found with C3435T polymorphism[77].
Although the Farrell’s hypothesis is attractive, data from our study strongly question the influence of MDR1 gene on response to steroid therapy in IBD patients. There are, indeed, also some caveats to the Farrell’s study; firstly, circulating lymphocytes consist of different subsets of lymphocytes which express MDR1 at varying levels, since it is now established that lymphocytes homing to the gut associated lymphoid tissue are phenotypically distinct from circulatory ones. Accordingly, Yacyshyn et al[78] have demonstrated different level of MDR1 expression and activity in intraepithelial, lamina propria, and PBL. Overall, intraepithelial MDR1 expression and function were lower in UC compared with CD and healthy controls. Accordingly, Langman et al found a down-regulation of MDR1 tissue expression in UC patients[79]. Moreover, the effect of corticosteroids or other treatment used in IBD on MDR1 expression it is not fully established. It remains therefore to be evaluated whether different expression of P-gp reflects a secondary phenomenon to therapy or a primary one, influencing the response to treatment. Finally, increased expression does not necessarily imply increased function in diseased states. Based on the available evidence, the contribution of MDR1 gene on IBD predisposition and response to medical therapy, although biologically plausible is still unproven.
META-ANALYSIS
In the attempt to evaluate the potential association of C3435T and G2677T/A polymorphisms with IBD, a meta-analysis has been performed. Because case-control studies were included, the odds ratios (ORs) were employed. For each outcome, the between-study heterogeneity across all the eligible comparisons has been performed by using the chi-square based Q statistic; heterogeneity was considered significant for p lower than 0.10. Data were combined by using both fixed and random effects models; random effects are more appropriate when heterogeneity is present. Analysis was conducted by Comprehensive Meta Analysis v.1.0.23, 1999 Biostat software (www.meta-analysis.com).
In Tables 2 and 3 are depicted the characteristics of the studies included in the meta-analysis, with the allele and genotype frequencies. The study by Gazouli et al[69] has been excluded due to failure of Hardy-Weinberg equilibrium. The study of Croucher et al[70] was also excluded due to lack of data concerning allele and genotype frequencies, also upon specific request to the authors. For the study of Brant et al[72] data are expressed as whole IBD, since no complete information for UC and CD subgroups were available.
When comparing allele (T vs C) and genotype frequencies (TT vs CC) of the C3435T SNP in CD and UC patients, a slight but significant difference in the pooled data was found in UC patients (Table 4). More specifically a significant association with T allele (OR 1.17, CI 1.06-1.31, P = 0.003) and TT genotype (OR=1.36, CI 1.05-1.76, P = 0.017) was demonstrated. In contrast no association in CD patients was found and in IBD patients as whole. Similarly no significant difference was found after pooling data of the available studies for allele and genotype frequencies of the G2677T/A SNP (Figures 1 and 2).
Table 4.
Summary of ORs and 95% CIs for different outcomes obtained at the meta-analysis
|
Fixed effects |
Random effects |
Heterogeneity test | ||||
| P value | OR (95% CI) | P value | OR (95% CI) | |||
| C3435T | Outcome CD T vs C (6 studies) | 0.519 | 0.968 (0.878-1.068) | 0.722 | 0.974 (0.842-1.126) | 0.09 |
| TT vs CC (5 studies) | 0.297 | 0.892 (0.720-1.106) | 0.472 | 0.900 (0.675-1.200) | NS | |
| G2677T/A | G vs T (4 studies) | 0.635 | 1.027 (0.920-1.145) | 0.635 | 1.027 (0.920-1.145) | NS |
| GG vs GT TT (3 studies) Outcome UC | 0.338 | 1.092 (0.912-1.308) | 0.338 | 1.092 (0.912-1.308) | NS | |
| C3435T | T vs C (6 studies) | 0.002 | 1.170 (1.062-1.289) | 0.003 | 1.178 (1.058-1.311) | NS |
| TT vs CC (5 studies) | 0.008 | 1.332 (1.080-1.644) | 0.017 | 1.367 (1.057-1.768) | NS | |
| G2677T/A | G vs T (4 studies) | 0.843 | 0.989 (0.887-1.103) | 0.862 | 0.986 (0.836-1.162) | NS |
| GG vs GT TT (3 studies) Outcome IBD | 0.947 | 0.994 (0.830-1.190) | 0.947 | 0.994 (0.830-1.190) | NS | |
| C3435T | T vs C (6 studies) | 0.083 | 1.074 (0.991-1.165) | 0.135 | 1.083 (0.976-1.201) | NS |
| TT vs CC (5 studies) | 0.225 | 1.116 (0.935-1.332) | 0.274 | 1.135 (0.904-1.426) | NS | |
| G2677T/A | G vs T (5 studies) | 0.351 | 1.041 (0.957-1.132) | 0.448 | 1.047 (0.930-1.178) | NS |
| GG vs GT TT (4 studies) | 0.366 | 1.065 (0.929-1.221) | 0.366 | 1.065 (0.929-1.221) | NS | |
Figure 1.
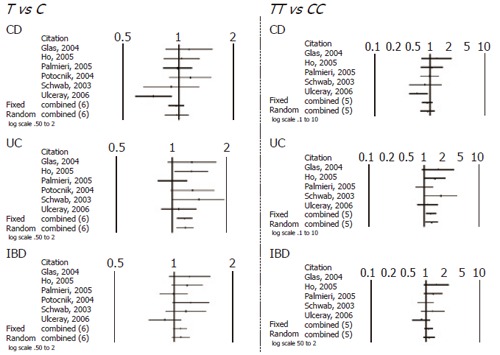
Schematic representation of studies included in the meta-analysis of C3435T SNP.
Figure 2.
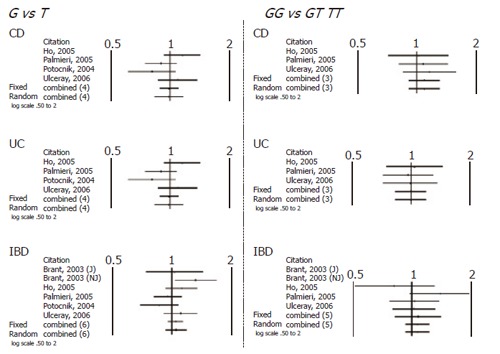
Schematic representation of studies included in the meta-analysis of G2677T/A SNP.
ACKNOWLEDGMENTS
Ms Ermelinda De Santo, Ms Tiziana Latiano, Ms Carla Zagaria and Mr Giuseppe Corritore for their skilful technical support; Dr. Lazzaro di Mauro of the Medicina Trasfusionale OO RR Foggia for providing blood donors DNA samples.
Footnotes
Supported by a grant from the Health Minister of Health N° RF03GA01, RC0503GA20
S- Editor Pan BR E- Editor Ma WH
References
- 1.Schinkel AH. The physiological function of drug-transporting P-glycoproteins. Semin Cancer Biol. 1997;8:161–170. doi: 10.1006/scbi.1997.0068. [DOI] [PubMed] [Google Scholar]
- 2.Higgins CF, Gottesman MM. Is the multidrug transporter a flippase. Trends Biochem Sci. 1992;17:18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- 3.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 4.Ueda K, Cardarelli C, Gottesman MM, Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987;84:3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugawara I, Kataoka I, Morishita Y, Hamada H, Tsuruo T, Itoyama S, Mori S. Tissue distribution of P-glycoprotein encoded by a multidrug-resistant gene as revealed by a monoclonal antibody, MRK 16. Cancer Res. 1988;48:1926–1929. [PubMed] [Google Scholar]
- 8.Klimecki WT, Futscher BW, Grogan TM, Dalton WS. P-glycoprotein expression and function in circulating blood cells from normal volunteers. Blood. 1994;83:2451–2458. [PubMed] [Google Scholar]
- 9.Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75:13–33. doi: 10.1016/j.clpt.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Chianale J, Vollrath V, Wielandt AM, Miranda S, Gonzalez R, Fresno AM, Quintana C, Gonzalez S, Andrade L, Guzman S. Differences between nuclear run-off and mRNA levels for multidrug resistance gene expression in the cephalocaudal axis of the mouse intestine. Biochim Biophys Acta. 1995;1264:369–376. doi: 10.1016/0167-4781(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 11.Fricker G, Drewe J, Huwyler J, Gutmann H, Beglinger C. Relevance of p-glycoprotein for the enteral absorption of cyclosporin A: in vitro-in vivo correlation. Br J Pharmacol. 1996;118:1841–1847. doi: 10.1111/j.1476-5381.1996.tb15612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987;84:265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satsangi J, Parkes M, Louis E, Hashimoto L, Kato N, Welsh K, Terwilliger JD, Lathrop GM, Bell JI, Jewell DP. Two stage genome-wide search in inflammatory bowel disease provides evidence for susceptibility loci on chromosomes 3, 7 and 12. Nat Genet. 1996;14:199–202. doi: 10.1038/ng1096-199. [DOI] [PubMed] [Google Scholar]
- 14.Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161:5733–5744. [PubMed] [Google Scholar]
- 15.Shomer NH, Dangler CA, Marini RP, Fox JG. Helicobacter bilis/Helicobacter rodentium co-infection associated with diarrhea in a colony of scid mice. Lab Anim Sci. 1998;48:455–459. [PubMed] [Google Scholar]
- 16.Kim R, Wilkinson G. Pharmacogenetics of drug transporters. In: Kalow W, Meyer UA, Tyndale RF, editors. Pharmacogenomics. New York: Marcel Dekker; 2001. pp. 81–108. [Google Scholar]
- 17.Lankas GR, Cartwright ME, Umbenhauer D. P-glycoprotein deficiency in a subpopulation of CF-1 mice enhances avermectin-induced neurotoxicity. Toxicol Appl Pharmacol. 1997;143:357–365. doi: 10.1006/taap.1996.8086. [DOI] [PubMed] [Google Scholar]
- 18.Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, Borst P, Nooijen WJ, Beijnen JH, van Tellingen O. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci U S A. 1997;94:2031–2035. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verschraagen M, Koks CH, Schellens JH, Beijnen JH. P-glycoprotein system as a determinant of drug interactions: the case of digoxin-verapamil. Pharmacol Res. 1999;40:301–306. doi: 10.1006/phrs.1999.0535. [DOI] [PubMed] [Google Scholar]
- 21.Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, Komano T, Hori R. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem. 1992;267:24248–24252. [PubMed] [Google Scholar]
- 22.Bourgeois S, Gruol DJ, Newby RF, Rajah FM. Expression of an mdr gene is associated with a new form of resistance to dexamethasone-induced apoptosis. Mol Endocrinol. 1993;7:840–851. doi: 10.1210/mend.7.7.8105374. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhary PM, Mechetner EB, Roninson IB. Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes. Blood. 1992;80:2735–2739. [PubMed] [Google Scholar]
- 24.Norris MD, De Graaf D, Haber M, Kavallaris M, Madafiglio J, Gilbert J, Kwan E, Stewart BW, Mechetner EB, Gudkov AV, et al. Involvement of MDR1 P-glycoprotein in multifactorial resistance to methotrexate. Int J Cancer. 1996;65:613–619. doi: 10.1002/(SICI)1097-0215(19960301)65:5<613::AID-IJC10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Callen DF, Baker E, Simmers RN, Seshadri R, Roninson IB. Localization of the human multiple drug resistance gene, MDR1, to 7q21.1. Hum Genet. 1987;77:142–144. doi: 10.1007/BF00272381. [DOI] [PubMed] [Google Scholar]
- 26.Chin JE, Soffir R, Noonan KE, Choi K, Roninson IB. Structure and expression of the human MDR (P-glycoprotein) gene family. Mol Cell Biol. 1989;9:3808–3820. doi: 10.1128/mcb.9.9.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kioka N, Tsubota J, Kakehi Y, Komano T, Gottesman MM, Pastan I, Ueda K. P-glycoprotein gene (MDR1) cDNA from human adrenal: normal P-glycoprotein carries Gly185 with an altered pattern of multidrug resistance. Biochem Biophys Res Commun. 1989;162:224–231. doi: 10.1016/0006-291x(89)91985-2. [DOI] [PubMed] [Google Scholar]
- 28.Kerb R, Hoffmeyer S, Brinkmann U. ABC drug transporters: hereditary polymorphisms and pharmacological impact in MDR1, MRP1 and MRP2. Pharmacogenomics. 2001;2:51–64. doi: 10.1517/14622416.2.1.51. [DOI] [PubMed] [Google Scholar]
- 29.Sparreboom A, Danesi R, Ando Y, Chan J, Figg WD. Pharmacogenomics of ABC transporters and its role in cancer chemotherapy. Drug Resist Updat. 2003;6:71–84. doi: 10.1016/s1368-7646(03)00005-0. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa T, Onishi Y, Hirano H, Oosumi K, Nagakura M, Tarui S. Pharmacogenomics of drug transporters: a new approach to functional analysis of the genetic polymorphisms of ABCB1 (P-glycoprotein/MDR1) Biol Pharm Bull. 2004;27:939–948. doi: 10.1248/bpb.27.939. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97:3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–199. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 33.Tanabe M, Ieiri I, Nagata N, Inoue K, Ito S, Kanamori Y, Takahashi M, Kurata Y, Kigawa J, Higuchi S, et al. Expression of P-glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)-1 gene. J Pharmacol Exp Ther. 2001;297:1137–1143. [PubMed] [Google Scholar]
- 34.Siegmund W, Ludwig K, Giessmann T, Dazert P, Schroeder E, Sperker B, Warzok R, Kroemer HK, Cascorbi I. The effects of the human MDR1 genotype on the expression of duodenal P-glycoprotein and disposition of the probe drug talinolol. Clin Pharmacol Ther. 2002;72:572–583. doi: 10.1067/mcp.2002.127739. [DOI] [PubMed] [Google Scholar]
- 35.Gaikovitch EA, Cascorbi I, Mrozikiewicz PM, Brockmöller J, Frötschl R, Köpke K, Gerloff T, Chernov JN, Roots I. Polymorphisms of drug-metabolizing enzymes CYP2C9, CYP2C19, CYP2D6, CYP1A1, NAT2 and of P-glycoprotein in a Russian population. Eur J Clin Pharmacol. 2003;59:303–312. doi: 10.1007/s00228-003-0606-2. [DOI] [PubMed] [Google Scholar]
- 36.Goto M, Masuda S, Saito H, Uemoto S, Kiuchi T, Tanaka K, Inui K. C3435T polymorphism in the MDR1 gene affects the enterocyte expression level of CYP3A4 rather than Pgp in recipients of living-donor liver transplantation. Pharmacogenetics. 2002;12:451–457. doi: 10.1097/00008571-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Illmer T, Schuler US, Thiede C, Schwarz UI, Kim RB, Gotthard S, Freund D, Schäkel U, Ehninger G, Schaich M. MDR1 gene polymorphisms affect therapy outcome in acute myeloid leukemia patients. Cancer Res. 2002;62:4955–4962. [PubMed] [Google Scholar]
- 38.Schaeffeler E, Eichelbaum M, Brinkmann U, Penger A, Asante-Poku S, Zanger UM, Schwab M. Frequency of C3435T polymorphism of MDR1 gene in African people. Lancet. 2001;358:383–384. doi: 10.1016/S0140-6736(01)05579-9. [DOI] [PubMed] [Google Scholar]
- 39.Ameyaw MM, Regateiro F, Li T, Liu X, Tariq M, Mobarek A, Thornton N, Folayan GO, Githang'a J, Indalo A, et al. MDR1 pharmacogenetics: frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics. 2001;11:217–221. doi: 10.1097/00008571-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Moriya Y, Nakamura T, Horinouchi M, Sakaeda T, Tamura T, Aoyama N, Shirakawa T, Gotoh A, Fujimoto S, Matsuo M, et al. Effects of polymorphisms of MDR1, MRP1, and MRP2 genes on their mRNA expression levels in duodenal enterocytes of healthy Japanese subjects. Biol Pharm Bull. 2002;25:1356–1359. doi: 10.1248/bpb.25.1356. [DOI] [PubMed] [Google Scholar]
- 41.Tang K, Ngoi SM, Gwee PC, Chua JM, Lee EJ, Chong SS, Lee CG. Distinct haplotype profiles and strong linkage disequilibrium at the MDR1 multidrug transporter gene locus in three ethnic Asian populations. Pharmacogenetics. 2002;12:437–450. doi: 10.1097/00008571-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Johne A, Köpke K, Gerloff T, Mai I, Rietbrock S, Meisel C, Hoffmeyer S, Kerb R, Fromm MF, Brinkmann U, et al. Modulation of steady-state kinetics of digoxin by haplotypes of the P-glycoprotein MDR1 gene. Clin Pharmacol Ther. 2002;72:584–594. doi: 10.1067/mcp.2002.129196. [DOI] [PubMed] [Google Scholar]
- 43.Verstuyft C, Schwab M, Schaeffeler E, Kerb R, Brinkmann U, Jaillon P, Funck-Brentano C, Becquemont L. Digoxin pharmacokinetics and MDR1 genetic polymorphisms. Eur J Clin Pharmacol. 2003;58:809–812. doi: 10.1007/s00228-003-0567-5. [DOI] [PubMed] [Google Scholar]
- 44.Gerloff T, Schaefer M, Johne A, Oselin K, Meisel C, Cascorbi I, Roots I. MDR1 genotypes do not influence the absorption of a single oral dose of 1 mg digoxin in healthy white males. Br J Clin Pharmacol. 2002;54:610–616. doi: 10.1046/j.1365-2125.2002.01691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horinouchi M, Sakaeda T, Nakamura T, Morita Y, Tamura T, Aoyama N, Kasuga M, Okumura K. Significant genetic linkage of MDR1 polymorphisms at positions 3435 and 2677: functional relevance to pharmacokinetics of digoxin. Pharm Res. 2002;19:1581–1585. doi: 10.1023/a:1020433422259. [DOI] [PubMed] [Google Scholar]
- 46.Sakaeda T, Nakamura T, Horinouchi M, Kakumoto M, Ohmoto N, Sakai T, Morita Y, Tamura T, Aoyama N, Hirai M, et al. MDR1 genotype-related pharmacokinetics of digoxin after single oral administration in healthy Japanese subjects. Pharm Res. 2001;18:1400–1404. doi: 10.1023/a:1012244520615. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura T, Sakaeda T, Horinouchi M, Tamura T, Aoyama N, Shirakawa T, Matsuo M, Kasuga M, Okumura K. Effect of the mutation (C3435T) at exon 26 of the MDR1 gene on expression level of MDR1 messenger ribonucleic acid in duodenal enterocytes of healthy Japanese subjects. Clin Pharmacol Ther. 2002;71:297–303. doi: 10.1067/mcp.2002.122055. [DOI] [PubMed] [Google Scholar]
- 48.Drescher S, Schaeffeler E, Hitzl M, Hofmann U, Schwab M, Brinkmann U, Eichelbaum M, Fromm MF. MDR1 gene polymorphisms and disposition of the P-glycoprotein substrate fexofenadine. Br J Clin Pharmacol. 2002;53:526–534. doi: 10.1046/j.1365-2125.2002.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chowbay B, Cumaraswamy S, Cheung YB, Zhou Q, Lee EJ. Genetic polymorphisms in MDR1 and CYP3A4 genes in Asians and the influence of MDR1 haplotypes on cyclosporin disposition in heart transplant recipients. Pharmacogenetics. 2003;13:89–95. doi: 10.1097/00008571-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Balram C, Sharma A, Sivathasan C, Lee EJ. Frequency of C3435T single nucleotide MDR1 genetic polymorphism in an Asian population: phenotypic-genotypic correlates. Br J Clin Pharmacol. 2003;56:78–83. doi: 10.1046/j.1365-2125.2003.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Ahsen N, Richter M, Grupp C, Ringe B, Oellerich M, Armstrong VW. No influence of the MDR-1 C3435T polymorphism or a CYP3A4 promoter polymorphism (CYP3A4-V allele) on dose-adjusted cyclosporin A trough concentrations or rejection incidence in stable renal transplant recipients. Clin Chem. 2001;47:1048–1052. [PubMed] [Google Scholar]
- 52.Min DI, Ellingrod VL. C3435T mutation in exon 26 of the human MDR1 gene and cyclosporine pharmacokinetics in healthy subjects. Ther Drug Monit. 2002;24:400–404. doi: 10.1097/00007691-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Yates CR, Zhang W, Song P, Li S, Gaber AO, Kotb M, Honaker MR, Alloway RR, Meibohm B. The effect of CYP3A5 and MDR1 polymorphic expression on cyclosporine oral disposition in renal transplant patients. J Clin Pharmacol. 2003;43:555–564. [PubMed] [Google Scholar]
- 54.Yamauchi A, Ieiri I, Kataoka Y, Tanabe M, Nishizaki T, Oishi R, Higuchi S, Otsubo K, Sugimachi K. Neurotoxicity induced by tacrolimus after liver transplantation: relation to genetic polymorphisms of the ABCB1 (MDR1) gene. Transplantation. 2002;74:571–572. doi: 10.1097/00007890-200208270-00024. [DOI] [PubMed] [Google Scholar]
- 55.Anglicheau D, Verstuyft C, Laurent-Puig P, Becquemont L, Schlageter MH, Cassinat B, Beaune P, Legendre C, Thervet E. Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol. 2003;14:1889–1896. doi: 10.1097/01.asn.0000073901.94759.36. [DOI] [PubMed] [Google Scholar]
- 56.Macphee IA, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, Goldberg L, Holt DW. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P-glycoprotein correlate with dose requirement. Transplantation. 2002;74:1486–1489. doi: 10.1097/00007890-200212150-00002. [DOI] [PubMed] [Google Scholar]
- 57.Zheng H, Webber S, Zeevi A, Schuetz E, Zhang J, Bowman P, Boyle G, Law Y, Miller S, Lamba J, et al. Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am J Transplant. 2003;3:477–483. doi: 10.1034/j.1600-6143.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 58.Morita N, Yasumori T, Nakayama K. Human MDR1 polymorphism: G2677T/A and C3435T have no effect on MDR1 transport activities. Biochem Pharmacol. 2003;65:1843–1852. doi: 10.1016/s0006-2952(03)00178-3. [DOI] [PubMed] [Google Scholar]
- 59.Kimchi-Sarfaty C, Gribar JJ, Gottesman MM. Functional characterization of coding polymorphisms in the human MDR1 gene using a vaccinia virus expression system. Mol Pharmacol. 2002;62:1–6. doi: 10.1124/mol.62.1.1. [DOI] [PubMed] [Google Scholar]
- 60.Fellay J, Marzolini C, Meaden ER, Back DJ, Buclin T, Chave JP, Decosterd LA, Furrer H, Opravil M, Pantaleo G, et al. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet. 2002;359:30–36. doi: 10.1016/S0140-6736(02)07276-8. [DOI] [PubMed] [Google Scholar]
- 61.Hitzl M, Drescher S, van der Kuip H, Schäffeler E, Fischer J, Schwab M, Eichelbaum M, Fromm MF. The C3435T mutation in the human MDR1 gene is associated with altered efflux of the P-glycoprotein substrate rhodamine 123 from CD56+ natural killer cells. Pharmacogenetics. 2001;11:293–298. doi: 10.1097/00008571-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 62.van der Heiden IP, van der Heuvel MM, Wioemer E, Pieters R, Lindemans J, van der Anker J. MDR1 C3435T gene polymorphism does not correlate with P-gp expression and function in acute myeloid leukemia. Clin Pharmacol Ther. 2003;75:58 (abstract). [Google Scholar]
- 63.Oselin K, Gerloff T, Mrozikiewicz PM, Pähkla R, Roots I. MDR1 polymorphisms G2677T in exon 21 and C3435T in exon 26 fail to affect rhodamine 123 efflux in peripheral blood lymphocytes. Fundam Clin Pharmacol. 2003;17:463–469. doi: 10.1046/j.1472-8206.2003.00163.x. [DOI] [PubMed] [Google Scholar]
- 64.Calado RT, Falcão RP, Garcia AB, Gabellini SM, Zago MA, Franco RF. Influence of functional MDR1 gene polymorphisms on P-glycoprotein activity in CD34+ hematopoietic stem cells. Haematologica. 2002;87:564–568. [PubMed] [Google Scholar]
- 65.Oselin K, Nowakowski-Gashaw I, Mrozikiewicz PM, Wolbergs D, Pähkla R, Roots I. Quantitative determination of MDR1 mRNA expression in peripheral blood lymphocytes: a possible role of genetic polymorphisms in the MDR1 gene. Eur J Clin Invest. 2003;33:261–267. doi: 10.1046/j.1365-2362.2003.01133.x. [DOI] [PubMed] [Google Scholar]
- 66.Ho GT, Moodie FM, Satsangi J. Multidrug resistance 1 gene (P-glycoprotein 170): an important determinant in gastrointestinal disease. Gut. 2003;52:759–766. doi: 10.1136/gut.52.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwab M, Schaeffeler E, Marx C, Fromm MF, Kaskas B, Metzler J, Stange E, Herfarth H, Schoelmerich J, Gregor M, et al. Association between the C3435T MDR1 gene polymorphism and susceptibility for ulcerative colitis. Gastroenterology. 2003;124:26–33. doi: 10.1053/gast.2003.50010. [DOI] [PubMed] [Google Scholar]
- 68.Glas J, Török HP, Schiemann U, Folwaczny C. MDR1 gene polymorphism in ulcerative colitis. Gastroenterology. 2004;126:367. doi: 10.1053/j.gastro.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 69.Gazouli M, Zacharatos P, Mantzaris GJ, Barbatis C, Ikonomopoulos I, Archimandritis AJ, Lukas JC, Papalambros E, Gorgoulis V. Association of NOD2/CARD15 variants with Crohn's disease in a Greek population. Eur J Gastroenterol Hepatol. 2004;16:1177–1182. doi: 10.1097/00042737-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 70.Croucher PJ, Mascheretti S, Foelsch UR, Hampe J, Schreiber S. Lack of association between the C3435T MDR1 gene polymorphism and inflammatory bowel disease in two independent Northern European populations. Gastroenterology. 2003;125:1919–20; author reply 1920-1921;. doi: 10.1053/j.gastro.2003.05.016. [DOI] [PubMed] [Google Scholar]
- 71.Ho GT, Nimmo ER, Tenesa A, Fennell J, Drummond H, Mowat C, Arnott ID, Satsangi J. Allelic variations of the multidrug resistance gene determine susceptibility and disease behavior in ulcerative colitis. Gastroenterology. 2005;128:288–296. doi: 10.1053/j.gastro.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 72.Brant SR, Panhuysen CI, Nicolae D, Reddy DM, Bonen DK, Karaliukas R, Zhang L, Swanson E, Datta LW, Moran T, et al. MDR1 Ala893 polymorphism is associated with inflammatory bowel disease. Am J Hum Genet. 2003;73:1282–1292. doi: 10.1086/379927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Potocnik U, Ferkolj I, Glavac D, Dean M. Polymorphisms in multidrug resistance 1 (MDR1) gene are associated with refractory Crohn disease and ulcerative colitis. Genes Immun. 2004;5:530–539. doi: 10.1038/sj.gene.6364123. [DOI] [PubMed] [Google Scholar]
- 74.Palmieri O, Latiano A, Valvano R, D'Incà R, Vecchi M, Sturniolo GC, Saibeni S, Bossa F, Latiano T, Devoto M, et al. Multidrug resistance 1 gene polymorphisms are not associated with inflammatory bowel disease and response to therapy in Italian patients. Aliment Pharmacol Ther. 2005;22:1129–1138. doi: 10.1111/j.1365-2036.2005.02701.x. [DOI] [PubMed] [Google Scholar]
- 75.Urcelay E, Mendoza JL, Martín MC, Mas A, Martínez A, Taxonera C, Fernandez-Arquero M, Díaz-Rubio M, de la Concha EG. MDR1 gene: susceptibility in Spanish Crohn's disease and ulcerative colitis patients. Inflamm Bowel Dis. 2006;12:33–37. doi: 10.1097/01.mib.0000194184.92671.78. [DOI] [PubMed] [Google Scholar]
- 76.Farrell RJ, Murphy A, Long A, Donnelly S, Cherikuri A, O'Toole D, Mahmud N, Keeling PW, Weir DG, Kelleher D. High multidrug resistance (P-glycoprotein 170) expression in inflammatory bowel disease patients who fail medical therapy. Gastroenterology. 2000;118:279–288. doi: 10.1016/s0016-5085(00)70210-1. [DOI] [PubMed] [Google Scholar]
- 77.McGovern DP, Ahmad T, Van Heel D, Negoro K, Jewell D. Cytochrome p450 and multidrug-resistance gene 1 (MDR-1) polymorphisms: predictors of the need for colectomy in ulcerative colitis. Gastroenterology. 2002;122:W1313. [Google Scholar]
- 78.Yacyshyn B, Maksymowych W, Bowen-Yacyshyn MB. Differences in P-glycoprotein-170 expression and activity between Crohn's disease and ulcerative colitis. Hum Immunol. 1999;60:677–687. doi: 10.1016/s0198-8859(99)00036-1. [DOI] [PubMed] [Google Scholar]
- 79.Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, Stremmel W, Schmitz G. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26–40. doi: 10.1053/j.gastro.2004.04.019. [DOI] [PubMed] [Google Scholar]


