Abstract
AIM: To clarify possible contributions of DNA mismatch repair (MMR) system in carcinogenesis of liver fluke infection-associated intrahepatic cholangiocarcinoma (ICC) by using immunohistochemical assay.
METHODS: A total of 29 ICC samples, which had been assessed for genomic instability by a PCR-based method, were used for study. They were examined immunohistochemically to demonstrate protein expression of two MMR genes, hMSH2 and hMLH1. Results obtained were compared with their mutator phenotype assessed previously.
RESULTS: Either hMSH2 or hMLH1 protein was obviously expressed in 28 of 29 (96.6%) ICC samples. Positive nuclear localization of hMSH2 or hMLH1 protein was observed in 86.2% (25/29) or 93.1% (27/29) ICC cases, respectively, while their negative nuclear reactivity was only detected in 13.8% (4/29) or 6.9% (2/29) ICC cases analyzed, respectively.
CONCLUSION: Our study, probably for the first time, showed through immunohistochemical detection of hMSH2 and hMLH1 gene that DNA MMR system does not play a prominent role in liver fluke infection-associated cholangiocarcinogenesis. These results confirm previous findings on mutational status of these genes assessed through a PCR-based method. The immunohistochemical analysis has proven to be an effective and sensitive approach for screening MMR deficiency regardless of somatic inactivation or promoter hypermethylation of hMSH2 and/or hMLH1 gene. Furthermore, immunohistochemistry is more advantageous compared to mutator phenotyping assay in terms of simplicity, less time consuming and cost effectiveness for screening possible involvements of target MMR genes in tumorigenesis.
Keywords: Liver fluke infection, Cholangiocarcinoma, Mismatch repair, hMSH2, hMLH1, Immunohistochemistry, MSI, Mutator phenotype
INTRODUCTION
The multistep process of carcinogenesis results from multiple genetic changes in oncogene(s), tumor suppressor gene(s) and/or DNA integrity gene(s)[1,2]. hMSH2 and hMLH1, the common human mutS homolog 2 and mutL homolog 1, respectively, are two key genes implicated in DNA mismatch repair (MMR) machinery[3]. They are among other stability genes that function as DNA mismatch restorers for the maintenance of genomic integrity[2]. Defects in this repair system are involved in the failure to recognize and/or repair spontaneous errors during the replication process. MMR deficiency leads to the accumulation of secondary mutations resulting from base-base mismatches and/or short insertion or deletion mispairings throughout the genome; this deficiency affects important growth regulatory genes and thereby leads to carcinogenesis[4]. Most cells that are deficient in this repair system often display a high level of microsatellite instability (MSI), which generates a mutator phenotype, a hallmark of human cancers, as a consequence of DNA replication errors and homologous recombination[5,7]. Defects in MMR genes have recently been described as an alternative pathway in the pathogenesis of and predisposition toward a significant proportion of certain inherited and sporadic human malignancies[8-11].
Intrahepatic cholangiocarcinoma (ICC), a primary adenocarcinoma and the second most common cancer of the liver, develops from the overgrowth of cholangiocytes, which are epithelial cells that line intrahepatic ducts. Patients with this disease have a poor prognosis: ICC is usually fatal because of the difficulty in detecting it at an early stage[12,13]. Based on an epidemiological study, the incidence of ICC is dependent on geography[14]. This disease is most common in Southeast Asia[15], especially Thailand[15-16], and its etiology is thought to be multifactorial. Documented risk factors include sclerosing cholangitis, hepatolithiasis, congenital cysts and, especially, liver fluke infection[17]. Furthermore, regular alcohol consumption has also recently been found to be one of the risk factors for this tumor[18]. Known predisposing conditions, such as mechanical irritation and chronic inflammation, exert marked and long-term effects on the development and progression of this tumor by inducing epithelial proliferation and promoting the subsequent susceptibility of these tissues to exogenous and/or endogenous carcinogenic exposure, leading to cholangiocarcinogenesis[19]. In Thailand, ICC is one of the most common cancers and lethal diseases in the North and Northeast regions[20]. In Khon Kaen province, where liver fluke (Opisthorchis viverrini) infection is prevalent[20,21], 90% of the local inhabitants are or have been infected with this parasite[22]. In this same region, ICC occurs at its highest rate worldwide[16,18,21,23].
Although the roles of hMSH2 and hMLH1 genes in the pathogenesis of colorectal cancer (CRC), especially the hereditary nonpolyposis colon cancer (HNPCC), have been described[24], little is known about their involvement in cholangiocarcinogenesis. An analysis of the mutational status of the hMSH2 and hMLH1 genes in ICC from Thai patients was carried out via assessments of genomic instability in repeat sequences of microsatellites. The low frequency of somatic mutations in hMSH2 and hMLH1 genes indicated a minor involvement of this repair system[25]. However, MMR gene inactivation is not always caused by somatic mutations, but rather can be achieved by hypermethylation at the gene promoter, as reported in the hMLH1 gene[26,27]. Currently, the identification of a mutator phenotype in tumors still requires molecular testing. As an alternative screening tool for detecting DNA MMR deficiency derived from either somatic mutations or promoter hypermethylation of target MMR genes, we used immunohistochemical staining for cellular expression of hMSH2 or hMLH1 protein in archival tissues from Thai ICC that had previously been assessed for mutator phenotype by mutational inactivation using the PCR-based method. Nuclear localization of encoded proteins evaluated from the current study and the mutator phenotype assessed from the previous investigation were comparatively analyzed.
MATERIALS AND METHODS
Tissue samples
A total of 29 cases consisting of 11 fresh-frozen and 18 formalin-fixed, paraffin-embedded ICC tissues were used for this study. Samples were obtained with informed consent and the project was approved by the Ethical Committee of the National Cancer Institute of Thailand. After surgery, parts of specimens were promptly fixed in neutral formalin and processed histologically to prepare tissue sections for further immunohistochemical analysis.
Immunohistochemical study
Immunohistochemical staining for hMSH2 and hMLH1 proteins was performed with a standard avidin-biotin-peroxidase complex technique[28], using diaminobenzidine (DAB) as a chromogen. Formalin-fixed, paraffin-embedded tissue sections (4-μm thick) of the ICC were processed immunohistochemically. Tissue sections were first dipped in three changes of xylene for 5 min each and three changes of absolute ethanol for 5 min each, followed by two washes with distilled water and a final wash in PBS for 5 min. Antigen retrieval was performed by immersing tissue sections in sodium citrate buffer (0.01 mol/L, pH 6.0), autoclaving for 10 min at 121°C, cooling and rewashing the sections in distilled water prior to incubating for 15 min in 30 mL/L H2O2 in methanol to block endogenous peroxidase activity. To eliminate non-specific protein binding, after three washes with PBS for 3 min each, tissue sections were incubated at room temperature for 10 min with 100 mL/L normal goat serum in PBS. Immunostaining was performed by incubating tissues with a primary antibody against hMSH2 protein (clone FE11, Oncogene Research, 1:50 dilution), hMLH1 protein (clone 14, Oncogene Research, 1:10 dilution) or PBS (as a negative control) in a humidified chamber at 4°C overnight. Tissue sections were washed three times with PBS, 3 min each, followed by incubation with biotinylated rabbit anti–mouse IgG (1:1000 dilution) for 30 min at room temperature. After three washes for 3 min each in PBS, sections were incubated in a streptavidin-peroxidase reagent for another 30 min. After three PBS washes, the peroxidase reaction was initiated by incubating tissue sections in DAB reagent for 5-20 min at room temperature. Sections were washed three times in distilled water for 3 min each. Immunostained tissue sections were then lightly counterstained with Mayer hematoxylin, washed with distilled water, dehydrated with ascending graded ethanol and xylene and mounted with mounting medium for further viewing under the light microscope.
The expression of hMSH2 or hMLH1 protein is normally observed in the nucleus. A case was considered immunoreactively positive or negative when nuclear staining for either target protein was present in or completely absent from tumor cells in ICC tissues, respectively.
Assessment of mutator phenotype
MS alterations were previously assessed in each normal DNA and paired tumor DNA in the majority of this same set of samples (except cases 26 and 27) using a panel of 12 known highly polymorphic MS loci in nuclear DNA: five Bethesda markers including BAT-25, BAT-26, D2S123, D5S346 and D17S250; and seven other markers including D2S119, D3S1277, D3S1298, D3S1561, D3S1611, D11S904 and TP53. Three different markers of repeat sequences in mitochondrial DNA, including the D-loop, NADH dehydrogenase subunit 1 and NADH dehydrogenase subunit 5, were also analyzed using a fluorescence PCR–based technique, as reported previously[25].
RESULTS
Immunohistochemical study
The immunohistochemical analysis for nuclear localization of two MMR genes, hMSH2 and hMLH1, in 29 ICC tissues demonstrated positive immunostaining in the majority of stained cases (Table 1). Intense nuclear expression of either hMSH2 or hMLH1 was observed in 96.6% (28/29) ICC cases. hMSH2 nuclear staining was readily visible in 86.2% (25/29) ICC cases (Figure 1 A and B), whereas positive immunostaining for the hMLH1 protein was detected in 93.1% (27/29) cases (Figure 1, C and D). Consequently, negative immunoreactivities for nuclear expression of hMSH2 and hMLH1 genes were verified in 4 (13.8%, cases 7, 8, 9 and 17) and 2 (6.9%, cases 7 and 19) of the 29 ICC cases, respectively (Table 1).
Table 1.
Immunohistochemical expression of hMSH2 and hMLH1 genes and microsatellite profile in ICC cases analyzed
| Case | Age/sex | Histology | pTMN stage | MS phenotype | hMSH2 | hMLH1 |
| 1 | 50/M | G3 | IVA | o | + | + |
| 2 | 60/F | G2 | IIIB | o | + | + |
| 3 | 60/F | G2 | IVA | o | + | + |
| 4 | 55/F | G2 | IVB | MSI (D17S250) | + | + |
| 5 | 57/M | G3 | IVA | o | + | + |
| 6 | 71/M | G2 | IIIB | o | + | + |
| 7 | 65/F | G3 | IIIA | MSI (D2S123) | - | - * |
| 8 | 41/F | G1 | II | MSI (D2S123) | - | + |
| 9 | 60/M | G2 | IIIB | o | - | + |
| 10 | 57/M | G2 | IVA | MSI (D17S250) | + | + |
| 11 | 55/M | G2 | U | o | + | + |
| 12 | 55/M | GX | IVB | MSI (D2S123) | + | + |
| 13 | 54/M | G1 | IVA | o | + | + |
| 14 | 59/F | G2 | IVB | o | + | + |
| 15 | 45/F | G3 | IVB | o | + | + |
| 16 | 42/M | G2 | IIIB | o | + | + |
| 17 | 57/M | G1 | IIIA | MSI (D2S123) | - | + |
| 18 | 51/F | GX | IVB | MSI (BAT-25) | + | + |
| 19 | 42/M | G2 | II | o | + | - |
| 20 | 51/M | G3 | IIIA | o | + | + |
| 21 | 59/M | G3 | IVA | MSI (D2S123) | + | + |
| 22 | 66/M | G3 | IVB | o | + | + |
| 23 | 64/F | G3 | IVA | o | + | + |
| 24 | 66/M | G1 | II | o | + | + |
| 25 | 42/M | G2 | IVB | o | + | + |
| 26 | 37/F | G3 | II | N | + | + |
| 27 | 57/M | G1 | III | N | + | + |
| 28 | 66/M | G2 | IVB | o | + | + |
| 29 | 52/M | G3 | IVB | o | + | + |
G1: well differentiated; G2: moderately differentiated; G3: poorly differentiated; GX: grade cannot be assessed; U: not known; M: male; F: female; +: positive nuclear staining; -: negative nuclear staining; MSI: microsatellite instability; o: microsatellite stable; N: MSI status was not available; *: harbouring LOH at D3S1298 and D3S1561.
Figure 1.
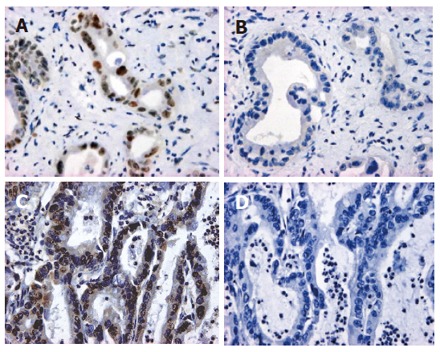
Representative data of the immunohistochemical expression of hMSH2 (case 26: A and B) or hMLH1 (case 22: C and D) protein in ICC. A and C: Stained with anti-hMSH2 and anti-hMLH1 antibodies, respectively; B and D: stained without the first antibodies as negative controls (immunoperoxidase, original magnification x 400).
Among positively stained cases, different levels of expression of either MMR gene were noted. An intense immunohistochemical reaction was observed in cases 2, 12, 14 and 16 (16.0%) for hMSH2 and cases 18, 26, 27 and 29 (14.8%) for hMLH1. A relatively reduced degree of nuclear expression was observed in cases 1, 4, 5, 10 and 15 (20.0%) for hMSH2 and cases 4, 5, 10 and 21 (14.8%) for hMLH1.
Mutator phenotype study
Among the 15 microsatellite markers that were assessed, 8 loci were found to be aberrant. Low-frequency MSI was detected for BAT-25, D2S123, D11S904 and D17S250, and loss of heterozygosity (LOH) was detected for D3S1298, D3S1561, D5S346 and TP53. Most ICC samples were therefore classified as microsatellite stable (MSS), and a subset was classified as low-frequency MSI (MSI-L), as reported previously[25].
DISCUSSION
The assessment of MMR deficiencies has become an important tool for the characterization of genomic instability and of the molecular pathogenesis of both inherited and sporadic human cancers. Molecular defects in known MMR genes, especially hMSH2 and hMLH1, have been well defined in the HNPCC syndrome[29-31]. In subsets of sporadic human cancers, these two genes were also found to be involved in the pathogenesis with varying frequency[3]. Previous studies demonstrated that in a majority of sporadic MSI tumors, hMLH1 inactivation was frequently caused by an epigenetic rather than genetic mechanism, whereas the rest displayed somatic mutations in these two genes[32-34]. Methylation of the hMLH1 gene promoter is usually biallelic[35]. Assessments of microsatellite alterations are characterizations of a mutator phenotype through somatic mutational inactivation, however, do not identify aberrations in specific genes involved, which requires further investigation.
In ICC from Thai patients, hMSH2 and hMLH1 genes do make a minor contribution to cholangiocarcinogenesis, as shown by the assessment of the mutator phenotype through their somatic mutations, using a panel of highly polymorphic MS markers that are mainly related to these genes[25]. However, the inactivation of these genes via other genetic mechanisms has not yet been excluded. In the present study, using an immunohistochemical analysis, aberrations either through somatic mutations and/or promoter hypermethylation of hMSH2 and hMLH1 gene were sought at a protein level by evaluating the nuclear expression of their proteins in ICC carcinogenesis. Our results clearly showed immunoreactivity of either protein with different degree of expression level in the majority of cases, thereby indicating the minor involvement of these genes in the pathogenesis of this tumor (Table 1), which is consistent with results that were obtained through molecular assessments[25]. In a few cases of them, however, expression of one or both proteins was lost (Table 1). Considering the different degree of MMR gene expression noted in positively stained cases, a relatively lower degree of hMSH2 and hMLH1 immunostaining was observed in 20.0% (5/25) and 14.8% (4/27) immunopositive cases, respectively, giving rise to 21.4% (6/28) cases that presented with reduced expression of at least one MMR gene, suggesting that the down-regulation of the encoding genes occurred during their carcinogenic process. This phenomenon might have been due to hypermethylation at the gene promoter, at least in the case of the hMLH1 gene, of which was often accompanied by the down-regulation or absence of hMLH1 gene expression[34,36,37]. Hypermethylation-associated reduced expression of the hMLH1 gene has already been described in lung[38], gastric[39,40] and colorectal cancers[34,36].
There was a correlation between the nuclear expression of hMSH2 or hMLH1 protein and a previously assessed MSI/mutator phenotype in a given ICC tissue (Table 1). In hMSH2 immunostaining, 3 of 4 cases (cases 7, 8 and 17) that displayed negative nuclear staining also had MSI at D2S123, a hMSH2-related MS locus[25]. The remaining case (case 9), which also exhibited loss of hMSH2 gene expression, showed stability at this locus, suggesting the presence of other hMSH2-related MS loci that mapped to 2p is involved in hMSH2 somatic inactivation. Simultaneously, in hMLH1 immunostaining, two cases (cases 7 and 19) showed a loss of this gene expression. One of them (case 7) also exhibited LOH at two hMLH1-related MS loci, D3S1298 and D3S1561, whereas the other one was MSS[25] (Table 2), indicating an availability of other hMLH1-related loci that mapped to 3p, whose MSI/LOH are involved in the loss of expression of this gene.
Table 2.
Correlation between hMSH2 and hMLH1 immunohistochemical expression and microsatellite profile in ICC studied n(%)
| MS status | Number of cases |
Pattern of nuclear expression/n (%) |
|||
| hMSH2+ hMLH1- | hMSH2- hMLH1+ | hMSH2+ hMLH1+ | hMSH2- hMLH1- | ||
| MSI | 8 | 0 (0) | 2 (25) | 5 (62.5) | 1 (12.5) |
| MSS | 19 | 1 (5.3) | 1 (5.3) | 17 (89.5) | 0 (0) |
| NP | 2 | 0 (0) | 0 (0) | 2 (100) | 0 (0) |
| Total | 29 | 1 (3.4) | 3 (10.3) | 24 (82.8) | 1 (3.4) |
MSI: Microsatellite instability; MSS: Microsatellite stable; NP: MSI assessment was not performed.
In the current study, the majority of ICC samples (89.5%), almost all of which was previously assessed as MSS/MSI-L[25], consistently displayed positive nuclear staining for both MMR proteins (Table 2). Five (cases 4, 10, 12, 18, 21) of eight MSI cases (62.5%) retained both hMSH2 and hMLH1 protein expression (Tables 1 and 2). These results are in agreement with previous findings describing the presence of MSI tumors with positive immunohistochemical staining and without detectable somatic mutations in either MMR gene[41]. On the other hand, three (cases 1, 5, 15) of the MSS cases in this study had reduced expression of either or both MMR proteins. Nevertheless, previous findings demonstrated that relatively low level of hMSH2 and hMLH1 proteins may be sufficient to retain the MSS phenotype[42], which might also be the case for the current study. Certain incompatibilities between MSI and protein expression in cases 4, 10, 12, 18 and 21 might have been due to the existence of other as yet unknown stability gene(s) or another related gene(s) that function in or outside the DNA MMR complex and play a major role in the development of this tumor type. The present findings undoubtedly demonstrated and confirmed the previous findings that only a small fraction of ICC cases was affected by hMSH2 and/or hMLH1 functional inactivation and that these two MMR genes do not seem to be implicated in carcinogenesis steps in most ICC cases.
In conclusion, to our best of knowledge, we are the first to report the pattern of immunohistochemical expression of MMR genes in ICC. Results from previous mutator phenotype assays and the current immunohistochemical screening of hMSH2 and hMLH1 genes in ICC from Thai patients consistently confirm the minor involvement of the DNA MMR system, through malfunctional activity of the hMSH2 and/or hMLH1 genes, in ICC carcinogenesis. The immunohistochemical analysis has proven to be an effective and useful approach for the screening of defects in the DNA MMR complex derived from either somatic mutational inactivation or promoter hypermethylation of target MMR genes. It is more advantageous, in terms of simplicity, less time consuming and cost effectiveness, to screen mutator phenotype through inactivation of target MMR genes in cholangiocarcinogenesis by using the immunohistochemical method.
ACKNOWLEDGMENTS
We thank Drs. K Chindavijak and S Deerasamee, former directors of the National Cancer Institute of Thailand, for their valuable support for our work. Thanks are also due to Dr. Katsumi Takano for his suggestion concerning the immunohistochemical study.
Footnotes
Supported by Ministry of Education, Culture, Sports, Science and Technology of Japan
S- Editor Wang J L- Editor Kumar M E- Editor Liu Y
References
- 1.Lairmore TC, Norton JA. Advances in molecular genetics. Am J Surg. 1997;173:37–41; discussion 42-43. doi: 10.1016/S0002-9610(96)00363-7. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 3.Eshleman JR, Markowitz SD. Mismatch repair defects in human carcinogenesis. Hum Mol Genet. 1996;5 Spec No:1489–1494. doi: 10.1093/hmg/5.supplement_1.1489. [DOI] [PubMed] [Google Scholar]
- 4.Peltomäki P, de la Chapelle A. Mutations predisposing to hereditary nonpolyposis colorectal cancer. Adv Cancer Res. 1997;71:93–119. doi: 10.1016/s0065-230x(08)60097-4. [DOI] [PubMed] [Google Scholar]
- 5.Kolodner RD. Mismatch repair: mechanisms and relationship to cancer susceptibility. Trends Biochem Sci. 1995;20:397–401. doi: 10.1016/s0968-0004(00)89087-8. [DOI] [PubMed] [Google Scholar]
- 6.Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu Rev Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulos N, Lindblom A. Molecular basis of HNPCC: mutations of MMR genes. Hum Mutat. 1997;10:89–99. doi: 10.1002/(SICI)1098-1004(1997)10:2<89::AID-HUMU1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 8.Jacob S, Praz F. DNA mismatch repair defects: role in colorectal carcinogenesis. Biochimie. 2002;84:27–47. doi: 10.1016/s0300-9084(01)01362-1. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima T, Akiyama Y, Shiraishi J, Arai T, Yanagisawa Y, Ara M, Fukuda Y, Sawabe M, Saitoh K, Kamiyama R, et al. Age-related hypermethylation of the hMLH1 promoter in gastric cancers. Int J Cancer. 2001;94:208–211. doi: 10.1002/ijc.1454. [DOI] [PubMed] [Google Scholar]
- 10.Tokunaga E, Oki E, Oda S, Kataoka A, Kitamura K, Ohno S, Maehara Y, Sugimachi K. Frequency of microsatellite instability inBreast cancer determined by high-resolution fluorescent microsatellite analysis. Oncology. 2000;59:44–49. doi: 10.1159/000012136. [DOI] [PubMed] [Google Scholar]
- 11.Hirose T, Kondo K, Takahashi Y, Ishikura H, Fujino H, Tsuyuguchi M, Hashimoto M, Yokose T, Mukai K, Kodama T, et al. Frequent microsatellite instability in lung cancer from chromate-exposed workers. Mol Carcinog. 2002;33:172–180. doi: 10.1002/mc.10035. [DOI] [PubMed] [Google Scholar]
- 12.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 13.Schlinkert RT, Nagorney DM, Van Heerden JA, Adson MA. Intrahepatic cholangiocarcinoma: clinical aspects, pathology and treatment. HPB Surg. 1992;5:95–101; discussion 101-102. doi: 10.1155/1992/93976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivatanakul P, Parkin DM, Jiang YZ, Khlat M, Kao-Ian UT, Sontipong S, Wild C. The role of infection by Opisthorchis viverrini, hepatitis B virus, and aflatoxin exposure in the etiology of liver cancer in Thailand. A correlation study. Cancer. 1991;68:2411–2417. doi: 10.1002/1097-0142(19911201)68:11<2411::aid-cncr2820681114>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Srivatanakul P, Sriplung H, Deerasamee S. Epidemiology of liver cancer: an overview. Asian Pac J Cancer Prev. 2004;5:118–125. [PubMed] [Google Scholar]
- 16.McGlynn KA, Tsao L, Hsing AW, Devesa SS, Fraumeni JF Jr. International trends and patterns of primary liver cancer. Int J Cancer. 2001;94:290–296. doi: 10.1002/ijc.1456. [DOI] [PubMed] [Google Scholar]
- 17.Holzinger F, Z'graggen K, Büchler MW. Mechanisms of biliary carcinogenesis: a pathogenetic multi-stage cascade towards cholangiocarcinoma. Ann Oncol. 1999;10 Suppl 4:122–126. [PubMed] [Google Scholar]
- 18.Honjo S, Srivatanakul P, Sriplung H, Kikukawa H, Hanai S, Uchida K, Todoroki T, Jedpiyawongse A, Kittiwatanachot P, Sripa B, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. Int J Cancer. 2005;117:854–860. doi: 10.1002/ijc.21146. [DOI] [PubMed] [Google Scholar]
- 19.Vatanasapt V, Sriamporn S, Vatanasapt P. Cancer control in Thailand. Jpn J Clin Oncol. 2002;32 Suppl:S82–S91. doi: 10.1093/jjco/hye134. [DOI] [PubMed] [Google Scholar]
- 20.Haswell-Elkins MR, Sithithaworn P, Elkins D. Opisthorchis viverrini and cholangiocarcinoma in Northeast Thailand. Parasitol Today. 1992;8:86–89. doi: 10.1016/0169-4758(92)90241-s. [DOI] [PubMed] [Google Scholar]
- 21.Watanapa P, Watanapa WB. Liver fluke-associated cholangiocarcinoma. Br J Surg. 2002;89:962–970. doi: 10.1046/j.1365-2168.2002.02143.x. [DOI] [PubMed] [Google Scholar]
- 22.Kullavanijaya P, Tangkijvanich P, Poovorawan Y. Current status of infection-related gastrointestinal and hepatobiliary diseases in Thailand. Southeast Asian J Trop Med Public Health. 1999;30:96–105. [PubMed] [Google Scholar]
- 23.Vatanasapt V, Martin N, Sriplung H, Chindavijak K, Sontipong S, Sriamporn H, Parkin DM, Ferlay J. Cancer incidence in Thailand, 1988-1991. Cancer Epidemiol Biomarkers Prev. 1995;4:475–483. [PubMed] [Google Scholar]
- 24.Peltomäki P. Deficient DNA mismatch repair: a common etiologic factor for colon cancer. Hum Mol Genet. 2001;10:735–740. doi: 10.1093/hmg/10.7.735. [DOI] [PubMed] [Google Scholar]
- 25.Liengswangwong U, Nitta T, Kashiwagi H, Kikukawa H, Kawamoto T, Todoroki T, Uchida K, Khuhaprema T, Karalak A, Srivatanakul P, et al. Infrequent microsatellite instability in liver fluke infection-associated intrahepatic cholangiocarcinomas from Thailand. Int J Cancer. 2003;107:375–380. doi: 10.1002/ijc.11380. [DOI] [PubMed] [Google Scholar]
- 26.Simpkins SB, Bocker T, Swisher EM, Mutch DG, Gersell DJ, Kovatich AJ, Palazzo JP, Fishel R, Goodfellow PJ. MLH1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Hum Mol Genet. 1999;8:661–666. doi: 10.1093/hmg/8.4.661. [DOI] [PubMed] [Google Scholar]
- 27.Jung HY, Jung KC, Shim YH, Ro JY, Kang GH. Methylation of the hMLH1 promoter in multiple gastric carcinomas with microsatellite instability. Pathol Int. 2001;51:445–451. doi: 10.1046/j.1440-1827.2001.01222.x. [DOI] [PubMed] [Google Scholar]
- 28.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 29.Aaltonen LA, Peltomäki P, Leach FS, Sistonen P, Pylkkänen L, Mecklin JP, Järvinen H, Powell SM, Jen J, Hamilton SR. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 30.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 31.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 32.Thibodeau SN, French AJ, Cunningham JM, Tester D, Burgart LJ, Roche PC, McDonnell SK, Schaid DJ, Vockley CW, Michels VV, et al. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998;58:1713–1718. [PubMed] [Google Scholar]
- 33.Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, Markowitz S, Willson JK, Hamilton SR, Kinzler KW, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuismanen SA, Holmberg MT, Salovaara R, de la Chapelle A, Peltomäki P. Genetic and epigenetic modification of MLH1 accounts for a major share of microsatellite-unstable colorectal cancers. Am J Pathol. 2000;156:1773–1779. doi: 10.1016/S0002-9440(10)65048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veigl ML, Kasturi L, Olechnowicz J, Ma AH, Lutterbaugh JD, Periyasamy S, Li GM, Drummond J, Modrich PL, Sedwick WD, et al. Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci U S A. 1998;95:8698–8702. doi: 10.1073/pnas.95.15.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menigatti M, Di Gregorio C, Borghi F, Sala E, Scarselli A, Pedroni M, Foroni M, Benatti P, Roncucci L, Ponz de Leon M, et al. Methylation pattern of different regions of the MLH1 promoter and silencing of gene expression in hereditary and sporadic colorectal cancer. Genes Chromosomes Cancer. 2001;31:357–361. doi: 10.1002/gcc.1154. [DOI] [PubMed] [Google Scholar]
- 37.Arai T, Esaki Y, Sawabe M, Honma N, Nakamura K, Takubo K. Hypermethylation of the hMLH1 promoter with absent hMLH1 expression in medullary-type poorly differentiated colorectal adenocarcinoma in the elderly. Mod Pathol. 2004;17:172–179. doi: 10.1038/modpathol.3800018. [DOI] [PubMed] [Google Scholar]
- 38.Xinarianos G, Liloglou T, Prime W, Maloney P, Callaghan J, Fielding P, Gosney JR, Field JK. hMLH1 and hMSH2 expression correlates with allelic imbalance on chromosome 3p in non-small cell lung carcinomas. Cancer Res. 2000;60:4216–4221. [PubMed] [Google Scholar]
- 39.Leung SY, Yuen ST, Chung LP, Chu KM, Chan AS, Ho JC. hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res. 1999;59:159–164. [PubMed] [Google Scholar]
- 40.Fleisher AS, Esteller M, Wang S, Tamura G, Suzuki H, Yin J, Zou TT, Abraham JM, Kong D, Smolinski KN, et al. Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res. 1999;59:1090–1095. [PubMed] [Google Scholar]
- 41.Thibodeau SN, French AJ, Roche PC, Cunningham JM, Tester DJ, Lindor NM, Moslein G, Baker SM, Liskay RM, Burgart LJ, et al. Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res. 1996;56:4836–4840. [PubMed] [Google Scholar]
- 42.Shin KH, Park JG. Microsatellite instability is associated with genetic alteration but not with low levels of expression of the human mismatch repair proteins hMSH2 and hMLH1. Eur J Cancer. 2000;36:925–931. doi: 10.1016/s0959-8049(00)00025-3. [DOI] [PubMed] [Google Scholar]


