Abstract
AIM: To evaluate the role of azathioprine (AZA) in Indian patients with ulcerative colitis over longer duration of time.
METHODS: One hundred fifty six patients with ulcerative colitis who were treated with AZA from January 1995 to December 2003 were reviewed. The indications for its use were as follows: (1) steroid dependent and steroid refractory disease; (2) Azathioprine monotherapy for naïve patients with severe disease; and (3) combination therapy (AZA + sulfasalazine or 5-aminosalicylates) for naïve patients with severe disease. The data included patient and disease demographics, efficacy and toxicity profile of AZA. Patients with a minimum duration of 6 mo use of AZA were included in this report.
RESULTS: Of a total of 156 patients treated with AZA, 45 were excluded from analysis for the following reasons- (follow up less than 6 mo, n = 9; poor follow up, n = 18; adverse affects, n = 18). In steroid refractory/dependent group the mean number of relapses prior to and post initiation of AZA therapy were 3.28 (± 0.81) and 0.94 (± 0.29) respectively. Discontinuation of steroids could be accomplished in 12 of the 15 steroid dependent patients. The proportion of patients with sustained remission of 1, 2, 3, 4 and 5 years duration were calculated. Eighteen patients experienced adverse effects necessitating withdrawal of AZA (pancreatitis, n = 7; hepatitis, n = 3; gastrointestinal intolerance, n = 2; alopecia, n = 2; and hematological, n = 4) while 13 patients needed dose reduction or temporary withdrawal of the drug.
CONCLUSION: Azathioprine is well tolerated and has therapeutic benefits lasting as long as 4 years. Adverse effects such as pancreatitis, hepatitis, cytopenias and gastrointestinal symptoms do occur but are controlled by drug withdrawal only.
Keywords: Ulcerative colitis, Azathioprine, Immuno-suppressive
INTRODUCTION
Immunosuppressive drugs were initially used clinically in the treatment of leukemias and solid organ transplantation. Over time, it was learned that these drugs were capable of long term modulation of the immune system in transplant recipients, a fact that was later extrapolated to change the course of many chronic diseases with presumed immune etiopathogenesis[1]. Based on the results of many controlled studies immunosuppressive drugs have obtained an obvious place in the medical armamentarium of ulcerative colitis in which there is growing evidence of dysregulation of the mucosal immune system[2].
Out of the various immunosuppressive drugs, the purine analogues, azathioprine (AZA) and 6-mercapto-purine (6-MP) have been most extensively used and are now considered as the first line of immunosuppressive agents for the maintenance of remission in patients with ulcerative colitis. Bowen and colleagues in 1966, first gave an encouraging report on the use of AZA in ulcerative colitis, which was followed by a number of uncontrolled observations in a series of patients. Finally, controlled double blind studies were done comparing AZA with placebo and other drugs[3-11]. These studies leave little doubt about the usefulness of AZA as a steroid sparing agent for maintenance of remission in chronic active disease. Its role in severe ulcerative colitis has been documented by our group earlier[11]. However, despite impressive data from clinical trials supporting the use of AZA, many clinicians may still be reluctant to use it because of fear of drug toxicity in the long term. Often, patients are put on this drug either too late in the course of the disease, i.e. after multiple courses of steroids or not started at all even when unable to taper steroids after many months. Recently, some studies tried to evaluate the long-term benefit and tolerability of AZA in patients with inflammatory bowel disease (IBD)[12-16]. However, the optimal duration of AZA, 6-MP therapy is unclear and has not been studied in ulcerative colitis. We report results of our experience with AZA in patients with ulcerative colitis with particular attention to clinical response and adverse events and this is probably the first such study from India.
MATERIALS AND METHODS
Patients and methods
The analysis included 156 patients with ulcerative colitis who were treated with AZA from January 1995 to December 2003. Some of these patients have been the subjects of our previously published reports. The indications for AZA included (1) steroid dependent/steroid refractory disease and frequent relapses, (2) AZA monotherapy in naïve patients with severe disease and (3) combination therapy [AZA + sulfasalazine or 5-aminosalicylates (5-ASA)] in naive patients with severe disease. Steroid-dependence was defined as a requirement for corticosteroids at a prednisolone-equivalent dose of more than 10 mg/d on an average for at least 6 mos to control disease activity after failure of at least one attempt to withdraw corticosteroids. Steroid-resistance was defined as evidence of persistent active IBD despite 6 wk treatment with corticosteroids at a dose ≥ 30 mg/d during the 6 preceding months. Each patient had been regularly examined as an outpatient and a record had been maintained detailing drug tolerability, course of the disease and side effects, if any. Reasons for modifying the dose or stopping the drug have been recorded. Patients had been continuing other conventional drugs like SLZ or 5-ASA derivatives (oral and/or enema preparations). Each relapse had been diagnosed with routine stool and sigmoidoscopic examination showing evidence of active colitis and had been treated with steroids. Patients with a minimum of 6 mo of AZA treatment were eligible for inclusion in the study. Patients with no follow-up for 1 year were taken as lost to follow-up.
Medical information collected included the following: (1) patient characteristics such as age, gender, age at diagnosis, personal habits such as smoking, family history of IBD and extent of disease etc.; (2) Disease activity assessed at each visit; remission being defined as absence of symptoms of active disease as rectal bleeding and normal bowel frequency and hence no need for steroids for at least 6 mo. Relapse was defined as presence of blood and mucus in stools, increased bowel frequency, active colitis on sigmoidoscopy and need for reintroduction of steroids or the need for colectomy. (3) AZA related data including age at initiation, duration of disease prior to AZA initiation, indication of its use and drug adverse effects which included fever, gastrointestinal intolerance (recurrent nausea, vomiting), pancreatitis, hepatitis, infectious complications, hematological abnormalities and malignancies. Laboratory test results were reviewed for evidence of hepatic dysfunction based on increase in alanine aminotransferase (more than two times upper limit of normal), leukopenia (less than 2500/cumm) and thrombocytopenia (< 1 lac/cumm).
Recommendations for laboratory monitoring included complete hemogram, alanine aminotransferase at 2, 4 and 8 wk after initiation of treatment and then every 3 mo thereafter. In some cases, noncompliance, had resulted in blood tests being performed at 4-8 mo intervals. When investigation abnormalities were found, the dose reduction or discontinuation was done depending upon the values and the side effects reported.
Statistical analysis
Data was analyzed as mean, median and range as suitable. Where appropriate, the median values (not the mean) were used to represent the data most accurately, given the limited sample size and survival curve analysis. A paired students t-test and standard deviation were used to compare the pre and post AZA change in the corticosteroid dose. The threshold of significance was set at 0.05. The probabilities of relapse were calculated using life table analysis. The effect of various variables on time to relapse was explored by Kaplan-Meier survival curves and compared using log rank test. Non-parametric Mann-Whitney test was used for comparing the mean relapse rate per year for the neutropenic and non-neutropenic group. The study was approved by the Hospital Drug Trial Ethical Committee.
RESULTS
A total of 156 patients treated with AZA for ulcerative colitis were enrolled. Forty-five patients were excluded from analysis because of either of the following reasons: (a) follow up period of less than 6 mo, n = 9; (b) poor compliance, n = 18; (c) adverse effects, n = 18. The study sample, thus, had 111 patients; 56 were males. The Median age of the patients was 34 years (range 11-73). The disease was pancolonic in 40%, left sided in 32% and proctosigmoiditis in 28%. The characteristics of the study patients are summarized in Table 1. The mean follow up period of patients on AZA was 18.70 ± 13.78 mo with a range of 6-73 mo.
Table 1.
Baseline characteristics of the patients at initiation of azathioprine treatment
| Characteristic | |
| Age (yr) (mean ± SD) | 34 (34.76 ± 11.70) |
| Sex (M/F) | 56/55 |
| Disease duration (yr) (range) | 0-7.3 |
| Disease extent n (%) | |
| Pancolitis | 51 (45.9) |
| Proctosigmoiditis | 29 (26.2) |
| Left sided | 31 (27.9) |
| Indication of use n (%) | |
| Steroid dependent/refractory | 54 (48.65) |
| Azathioprine monotherapy for naïve severe disease | 16 (14.41) |
| Combination therapy for naive severe disease | 41 (36.94) |
In the steroid refractory steroid dependent and frequent relapsers group, the mean numbers of relapses prior and post initiation of AZA therapy were 3.28 (± 0.81) and 0.94 (± 0.29) respectively (P < 0.01). Discontinuation of steroids could be accomplished in 12 of the 15 patients who were on steroids for long time; in 1 patient the dose was decreased to half while the other two patients did not improve and underwent proctocolectomy.
Our analysis revealed that the proportion of patients in remission at 12, 24, 36, 48 and 60 mo were 0.91, 0.88, 0.76, 0.53 and 0.38 respectively. Life table analysis in our study showed that maintenance AZA treatment is effective for at least until 42 mo of treatment. Figure 1. As the number of patients on AZA beyond 42 mo is small we cannot comment on the efficacy beyond 42 mo. Various parameters, namely age, gender, disease activity at onset, extent of disease and indication of treatment were evaluated for predicting response by Kaplan Meier survival analysis. None of the above mentioned factors emerged as predictors of maintenance of remission. Thirteen patients became neutropenic (i.e. neutrophil count ≤ 2.5 × 109) while taking AZA. The mean number of yearly relapses for the neutropenic group was 0.2023 (± 0.3930) as compared to 0.1742 (± 0.3886) for the non-neutropenic group; the difference not being statistically significant (P > 0.59).
Figure 1.
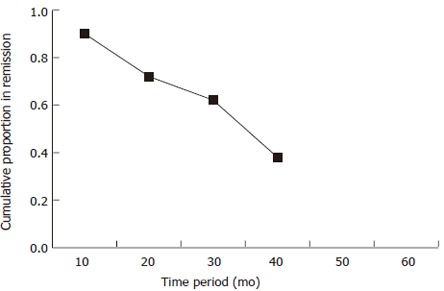
Cumulative proportion of patients with ulcerative colitis in remission with azathioprine
All patients necessitating drug withdrawal had experienced side effects within 6 mos of initial therapy. Hematological toxicity requiring drug withdrawal was seen in 4 patients; 3 had bicytopenia, 1 had thrombocytopenia. Dose reduction was required in 5 patients for bicytopenia and 5 for leukopenia. Persistent upper respiratory tract infection in 2 patients and pneumonia in 1 resulted in temporary discontinuation of the drug. Five patients had to undergo surgery, the indications being, unsatisfactory response resulting in chronic active disease, n = 2; malignancy, n = 1 and fulminant colitis, n = 2. The reasons for cessation of therapy included acute pancreatitis, n = 7; alanine aminotransferase, hepatotoxicity, in 3 gastrointestinal intolerance such as nausea, recurrent vomiting in 2 patients and alopecia in 2 patients.
DISCUSSION
Ulcerative colitis treatment has undergone a significant change in the last 20 years. In particular, the use of immunomodulatory drugs like AZA and 6-MP has become widespread, as increasing familiarity and clinical trials have led to a clearer understanding of their benefits and potential risks. The results of our analysis show that AZA is efficacious and well tolerated in most patients with ulcerative colitis. Although ours is an analysis of results of all patients where AZA was used without randomization, it perhaps provides the ‘best available’ and the ‘only’ evidence of its use from our country to date. The scarcity of data on its use from our country is possibly related to fear of drug toxicity as AZA has been commonly viewed as a ‘cancer drug’ with high incidence of bone marrow toxicity, increased predisposition to infections, teratogenicity and malignancies, especially myeloproliferative disorders. However, it needs to be realized that this perception is derived in part from the side effect profile when the drug was originally used in preventing transplant rejection and in treatment of leukemia where significantly higher dosages were used.
Our analysis revealed that AZA is well tolerated as only 16.22% (18/111) experienced side effects requiring discontinuation of the drug. This frequency is comparable to the previously reported rate of approximately 15%. Pancreatitis, hepatitis, alopecia and gastrointestinal intolerance were the reasons for drug withdrawal. Nevertheless, all these adverse effects responded promptly to just drug withdrawal. Infections occurred in 3 cases resulting in temporary withdrawal of the drug; no patient died due to severe sepsis. In a large series by Connell et al[16] asymptomatic leukopenia was reported in 5% of patients and associated infections in only 1% patients with severe leukopenia. Fear of neoplasia, both intestinal and extraintestinal, has been cited as a major barrier for physicians choosing to use this drug in IBD[17,18]. We did come across one patient with rectal carcinoma who had been on AZA for 17 mo. However, an important confounding factor in the evaluation of the drugs oncogenic potential was the predisposition towards spontaneous neoplasm in ulcerative colitis. This patient had disease duration of 7 years and finally underwent surgery.
The spectrum of adverse events associated with AZA provides the most cogent argument for optimizing and individualizing drug dosing by determination of TPMT activity (phenotype or genotype) prior to initiation of therapy[19]. However, the validity of this recommendation still remains a debated topic. Nevertheless, careful regular clinical follow-up and monitoring of laboratory tests are fundamental to prevent and control adverse effects.
The effectiveness of AZA has been demonstrated by both uncontrolled and controlled trials in patients with ulcerative colitis. Our data corroborates previous clinical studies suggesting efficacy of AZA in maintenance of remission in steroid dependent and steroid refractory cases. Discontinuation of the steroids could be accomplished in 12 of the 15 patients who were taking low doses of steroids as a maintenance dose; in one patient steroid requirement decreased and two patients who did not respond underwent proctocolectomy. There was a significant decline in the mean number of relapses pre and post initiation of AZA therapy. The most convincing controlled trial to date evaluating the maintenance benefits of AZA showed a 12 mo relapse rate of 36% for patients on AZA versus 59% for patients randomized to placebo. In a metaanalysis, AZA was found to be useful for maintenance of remission in ulcerative colitis, the pooled odd’s ratio (OR) being 2.26 (95% CI: 1.27-4.01). The number needed to treat (NNT) to prevent one recurrence was 6 patients. For induction of remission, the pooled OR of the response to AZA therapy compared with placebo in active ulcerative colitis was 1.45 (95% CI: 0.68-3.08)[20]. In all these trials most patients were maintained on additional aminosalicylate therapy. However, the role of concurrent use of 5-ASA in patients effectively maintained on AZA has been questioned[21]. We reported earlier the role of AZA as monotherapy versus sulfasalazine monotherapy in the maintenance of disease remission. Although relapse rates were comparable in both groups, a trend towards earlier treatment failure was seen in patients on AZA monotherapy[22]. We have tried to shift the paradigm from ‘step up’ approach to combination therapy in naïve patients with severe ulcerative colitis[11]. Unfortunately the published experience with combination therapy in naïve patients is not large but we expect future studies to comment on this. It might be intuitive that early use of AZA may reset the immunostat of the body and influence the course of the disease. The results of our earlier study have demonstrated a trend in favor of combination therapy in preventing relapses but more information needs to be accrued over a longer follow-up. Life table analysis in our present study shows that AZA treatment is effective for as long as 42 mo of treatment. Although there is a gradual but variable decline in the proportion of patients who remained relapse free over the follow-up time period, it does not suggest that the effectiveness ‘wears out’ after this period. However, a recent retrospective study has suggested that the efficacy of AZA declines with time in patients with ulcerative colitis[20].
Over the past few years, great interest has surfaced in the potential therapeutic levels of leukopenia and neutropenia in achieving remission. Anecdotal reports have suggested that these may now be desirable end points of AZA therapy[23,24]. However, the cumulative remission percent determined by Kaplan Meier survival analysis showed no difference between neutropenic and non-neutropenic groups by log rank analysis in our study, suggesting that AZA dose titration to achieve neutropenia is not necessary for optimizing the dose.
Although a large array of therapeutic options exists, we have analyzed the therapeutic effects and toxicity of this not very new drug among patients with ulcerative colitis in the Indian population. We are of the opinion that the data is suggestive of the effectiveness of AZA in maintenance of remission and the drug, if indicated, should not be withheld for fear of toxicity.
Footnotes
S- Editor Wang GP L- Editor Alpini GD E- Editor Ma WH
References
- 1.Hong JC, Kahan BD. Immunosuppressive agents in organ transplantation: past, present, and future. Semin Nephrol. 2000;20:108–125. [PubMed] [Google Scholar]
- 2.Sartor RB. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn's disease. Gastroenterol Clin North Am. 1995;24:475–507. [PubMed] [Google Scholar]
- 3.Bean RH. The treatment of chronic ulcerative colitis with 6-mercaptopurine. Med J Aust. 1962;49(2):592–593. [PubMed] [Google Scholar]
- 4.Bowen GE, Irons GV Jr, Rhodes JB, Kirsner JB. Early experiences with azathioprine in ulcerative colitis; a note of caution. JAMA. 1966;195:460–464. [PubMed] [Google Scholar]
- 5.Lamers CB, Griffioen G, van Hogezand RA, Veenendaal RA. Azathioprine: an update on clinical efficacy and safety in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1999;230:111–115. doi: 10.1080/003655299750025633. [DOI] [PubMed] [Google Scholar]
- 6.Jewell DP, Truelove SC. Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. Br Med J. 1974;4:627–630. doi: 10.1136/bmj.4.5945.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawthorne AB, Logan RF, Hawkey CJ, Foster PN, Axon AT, Swarbrick ET, Scott BB, Lennard-Jones JE. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ. 1992;305:20–22. doi: 10.1136/bmj.305.6844.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg JL, Wall AJ, Levin B, Binder HJ, Kirsner JB. A controlled trial of azathioprine in the management of chronic ulcerative colitis. Gastroenterology. 1975;69:96–99. [PubMed] [Google Scholar]
- 9.Sood A, Midha V, Sood N, Kaushal V. Role of azathioprine in severe ulcerative colitis: one-year, placebo-controlled, randomized trial. Indian J Gastroenterol. 2000;19:14–16. [PubMed] [Google Scholar]
- 10.Kirk AP, Lennard-Jones JE. Controlled trial of azathioprine in chronic ulcerative colitis. Br Med J (Clin Res Ed) 1982;284:1291–1292. doi: 10.1136/bmj.284.6325.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sood A, Kaushal V, Midha V, Bhatia KL, Sood N, Malhotra V. The beneficial effect of azathioprine on maintenance of remission in severe ulcerative colitis. J Gastroenterol. 2002;37:270–274. doi: 10.1007/s005350200034. [DOI] [PubMed] [Google Scholar]
- 12.Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989;111:641–649. doi: 10.7326/0003-4819-111-8-641. [DOI] [PubMed] [Google Scholar]
- 13.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50:485–489. doi: 10.1136/gut.50.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirschner BS. Safety of azathioprine and 6-mercaptopurine in pediatric patients with inflammatory bowel disease. Gastroenterology. 1998;115:813–821. doi: 10.1016/s0016-5085(98)70251-3. [DOI] [PubMed] [Google Scholar]
- 15.George J, Present DH, Pou R, Bodian C, Rubin PH. The long-term outcome of ulcerative colitis treated with 6-mercaptopurine. Am J Gastroenterol. 1996;91:1711–1714. [PubMed] [Google Scholar]
- 16.Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081–1085. doi: 10.1136/gut.34.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korelitz BI, Mirsky FJ, Fleisher MR, Warman JI, Wisch N, Gleim GW. Malignant neoplasms subsequent to treatment of inflammatory bowel disease with 6-mercaptopurine. Am J Gastroenterol. 1999;94:3248–3253. doi: 10.1111/j.1572-0241.1999.01530.x. [DOI] [PubMed] [Google Scholar]
- 18.Connell WR, Kamm MA, Dickson M, Balkwill AM, Ritchie JK, Lennard-Jones JE. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet. 1994;343:1249–1252. doi: 10.1016/s0140-6736(94)92150-4. [DOI] [PubMed] [Google Scholar]
- 19.Yates CR, Krynetski EY, Loennechen T, Fessing MY, Tai HL, Pui CH, Relling MV, Evans WE. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997;126:608–614. doi: 10.7326/0003-4819-126-8-199704150-00003. [DOI] [PubMed] [Google Scholar]
- 20.Kull E, Beau P. [Compared azathioprine efficacy in ulcerative colitis and in Crohn's disease] Gastroenterol Clin Biol. 2002;26:367–371. [PubMed] [Google Scholar]
- 21.Campbell S, Ghosh S. Effective maintenance of inflammatory bowel disease remission by azathioprine does not require concurrent 5-aminosalicylate therapy. Eur J Gastroenterol Hepatol. 2001;13:1297–1301. doi: 10.1097/00042737-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Sood A, Midha V, Sood N, Avasthi G. Azathioprine versus sulfasalazine in maintenance of remission in severe ulcerative colitis. Indian J Gastroenterol. 2003;22:79–81. [PubMed] [Google Scholar]
- 23.Campbell S, Ghosh S. Is neutropenia required for effective maintenance of remission during azathioprine therapy in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2001;13:1073–1076. doi: 10.1097/00042737-200109000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Korelitz BI, Zlatanic J, Smith MJ, Lipe RJ, Baiocco PJ, Gleim GW. Significance of WBC differential when leukopenia is induced by 6-MP for IBD. Gastroenterology. 1997;113:1810–1811. doi: 10.1053/gast.1997.v113.agast971131810b. [DOI] [PubMed] [Google Scholar]


