Abstract
AIM: To evaluate the long-term outcome of standard 5-FU based adjuvant or neoadjuvant radiochemotherapy and to identify the predictive factors, especially anemia before and after radiotherapy as well as hemoglobin increase or decrease during radiotherapy.
METHODS: Two hundred and eighty-six patients with Union International Contre Cancer (UICC) stage II and III rectal adenocarcinomas, who underwent resection by conventional surgical techniques (low anterior or abdominoperineal resection), received either postoperative (n = 233) or preoperative (n = 53) radiochemotherapy from January 1989 until July 2002. Overall survival (OAS), cancer-specific survival (CSS), disease-free survival (DFS), local-relapse-free (LRS) and distant-relapse-free survival (DRS) were evaluated using Kaplan-Meier, Log-rank test and Cox’s proportional hazards as statistical methods. Multivariate analysis was used to identify prognostic factors. Median follow-up time was 8 years.
RESULTS: Anemia before radiochemotherapy was an independent prognostic factor for improved DFS (risk ratio 0.76, P = 0.04) as well as stage, grading, R status (free radial margins), type of surgery, carcinoembryonic antigen (CEA) levels, and gender. The univariate analysis revealed that anemia was associated with impaired LRS (better local control) but with improved DFS. In contrast, hemoglobin decrease during radiotherapy was an independent risk factor for DFS (risk ratio 1.97, P = 0.04). During radiotherapy, only 30.8% of R0-resected patients suffered from hemoglobin decrease compared to 55.6% if R1/2 resection was performed (P = 0.04). The 5-year OAS, CSS, DFS, LRS and DRS were 47.0%, 60.0%, 41.4%, 67.2%, and 84.3%, respectively. Significant differences between preoperative and postoperative radiochemotherapy were not found.
CONCLUSION : Anemia before radiochemotherapy and hemoglobin decrease during radiotherapy have no predictive value for the outcome of rectal cancer. Stage, grading, R status (free radial margins), type of surgery, CEA levels, and gender have predictive value for the outcome of rectal cancer.
Keywords: Rectal cancer, Adjuvant radiotherapy, Adjuvant radiochemotherapy, Anemia, Prognostic factor
INTRODUCTION
An improved therapeutic strategy for stage II and III rectal adenocarcinomas is urgently needed because up to 30% of patients still develop recurrent disease after curative surgical resection[1]. Several studies are ongoing aiming at evaluation of new multimodality treatment strategies[2]. However, pelvic radiotherapy per se is nowadays undisputed and accepted as standard therapy in all trials concerning locally advanced rectal cancer. The current standard treatment is the result of two independent, multi-institutional, prospective randomized trials more than 12 years ago by the Gastrointestinal Tumor Study Group (GITSG 7175) in 1985[3] and the North Central Cancer Treatment Group (NCCTG 79-47-51) in 1991[4]. Both studies demonstrated that combined radiotherapy and chemotherapy following surgical resection of stage II and III rectal cancer can improve the overall survival. These results have prompted the National Institute of Health to publish the NIH Consensus recommending postoperative radiochemotherapy for stage II and III tumors.
Anemia has been shown to have impact on the rate of local control or distant metastasis of other tumor entities[5,6]. To identify such prognostic factors and to evaluate the outcome of preoperative or postoperative radiochemotherapy, we conducted a retrospective study including 286 patients with stage II and III rectal carcinoma treated with radiochemotherapy in the Department of Radiotherapy at the University Hospital Freiburg from 1989 until 2002.
MATERIALS AND METHODS
Patient cohort
The retrospective study enrolled patients with stage II or III rectal cancer who were treated with pre- or postoperative adjuvant radiochemotherapy between January 1989 and July 2002. Pretreatment evaluation included complete blood test, chemistry profile, determination of carcinoembryonic antigen (CEA), chest radiography, liver ultrasonography and computer tomography (CT) of abdomen and pelvis[7]. Tumor location was divided in to the lower third (less than 7 cm from the anal verge), the middle third (less than 12 cm) and the upper third (12 or more than 12 cm) according to Phang et al[8]. However, there are other definitions[9], and even the anatomic length of the rectum is unclear[10]. If patients suffered from other diseases leading to a Karnofski lower than 80, these patients were counted as having “other serious disease(s)”. Concerning survival a complete set of data were available. But due to the missing data about hemoglobin during radiotherapy, the effect of anemia (haemoglobin <120 g/L in women or <130 g/L in men) was analyzed using a subgroup of 192 patients with complete patient documentation.
Multi-modal therapy
According to the surgical reports from the different hospitals, all patients were treated with standard surgical technique. Total mesorectal excision (TME) was performed in all patients with abdominal-perineal resection (APR) but less consequently and not quality-controlled in patients with low anterior resection (LAR). If proximal and distal surgical margins were microscopically free of tumor, the patients were defined as “radically resected” (R0). According to the patient documentation analyzed, the circumferential margins were not systematically assessed. The scheduled radiotherapy delivered 45 - 56 Gy in 25 - 31 sessions using 6 / 18 MeV linear accelerator. The treatment included two parallel opposing right and left portals (using wedges with 40 or 50 % absorption) and a dorsal field. These three (or four, if external iliac lymph nodes were included) field box arrangements were used, representing the generally approved radiotherapeutic scheme during the retrospective study[11]. The upper margin was fixed 1.5 cm cranial of the promontorium whereas the lower margin was chosen depending on the exact tumor localization. The lower margin including the perineal scar (if the tumor was located less than 8 cm from the anal verge or if abdominoperineal resection was performed), was marked by the tuber ischiadicum (between 8 and 12 cm above the anal verge), or covered by the lower border of the obturator foramen (12 cm above the anal verge). The width of posteroanterior portals covered the pelvic inlet with 2 cm margin. Radiotherapy was administered with cycles 1 and 2 instead of cycles 3 and 4 as recommended by the NIH[12], renewed by the 1996 Patterns of Care Rectal Cancer Committee[13]. In the NCCTG study, cycles 1 and 2 of 5-FU plus semustine were given, followed by pelvic radiotherapy plus chemotherapy.
Depending on their R status, patients were treated with combined modality therapy either according to the NIH recommendations[12] or to the protocol[14] of the Arbeitsgemeinschaft Radioonkologie (ARO) of the German Cancer Society. Patients with no evidence of microscopical residuals of the disease (R0 resection) were treated according to the NIH protocol[12]. Following R1 or R2 resection, patients were treated according to the ARO protocol. When the NIH protocol was used, the concurrent bolus 5-FU was given at a daily dose of 500 mg/m² for 3 d during cycles 1-3, the following three cycles were given for 5 d. When the ARO protocol was used, patients received 350 mg/m² 5-FU iv continuously during 24 h for 14 d. Additionally, bolus of 200 mg/m² leucovorin and 4 mg/m² mitomycin C, was given daily for 1 h. In both protocols radiotherapy and chemotherapy were started simultaneously.
Statistical analysis
The data were analyzed using SAS. The statistical methods included Student’s t-test, Chi-square test, and Kruskal-Wallis-test. Survival was analyzed using univariate and multivariate methods (step down analysis). Kaplan-Meier curves[15] were used to estimate the distribution of overall survival (OAS), cancer-specific survival (CSS) and disease-free survival (DFS). For analysis, the rates of treatment failure were adjusted, local-relapse-free survival (LRS) and distant-relapse-free survival (DRS) were determined as life table analysis referring to freedom of locoregional relapse and freedom of distant recurrence (metastasis). LRS was defined as the rate of local control. Log-rank test (Cox-Mantel) was used to compare the survival distributions between different patient subgroups[16]. Multivariate analysis and proportional hazard models[17] were used to determine the prognostic factors with significant impact on survival, including hemoglobin effect, grading, staging, adjuvant therapy, surgical method and tumor marker.
RESULTS
Two hundred and eighty-six patients (186 men and 100 women) fulfilled the inclusion criteria and were enrolled in the study (Table 1). Their age ranged from 30 to 84 years (median 62 years). The distribution of stages was as follows: 92 patients (32.2%) were assigned to stage II (lymph node negative) and 194 (67.8%) to stage III (lymph node positive). One hundred and forty-six patients (51.0%) were treated with low anterior resection (LAR), 140 patients (49.0 %) with abdominoperineal resection (APR). According to the surgical reports from different hospitals, all patients were treated with standard surgical technique. Total mesorectal excision (TME) was performed in all patients with APR but less consequently and not quality-controlled in patients after LAR. Two hundred and thirty four patients (81.8 %) were defined as R0 (Table 1). Sixteen patients were known to have oncological diseases in their histories. Other severe diseases referred to cardial (n = 62), pulmonary (n = 5), hepatic or gastric (n = 18), psychiatric diseases (n = 8), or diabetes (n = 6).
Table 1.
Patient characteristics
| Adjuvant radiochemotherapy n (%) | Neoadjuvant radiochemotherapy n (%) | All n (%) | |
| Gender | |||
| Female | 148 (63.5) | 38 (71.7) | 186 (65.0) |
| Male | 85 (36.5) | 15 (28.3) | 100 (35.0) |
| Age | |||
| ≤Median age of 62 yr | 134 (57.5) | 32 (60.4) | 166 (58.0) |
| > Median age of 62 yr | 99 (42.5) | 21 (39.6) | 120 (42.0) |
| Tumor location | |||
| Upper third | 35 (15.1) | 0 (0.0) | 35 (12.2) |
| Middle third | 100 (42.9) | 23 (43.4) | 123 (43.0) |
| Lower third | 87 (37.3) | 25 (47.2) | 112 (39.2) |
| Not known | 11 (4.7) | 5 (9.4) | 16 (5.6) |
| Surgical Resection | |||
| Anterior (LAR) | 133 (56.8) | 13 (24.6) | 146 (51.0) |
| Abdominoperineal (APR) | 100 (43.2) | 40 (75.4) | 140 (49.0) |
| R status | |||
| R0 | 197 (84.5) | 37 (69.8) | 234 (81.8) |
| R1 | 17 (7.3) | 5 (9.4) | 22 (7.7) |
| R2 | 4 (1.8) | 2 (3.8) | 6 (2.1) |
| not known | 15 (6.4) | 9 (17.0) | 24 (8.4) |
| Stage | |||
| II | 75 (32.2) | 17 (32.1) | 92 (32.2) |
| III | 158 (67.8) | 36 (67.9) | 194 (67.8) |
| N stage | |||
| N0 | 74 (31.8) | 17 (32.1) | 91 (31.8) |
| N1 | 81 (34.8) | 27 (50.9) | 108 (37.8) |
| N2 | 78 (33.4) | 9 (17.0) | 87 (30.4) |
| Grading | |||
| G I | 11 (4.7) | 9 (17.0) | 20 (7.0) |
| G II | 161 (69.1) | 29 (54.7) | 190 (66.5) |
| G III | 44 (18.9) | 9 (17.0) | 53 (18.5) |
| Not known | 17 (7.3) | 6 (11.3) | 23 (8.0) |
| CEA | |||
| < 3 ng/mL | 102 (43.8) | 16 (30.2) | 118 (41.3) |
| ≥ 3 ng/mL | 102 (43.8) | 16 (30.2) | 118 (41.3) |
| Not known | 29 (12.4) | 21 (39.6) | 50 (17.4) |
| RTOG | |||
| RTOG 0 | 96 (41.2) | 30 (56.6) | 126 (44.1) |
| RTOG I | 75 (32.2) | 14 (26.4) | 89 (31.1) |
| RTOG II | 46 (19.7) | 9 (17.0) | 55 (19.2) |
| RTOG III | 13 (5.6) | 0 (0.0) | 13 (4.6) |
| RTOG IV | 3 (1.3) | 0 (0.0) | 3 (1.0) |
| RTOG V | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Survival and life table analysis
The calculated overall 5-year survival (OAS) of all stage II and III patients was 47% (Figure 1 and Figure 2), the 10-year survival was 36.3%. The cancer-specific survival (CSS) and disease-free survival (DFS) decreased from 60.0% and 41.4% respectively to 52.0% and 34.9 % between 5 and 10 years (Table 3). Local-relapse-free survival (LRS) was 67.2% (5 years) and 65.7% (10 years), distant-relapse-free survival (DRS) was 84.3% (5 and 10 years). The mean overall survival time was 4.4 years and the mean disease-free survival time was 2.73 years. The mean follow-up time was 8 years.
Figure 1.
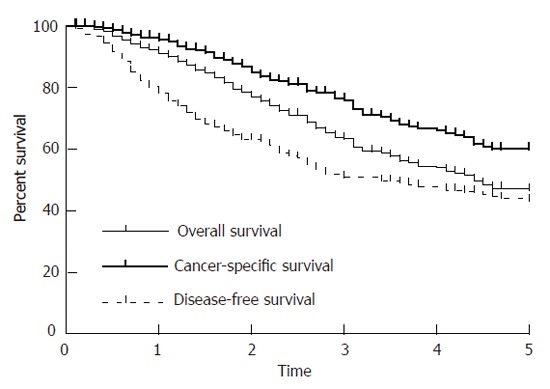
Kaplan-Meier curves of OAS, CSS and DSF.
Figure 2.
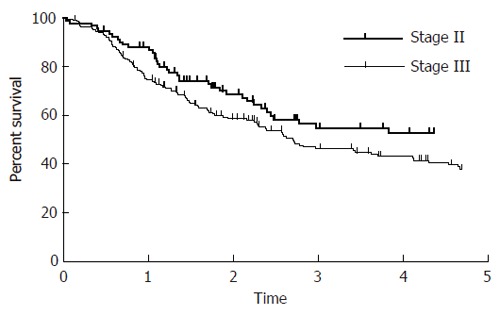
Log-rank test of staging (5-year disease-free survival, P = 0.015).
Table 3.
Survival rates and results of uni- and multivariate analysis (patient-related variables)
| OAS (%) | CSS (%) | DFS(%) | LRS (%) | DRS (%) | ||
| All stages II & III | 5 yr / 10 yr: | 47.0 / 36.0 | 60.0 / 52.0 | 41.4 /34.9 | 67.2 / 65.7 | 84.3 / 84.3 |
| at risk (5 yr / 10 yr): | 78 / 34 | 81 / 36 | 69 / 34 | 72 / 46 | 74 / 34 | |
| median survival: | 1602 | 996 | ||||
| Gender | ||||||
| Male | 5 yr / 10 yr: | 49.2 / 36.3 | 64.9 / 56.8 | 24.7 / 12.9 | 73.1 / 73.1 | 85.1 / 85.1 |
| Female | 5 yr / 10 yr: | 43.2 / 37.0 | 51.7 / 44.3 | 21.0 / 8.0 | 56.8 / 53.0 | 83.2 / 83.2 |
| uni- / multivariate: P = | 0.93 / NS | 0.16 / NS | 0.17 / NS | 0.01 / 0.018 | 0.66 / NS | |
| multivariate risk ratio: | male: 0.75 | |||||
| (CI 0.59 – 0.95) | ||||||
| Age | ||||||
| < 62 yr | 5 yr / 10 yr: | 52.2 / 42.0 | 62.0 / 55.8 | 31.1 / 16.0 | 65.3 / 63.4 | 85.9 / 85.9 |
| > 62 yr | 5 yr / 10 yr: | 41.0 / 27.0 | 57.1 / 42.6 | 13.6 / 5.1 | 70.4 / 70.4 | 80.2 / 80.2 |
| *Median age | uni- / multivariate: P = | 0.02 / NS | 0.22 / 0.005 | < 0.01 / NS | 0.47 / NS | 0.59 / NS |
| multivariate risk ratio: | < 61 yr: 0.48 | |||||
| (CI 0.28 – 0.80) | ||||||
| Staging | ||||||
| II | 5 yr / 10 yr: | 57.0 / 49.9 | 65.8 / 62.5 | 53.3 / 45.5 | 75.1 / 75.1 | 85.5 / 85.5 |
| III | 5 yr / 10 yr: | 42.6 / 30.2 | 57.2 / 47.4 | 36.3 / 28.7 | 63.3 / 61.0 | 83.8 / 83.8 |
| uni- / multivariate: P = | 0.02 / NS | 0.09 / 0.009 | 0.02 / NS | 0.06 / (0.07) | 0.7 / NS | |
| multivariate risk ratio: | stage II: 0.45 | stage II: 0.79 | ||||
| (CI 0.21 – 0.84) | (CI 0.59 – 1.03) | |||||
| Grading | ||||||
| II | 5 yr / 10 yr: | 50.6 / 39.7 | 62.0 / 55.1 | 49.3 / 41.7 | 73.3 / 70.7 | 86.8 / 86.8 |
| III | 5 yr / 10 yr: | 40.7 / 36.6 | 55.3 / 49.8 | 23.3 / 23.3 | 58.8 / 58.8 | 64.4 / 64.4 |
| uni- / multivariate: P = | 0.023 / (0.06) | 0.07 / NS | < 0.01 / NS | 0.04 / NS | < 0.01 / 0.04 | |
| multivariate risk ratio: | G III: 1.68 | G III: 2.87 | ||||
| (CI 0.97 – 2.78) | (CI 1.08 – 7.01) | |||||
Risk ratio with confidence interval (CI) is given if multivariate analysis (step down analysis) revealed a significant result. Five- and 10- year survival rates are given as OAS, CSS, DFS, LRS and DRS.
Survival and patient characteristics
Complete data about hemoglobin were available from 192 patients. When this subgroup (Table 2) was analyzed, anemia was found in 52.1% of the patients. Of the patients who received adjuvant radiotherapy, 64.6% showed increased hemoglobin during radiotherapy compared to 35.7% of the patients who were treated neoadjuvantly. Patients showed more frequently anemia if they were older than the median age or if R0 resection was performed. Anemia after radiotherapy was less frequently seen if postoperative radiotherapy was given instead of preoperative radiotherapy (37.8% vs 71.4%, respectively) or if low anterior resection (LAR) instead of abdominoperineal resection (APR) was performed (36.8% vs 51.4%, respectively), while 43.8% of the patients with stage III tumors showed increased hemoglobin during radiotherapy compared to 30.0% of the patients with stage II tumors (Table 7). Furthermore, patients less frequently suffered from increased hemoglobin if they received postoperative radiochemotherapy or their tumors underwent R0-resection. Decrease of hemoglobin was found to be an independent risk factor for DFS with a risk ratio of 1.97 (CI 1.02 - 3.43). Conversely anemia before radiotherapy had a risk ratio of 0.76 (CI 0.57 - 0.99) (Table 6).
Table 2.
Anemia and hemoglobin values during radiotherapy (subgroup analysis of 192 patients)
| Adjuvant radiochemotherapy n (%) | Neoadjuvant radiochemotherapy n (%) | All n (%) | |
| Anemia before RT | |||
| No | 78 (47.6) | 14 (50.0) | 92 (47.9) |
| Yes | 86 (52.4) | 14(50.0) | 100 (52.1) |
| Anemia after RT | |||
| No | 102 (62.2) | 8 (28.6) | 110 (57.3) |
| Yes | 62 (37.8) | 20 (71.4) | 82 (42.7) |
| Hemoglobin during RT | |||
| Hb increase | 106 (64.6) | 10 (35.7) | 116 (60.4) |
| Hb decrease | 58 (35.4) | 18 (64.3) | 76 (39.6) |
Table 7.
Anemia and patient characteristics.
| Hb decrease during RT n (%) | P value | |
| Age | ||
| ≤ 621yr | 42 (42.9) | 0.31 |
| > 621yr | 32 (35.6) | |
| Stage | ||
| II | 18 (30.0) | 0.07 |
| III | 56 (43.8) | |
| Other serious diagnoses | ||
| No | 38 (35.2) | 0.17 |
| Yes | 36 (45.0) | |
| BMI | ||
| ≤ 24.22 | 44 (50.0) | 0.03 |
| > 24.22 | 18 (26.5) | |
| Radiochemotherapy | ||
| Postoperative | 56 (35.0) | 0.03 |
| Preoperative | 18 (64.3) | |
| Surgical Resection | ||
| LAR | 42 (38.2) | 0.96 |
| APR | 28 (37.8) | |
| R status | ||
| R 0 | 24 (30.8) | 0.04 |
| R 1/2 | 10 (55.6) | |
| Side effects | ||
| RTOG 0 | 8 (80.0) | 0.27 |
| RTOG I / II | 62 (38.8) | |
| RTOG III / IV | 4 (22.2) | |
Results of chi-square test.
Median age,
median BMI (body mass index).
Table 6.
Anemia and hemoglobin increase versus decrease during radiotherapy (subgroup analysis of 199 patients with documented hemoglobin values before and after radiotherapy)
| OAS (%) | CSS (%) | DFS(%) | LRS (%) | DRS (%) | ||
| Anemia before RCT | ||||||
| No | 5 yr / 10 yr: | 44.6 / 31.4 | 66.4 / 66.4 | 41.5 / 33.7 | 76.6 / 76.6 | 74.9 / 74.9 |
| Yes | 5 yr / 10 yr: | 49.5 / 47.2 | 55.4 / 45.6 | 48.5 / 39.2 | 61.5 / 59.6 | 88.1 / 88.1 |
| uni- / multivariate: P = | 0.39 / NS | 0.14 / NS | 0.83 / 0.04 | 0.04 / NS | 0.01 / NS | |
| multivariate risk ratio: | anemia present: 0.76 | |||||
| (CI 0.57 – 0.99) | ||||||
| Anemia after RCT | ||||||
| No | 5 yr / 10 yr: | 47.5 / 34.0 | 57.4 / 48.4 | 42.5 / 34.7 | 87.6 / 87.6 | 62.7 / 60.7 |
| Yes | 5 yr / 10 yr: | 47.0 / 41.5 | 64.7 / 59.3 | 39.6 / 35.9 | 80.0 / 80.0 | 73.0 / 73.0 |
| uni- / multivariate: P = | 0.76 / NS | 0.56 / NS | 0.83 / NS | 0.12 / NS | 0.06 / NS | |
| multivariate risk ratio: | ||||||
| Hemoglobin (Hb) increase or decrease during RT | ||||||
| Hb increase | 5 yr / 10 yr: | 67.7 / 59.3 | 58.8 / 51.4 | 45.2 / 45.2 | 83.8 / 83.8 | 62.8 / 62.8 |
| Hb decrease | 5 yr / 10 yr: | 35.3 / 35.3 | 38.7 / 33.9 | 36.2 / 36.2 | 73.9 / 73.9 | 75.9 / 75.9 |
| uni- / multivariate: P = | < 0.01 / NS | < 0.01 / NS | 0.08 / 0.04 | 0.38 / NS | 0.79 / NS | |
| multivariate risk ratio: | Hb decrease: 1.97 | |||||
| (CI 1.02 – 3.43) | ||||||
Survival rates and results of the uni- and multivariate analysis as well as risk ratio with confidence interval (CI) are given if multivariate analysis (step down analysis) revealed a significant result. Five- and 10-year survival rates are given as OAS, CSS, DFS, LRS and DRS
Tumor grading was found to be an independent prognostic factor for CSS and DFS (Table 3). The overall 5-year survival rate of patients with stage III tumors was about 10% compared to patients with stage II tumors (50.6% vs 40.7%; P = 0.023) (Table 3). Patients with stage II tumors showed a 5-year overall survival of 57 % (CSS: 65.8%; DFS: 53.3%) whereas patients with stage III tumors showed a 5-year overall survival of 42.6% (CSS: 57.2 %; DFS: 36.3%). Univariate analysis showed that the N stage was found to be significant for OAS and CSS (P = 0.06). The patients’ gender was found to be an independent factor for LRS. The 5-year LRS for female was significantly worse than that for males (56.8% vs 73.1%). No dependency between tumor location and outcome could be seen (Table 5). Tumor adherence to adjacent structures predicted survival (OAS P < 0.001, CSS P = 0.004, DFS P < 0.001, DRS P = 0.019) regardless of the surgical method, but was not significant for LRS (P = 0.13).
Table 5.
Results of uni- and multivariate analysis (other variables)
| OAS | CSS | DFS | LRS | DRS | |
| N stage | |||||
| uni- / multivariate: P = | 0.009 / NS | 0.05 / NS | 0.06 / NS | 0.09 / NS | 0.15 / NS |
| CEA | |||||
| uni- / multivariate: P = | < 0.001 / 0.007 | < 0.001 / NS | < 0.001 / 0.001 | < 0.001 / NS | 0.55 / 0.055 |
| multivariate risk ratio: | CEA increase: 1.37 | CEA increase: 3.21 | CEA decrease: 0.91 | ||
| (CI 1.09 – 1.73) | (CI 1.61 – 6.82) | (CI 0.76 – 1.00) | |||
| CA 19-9 | |||||
| uni- / multivariate: P = | < 0.001 / NS | 0.033 / NS | 0.22 / NS | 0.79 / NS | 0.62 / NS |
| Tumor location | |||||
| uni- / multivariate: P = | 0.34 / NS | 0.43 / NS | 0.4 / NS | 0.83 / NS | 0.37 / NS |
| Adherence to adjacent structures | |||||
| uni- / multivariate: P = | < 0.001 / NS | 0.004 / NS | < 0.001 / NS | 0.13 / NS | 0.019 / NS |
| BMI before RT | |||||
| uni- / multivariate: P = | 0.47 / NS | 0.55 / NS | 0.83 / NS | 0.83 / NS | 0.15 / NS |
| Smoking | |||||
| uni- / multivariate: P = | 0.79 / NS | 0.49 / NS | 0.71 / NS | 0.22 / NS | 0.31 / NS |
| Hkt before vs after RT | |||||
| uni- / multivariate: P = | 0.047 / NS | 0.35 / NS | 0.31 / NS | 0.87 / NS | 0.99 / NS |
| LDH before vs after RT | |||||
| uni- / multivariate: P = | 0.32 / NS | 0.69 / NS | 0.77 / NS | 0.37 / NS | 0.21 / NS |
Risk ratio with confidence interval (CI) is given if multivariate analysis (step down analysis) revealed a significant result. Five- and 10- year survival rates are given as OAS, CSS, DFS, LRS and DRS
Survival and treatment characteristics
The overall survival did not vary depending on the radiotherapy treatment type (either pre- or post-operative radiotherapy) (Table 4). Neither gross nor microscopic evidence of disease could be achieved in 37 of 53 (69.8%) preoperatively irradiated patients, and in 197 of 233 (84.5%) of postoperatively irradiated patients. Sphincter-saving surgery could be performed in 24.6% of patients receiving preoperative radiotherapy and in 56.8% of the patients receiving postoperative radiochemotherapy.
Table 4.
Survival rates and results of uni- and multivariate analysis (treatment-related variables)
| OAS (%) | CSS (%) | DFS(%) | LRS (%) | DRS (%) | ||
| Adjuvant therapy | ||||||
| Postop RCT | 5 yr / 10 yr: | 47.6 / 35.6 | 59.0 / 48.4 | 40.2 / 34.0% | 66.1 / 64.1 | 82.2 / 82.2 |
| Preop RCT | 5 yr / 10 yr: | 43.9 / 38.0 | 62.6 / 62.6 | 45.4 / 39.3 | 69.8 / 69.8 | 92.8 / 92.8 |
| uni- / multivariate: P = | 0.82 / NS | 0.56 / NS | 0.61 / NS | 0.96 / NS | 0.12 / NS | |
| Protocol | ||||||
| NIH | 5 yr / 10 yr: | 60.7 / 45.2 | 72.5 / 59.4 | 49.8 / 44.2 | 84.1 / 84.1 | 73.2 / 73.2 |
| ARO | 5 yr / 10 yr: | 26.9 / 21.5 | 45.6 / 36.5 | 24.5 / 16.3 | 67.6 / 67.6 | 64.0 / 64.0 |
| uni- / multivariate: P = | < 0.001 / NS | 0.003 / 0.004 | 0.002 / NS | 0.024 / NS | 0.57 / NS | |
| multivariate risk ratio: | ARO: 2.66 | |||||
| (CI 1.37 – 5.21) | ||||||
| Surgery | risk ratio: | |||||
| LAR | 5 yr / 10 yr: | 36.4 / 25.9 | 49.5 / 41.1 | 27.8 / 22.7 | 64.7 / 64.7 | 81.3 / 81.3 |
| APR | 5 yr / 10 yr: | 35.2 / 26.0 | 46.7 / 39.9 | 27.6 / 22.9 | 52.4 / 51.1 | 86.4 / 86.4 |
| uni- / multivariate: P = | 0.4 / NS | 0.51 / NS | 0.27 / NS | 0.015 / 0.039 | 0.6 / NS | |
| multivariate risk ratio: | LAR: 1.3 | |||||
| (CI 1.01 – 1.60) | ||||||
| R status | ||||||
| R0 | 5 yr / 10 yr: | 53.2 / 40.3 | 64.2 / 55.1 | 45.6 / 39.5 | 66.2 / 64.5 | 91.2 / 91.2 |
| R1 or R2 | 5 yr / 10 yr: | 14.2 / 14.2 | 20.7 / 20.7 | 17.3 / 17.3 | 62.9 / 62.9 | 51.2 / 51.2 5 |
| uni- / multivariate: P = | < 0.001 / NS | 0.011 / (0.09) | < 0.001 / 0.026 | 0.95 / NS | < 0.001 / NS | |
| multivariate risk ratio: | R1/R2: 1.65 | R1/R2: 3.45 | ||||
| (CI 0.91 – 3.10) | (1.56 – 7.96) | |||||
Risk ratio with confidence interval (CI) is given if multivariate analysis (step down analysis) revealed a significant result. Five- and 10- year survival rates are given as OAS, CSS, DFS, LRS and DRS.
Multivariate analysis showed that R status (R0 vs R1/2) was an independent prognostic factor for disease-free survival (risk ratio 3.45, CI 1.56 -7.96) whereas surgical method (low anterior or abdominoperineal resection) was an independent prognostic factor for local control, determined as LRS (risk ratio 1.3, CI 1.01-1.60) (Table 4). Chi-square test showed no significant relationship between surgical method and staging (P = 0.93) or R status (P = 0.07) but a significant relationship between R status and grading (P = 0.01). The chemotherapy protocol was proved to be an independent prognostic factor (risk ratio 2.66, CI 1.37-5.21), showing a higher 5-year cancer-specific survival rate for patients treated with NIH protocol (72.5%) compared to ARO protocol (45.6%). Elevated CEA (> 3 ng/mL) levels were proved to be an independent prognostic factor for OAS and DFS (Table 5). The risk ratios were 1.37 (CI 1.09- 1.73) for OAS and 3.21 (CI 1.61 - 6.82) for DFS, respectively. According to our patient documentation, 126 patients (44%) had no documented side effects (Table 1), while 89 (31.1%), 55 (19.2%), 13 (4.5%), and 3 (0.7%) patients had RTOG grade I, II, III or IV side effects, respectively. Diarrhoea, dysuria and skin reaction were the leading problems whereas other side effects were rarely reported.
DISCUSSION
Concerning the outcome of rectal cancer, patients with younger age, lower stage, tumor grading GII or better, no elevated tumor markers, and a higher distance from the anal verge may fare better[8,18,19]. There is also some evidence that R0 resection, no adherence to adjacent structures, and lower anterior resection (LAR), performed with quality-controlled total mesorectal excision (TME), are beneficial factors for the local control whereas positive lymph nodes are risk factors mainly for distant metastases. The impact of radiotherapy is still debated. Moreover, valid data from other tumor entities suggest that anemia may have predictive value for local relapse or distant recurrence[5,6]. At the Department of Radiotherapy, University Hospital Freiburg from 1989 to 2002, 286 patients with stage II or III cancer were treated and qualified for our retrospective study analyzing patient- or treatment-related variables and their impact on treatment outcome. This study, however, has some limitations mainly due to lack of data about the quality of surgery and incomplete patient documentation. Therefore, the extent and quality of total mesorectal excision could not be assessed. The impact of anemia on 192 patients with complete data about hemoglobin during radiochemotherapy was analyzed.
The overall 5-year survival rate (47.0%) and disease-free survival rate (41.1%) were lower than those of other large randomized trials[3,4]. It is known that the outcome of therapeutic interventions obtained in studies usually exceed “the reality” of population-based reports of cancer treatment[21]. The rate of distant recurrence (16.7%) was slightly lower in our patient cohort than that reported in literature (Table 4), which may be due to the modified sequence of radiochemotherapy. This scheme of early radiochemotherapy can achieve significantly improved rates of local recurrence and distant metastasis[22], suggesting that early radiotherapy as performed in our study is more effective concerning local control and consequently more effective concerning distant outcome.
The Mayo study and other studies are influenced by a local relapse rate of about 30 %[3,4]. These studies could reflect the situation before the “era” of trained and quality-controlled total mesorectal excision. Similarly, we found that a local recurrence rate of 32.8% (local-relapse-free survival of 67.2%) is consistent with current literature[23]. After conventional and non-standardized surgery, local recurrence was 15%-55%[24-26]. However, it was reported that the 5-year local control rate was higher than 90 %, even without chemotherapy or any adjuvant treatment[27-29].
Tumor adherence to the adjacent structures can predict local recurrence and survival[30-32]. Consistent with these data, the Freiburg patient cohort including 286 cases showed that all survival rates (OAS, CSS, DFS) were associated with tumor adherence. This may be due to the effect of sample size or support the contention that out-spread of the gross tumor should be estimated as an important indicator for systemic tumor progression leading to worse outcome. According to this, it has been recently shown that even among N2 patients (4 or more positive lymph nodes), T stage influences overall 5-year survival[22]. Furthermore, T and N classifications can predict survival, but only N stage is correlated with local recurrence[30]. On the contrary, in our retrospective study N stage was significant for overall survival (P = 0.009) and cancer-specific survival (P =0.05), whereas a trend for local recurrence (P =0.09) and disease-free survival (P = 0.06) could be seen.
When an average radiation dose of 50 Gy is used for preoperative or postoperative radiochemotherapy, patients classified as N0 have better results than N positive ones[33]. Similarly, analysis of eligible patients has confirmed the effect of N stage[19]. Notably our data suggest that N positive patients are even more substantially at risk for local failure rather than for distant metastasis. Apparently, spread-out to lymph nodes indicates a more aggressive nature of local tumor growth. Concerning treatment intervention, these results support the hypothesis that sterilization of tumor cells in lymph nodes could be achieved in many patients, preventing them from distant metastasis. As a consequence, this contradicts to any efforts to minimize radiation fields. Only if adequately included in the radiation field, the tumor cells in regional lymph nodes can be killed.
In the present study, when anemia and hemoglobin were analyzed, conflicting results were obtained from the multivariate analysis. When hemoglobin values decreased during radiotherapy it meant to be a risk factor for patients, possibly indicating bad performance status, prolonged convalescence, side effects of therapy or progressive disease (Table 7). But anemia had a risk ratio of 0.76 for DFS (Table 6), suggesting that rectal cancer patients with anemia before radiotherapy have better results than patients without anemia. Considering the adjuvant setting at a time interval of 3 - 5 wk between surgery and radiochemotherapy, anemia can be regarded as a consequence of a more radical surgical intervention. This hypothesis is supported by the analysis of 192 patients with complete data about hemoglobin during radiochemotherapy: 60.0% of patients after R0-resection had anemia compared to 55.6% of patients after R1/2-resection. After radiotherapy only 42.5% patients after R0-resection still had anemia compared to 44.4% patients after R1/2-resection. Only 30.8% patients after R0-resection suffered from hemoglobin decrease during radiotherapy compared to 55.6 % patients after the R1/2-resection, suggesting that R status is an independent prognostic factor for disease-free survival. According to our data, anemia before radiochemotherapy or hemoglobin decrease during radiotherapy does not imply any predictive value for the outcome of rectal cancer. It should be considered as a readout of age, stage, type of surgical resection, or performance status. Taken together, if there is any hemoglobin effect on the outcome of rectal cancer, it is completely biased by the extent of surgical intervention, which is one of the most important predictors for the outcome of rectal cancer.
Multivariate analysis of our data revealed that tumor grading was the most important prognostic factor for CSS and DFS. In a retrospective study of 214 patients with primary rectal carcinoma, Luna-Perez et al[33] have identified well-differentiated cancer as a prognostic factor for achieving local control. Martijn et al[31] also found that tumor grading has a prognostic value, which is consistent with our study. We found that there was no significant difference in survival rate regarding tumors in the lower, middle or upper third. Thus, the hypothesis that tumor location influences response rate or survival is not supported by our data. Phang et al[8] reported that survival is affected by tumor distance from the anus. Lower distance significantly can worsen survival and distant recurrence rate but not the rate of local recurrence. Finally, an independent detrimental influence on local recurrence has been proved by a retrospective study of 197 patients, using conventional resection technique[32].
By analyzing the influence of the resection type, we revealed a better control rate of local relapse (34%) if the patients were treated by low anterior resection (LAR) compared to abdominoperineal resection (APR). Out data are consistent with a study of Stocchi et al[30] showing a better rate for LAR (28%) but not with a study of Kuru et al[32] who identified anterior resection as a negative independent prognostic factor for local control. However, Stocchi et al[30] stated that the overall rate of local recurrence (16%) is unexpectedly low in their study, considering the high-risk patient cohort and the data from literature. Unlike this, our data (32.8 % local recurrence in five years) could obviously reflect the “surgical reality today”[34] or, more accurately, the surgical results in the era (Table 6) before comprehensive surgical quality control of TME[36].
In the Freiburg cohort male gender was found to be an independent prognostic factor for local recurrences. No difference was found in OAS, CSS, DFS and DRS. It was reported that DFS and local recurrence do not differ significantly in gender, but differ significantly in OAS with a risk ratio 1.2 (P = 0.03) for men. Furthermore, increased grade III or IV toxicity in females has been described. As in our study, no interaction between sex and age was seen. Therefore sex as an indicator for higher age can be ruled out. Studies evaluating psycho-oncological aspects have identified gender-related factors which influence cancer treatment and outcome[38]. However, if sex difference has any significant impact on outcome, this effect seems to be inconsistent and may be influenced by local differences in lifestyle or environmental factors.
Among the patients-related factors, age is known to be crucial, because administration of adjuvant chemotherapy to patients aged over 70 years remains a difficult choice for the clinicians with respect to the expected benefit versus toxicity. Sargant et al[39] and Popescu et al [40] showed that old patients (with good performance status and renal and hepatic function) share the same benefit from adjuvant chemotherapy as younger patients without increasing toxicity. CEA and CA 19-9 levels are reliable tumor markers for rectal cancer[18,41,42]. In our study, the prognostic value of CEA could be shown by uni- and multivariate analysis. Additionally, the increase of CEA during radiotherapy indicated a decreased survival rate. Our results are consistent with that of Behbehani et al[43], who demonstrated that patients with preoperatively elevated CEA levels have a 2-year DFS of 23% whereas patients without CEA elevation have a 2-year DFS of 71%.
Our retrospective review of the Freiburg patient cohort did not detect any difference between preoperative and postoperative radiochemotherapy. Cancer-specific survival was 62.6% and 59.0%, respectively. Our data correspond to Rinkus et al[34] who reviewed 292 patients showing no significant difference in survival between preoperative versus postoperative radiotherapy (combined with chemotherapy in 66% and 48% of the cases, respectively).
It was reported that preoperative radiation can significantly decrease local failure rate. The 5-year survival curve does not differ significantly between both strategies. Until now the German trial (Protocol CAO/ARO/AIO 94) is the only prospective study with patients randomized to conventional preoperative and postoperative radiochemotherapy[20]. This study showed that preoperative radiochemotherapy could improve local control and reduce toxicity but overall survival was not different between pre- and postoperative radiochemotherapy. However, our retrospective, non-randomized data might be biased as preoperative radiochemotherapy was mainly given if the tumor was inoperable. Therefore these patients were in more advanced tumor stages. Supposing an advantage of preoperative radiochemotherapy, as shown by Sauer et al[20], this may explain why preoperative and postoperative radiochemotherapy seem to be equivalent according to our data.
In conclusion, new therapeutic strategies are urgently needed, preferably based on a better understanding of beneficial or disadvantageous factors determining the outcome of rectal cancer. Tumor stage, grading, R status (free radial margins), type of surgery, CEA levels, and gender are predictive factor for the outcome of rectal cancer. However, anemia before radiochemotherapy or hemoglobin decrease during radiotherapy does not imply any predictive value for the outcome of rectal cancer. It should be considered as an indicator for higher age, stage, type of surgical resection, or performance status. Any hemoglobin effect of radiotherapy on outcome of rectal cancer is completely biased by the extent of surgical intervention.
Footnotes
S- Editor Wang J L- Editor Wang XL E- Editor Bai SH
References
- 1.Saltz LB, Minsky B. Adjuvant therapy of cancers of the colon and rectum. Surg Clin North Am. 2002;82:1035–1058. doi: 10.1016/s0039-6109(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 2.Kachnic LA, Willett CG. Radiation therapy in the management of rectal cancer. Curr Opin Oncol. 2001;13:300–306. doi: 10.1097/00001622-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Radiation therapy and fluorouracil with or without semustine for the treatment of patients with surgical adjuvant adenocarcinoma of the rectum. Gastrointestinal Tumor Study Group. J Clin Oncol. 1992;10:549–557. doi: 10.1200/JCO.1992.10.4.549. [DOI] [PubMed] [Google Scholar]
- 4.Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, Kubista TP, Poon MA, Meyers WC, Mailliard JA. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 5.Henke M, Sindlinger F, Ikenberg H, Gerds T, Schumacher M. Blood hemoglobin level and treatment outcome of early breast cancer. Strahlenther Onkol. 2004;180:45–51. doi: 10.1007/s00066-004-1123-7. [DOI] [PubMed] [Google Scholar]
- 6.Frommhold H, Guttenberger R, Henke M. The impact of blood hemoglobin content on the outcome of radiotherapy. The Freiburg experience. Strahlenther Onkol. 1998;174 Suppl 4:31–34. [PubMed] [Google Scholar]
- 7.Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9–20. doi: 10.1007/s003840050002. [DOI] [PubMed] [Google Scholar]
- 8.Phang PT, MacFarlane JK, Taylor RH, Cheifetz RE, Davis N, Hay JH, McGregor G, Speers C, Sullivan BJ, Pitts J, et al. Effects of positive resection margin and tumor distance from anus on rectal cancer treatment outcomes. Am J Surg. 2002;183:504–508. doi: 10.1016/s0002-9610(02)00840-1. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E. Report of the expert opinion on the treatment of rectal cancer. 5th International Congress: Perspectives in Colorectal Cancer; 2003. [Google Scholar]
- 10.Boyle P, McArdle CS and Kerr DJ. Colorectal Cancer. 2002 [Google Scholar]
- 11.Kline RW, Smith AR, Coia LR, Owen JB, Hanlon A, Wallace M, Hanks G. Treatment planning for adenocarcinoma of the rectum and sigmoid: a patterns of care study. PCS Committee. Int J Radiat Oncol Biol Phys. 1997;37:305–311. doi: 10.1016/s0360-3016(96)00532-9. [DOI] [PubMed] [Google Scholar]
- 12.NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 13.Coia LR, Gunderson LL, Haller D, Hoffman J, Mohiuddin M, Tepper JE, Berkey B, Owen JB, Hanks GE. Outcomes of patients receiving radiation for carcinoma of the rectum. Results of the 1988-1989 patterns of care study. Cancer. 1999;86:1952–1958. doi: 10.1002/(sici)1097-0142(19991115)86:10<1952::aid-cncr11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Deutsche Krebsgesellschaft. Colon carcinoma and rectal carcinoma. Interdisciplinary guidelines. Onkologe. 1999;5:348–358. [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Peto R, Peto J. Asymptocally efficient rank invariant test procedures. J R Stat Soc. 1972;A 135:185–206. [Google Scholar]
- 17.Cox DR. Regression models and life tables. J R Stat Soc. 1972;B 34:187–220. [Google Scholar]
- 18.Nakayama T, Watanabe M, Teramoto T, Kitajima M. Slope analysis of CA19-9 and CEA for predicting recurrence in colorectal cancer patients. Anticancer Res. 1997;17:1379–1382. [PubMed] [Google Scholar]
- 19.Gunderson LL, Sargent DJ, Tepper JE, O'Connell MJ, Allmer C, Smalley SR, Martenson JA, Haller DG, Mayer RJ, Rich TA, et al. Impact of T and N substage on survival and disease relapse in adjuvant rectal cancer: a pooled analysis. Int J Radiat Oncol Biol Phys. 2002;54:386–396. doi: 10.1016/s0360-3016(02)02945-0. [DOI] [PubMed] [Google Scholar]
- 20.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 21.Gnant M. Impact of participation in randomized clinical trials on survival of women with early-stage breast cancer - an analysis of 7985 patients. Proc Am Soc Oncol. 2000;19:287. [Google Scholar]
- 22.Lee JH, Lee JH, Ahn JH, Bahng H, Kim TW, Kang YK, Lee KH, Kim JC, Yu CS, Kim JH, et al. Randomized trial of postoperative adjuvant therapy in stage II and III rectal cancer to define the optimal sequence of chemotherapy and radiotherapy: a preliminary report. J Clin Oncol. 2002;20:1751–1758. doi: 10.1200/JCO.2002.07.037. [DOI] [PubMed] [Google Scholar]
- 23.Arnaud JP, Nordlinger B, Bosset JF, Boes GH, Sahmoud T, Schlag PM, Pene F. Radical surgery and postoperative radiotherapy as combined treatment in rectal cancer. Final results of a phase III study of the European Organization for Research and Treatment of Cancer. Br J Surg. 1997;84:352–357. [PubMed] [Google Scholar]
- 24.McCall JL, Cox MR, Wattchow DA. Analysis of local recurrence rates after surgery alone for rectal cancer. Int J Colorectal Dis. 1995;10:126–132. doi: 10.1007/BF00298532. [DOI] [PubMed] [Google Scholar]
- 25.Phillips RK, Hittinger R, Blesovsky L, Fry JS, Fielding LP. Local recurrence following 'curative' surgery for large bowel cancer: I. The overall picture. Br J Surg. 1984;71:12–16. doi: 10.1002/bjs.1800710104. [DOI] [PubMed] [Google Scholar]
- 26.Harnsberger JR, Vernava VM 3rd, Longo WE. Radical abdominopelvic lymphadenectomy: historic perspective and current role in the surgical management of rectal cancer. Dis Colon Rectum. 1994;37:73–87. doi: 10.1007/BF02047218. [DOI] [PubMed] [Google Scholar]
- 27.Enker WE, Merchant N, Cohen AM, Lanouette NM, Swallow C, Guillem J, Paty P, Minsky B, Weyrauch K, Quan SH. Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service. Ann Surg. 1999;230:544–52; discussion 552-554. doi: 10.1097/00000658-199910000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–460. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 29.Tang R, Wang JY, Chen JS, Chang-Chien CR, Lin SE, Leung S, Fan HA. Postoperative adjuvant radiotherapy in Astler-Coller stages B2 and C rectal cancer. Dis Colon Rectum. 1992;35:1057–1065. doi: 10.1007/BF02252996. [DOI] [PubMed] [Google Scholar]
- 30.Stocchi L, Nelson H, Sargent DJ, O'Connell MJ, Tepper JE, Krook JE, Beart R Jr. Impact of surgical and pathologic variables in rectal cancer: a United States community and cooperative group report. J Clin Oncol. 2001;19:3895–3902. doi: 10.1200/JCO.2001.19.18.3895. [DOI] [PubMed] [Google Scholar]
- 31.Martijn H, de Neve W, Lybeert ML, Crommelin MA, Ribot JG. Adjuvant postoperative radiotherapy for adenocarcinoma of the rectum and rectosigmoid. A retrospective analysis of locoregional control, survival, and prognostic factors on 178 patients. Am J Clin Oncol. 1995;18:277–281. doi: 10.1097/00000421-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Kuru B, Camlibel M, Dinç S, Erdem E, Alagöl H. Prognostic factors affecting local recurrence and survival for operable rectal cancers. J Exp Clin Cancer Res. 2002;21:329–335. [PubMed] [Google Scholar]
- 33.Luna-Pérez P, Rodríguez-Ramírez S, Vega J, Sandoval E, Labastida S. Morbidity and mortality following abdominoperineal resection for low rectal adenocarcinoma. Rev Invest Clin. 2001;53:388–395. [PubMed] [Google Scholar]
- 34.Rinkus KM, Russell GB, Levine EA. Prognostic significance of nodal disease following preoperative radiation for rectal adenocarcinoma. Am Surg. 2002;68:482–487. [PubMed] [Google Scholar]
- 35.Link KH, Staib L, Schatz M, Suhr P, Röttinger E, Beger HG. Adjuvant radiochemotherapy--what is the patients benefit. Langenbecks Arch Surg. 1998;383:416–426. doi: 10.1007/s004230050154. [DOI] [PubMed] [Google Scholar]
- 36.Kapiteijn E, van de Velde CJ. Developments and quality assurance in rectal cancer surgery. Eur J Cancer. 2002;38:919–936. doi: 10.1016/s0959-8049(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 37.Tepper JE, O'Connell M, Niedzwiecki D, Hollis DR, Benson AB, Cummings B, Gunderson LL, Macdonald JS, Martenson JA, Mayer RJ. Adjuvant therapy in rectal cancer: analysis of stage, sex, and local control--final report of intergroup 0114. J Clin Oncol. 2002;20:1744–1750. doi: 10.1200/JCO.2002.07.132. [DOI] [PubMed] [Google Scholar]
- 38.Moynihan C. Men, women, gender and cancer. Eur J Cancer Care (Engl) 2002;11:166–172. doi: 10.1046/j.1365-2354.2002.00348.x. [DOI] [PubMed] [Google Scholar]
- 39.Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, Shepherd LE, Seitz JF, Francini G. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 40.Popescu RA, Norman A, Ross PJ, Parikh B, Cunningham D. Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol. 1999;17:2412–2418. doi: 10.1200/JCO.1999.17.8.2412. [DOI] [PubMed] [Google Scholar]
- 41.Reiter W, Stieber P, Reuter C, Nagel D, Lau-Werner U, Pahl H, Fateh-Moghadam A. Preoperative serum levels of CEA and CA 19-9 and their prognostic significance in colorectal carcinoma. Anticancer Res. 1997;17:2935–2938. [PubMed] [Google Scholar]
- 42.Nelson RL. The usefulness of carcinoembryonic antigen in postoperative colorectal cancer patients. Dis Colon Rectum. 1997;40:866–867. doi: 10.1007/BF02055448. [DOI] [PubMed] [Google Scholar]
- 43.Behbehani AI, Al-Sayer H, Farghaly M, Kanawati N, Mathew A, al-Bader A, Van Dalen A. Prognostic significance of CEA and CA 19-9 in colorectal cancer in Kuwait. Int J Biol Markers. 2000;15:51–55. doi: 10.1177/172460080001500109. [DOI] [PubMed] [Google Scholar]
- 44.Frykholm GJ, Glimelius B, Påhlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum. 1993;36:564–572. doi: 10.1007/BF02049863. [DOI] [PubMed] [Google Scholar]
- 45.Påhlman L, Glimelius B. Pre-operative and post-operative radiotherapy and rectal cancer. World J Surg. 1992;16:858–865. doi: 10.1007/BF02066982. [DOI] [PubMed] [Google Scholar]


