Abstract
AIM: To investigate the expression of different cytokeratins (CKs) in gastric epithelium of adult patients with chronic gastritis infected with Helicobacter pylori (H pylori) cagA+ strains.
METHODS: The expression of CK 7, 8, 18, 19 and 20 was studied immunohistochemically in antral gastric biopsies of 84 patients. All the CKs were immunostained in cagA+H pylori gastritis (57 cases), non-H pylori gastritis (17 cases) and normal gastric mucosa (10 cases).
RESULTS: In cagA+ H pylori gastritis, CK8 was expressed comparably to the normal antral mucosa from surface epithelium to deep glands. Distribution of CK18 and CK 19 was unchanged, i.e. transmucosal, but intensity of the expression was different in foveolar region in comparison to normal gastric mucosa. Cytokeratin 18 immunoreactivity was significantly higher in the foveolar epithelium of H pylori-positive gastritis compared to both H pylori-negative gastritis and controls. On the contrary, decrease in CK19 immunoreactivity occurred in foveolar epithelium of H pylori-positive gastritis. In both normal and inflamed antral mucosa without H pylori infection, CK20 was expressed strongly/moderately and homogenously in surface epithelium and upper foveolar region, but in H pylori -induced gastritis significant decrease of expression in foveolar region was noted. Generally, in both normal antral mucosa and H pylori-negative gastritis, expression of CK7 was not observed, while in about half cagA+ H pylori-infected patients, moderate focal CK7 immunoreactivity of the neck and coiled gland areas was registered, especially in areas with more severe inflammatory infiltrate.
CONCLUSION: Alterations in expression of CK 7, 18, 19 and 20 together with normal expression of CK8 occur in antral mucosa of H pylori-associated chronic gastritis in adult patients infected with cagA+ strains. Alterations in different cytokeratins expression might contribute to weakening of epithelial tight junctions observed in H pylori-infected gastric mucosa.
Keywords: cytokeratin 7, cytokeratin 8, cytokeratin18, cytokeratin19, cytokeratin20, Helicobacter pylori, Gastritis, CagA
INTRODUCTION
Cytokeratins (CKs), a family of important cytoskeleton structural proteins, have specific spatial and temporal dynamic locations along the epithelial axis of the gastrointestinal tract (GIT), and their expression is linked to the degree of epithelial differentiation[1-4]. Cytokeratins 7, 8 (intermediate-sized and basic), 18 and 19 (smallest in size and acidic) are exclusively expressed in nearly all simple epithelia, pseudostratified respiratory epithelium and transitional epithelium. CK8 and CK18 pair together and have a similar distribution, while CK19 can be detected in a broad range of epithelial tissues, including many simple epithelia, diverse stratified epithelia, and cultured keratinocytes. CK20 (intermediate sized and acidic) is expressed in gastric foveolar epithelium, intestinal villi and crypt epithelium, cutaneous and oral Merkel cells[4]. Various changes in CK expression and distribution profile have been noted in inflammatory[5], preneoplastic[6-10] and neoplastic[2,11-13] disorders along GIT, including gastric mucosa. Structural changes in the gastric epithelium of adult and pediatric H pylori-infected patients with chronic gastritis have been recently demonstrated using cytokeratin immunohistochemistry[14-19].
Results of previous studies postulated that only a subset of individuals infected with H pylori develop severe gastritis and/or metaplasia, peptic ulcer or gastric cancer [14,20]. Bacterial strain, environmental and host factors can converge in the gastroduodenal milieu and control the final outcome of H pylori infection. However, to the best of our knowledge, relationship between cagA+ H pylori and changes in CK expression in the gastric epithelium has not yet been studied in patients with chronic gastritis. Since we have previously demonstrated high seroprevalence of antibodies to cagA in H pylori-infected patients in Serbia and Montenegro[21], this study aimed to identify and describe immunohistochemical pattern of antral CK expression in H pylori –associated chronic gastritis of adult patients infected with cagA+ strains.
MATERIALS AND METHODS
Subjects
We conducted an outpatient-based prospective study at the Clinic for Gastroenterology and Hepatology (Clinical Center of Serbia, University of Belgrade). All patients gave informed consent for participation in the study and the study protocol was approved by the local Ethics Committee. Adult patients with dyspeptic symptoms and histological signs of gastritis entered the study. Dyspepsia was defined as upper abdominal or retrosternal pain, discomfort, nausea, vomiting or other symptoms referable to the upper abdominal tract lasting for at least one month[22]. Exclusion criteria were in concordance with the recommendations from European H pylori Study Group[20].
Ninety-one patients entered the study. After histological examination, we excluded 17 patients (15 with and 2 without H pylori infection) due to the presence of intestinal metaplasia (IM) in the antral mucosa. Since specific IM- related changes in CK expression were reported, we aimed to identify changes related exclusively to the presence of H pylori infection. Therefore, data from 74 patients (57 H pylori-positive and 17 H pylori-negative) with chronic gastritis were analyzed. Control group (CG) consisted of 10 asymptomatic healthy volunteers with no histological changes in the gastric mucosa (mean age 32±11 years, 3 males, 7 females). In the H pylori-positive group mean age was 44 ± 13 years (26 males, 31 females), while in the non-infected group mean age was 47 ± 17 years (5 males, 12 females). Esophagogastroduodenoscopy with biopsies from antral and corpus mucosa was performed in all patients and blood was taken for serology and immunoblot.
H pylori infection was diagnosed by rapid urease test (RUT), histology, immunohistochemistry and serology. A patient was defined as H pylori positive if histology and at least one of the other applied diagnostic methods were positive.
Routine endoscopy and H pylori status
Each patient underwent upper endoscopy and testing for the presence of H pylori by RUT and histology. Six biopsies were taken, 3 from the antrum and 3 from the corpus (2 for histology and 1 for RUT). Biopsies selected for histological examination were stained with hematoxylin-eosin, alcian blue pH 2.5/periodic acid Schiff (AB/PAS) and high iron diamine/alcian blue pH2.5 (HID/PAS). Both Giemsa and immunohistochemical stainings (polyclonal antibody to H pylori, dilution 1:10, DAKO A/S, Glostrup, Denmark) were applied for the detection of H pylori.
H pylori serology and immunoblot assay
Blood samples were taken from all patients after endoscopic examination and sera were separated by centrifugation and stored at -20 oC until analyzed. The presence of anti-cagA antibodies was detected using the Helicobacter pylori Vira blot test kit™ (Viramed Biotech AG, Lich, Germany). The concentration of anti-H pylori IgG antibodies was analyzed using Pyloriset EIA-G III™ (Orion Diagnostica, Finland)[23]. Both tests were performed according to the manufacturer’s instructions.
Immunohistochemistry
Immunohistochemical reactions were performed on consecutive sections of one selected antral biopsy of each patient to detect cytokeratins 7, 8, 18, 19 and 20. Only well-oriented biopsies allowing assessment of full mucosal thickness, were selected for immunohistochemical study. The sections were stained with monoclonal antibodies to CK7 (dilution 1:25, DAKO A/S, Glostrup, Denmark), CK8 (dilution 1:20, DAKO Carpinteria, CA, USA), CK18 (dilution 1:25, DAKO A/S, Glostrup, Denmark), CK19 (dilution 1:50, DAKO A/S, Glostrup, Denmark) and CK20 (dilution 1:25, DAKO A/S, Glostrup, Denmark). Immunohistochemical staining was performed according to the manufacturer’s instructions using streptavidin-biotin/HRP detection system (DAKO LSAB+/HRP kit, Glostrup, Denmark), followed by counterstaining with hematoxylin. For negative controls, no staining was detectable when the pre-immune serum was used instead of primary antibodies. In addition, human fetal esophagus of the 13th gestation week was used as positive control for evaluation of CK7 immunoreactivity.
Histological and immunohistochemical evaluation
Mucosal biopsies were evaluated by an experienced pathologist blinded to clinical and endoscopic data. In addition, an experienced gastrointestinal pathologist and a cytologist independently evaluated immunohistochemistry. Differences in immunohistochemical evaluation were resolved by re-examination and consensus. Chronic gastritis was diagnosed and graded according to the updated Sydney system[24]. Biopsies showing intestinal metaplasia classified according to previous proposals [13] were not included in this study. Mucosal distribution (surface epithelium, foveolar region, glandular necks and deep glands) and intensity of cytoplasm staining (without staining, weak or moderate/strong staining) were registered together with the expression pattern (focal or diffuse) for each analyzed CK, while an additional semi-quantitative assessment of percentages of immunoreactive cells in each mucosal compartment was performed and graded for CK7 in 3 groups (<10%, 10-20%, and >20% immunoreactive cells).
Statistical analysis
Results were analyzed using non-parametric tests, Kruskall-Wallis, chi-square or Fisher’s exact test for independent samples. P < 0.05 was considered statistically significant.
RESULTS
Results of our study showed that presence of intestinal metaplasia could be detected in 18.6% (17/91) of dyspeptic patients with histological signs of gastritis (15 with and 2 without H pylori infection). These patients were excluded from further analysis, since we aimed to investigate the influence of H pylori infection on normal gastric epithelium. Out of the remaining 74 patients, 57 (77%) were infected with H pylori, while infection was not found in 17. All infected patients harbored cagA+ bacterial strain in the gastric mucosa.
Histology evaluation
Histological examination of antral and corpus mucosa in H pylori-infected individuals revealed signs of antral gastritis in 5 (9%), while pangastritis was diagnosed in the remaining 52 (91%) patients. In the H pylori negative group antral gastritis was diagnosed in 7 (42%) patients and histological signs of pangastritis were found in 10 (58%). Histological changes in the gastric mucosa were graded using Sydney classification both for antral (Table 1) and corpus mucosa (data not shown).
Table 1.
Antral histology according to Sydney classification of gastritis in patients with and without Helicobacter pylori infection
| Antral mucosa (Sydney classification) | H pylori+ (n=57) | H pylori- (n=17) | P |
| Inflammatory infiltrate | |||
| 0 | 0 | 1 | 0.000 |
| 1 | 19 | 14 | |
| 2 | 31 | 2 | |
| 3 | 7 | 0 | |
| Activity of inflammation | |||
| 0 | 2 | 9 | 0.000 |
| 1 | 39 | 6 | |
| 2 | 15 | 2 | |
| 3 | 1 | 0 | |
| Atrophy | |||
| 0 | 45 | 13 | NS |
| 1 | 12 | 4 | |
| H pylori-colonization density | |||
| 0 | 0 | 17 | |
| 1 | 19 | 0 | |
| 2 | 25 | 0 | |
| 3 | 13 | 0 | |
H pylori+: Helicobacter pylori positive patients; H pylori -: Helicobacter pylori negative patients; NS: P > 0.05.
In the antrum of H pylori-infected individuals, inflammatory infiltrate density and activity of inflammation were higher than in the uninfected group (P < 0.001 for both histological parameters). Presence of atrophic changes was not different between the two groups, while moderate density of H pylori-colonization was most frequent in infected patients.
Immunohistochemical evaluation of CK expression and distribution
Cytokeratin 8 was identified immunohistochemically in antral mucosa of both H pylori positive and negative patients with gastritis and controls (Table 2). Diffuse immunoreactivity to CK8 was the predominant expression pattern in surface epithelium, foveolae and glandular neck (moderate/strong immunoreactivty was observed in 90% of controls, 70.6% of H pylori negative and 63.1% of H pylori+ gastritis and weak in only 11.8% with and 12.3% without H pylori infection). On the other hand, deeper glandular structures did not express CK8 in about 10-30% of cases. No significant difference was found in any of the examined antral mucosa regions between the three groups (Figure 1).
Table 2.
Expression of CK8 in antral mucosa of patients with gastritis (H pylori positive and negative) compared to the control group
| Cytokeratin 8 expression |
Antral mucosa epithelium |
||||||
| H pylori+G | H pylori-G | CG | P | ||||
| n | % | n | % | n | % | ||
| Surface epithelium, foveolae and glandular necks | |||||||
| Diffuse immunoreactivity | 43 | 75.4 | 14 | 82.4 | 9 | 90 | NS |
| Focal immunoreactivity | 14 | 24.6 | 3 | 17.6 | 1 | 10 | NS |
| Without staining | 0 | 0 | 0 | 0 | 0 | 0 | NS |
| n (%) | 57 | 100 | 17 | 100 | 10 | 100 | |
| Deep glands | |||||||
| Diffuse immunoreactivity | 24 | 42.1 | 11 | 64.7 | 5 | 50 | NS |
| Focal immunoreactivity | 15 | 26.3 | 2 | 11.8 | 4 | 40 | NS |
| Without staining | 18 | 31.6 | 4 | 23.5 | 1 | 10 | NS |
| n (%) | 57 | 100 | 17 | 100 | 10 | 100 | |
CG: control group; H pylori+G: H pylori positive patients with gastritis; H pylori-G -: H pylori negative patients with gastritis; NS: P > 0.05
Figure 1.
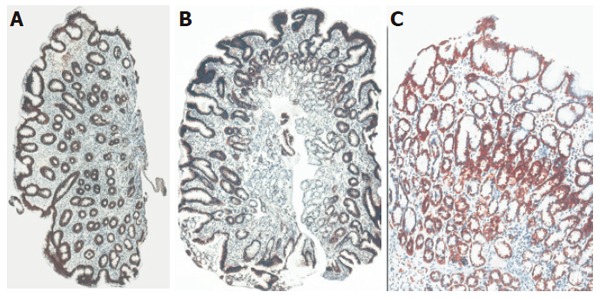
CK8 immunoreactivity in antral mucosa of patients with cagA+ H pylori chronic gastritis (A), non-H pylori chronic gastritis (B) and control subjects with normal gastric mucosa (C) with differences of CK8 immunoreactivity in antral mucosa. Original magnification: x10 (A,C); x20 (B).
Normal antral mucosa was in general immunostained transmucosal (from the surface to the gland region) when antibodies to CK18 and CK19 were applied (Table 3 and Table 4), while in foveolar region inconsistent CK18 immunoreactivity with the expression rate of only 10% was observed. In the foveolar epithelium of H pylori+ patients more intense diffuse (P < 0.05) and focal (P < 0.01) CK18 immunoreactivity was detected when compared to H pylori-negative gastritis and controls (Figure 2). As opposed to these results, lower antral CK19 patchy immunoreactivity was noted in foveolar epithelium of H pylori+ gastritis compared to H pylori-negative gastritis and controls (P < 0.05) (Figure 3).
Table 3.
Expression of CK18 in antral mucosa of patients with gastritis (H pylori positive and negative) compared to the control group
| Cytokeratin 18 expression |
Antral mucosa epithelium |
||||||
| H pylori+G | H pylori-G | CG | P | ||||
| n | % | n | % | n | % | ||
| Surface epithelium | |||||||
| Diffuse immunoreactivity | 32 | 56.1 | 14 | 82.3 | 5 | 50.0 | NS |
| Focal immunoreactivity | 24 | 42.1 | 2 | 11.8 | 3 | 30.0 | NS |
| Without staining | 1 | 1.7 | 1 | 5.9 | 2 | 20.0 | 0.043 |
| n (%) | 57 | 100 | 17 | 100 | 10 | 100 | |
| Foveolar epithelium | |||||||
| Diffuse immunoreactivity | 31 | 54.4 | 8 | 47.1 | 1 | 10 | 0.035 |
| Moderate/Strong | 30 | 52.7 | 8 | 47.1 | 1 | 10 | 0.045 |
| Weak | 1 | 1.7 | 0 | 0 | 0 | 0 | NS |
| Focal immunoreactivity | 20 | 35.1 | 1 | 5.8 | 0 | 0 | 0.008 |
| Moderate/Strong | 5 | 8.8 | 0 | 0 | 0 | 0 | NS |
| Weak | 15 | 26.3 | 1 | 5.8 | 0 | 0 | 0.045 |
| Without staining | 6 | 10.5 | 8 | 47.1 | 9 | 90 | <0.001 |
| n (%) | 57 | 100 | 17 | 100 | 10 | 100 | |
| Glandular necks | |||||||
| Diffuse immunoreactivity | 36 | 63.2 | 13 | 76.5 | 6 | 60 | NS |
| Focal immunoreactivity | 18 | 31.6 | 2 | 11.8 | 2 | 20 | NS |
| Without staining | 3 | 5.2 | 1 | 5.9 | 2 | 20 | NS |
| n (%) | 57 | 100 | 17 | 100 | 10 | 100 | |
| Deep glands | |||||||
| Diffuse immunoreactivity | 15 | 26.3 | 8 | 47.0 | 4 | 40 | NS |
| Moderate/Strong | 15 | 26.3 | 5 | 29.4 | 3 | 30 | NS |
| Weak | 0 | 0 | 3 | 17.6 | 1 | 10 | 0.008 |
| Focal immunoreactivity | 18 | 31.6 | 3 | 17.7 | 4 | 40 | NS |
| Without staining | 24 | 42.1 | 6 | 10.3 | 2 | 20 | NS |
| n (%) | 57 | 100 | 17 | 100 | 10 | 100 | |
CG:control group; H pylori+G -:Helicobacter pylori positive patients with gastritis; H pylori-G: Helicobacter pylori negative patients with gastritis; NS: P > 0.05
Table 4.
Expression of CK19 in antral mucosa of patients with gastritis (H pylori positive and negative) compared to the control group
| Cytokeratin 19 expression |
Antral mucosa epithelium |
||||||
| H pylori+G | H pylori-G | CG | P | ||||
| n | % | n | % | n | % | ||
| Surface epithelium | |||||||
| Expression in all cells | 34 | 60.6 | 12 | 70.6 | 5 | 55.6 | NS |
| Patchy staining | 21 | 37.4 | 5 | 29.4 | 4 | 44.4 | |
| Without staining | 1 | 2.0 | 0 | 0 | 0 | 0 | |
| n (%) | 56 | 100 | 17 | 100 | 9 | 100 | |
| Foveolar epithelium | |||||||
| Expression in all cells | 39 | 69.7 | 9 | 53.0 | 5 | 55.6 | NS |
| Patchy staining | 7 | 12.5 | 5 | 29.4 | 4 | 44.4 | 0.027 |
| Moderate/Strong | 2 | 3.6 | 2 | 11.8 | 2 | 22.2 | |
| Weak | 5 | 8.9 | 3 | 17.6 | 2 | 22.2 | |
| Without staining | 10 | 17.8 | 3 | 17.6 | 0 | 0 | NS |
| n (%) | 56 | 100 | 17 | 100 | 9 | 100 | |
| Glandular neck | |||||||
| Expression in all cells | 37 | 58.1 | 9 | 53 | 6 | 66.7 | NS |
| Patchy staining | 10 | 17.8 | 6 | 25.2 | 3 | 33.3 | |
| Without staining | 9 | 16.1 | 2 | 11.8 | 0 | 0 | |
| n (%) | 56 | 100 | 17 | 100 | 9 | 100 | |
| Deep gland | |||||||
| Expression in all cells | 22 | 39.2 | 6 | 35.2 | 3 | 33.3 | NS |
| Patchy staining | 10 | 17.8 | 5 | 29.5 | 2 | 22.2 | |
| Without staining | 24 | 43.0 | 6 | 35.3 | 4 | 44.5 | |
| n (%) | 56 | 100 | 17 | 100 | 9 | 100 | |
CG: control group; H pylori+G: Helicobacter pylori positive patients with gastritis; H pylori-G: Helicobacter pylori negative patients with gastritis; NS: P > 0.05.
Figure 2.
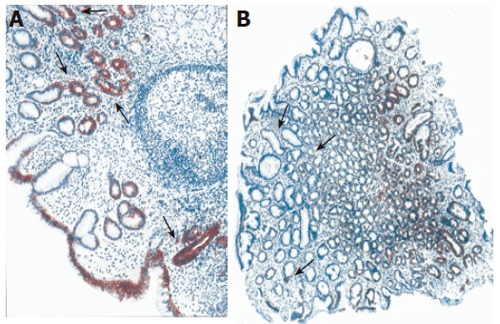
CK18 immunoreactivity in antral mucosa of patients with cagA+ H pylori chronic gastritis (A) and control subjects without gastritis (B). Original magnification: x10. Cytokeratin 18 immunoreactivity was significantly higher in the foveolar epithelium of H pylori-positive gastritis compared with H pylori-negative gastritis.
Figure 3.
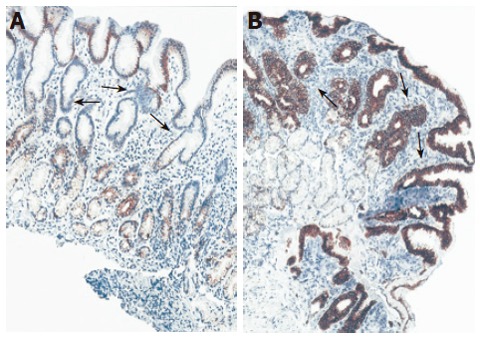
CK19 immunoreactivity in antral mucosa of patients with cagA+ H pylori chronic gastritis (A) and non-H pylori chronic gastritis (B). Decrease in CK19 immunoreactivity was found in foveolar epithelium of antral mucosa of H pylori-positive gastritis compared to non-H pylori chronic gastritis. Original magnification: x20 (A, B)
All examined antral biopsies showed positive immunostaining of CK20 (Table 5) restricted to the surface and upper foveolar epithelium. In the surface epithelium strong and homogenous CK20 immunoreactivity was predominant (moderate/strong diffuse immunoreactivity was observed in 90% of controls, 70.6% of H pylori negative and 64.9% of H pylori+ gastritis, as opposed to the patchy staining observed in 10%, 29.4% and 35.1% of individuals, respectively). No significant differences were noted between controls and patients with gastritis irrespective of the presence of H pylori infection. In the antral mucosa of the majority of H pylori negative patients with gastritis and controls CK20 was expressed strongly/moderately in all foveolar cells while in H pylori positive gastritis patients a significantly higher percentage of patients had focal expression of CK20 (Figure 4). Further analysis revealed different CK20 expressions in upper foveolar region related to the presence of gastritis but not H pylori infection. Namely, patchy staining pattern was detected in 44% of H pylori+ and 29% of H pylori negative gastritis patients and only 10% of controls (P < 0.05).
Table 5.
Expression of CK20 in antral mucosa of patients with gastritis (H pylori positive and negative) compared to the control group
| Cytokeratin 20 expression |
Antral mucosa epithelium |
||||||
| H pylori+G | H pylori-G | CG | P | ||||
| n | % | n | % | n | % | ||
| Surface epithelium | |||||||
| Expression in all cells | 37 | 64.9 | 12 | 70.6 | 9 | 90 | |
| Patchy staining | 20 | 35.1 | 5 | 29.4 | 1 | 10 | NS |
| Without staining | 0 | 0 | 0 | 0 | 0 | 0 | |
| n (%) | 57 | 100 | 17 | 100 | 10 | 100 | |
| Upper foveolar region | |||||||
| Expression in all cells | 32 | 56.1 | 12 | 70.6 | 9 | 90 | NS |
| Patchy staining | 25 | 43.9 | 5 | 29.4 | 1 | 10 | |
| Moderate/Strong | 20 | 35.1 | 4 | 23.5 | 1 | 10 | |
| Weak | 5 | 8.8 | 1 | 5.9 | 0 | 0 | 0.044 |
| Without staining | 0 | 0 | 0 | 0 | 0 | 0 | |
| n (%) | 57 | 100 | 17 | 100 | 10 | 100 | |
CG:control group; H pylori+G: Helicobacter pylori positive patients with gastritis; H pylori-G: Helicobacter pylori negative patients with gastritis; NS: P > 0.05.
Figure 4.
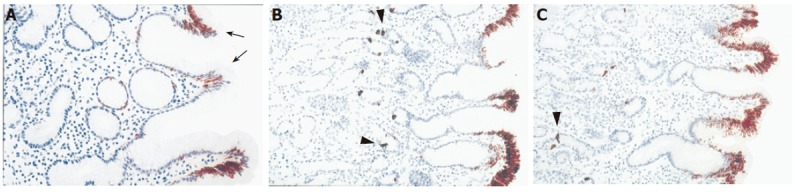
CK20 immunoreactivity in antral mucosa of patients with cagA+ H pylori chronic gastritis (A), non-H pylori chronic gastritis (B) and control subjects with normal gastric mucosa (C). In the cagA+ H pylori chronic gastritis, CK20 expression was decreased in upper foveolar epithelium in comparison to non-H pylori chronic gastritis and control subjects with normal gastric mucosa. Numerous CK20 strong immunoreactive endocrine cells could be observed (() in the glandular epithelium. Original magnification: x20 (A-C)
The main findings of CK7 immunoreactivity are listed in Table 6. In both controls and H pylori-negative gastritis patients almost no immunoreactivity of CK7 was found in the antral mucosa, with the exception of some inconsistent and faint CK7 immunoreactivity observed in single cells of glandular necks and deep glands in about 2/3 of cases (Figure 5). However, moderate focal CK7 immunoreactivity in the same area was more frequently observed in H pylori-positive gastritis compared to both H pylori-negative gastritis and control group (P < 0.01). Namely, about half in H pylori-infected patients (28/55, 50.1%) moderate focal immunoreactivity of CK7 in more than 10% of cells in neck and coiled gland areas was registered, especially in individuals with more severe inflammatory infiltrate (Figure 6).
Table 6.
Expression of CK7 in antral mucosa of patients with gastritis (H pylori positive and negative) compared to the control group
| Cytokeratin 7 expression |
Antral mucosa epithelium |
||||||
| H pylori+G | H pylori-G | CG | P | ||||
| n | % | n | % | n | % | ||
| Surface and foveolar epithelium | |||||||
| Weak focal | |||||||
| immunoreactivity | 0 | 0 | 0 | 0 | 0 | 0 | NS |
| Moderate focal | |||||||
| immunoreactivity | 5 | 9.1 | 0 | 0 | 0 | 0 | NS |
| <10% cells | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10-20% cells | 3 | 5.5 | 0 | 0 | 0 | 0 | |
| >20% cells | 2 | 3.6 | 0 | 0 | 0 | 0 | |
| Without staining | 50 | 90.9 | 16 | 100 | 10 | 100 | NS |
| n (%) | 55 | 100 | 16 | 100 | 10 | 100 | |
| Glandular necks and deep glands | |||||||
| Weak focal | |||||||
| immunoreactivity | 6 | 10.9 | 1 | 6.3 | 3 | 30.0 | |
| <10% cells | 3 | 5.5 | 1 | 6.3 | 3 | 30.0 | 0.01 |
| 10-20% cells | 2 | 3.6 | 0 | 0 | 0 | 0 | NS |
| >20% cells | 1 | 1.8 | 0 | 0 | 0 | 0 | NS |
| Moderate focal | |||||||
| immunoreactivity | 38 | 69.1 | 10 | 62.5 | 7 | 70.0 | |
| <10% cells | 10 | 18.3 | 10 | 62.5 | 7 | 70.0 | 0.002 |
| 10-20% cells | 14 | 25.4 | 0 | 0 | 0 | 0 | 0.002 |
| >20% cells | 14 | 25.4 | 0 | 0 | 0 | 0 | 0.002 |
| Without staining | 11 | 20.0 | 5 | 31.2 | 0 | 0 | |
| n (%) | 55 | 100 | 16 | 100 | 10 | 100 | |
CG: control group; H pylori+G: Helicobacter pylori positive patients with gastritis; H pylori-G: Helicobacter pylori negative patients with gastritis; NS: P > 0.05.
Figure 5.
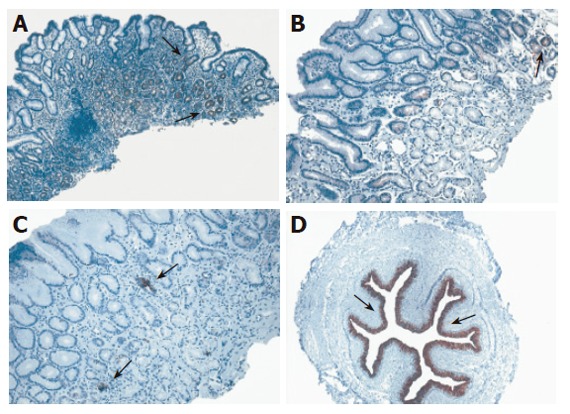
CK7 immunoreactivity in antral mucosa of patients with cagA+ H pylori chronic gastritis (A), non-H pylori chronic gastritis (B), control subjects with normal gastric mucosa (C), and control specimen of human fetal esophagus (D). Moderate focal CK7 positivity (→) in the pits and the glands of antral mucosa in patients with cagA+ H pylori chronic gastritis could be observed. CK7 positive cells covering a single gland (→) of antral mucosa could be found in patients with non-H pylori chronic gastritis. Normal antral mucosa with a few single cells or cell clusters (→) were strongly decorated with antibody to CK7. Strong CK7 immunoreactivity was displayed in the stratified columnar epithelium (→) of human fetal esophagus of the 13th gestation week. Original magnification: x10 (A-D).
Figure 6.
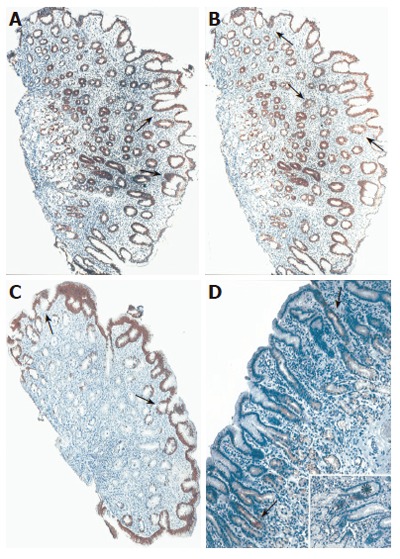
Typical expression pattern of CK18 (A), CK19 (B), CK20 (C) and CK7 (D) in antral mucosa of patients with cagA+ H pylori chronic gastritis. Strong diffuse cytokeratin 18, moderate focal CK19 and CK20 (C) immunoreactivities were demonstrated in upper foveolar epithelium (→) of the antral mucosa. Moderate focal CK7 positivity (→) was revealed in the pits and the glands of antral mucosa. CK7 immunoreactivity was prominent (*) in some cell clusters of the foveolar epithelium (insert). Original magnification: x10 (A-C), insert x20.
DISCUSSION
H pylori-induced proinflammatory cytokine expression and inflammation affects various gastric cell populations controlling gastric acid secretion[25], including somatostatin-producing D cells[26], gastrin-producing G cells[27], and parietal cells[28]. In addition, in the course of H pylori-induced gastritis, acute foveolitis of the pit proliferative zone with characteristic morphological change of epithelial cells, which is often described as the malgun (clear) cell change[29] occurs. It is postulated that malgun cell change in the course of H pylori gastritis reflects epithelial genomic damage and repair processes. In addition, various structural changes of mucosal epithelial cells in H pylori gastritis have been reported in both adult[14-16,19] and pediatric patients[18].
Strain-specific H pylori gene, cagA, has been recognized as a marker of strains that confer increased risk for peptic ulcer disease and gastric cancer[25]. Since the seroprevalence of cagA+ H pylori strains was high in chronic gastritis patients of Serbia and Montenegro[21] and there is no evidence that bacterial strain is related to alterations in cytokeratin expression in gastric epithelium, we aimed in the current study to evaluate the changes in distribution and expression pattern of different CKs in the course of cagA+ H pylori -associated chronic gastritis in adult patients.
In normal antral gastric mucosa, results of our study revealed broad panmucosal (from the surface to the gland) expressions of CKs8,18 and 19, while CK20 immunoreactivity was strong and homogenous in the tip and upper portion cells of foveolae. As opposed to other examined cytokeratins, CK7 was mostly undetectable. Similar results were obtained for gastritis patients without H pylori infection and overall these findings are in line with previous reports[1,2,14,15,17].
Several immunohistochemical studies in the past few years demonstrated that altered gastric cytokeratin expression is closely related with H pylori infection in adult patients[1,2,14-17,19]. However only two studies by Schwerer et al[15] and Louwers et al[17] have investigated multiple CKs simultaneously. To the best of our knowledge, all other studies are focused on particular CK. None of these studies however have provided data concerning bacterial strain.
According to previous observations, CK7 is present in fetal but largely absent in normal adult and is transiently de novo expressed in metaplastic and neoplastic epithelial cells[2,11]. Our results suggest that CK7, largely absent in normal adult antral mucosa, is expressed in H pylori chronic gastritis patients, which is in line with reports in adults describing slight[17] and markedly increased CK7 expression in H pylori-associated chronic gastritis in children[18]. As opposed to these findings, a study by Schwerer and Baczako[14] when investigating CK7 expression in normal foveolar epithelium found that H pylori can induce gastritis and intestinal metaplasia with CK7 immunoreactivity detected only in intestinal metaplasia.
Study by Kirchner et al[2] using animal experimental model of gastritis-cancer sequence in Mongolian gerbils revealed signs of mild gastritis 2 mo after H pylori infection together with CK7 expression in epithelial cells of basal glands followed by loss of specific differentiation and changes to duct-like appearance, while after 6, 12 and 24 mo moderate to severe gastritis with loss of differentiated gastric glands and switch to CK7 positive duct-like structures in large mucosal segments was observed. These results may provide evidence that non-neoplastic stomach with non-atrophic H pylori gastritis constantly exhibits low score of CK7 positive cells in antrum and corpus, thus supporting our findings. Furthermore, there is evidence that CK7 expression in metaplastic and neoplastic stomach is related to dedifferentiated epithelial cells that can phenotypically be linked to fetal cells at the beginning of gastric pit development[2]. The dedifferentiated cells exhibit low proliferation and beta-catenin accumulation similar to stem cells. Therefore, observations of Kirchner et al[2] imply that metaplasia, gastric intraepithelial neoplasia, early gastric cancer and dedifferentiated epithelial cells defined by CK7 expression are related with each other in H pylori induced gastritis. Based on the above stated findings, we speculate that CK7 de novo expression in gastric mucosa of patients infected with cagA+ H pylori strains represents the proliferative/regenerative cells rather than pure dedifferentiated cells because CK7 positive flat duct-like structures have not been identified.
Our results did not reveal any significant difference in CK8 expression between patients with cagA+ H pylori-induced gastritis and normal mucosa. These findings are supported by results of other authors[15,17], but not by study of Baek et al[19] that described under-expressed CK8 in the gastric mucosa of H pylori infected-individuals as a result of oxidative stress-induced cytoskeleton damage.
Higher expression of CK 18 in foveolar epithelium together with a decrease in CK19 expression was noted in patients with cagA+ H pylori-induced gastritis. These findings differ from previous studies, since two independent studies have revealed unchanged CK18 and CK19 expression in gastric mucosa of H pylori-infected patients[15,17]. Normal stomach expresses less CK19 in the upper mucosal compartment in comparison to other mucosal compartments[1]. CK19 expression is thought to be inversely related to cell proliferation, strong CK19 expression implying weak proliferation and vice versa[1]. Intestinal metaplasia of the stomach however exhibits more intense CK19-immunoreactivity than gastric cancer tissue[1]. If CK19 immunoreactivity is negatively correlated with cell proliferation and differentiation in fetal, normal and pathologically transformed adult gastric mucosa, our results may suggest good differentiation and enhanced proliferation of upper foveolar cells in patients with cagA+ H pylori-induced gastritis.
Botta et al[3] studied CK20 immunoreactivity in fetal and neonatal human gut, including stomach and demonstrated that CK20 expression is progressive increased during gestation, suggesting that the degree of CK20 positivity is related to the epithelial maturation stage in gastric mucosa. CK20 expression in adults is restricted to the surface foveolar epithelium and is not detectable in gastric pit or glandular region. Previous investigations revealed that CK20 expression is significantly lower in foveolar epithelium of H pylori-induced chronic gastritis[15-17] supporting our results, while available studies suggest that this is a reversible change and CK20 expression is normalized within 6 mo after eradication of H pylori infection[15].
Taken together, all these findings imply alterations in epithelial cell maturation in the course of H pylori-induced chronic gastritis.
Our results suggest that bacterial strain is of importance in inducing alterations of CK expression. It is well known that cagA present in 50-60% of all strains is a part of H pylori genome termed cag pathogenicity island (cagPAI) and that proteins encoded by cagPAI are responsible for both NFkB and MAPK activation in gastric epithelial cells. It has also been demonstrated that infection with cagA+ strains is more likely to result in peptic ulceration, atrophic gastritis and gastric carcinoma[25]. Therefore, presence of cagPAI in H pylori genome might play a role in signal transduction leading to H pylori-induced host gene expression, thus regulating inflammation, proliferation and carcinogenesis. In addition, bacterial strain-related differences in host gene expression have been reported, implying that protein expression profile including CKs, depends on bacterial strains and is related to the presence of cagA+ H pylori strains. Amieva et al.[31] reported that cagA appears to target H pylori host cell intercellular junctions and to disrupt junction-mediated functions. Since predominant genotype of H pylori in Serbia and Montenegro has been reported to be the cagA+ genotype[21], it is very important to further investigate the presence and reversibility of different epithelial alterations induced by different H pylori strains.
Footnotes
Supported by a grant from Serbian Ministry for Science and Environmental Protection, No. 1752
S- Editor Guo SY L- Editor Wang XL E- Editor Bai SH
References
- 1.Stammberger P, Baczako K. Cytokeratin 19 expression in human gastrointestinal mucosa during human prenatal development and in gastrointestinal tumours: relation to cell proliferation. Cell Tissue Res. 1999;298:377–381. doi: 10.1007/s004419900085. [DOI] [PubMed] [Google Scholar]
- 2.Kirchner T, Müller S, Hattori T, Mukaisyo K, Papadopoulos T, Brabletz T, Jung A. Metaplasia, intraepithelial neoplasia and early cancer of the stomach are related to dedifferentiated epithelial cells defined by cytokeratin-7 expression in gastritis. Virchows Arch. 2001;439:512–522. doi: 10.1007/s004280100477. [DOI] [PubMed] [Google Scholar]
- 3.Botta MC, Ambu R, Liguori C, Van Eyken P, Pisanu A, Cabras A, Hofler H, Werner M, Faa G. [CK20 expression in the gastrointestinal tract of the embryo and fetus] Pathologica. 2001;93:640–644. [PubMed] [Google Scholar]
- 4.Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403. doi: 10.1046/j.1365-2559.2002.01387.x. [DOI] [PubMed] [Google Scholar]
- 5.Stosiek P, Kasper M. [Neo-expression of cytokeratin 7 in chronic atrophic gastritis with pernicious anemia] Pathologe. 1990;11:14–17. [PubMed] [Google Scholar]
- 6.Couvelard A, Cauvin JM, Goldfain D, Rotenberg A, Robaszkiewicz M, Fléjou JF. Cytokeratin immunoreactivity of intestinal metaplasia at normal oesophagogastric junction indicates its aetiology. Gut. 2001;49:761–766. doi: 10.1136/gut.49.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeMeester SR, Wickramasinghe KS, Lord RV, Friedman A, Balaji NS, Chandrasoma PT, Hagen JA, Peters JH, DeMeester TR. Cytokeratin and DAS-1 immunostaining reveal similarities among cardiac mucosa, CIM, and Barrett's esophagus. Am J Gastroenterol. 2002;97:2514–2523. doi: 10.1111/j.1572-0241.2002.06033.x. [DOI] [PubMed] [Google Scholar]
- 8.Jovanovic I, Tzardi M, Mouzas IA, Micev M, Pesko P, Milosavljevic T, Zois M, Sganzos M, Delides G, Kanavaros P. Changing pattern of cytokeratin 7 and 20 expression from normal epithelium to intestinal metaplasia of the gastric mucosa and gastroesophageal junction. Histol Histopathol. 2002;17:445–454. doi: 10.14670/HH-17.445. [DOI] [PubMed] [Google Scholar]
- 9.Balaji NS, DeMeester SR, Wickramasinghe KS, Hagen JA, Peters JH, DeMeester TR. Etiology of intestinal metaplasia at the gastroesophageal junction. Surg Endosc. 2003;17:43–48. doi: 10.1007/s00464-002-8944-1. [DOI] [PubMed] [Google Scholar]
- 10.Piazuelo MB, Haque S, Delgado A, Du JX, Rodriguez F, Correa P. Phenotypic differences between esophageal and gastric intestinal metaplasia. Mod Pathol. 2004;17:62–74. doi: 10.1038/sj.modpathol.3800016. [DOI] [PubMed] [Google Scholar]
- 11.Stosiek P, Kasper M. [Transitory appearance of cytokeratin 7 in development of stomach cancer] Pathologe. 1993;14:71–73. [PubMed] [Google Scholar]
- 12.Cameron AJ, Souto EO, Smyrk TC. Small adenocarcinomas of the esophagogastric junction: association with intestinal metaplasia and dysplasia. Am J Gastroenterol. 2002;97:1375–1380. doi: 10.1111/j.1572-0241.2002.05669.x. [DOI] [PubMed] [Google Scholar]
- 13.Shen B, Ormsby AH, Shen C, Dumot JA, Shao YW, Bevins CL, Gramlich TL. Cytokeratin expression patterns in noncardia, intestinal metaplasia-associated gastric adenocarcinoma: implication for the evaluation of intestinal metaplasia and tumors at the esophagogastric junction. Cancer. 2002;94:820–831. doi: 10.1002/cncr.10215. [DOI] [PubMed] [Google Scholar]
- 14.Schwerer MJ, Baczako K. Expression of cytokeratins typical for ductal and squamous differentiation in the human stomach: an immunohistochemical study of normal foveolar epithelium, Helicobacter pylori gastritis and intestinal metaplasia. Histopathology. 1996;29:131–137. doi: 10.1046/j.1365-2559.1996.d01-496.x. [DOI] [PubMed] [Google Scholar]
- 15.Schwerer MJ, Kraft K, Baczako K. Structural changes in the gastric foveolar epithelium in Helicobacter pylori-positive gastritis revealed by keratin immunohistochemistry. Hum Pathol. 1997;28:1260–1267. doi: 10.1016/s0046-8177(97)90199-4. [DOI] [PubMed] [Google Scholar]
- 16.Sales MG, Nasciutti LE, Lorena DE, Muzzi M, Porto LC. Differential expression of laminin isoform (alpha2), integrins (alpha3beta1 and alpha6beta4) and cytokeratin 20 in H. pylori gastritis. Histol Histopathol. 2001;16:1021–1029. doi: 10.14670/HH-16.1021. [DOI] [PubMed] [Google Scholar]
- 17.Lauwers GY, Furman J, Michael LE, Balis UJ, Kubilis PS. Cytoskeletal and kinetic epithelial differences between NSAID gastropathy and Helicobacter pylori gastritis: an immunohistochemical determination. Histopathology. 2001;39:133–140. doi: 10.1046/j.1365-2559.2001.01185.x. [DOI] [PubMed] [Google Scholar]
- 18.Cohen M, Cueto Rúa E, Balcarce N, Drut R. Expression of cytokeratins 7 and 20 in Helicobacter pylori-associated chronic gastritis in children. Pediatr Dev Pathol. 2004;7:180–186. doi: 10.1007/s10024-003-1006-4. [DOI] [PubMed] [Google Scholar]
- 19.Baek HY, Lim JW, Kim H, Kim JM, Kim JS, Jung HC, Kim KH. Oxidative-stress-related proteome changes in Helicobacter pylori-infected human gastric mucosa. Biochem J. 2004;379:291–299. doi: 10.1042/BJ20031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malfertheiner P, Mégraud F, O'Morain C, Bell D, Bianchi Porro G, Deltenre M, Forman D, Gasbarrini G, Jaup B, Misiewicz JJ, et al. Current European concepts in the management of Helicobacter pylori infection--the Maastricht Consensus Report. The European Helicobacter Pylori Study Group (EHPSG) Eur J Gastroenterol Hepatol. 1997;9:1–2. doi: 10.1097/00042737-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Sokic-Milutinovic A, Wex T, Todorovic V, Milosavljevic T, Malfertheiner P. Anti-CagA and anti-VacA antibodies in Helicobacter pylori-infected patients with and without peptic ulcer disease in Serbia and Montenegro. Scand J Gastroenterol. 2004;39:222–226. doi: 10.1080/00365520310008403. [DOI] [PubMed] [Google Scholar]
- 22.Colin-Jones DG. Acid-related disorders: what are they. Scand J Gastroenterol Suppl. 1988;155:8–11. doi: 10.3109/00365528809096272. [DOI] [PubMed] [Google Scholar]
- 23.Weijnen CF, Hendriks HA, Hoes AW, Verweij WM, Verheij TJ, de Wit NJ. New immunoassay for the detection of Helicobacter pylori infection compared with urease test, 13C breath test and histology: validation in the primary care setting. J Microbiol Methods. 2001;46:235–240. doi: 10.1016/s0167-7012(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 24.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milutinovic AS, Todorovic V, Milosavljevic T, Micev M, Spuran M, Drndarevic N. Somatostatin and D cells in patients with gastritis in the course of Helicobacter pylori eradication: a six-month, follow-up study. Eur J Gastroenterol Hepatol. 2003;15:755–766. doi: 10.1097/01.meg.0000059153.68845.1a. [DOI] [PubMed] [Google Scholar]
- 27.Sokic-Milutinovic A, Todorovic V, Milosavljevic T, Micev M, Drndarevic N, Mitrovic O. Gastrin and antral G cells in course of Helicobacter pylori eradication: six months follow up study. World J Gastroenterol. 2005;11:4140–4147. doi: 10.3748/wjg.v11.i27.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227–234. doi: 10.1136/gut.42.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang J, Lee S, Jung Y, Song K, Fukumoto M, Gould VE, Lee I. Malgun (clear) cell change in Helicobacter pylori gastritis reflects epithelial genomic damage and repair. Am J Pathol. 2003;162:1203–1211. doi: 10.1016/S0002-9440(10)63916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stosiek P, Bräutigam E, Kasper M. Expression of cytokeratin 7 in human glandular epithelium of fetal stomach. Acta Histochem. 1991;91:21–23. doi: 10.1016/S0065-1281(11)80286-2. [DOI] [PubMed] [Google Scholar]
- 31.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]


