Abstract
AIM:To evaluate the method of noninvasive transient elastography for assessment of histological stage of liver fibrosis in patients with chronic hepatitis C (CHC).
METHODS: Two hundred and thirty-seven patients with CHC were included in this study. Liver biopsy was performed under ultrasonography on 217 of the patients, excluding twenty with clear clinical evidence of liver cirrhosis. Fifty subjects without liver disease were enrolled as a control group (stage 0). Twenty-five patients with sustained virological response (SVR) to interferon (IFN) therapy were also enrolled. These patients underwent liver biopsy before IFN therapy. Examination of liver stiffness (LS) was performed by elastography.
RESULTS: Medians (50% levels) of LS were 4.1 (3.5-4.9), 6.3 (4.8-8.5), 8.8 (6.8-12.0), 14.6 (10.5-18.6), and 22.2 (15.4-28.0), respectively, in the fibrosis stages 0-4 (P < 0.001). LS was significantly correlated with four serum fibrosis markers. LS values in patients with SVR were 3.8 (3.5-5.6), 5.2 (4.4-6.8), 6.8 (6.1-7.6), and 6.1 (3.6-7.9), respectively, in the fibrosis stages 1-4. In all stages, LS for patients with SVR was significantly lower than that for patients who did not undergo IFN therapy. LS was significantly correlated with serum concentrations of hyaluronic acid, type IV collagen, type IV collagen 7S, and type III procollagen N peptide.
CONCLUSION: LS correlated well with the histological stage of fibrosis. Changes in liver fibrosis stage may thus be estimated noninvasively using transient elastography.
Keywords: Hepatitis C virus, Echography, Fibrosis, Stiffness, Interferon
INTRODUCTION
Hepatic fibrosis deeply involves in the advance of stage of chronic hepatitis C (CHC), eventually leading to liver cirrhosis. In addition, the incidence of hepatocellular carcinoma (HCC) increases as the stage of fibrosis associated with CHC progresses[1]. It has been reported that patients with CHC with low-stage fibrosis respond better to interferon (IFN) therapy than those with higher-stage fibrosis[2]. Accordingly, evaluation of the stage of liver fibrosis is important when treating CHC. Although liver biopsy has been considered a gold standard for evaluation of liver fibrosis stage, it is invasive, stressful and is sometimes refused by patients or causes complications. In addition, liver biopsy can have life-threatening complications[3-5]. For these reasons, it is not possible to perform liver biopsy for all patients with CHC. Furthermore, the tissue samples obtained by needle biopsy are sometimes inadequate in quantity for accurate diagnosis[6,7]. Ratings based on examination of the liver tissue specimens may vary even among the specialists in pathology[8]. In addition, staging of fibrosis based on liver tissue specimens allows only step-wise evaluation (rather than as a continuous variable). While several serum markers (hyaluronic acid, type IV collagen, type IV collagen 7S, and P-III-P) are known to be useful for quantitative evaluation of the liver fibrosis, and are utilized for indirect testing using serum samples[9-12].
In general, liver stiffness (LS) increases as liver fibrosis progresses[13]. The FibroScan 502 (FS, EchoSens, Paris, France) for transient elastography is a new modality developed for noninvasive evaluation of liver stiffness based on the following principle. Waves including elastic shear waves are emitted from the vibrator attached to the ultrasound transducer probe. Pulse-echo ultrasound acquisitions follow the shear waves, and the velocity of such waves through the liver can be determined. LS is calculated from the shear wave velocity using Young's modulus. Use of transient vibration presents several advantages. First, the transmitted elastic waves can be temporally separated from reflected elastic waves. Second, the acquisition time is short, enabling measurements to be made on moving organs. Transient elastography is thus well adapted to the study of the liver.
We examined the relationships between the liver fibrosis of patients with CHC and LS determined by FS as well as serum markers of fibrosis.
MATERIALS AND METHODS
Patients
For initial examination, 237 subjects with CHC managed as patients at the Osaka City University Hospital were enrolled. Of these patients, 214 underwent liver biopsy under ultrasonic guidance. All the 237 patients satisfied the following criteria: (1) they were HCV RNA-positive, (2) free of ascites, and (3) liver disease due to HBV or alcohol could be ruled out for them. The control group (n = 50) was composed of 30 healthy volunteers and 20 patients without hepatic diseases. The mean age was lower in the control group than in any group of patients with CHC (Table 1). The liver biopsy was carried out using a 15-gauge needle biopsy apparatus (Hakko Inc., Tokyo, Japan). The specimens were fixed, paraffin-embedded, and stained with hematoxylin and eosin (H&E). Histological evaluation of the liver specimens was performed by two senior pathologists specialized in liver pathology. Stage of fibrosis and grade of activity in the liver were estimated according to the classification of Desmet et al[14]. The interval between the liver biopsy and FS measurement ranged from 0 d (on the same day) to 6 mo. The stage of fibrosis in the control group was rated stage 0. Patients clinically diagnosed with liver cirrhosis on the basis of diagnostic imagings (including computed tomography and ultrasonography) and hematological tests (although liver biopsy had not been performed) were also included in the analysis as stage 4 patients. These patients were included since, if analysis had been confined to the patients who had undergone liver biopsy, results might have been biased, because the liver biopsy is seldom performed in patients with liver cirrhosis (since IFN therapy is not indicated for these patients) and is not possible in cirrhotic patients with thrombocytopenia. Furthermore, 25 patients with CHC exhibiting sustained viral response (both disappearance of serum HCV RNA and normalization of alanine aminotransferase in response to previous IFN therapy) were also included for the next stage of analysis. These 25 patients had undergone liver biopsy and histological evaluation of the liver tissue before IFN therapy (Table 2). The study protocol accorded with the Helsinki Declaration. Patients were enrolled after provision of informed consent.
Table 1.
Characteristics of patients without SVR
| Stage | 0 | 1 | 2 | 3 | 4 | P |
| n | 50 | 113 | 68 | 18 | 38 | |
| Age (yr) | 46.2 ± 17.9 | 55.9 ± 12.8 | 59.7 ± 9.9 | 57.2 ± 11.6 | 65.0 ± 10.2 | < 0.001 |
| M/F | 25/25 | 54/59 | 19/49 | 7/11 | 21/17 | 0.031 |
| HCV serotype | ||||||
| 1 | 48 | 34 | 7 | 17 | ||
| 2 | 23 | 12 | 1 | 3 | ||
| ND | 42 | 22 | 10 | 18 | NS | |
| Histological -grading | ||||||
| Minimal | 57 | 11 | 2 | |||
| Mild | 50 | 38 | 8 | 6 | ||
| Moderate | 6 | 19 | 10 | 6 | ||
| Severe | 1 | |||||
| ND | 23 |
Results for age are given as mean ± SD deviation. SVR: sustained viral response; ND: not done.
Table 2.
Characteristics of patients with SVR
| Stage | 1 | 2 | 3 | 4 | P |
| n | 9 | 8 | 3 | 5 | |
| Age (yr) | 60.0 ± 8.6 | 60.2 ± 10.3 | 66.0 ± 6.1 | 58.5 ± 8.1 | NS |
| M/F | 5/4 | 3/4 | 2/1 | 2/3 | NS |
| Serotype | |||||
| 1 | 4 | 3 | 1 | 2 | |
| 2 | 3 | 4 | 2 | 2 | |
| ND | 2 | 1 | 1 | NS | |
| Histological -grading | |||||
| Minimal | 1 | 2 | |||
| Mild | 3 | 4 | 1 | 1 | |
| Moderate | 4 | 3 | 2 | 1 | |
| Severe | 1 | 1 | 0 | 1 | |
| Period from liver | 2141 | 981 | 2187 | 2240 | |
| biopsy (d) | (626-5667) | (282-2285) | (1191-2946) | (970-3074) | NS |
Results for age are given as mean ± SD. SVR: sustained viral response; ND: not done.
Liver stiffness measurement
LS was measured by transient elastography using an FS. Briefly, the subject lay on the bed in the horizontally supine position, and a probe was placed on the skin above the right intercostal space. The velocity of shear waves, generated temporarily and passing though the liver, was combined with Young’s modulus for automated calculation of elasticity[15]. The median of 10 consecutive measurements was used as the LS for a given subject, and expressed in units of kilopascals (kPa).
Serum markers of fibrosis
Blood for measurement of serum markers of fibrosis was sampled on the day of LS measurement. Of the markers, serum concentrations of type IV collagen (IV collagen) were measured with latex agglutination turbidimetry (PANASSAY IV C; Daiichi Fine Chemical Co., Ltd., Tokyo, Japan), with a normal range of not more than 150 ng/mL. Serum concentrations of type IV collagen 7S (IV collagen 7S) were measured by radioimmunoassay (type IV collagen-7S kit, Mitsubishi Kagaku Iatron Inc., Tokyo, Japan), with a normal range of not more than 6 ng/mL. Serum concentrations of type III procollagen N peptide (P-III-P) were measured by radioimmunoassay (RIA-gnost PIII P c.t, Nihon Shering K.K., Osaka, Japan), with a normal range of 0.3-0.8 U/mL. Serum hyaluronic acid (HA) concentrations were measured by latex agglutination immunoturbidimetry (LPIA Ace HA, Fujirebio Inc., Tokyo Japan), with a normal range of not more than 50 ng/mL.
Statistical analysis
Biochemical data were expressed as mean ± SD. Elastography data were expressed as median values. Box plots were used to study the LS value distribution according to the stage of fibrosis. Differences in mean values were tested by one-way analysis of variance (ANOVA), followed by the Kruskal-Wallis test. The Mann-Whitney U-test was used to compare the data between the two groups. The χ2 test was used to compare the distribution of individual variables among the patient groups. Correlations between two variables were examined using Spearman’s correlation coefficient. Differences were considered statistically significant when P values were less than 0.05. All analyses were performed using SPSS 11.0J (SPSS Japan Inc. Tokyo, Japan).
RESULTS
Biochemistry
Table 3 shows the results of biochemical tests at each stage of CHC. Platelet count decreased as the stage progressed. Albumin level at stage 4 was significantly lower than at stage 1 (P = 0.006). Type IV collagen 7S level at stage 4 was significantly lower than at stage 1 (P = 0.032). However, HA level at stage 4 was significantly higher than at stage 1 (P = 0.041).
Table 3.
Results of biochemical examination of patients
| Stage | 1 | 2 | 3 | 4 | P |
| n | 113 | 68 | 18 | 38 | |
| Platelet (× 104 μL) | 18.0 ± 8.0 | 13.9 ± 6.6 | 12.1 ± 5.7 | 9.9 ± 5.6 | < 0.001 |
| AST (IU) | 47.3 ± 31.9 | 51.6 ± 27.2 | 63.8 ± 33.4 | 95.9 ± 142.2 | 0.001 |
| ALT (IU) | 60.9 ± 59.3 | 61.0 ± 40.5 | 75.8 ± 51.0 | 86.8 ± 135.7 | 0.023 |
| ALB (g/dL) | 3.9 ± 1.0 | 3.6 ± 1.3 | 3.8 ± 1.0 | 3.3 ± 1.1 | 0.036 |
| HA (ng/mL)1 | 56.7 ± 65.8 | 89.2 ± 112.0 | 113.8 ± 125.8 | 509.3 ± 404.7 | < 0.001 |
| IV collagen (ng/mL)2 | 121.0 ± 57.5 | 149.9 ± 67.9 | 176.8 ± 129.2 | 229.7 ± 108.8 | 0.020 |
| IV collagen 7S (ng/mL)2 | 5.5 ± 1.8 | 6.0 ± 1.6 | 8.9 ± 2.9 | 8.0 ± 2.1 | 0.001 |
| P-III-P (U/mL)2 | 0.79 ± 0.19 | 0.88 ± 0.30 | 1.23 ± 0.60 | 1.02 ± 0.29 | 0.023 |
Results are given as mean ± SD.
HA was measured in 57 patients;
IVcollagen, IVcollagen 7S, and P-III-P were measured in 52 patients. AST: asparate aminotransferase; ALT: alanine aminotransferase; ALB: albumin; HA: hyaluronic acid.
Relationship between the histological stage and the liver stiffness
Figure 1 shows LS determined with FS for the control group (stage 0) and serum HCV RNA-positive patients with CHC. Median LS (50% level) was 4.1 (3.5-4.9) at stage 0, 6.3 (4.8-8.5) at stage 1, 8.8 (6.8-12.0) at stage 2, 14.6 (10.5-18.6) at stage 3, and 22.2 (15.4-28.0) at stage 4 (Kruskal-Wallis test, P < 0.001). Mean LS differed significantly between each two of the five stages. The differences between the groups were as follows: stage 1 versus stages 2 (P = 0.011), 3 (P < 0.001), and 4 (P < 0.001); stage 2 versus stages 3 (P = 0.013) and 4 (P < 0.001); stage 3 versus stage 4 (P = 0.004). These differences were much superior to those obtained using the biochemical markers. Figure 2 shows the examples of findings for Azan-Mallory-stained liver tissue and LS. Samples A and B were classified as stage 1. However, degrees of fibrosis noted on microscopic examination differed between the two samples.
Figure 1.
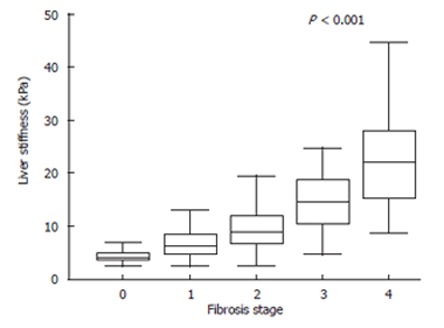
Liver stiffness measurements for each fibrosis stage. Fibrosis stage 0 is offered to the control group. The top and bottom of the boxes are the 1st and 3rd quartiles. The length of the box thus represents the interquartile range (IQR) within which 50% of values are located. The lines through the middle of the boxes represent the median. The error bars represent the minimum and maximum values (measurement range). Significant correlation was found between stage of fibrosis and liver stiffness (P < 0.001, Kruaskal-wallis test).
Figure 2.
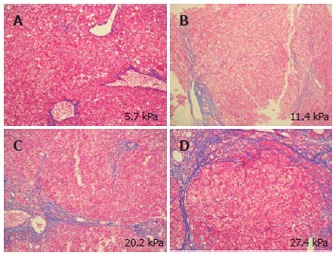
Azan-Mallory-stained liver tissue and liver stiffness measurement. A and B: stage 1 liver tissue samples, showing that the grade of fibrosis and elasticity was higher for B than for A; C: stage 3 liver tissue sample; D: stage 4 liver tissue sample. The elasticity thus increased as the fibrosis progressed.
At each stage of fibrosis, LS was significantly lower in SVR than in HCV RNA-positive patients not treated with interferon (NT) (Figure 3). The median LS of SVR was 3.8 (3.5-5.6) (P = 0.030) at stage 1, 5.7 (4.4-6.8) (P = 0.004) at stage 2, 6.8 (6.1-7.6) (P = 0.047) at stage 3, and 6.1 (3.6-7.9) (P = 0.001) at stage 4 (compared with NT patients).
Figure 3.
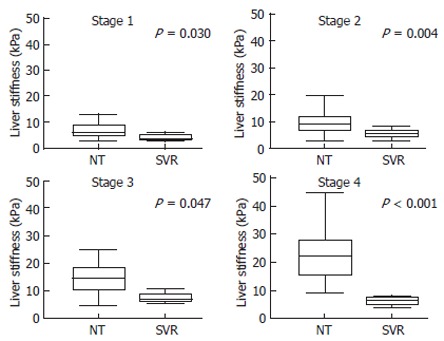
Liver stiffness measurements for each fibrosis stage in the patients not treated with IFN and the patients with sustained response for IFN therapy. Fibrosis stage 0 indicates the control group. The top and bottom of the boxes are the 1st and 3rd quartiles. The length of the box thus represents the interquartile range (IQR) within which 50% of values are located. The lines through the middle of the boxes represent the median. The error bars represent the minimum and maximum values (measurement range). In each stage, liver stiffness differed between NT and SVR. At each stage of fibrosis, elasticity was significantly lower in SVR than in HCV RNA-positive cases without IFN therapy (NT). NT: Patients without IFN therapy; SVR: Patients with sustained viral response to IFN therapy.
Correlations with serum markers of fibrosis
When correlations between the liver stiffness and serum markers of fibrosis were determined, the coefficient of correlation (r) was found to be 0.474 with HA (n = 70, P < 0.001), 0.581 with type IV collagen (n = 70, P < 0.001), 0.581 with type IV collagen 7S (n = 69, P < 0.001), and 0.233 with P-III-P (n = 70, P = 0.064), respectively (Figure 4). Each of the four serum markers of fibrosis were significantly correlated with LS measured using FS.
Figure 4.
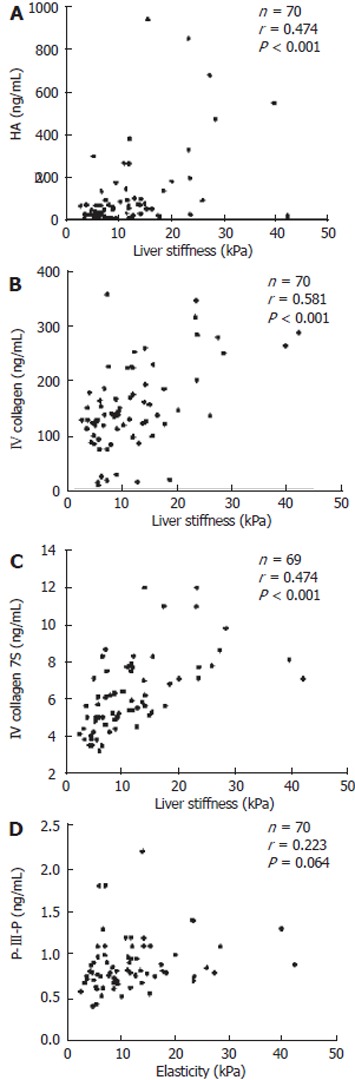
Correlation between liver stiffness and serum fibrosis markers. Correlations between elasticity measured by the Fibroscan502 and HA (A), type IV collagen (B), IV collagen 7S (C), and P-III-P (D) are indicated. HA: Hyaluronic acid; P-III-P: Type III procollagen N peptide.
DISCUSSION
HCC is the most frequent cause of death among patients with CHC. As liver fibrosis associated with CHC advances, HCC develops with high incidence[1]. It has been reported that the rate of viral eradication in response to IFN therapy is high in the patients with low-stage liver fibrosis[2,16]. Evaluation of the liver tissue is thus quite important when selecting a method of treatment and predicting prognosis for the individual patients with CHC. The stage of CHC is usually evaluated based on general assessment of hematological data and findings of diagnostic imagings. However, with these indirect tests, it is difficult to precisely determine the stage of liver fibrosis. Liver biopsy is a gold standard for direct evaluation of liver fibrosis. However, since it is invasive and stressful, and carries certain risks, it cannot be performed for all patients with chronic liver disease[17,18]. Furthermore, it is difficult to perform liver biopsy several times in the same patient. Regev et al[19] found that severity of fibrosis differed by at least one stage between the right and left lobes of the liver in 41 of 124 (33%) patients studied. Bedossa et al[7] reported that accurate staging by the METAVIR fibrosis staging method was possible in only 75% of cases when liver biopsy specimens of at least 25 mm in length were used for evaluation. Siddique et al[20] found that degree of fibrosis differed by at least one stage in 45% of cases when tissue specimens collected from the same puncture site were evaluated. With liver biopsy, precise determination of the stage of liver fibrosis is sometimes impossible, depending on the amount of tissue sample available. Furthermore, results of evaluation can differ among pathologists[21,22]. Correlations between fibrosis markers and the stage of fibrosis due to liver disease (including liver cirrhosis) have also been reported[9-12]. In the liver, HA is synthesized and secreted by fat-storing cells, which are liver-specific pericytes considered to be major contributors to liver fibrosis[23]. Elevated serum HA concentrations result not only from reduced catabolism of HA in the liver but also from excess hepatic production of HA. Type IV collagen is one of the major constituents of the basement membrane progressively laid down in fibrotic liver as a continuous subendothelial layer along the space of Disse. Appearance of type IV collagen 7S in serum is thought to be due primarily to degradation of existing basement membrane rather than newly synthesized type IV collagen[24]. Serum P-III-P concentrations are thought to reflect mainly the degree of fibrosis and fibrogenic activity in chronic liver disease[25]. However, P-III-P may also be derived from degradation of tissue type III collagen still containing the amino-terminal peptide[26]. In addition, circulating P-III-P is metabolized by the liver endothelial cells[24]. These factors may complicate interpretation of changes of serum P-III-P, obscuring relationships between serum concentration and fibrogenic activity in the liver. Almost all human studies have used biopsy specimens for histological evaluation. In the present study, positive correlations between measurement with FS and serum markers of fibrosis were found.
Transient elastography using FS permits noninvasive measurement of LS from the body surface with a high degree of reproducibility. According to a recent report, LS determined with FS increased as the stage of liver fibrosis advanced in the patients with CHC. Liver biopsy permits only semi-quantitative evaluation of fibrosis, since degree of fibrosis can be expressed only in steps and not as a continuous variable. FS, on the other hand, may enable more quantitative evaluation of fibrosis. Saito et al[27] reported that platelet count correlated well with the stage of fibrosis, and that variation was large for platelet count but small for FS measurement. Measurement of liver stiffness with FS is superior to liver biopsy, in that the former does not cause pain or other adverse events and thus can be repeated. Furthermore, FS measurement features little inter-observer variation and is hence highly reproducible. Since LS determined with FS correlated positively with the stage of liver fibrosis, it should be possible to utilize FS to estimate the degree of liver fibrosis in patients with CHC[28,29]. Data from patients who underwent several liver biopsies indicate that liver fibrosis in the patients with CHC advances at a rate of approximately one stage per 10 years[30]. However, the rate of progression of fibrosis can vary depending on sex, alcohol consumption, and certain other factors[31]. In the present study, degree of liver fibrosis was significantly lower in the patients with CHC who had become SVR in response to IFN therapy than in HCV RNA-positive patients with CHC. This finding indicates that liver fibrosis was alleviated in the patients with CHC who had exhibited viral eradication in response to IFN therapy. A long-term prospective follow-up study of determination of liver stiffness by FS is now needed. The findings of the present study suggest that FS is a promising means of quantitative evaluation of the degree of liver fibrosis associated with CHC.
Footnotes
S- Editor Liu Y L- Editor Kumar M E- Editor Liu WF
References
- 1.Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174–181. doi: 10.7326/0003-4819-131-3-199908030-00003. [DOI] [PubMed] [Google Scholar]
- 2.Poynard T, McHutchison J, Davis GL, Esteban-Mur R, Goodman Z, Bedossa P, Albrecht J. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology. 2000;32:1131–1137. doi: 10.1053/jhep.2000.19347. [DOI] [PubMed] [Google Scholar]
- 3.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 4.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF) Hepatology. 2000;32:477–481. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 5.Poynard T, Ratziu V, Bedossa P. Appropriateness of liver biopsy. Can J Gastroenterol. 2000;14:543–548. doi: 10.1155/2000/107982. [DOI] [PubMed] [Google Scholar]
- 6.Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–525. doi: 10.1016/s0140-6736(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 7.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 9.Engström-Laurent A, Lööf L, Nyberg A, Schröder T. Increased serum levels of hyaluronate in liver disease. Hepatology. 1985;5:638–642. doi: 10.1002/hep.1840050420. [DOI] [PubMed] [Google Scholar]
- 10.Niemelä O, Risteli L, Sotaniemi EA, Risteli J. Type IV collagen and laminin-related antigens in human serum in alcoholic liver disease. Eur J Clin Invest. 1985;15:132–137. doi: 10.1111/j.1365-2362.1985.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamada S, Suou T, Kawasaki H, Yoshikawa N. Clinical significance of serum 7S collagen in various liver diseases. Clin Biochem. 1992;25:467–470. doi: 10.1016/0009-9120(92)90150-q. [DOI] [PubMed] [Google Scholar]
- 12.Rohde H, Vargas L, Hahn E, Kalbfleisch H, Bruguera M, Timpl R. Radioimmunoassay for type III procollagen peptide and its application to human liver disease. Eur J Clin Invest. 1979;9:451–459. doi: 10.1111/j.1365-2362.1979.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 13.Yeh WC, Li PC, Jeng YM, Hsu HC, Kuo PL, Li ML, Yang PM, Lee PH. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med Biol. 2002;28:467–474. doi: 10.1016/s0301-5629(02)00489-1. [DOI] [PubMed] [Google Scholar]
- 14.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 15.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Tsubota A, Chayama K, Ikeda K, Yasuji A, Koida I, Saitoh S, Hashimoto M, Iwasaki S, Kobayashi M, Hiromitsu K. Factors predictive of response to interferon-alpha therapy in hepatitis C virus infection. Hepatology. 1994;19:1088–1094. [PubMed] [Google Scholar]
- 17.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–173. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 18.Garcia G, Keeffe EB. Liver biopsy in chronic hepatitis C: routine or selective. Am J Gastroenterol. 2001;96:3053–3055. doi: 10.1111/j.1572-0241.2001.05253.x. [DOI] [PubMed] [Google Scholar]
- 19.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 20.Siddique I, El-Naga HA, Madda JP, Memon A, Hasan F. Sampling variability on percutaneous liver biopsy in patients with chronic hepatitis C virus infection. Scand J Gastroenterol. 2003;38:427–432. doi: 10.1080/00365520310000825. [DOI] [PubMed] [Google Scholar]
- 21.Soloway RD, Baggenstoss AH, Schoenfield LJ, Summerskill WH. Observer error and sampling variability tested in evaluation of hepatitis and cirrhosis by liver biopsy. Am J Dig Dis. 1971;16:1082–1086. doi: 10.1007/BF02235164. [DOI] [PubMed] [Google Scholar]
- 22.Abdi W, Millan JC, Mezey E. Sampling variability on percutaneous liver biopsy. Arch Intern Med. 1979;139:667–669. [PubMed] [Google Scholar]
- 23.Gressner AM, Schäfer S. Comparison of sulphated glycosaminoglycan and hyaluronate synthesis and secretion in cultured hepatocytes, fat storing cells, and Kupffer cells. J Clin Chem Clin Biochem. 1989;27:141–149. doi: 10.1515/cclm.1989.27.3.141. [DOI] [PubMed] [Google Scholar]
- 24.Murawaki Y, Ikuta Y, Koda M, Kawasaki H. Serum type III procollagen peptide, type IV collagen 7S domain, central triple-helix of type IV collagen and tissue inhibitor of metalloproteinases in patients with chronic viral liver disease: relationship to liver histology. Hepatology. 1994;20:780–787. doi: 10.1002/hep.1840200403. [DOI] [PubMed] [Google Scholar]
- 25.Frei A, Zimmermann A, Weigand K. The N-terminal propeptide of collagen type III in serum reflects activity and degree of fibrosis in patients with chronic liver disease. Hepatology. 1984;4:830–834. doi: 10.1002/hep.1840040505. [DOI] [PubMed] [Google Scholar]
- 26.Trinchet JC, Hartmann DJ, Pateron D, Laarif M, Callard P, Ville G, Beaugrand M. Serum type I collagen and N-terminal peptide of type III procollagen in chronic hepatitis. Relationship to liver histology and conventional liver tests. J Hepatol. 1991;12:139–144. doi: 10.1016/0168-8278(91)90929-6. [DOI] [PubMed] [Google Scholar]
- 27.Saito H, Tada S, Nakamoto N, Kitamura K, Horikawa H, Kurita S, Saito Y, Iwai H, Ishii H. Efficacy of non-invasive elastometry on staging of hepatic fibrosis. Hepatol Res. 2004;29:97–103. doi: 10.1016/j.hepres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 29.Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 31.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]


